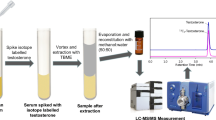
Abstract
Simultaneous measurements of the circulating testosterone (TS) and dehydroepiandrosterone sulfate (DHEAS) are deemed to be helpful for the assessment of men’s health. Liquid chromatography/electrospray ionization–tandem mass spectrometry (LC/ESI–MS/MS) is the most reliable methodology for this purpose; however, it has room for improvement in analysis throughput. In this study, a quadruplicate of the Girard reagents was used to develop an LC/ESI–MS/MS method capable of quantifying TS and DHEAS in four different serum samples in a single run. The four serum samples were separately pretreated, derivatized with one of four Girard reagents, and then combined. The LC/ESI–MS/MS analysis of the combined sample provided the androgen concentrations of four serum samples in parallel. The method had practical measuring ranges, in which good precision and accuracy, as well as negligible matrix effects were verified. The speed-up capability of the developed method was evaluated through the analysis of ten batches of serum samples (total 40 samples); the method saved a 60% post-pretreatment analysis time compared to the non-derivatization method for 40 samples.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testosterone (TS) is the primary androgen essential for the development and maintenance of male characteristics. It is well known that the abnormal production of TS is associated with hypogonadism (impaired production), as well as testis and prostate tumors (over-production). Recent studies have also demonstrated that the TS deficiency might cause many disorders including diabetes [1], cardiovascular diseases [2], osteoporosis [3], and depression [4]. The measurement of the circulating TS is indispensable for assessing the androgen status in men, and, consequently, in the diagnosis and treatment of these disorders.
Dehydroepiandrosterone (DHEA) is an adrenal androgen and most (over 99%) of DHEA circulates in the blood as the sulfated form, i.e., DHEA sulfate (DHEAS). Although it is still debated whether DHEAS, in itself, has specific biological functions, many studies have shown that the decline in the circulating DHEAS level is associated with diabetes [5], cardiovascular diseases [6], osteoporosis [7], and depression [8]. Thus, common disorders are found in patients with the TS deficiency and those with low circulating DHEAS concentrations. It is therefore expected that measuring the serum/plasma DHEAS together with TS proves to be more helpful for assessing men’s health.
In most clinical laboratories, commercially available immunoassay-based test kits are used for both the TS and DHEAS measurements. However, immunoassay occasionally has a problem of inaccuracy of the measured results due to interference from structurally similar steroids (cross-reactivity of the used antibodies) [9, 10]. Furthermore, simultaneous measurements of TS and DHEAS are impossible using the immunoassay kits. Liquid chromatography/electrospray ionization–tandem mass spectrometry (LC/ESI–MS/MS) can provide more reliable results due to its high specificity based on the LC separation and MS/MS discrimination. LC/ESI–MS/MS also has the capability of simultaneous quantification of TS and DHEAS in a single injection. However, LC/ESI–MS/MS has a challenge to overcome for routine clinical laboratory use; it is inferior to immunoassay regarding the analysis throughput, because LC/ESI–MS/MS generally analyzes one sample at a time. As one of the solutions for this challenge, Kishi et al. reported on a novel multiplex LC/MS system, in which multiple liquid chromatographs and one mass spectrometer were connected; in this system, an individual chromatogram for each sample was extracted from the mixed chromatograms of the multiple samples by the frequency division multiplexing technique [11]. Although this system will be put to practical use in the future, currently derivatization-based sample-multiplexing can be a more practical solution to enhance the analysis throughput of LC/ESI–MS/MS [12]. In the sample-multiplexing strategy, multiple samples are derivatized with multiple well-designed reagents, and then, the derivatized samples are combined and injected into the LC/ESI–MS/MS. Because the multiple sample quantification is achieved during a single LC/ESI–MS/MS run, the total LC/ESI–MS/MS run time can be considerably reduced. Several successful examples of the derivatization-based sample-multiplexing strategy have been reported for the quantification of various low-molecular-weight compounds including a vitamin D metabolite [13], fatty acids [14], sphingolipids [15], and chiral carboxylic acids [16] in biological samples. This strategy was also applied to steroid quantification [17,18,19,20]. A set of structurally analogous derivatization reagents with different masses is indispensable for the sample-multiplexing strategy; the resulting derivatives closely elute from the LC, show similar ESI–MS/MS behaviors, and are separately quantified by different selected reaction monitoring (SRM) transitions. Both TS and DHEAS have a carbonyl group; therefore, Girard reagents can be used to derivatize these androgens. We have reported on the sample-quadruplex LC/ESI–MS/MS method for the high-throughput quantification of serum DHEAS using the Girard reagents [19]. It is of significance to expand this strategy to the quantification of the most clinically important androgen, TS, for assessing the androgen status.
Based on this background information, the objective of this study was to develop and validate an LC/ESI–MS/MS method capable of quantifying TS and DHEAS in four different serum samples during a single run. This method employed a quadruplicate of the Girard reagents, i.e., Girard T (GT), Girard P (GP) and their isotopologues [2H3-GT (dGT) and 2H5-GP (dGP)] (Fig. 1). To demonstrate the advantage of the derivatization-based sample-multiplexing strategy, the analysis time was compared between the developed method and a non-derivatization LC/ESI–MS/MS method, which analyzed only one sample per injection.
Experimental
Materials and chemicals
TS and 2H3-TS [internal standard (IS) for TS] were obtained from Tokyo Chemical Industry (Tokyo, Japan) and Sigma-Aldrich Japan (Tokyo), respectively. DHEAS and 2H4-DHEAS (IS for DHEAS) were synthesized in our laboratories and the same as those used in a previous study [19]. Stock solutions were prepared by dissolving a precisely weighed quantity of the steroids with ethanol. Working solutions were prepared by serial dilutions of the stock solutions; 1.6, 3.2, 6.4, 16, 32, and 64 ng mL–1 for TS, 1.0, 2.0, 4.0, 10, 20, and 40 μg mL–1 for DHEAS, 32 ng mL–1 for 2H3-TS and 20 μg mL–1 for 2H4-DHEAS. GT and GP were the products of Tokyo Chemical Industry. dGT and dGP were synthesized in our laboratories [19, 21]. An Oasis® HLB solid-phase extraction (SPE) cartridge (30 mg adsorbent; Waters, Milford, MA) was used to purify the serum samples. All other reagents and solvents were of analytical grade or LC/MS grade.
Serum samples
The serum samples were collected from apparently healthy adult male volunteers (n = 40) at the Chiba University Hospital (Chiba, Japan) and stored at −30 °C until used. The experimental procedures were approved by the Ethics Committees of Tokyo University of Science and Chiba University. Written forms of informed consent were obtained from all the volunteers.
Pretreatment procedure
The serum sample (40 μL) was dropped into ethanol (60 μL) containing the ISs (2H3-TS; 320 pg and 2H4-DHEAS; 200 ng), vortex-mixed for 30 s, and then centrifuged at 2000 × g for 5 min. The supernatant was diluted with water (0.9 mL) and then loaded on an Oasis® HLB cartridge. After sequential washing the cartridge with water (1 mL) and 45% (v/v) methanol (1 mL), the steroids were eluted with methanol (1 mL). The solvent was evaporated off under a N2 gas stream, and then, the residue was dissolved in methanol-acetic acid (9:1, v/v, 50 μL) containing one of the reagents (GT, dGT, GP, or dGP, 20 μg). This reaction mixture was left in a dry bath incubator at 80 °C for 15 min. Four serum samples derivatized with different reagents were mixed and the solvent was evaporated. The residue was re-dissolved in the mobile phase (60 μL). The procedure to determine the recovery rates of TS, DHEAS and their ISs during the purification steps is described in Supporting Information.
LC/ESI–MS/MS
The LC/ESI–MS/MS quantification was performed by a Shimadzu LCMS-8030 triple quadrupole mass spectrometer with a Shimadzu LC-30AD chromatograph (Kyoto, Japan) and LabSolutions software (version 5.53 SP3, Shimadzu) for the system control and data processing. The derivatized androgens were separated on an ACQUITY UPLC BEH C18 column (1.7 μm, 100 × 2.1 mm i.d., Waters) with a mobile phase of methanol–10 mM ammonium formate–formic acid (45:55:0.1, v/v/v) at 40 °C. The flow rate was 0.3 mL min–1 and the injection volume was 15 μL. The ESI–MS/MS parameters are described in Supporting Information. The SRM transitions for the respective compounds are summarized in Table 1.
Calibration curves
The serum was treated with activated charcoal to remove the androgens [19] and then used as a surrogate matrix for the calibration curves. This surrogate matrix (40 μL) was dropped into ethanol containing TS (16, 32, 64, 160, 320, or 640 pg; corresponding to 0.40, 0.80, 1.6, 4.0, 8.0, or 16 ng mL–1, respectively, 6 points), DHEAS (10, 20, 40, 100, 200, or 400 ng; corresponding to 0.25, 0.50, 1.0, 2.5, 5.0, or 10 μg mL–1, respectively, 6 points), and ISs (2H3-TS; 320 pg and 2H4-DHEAS; 200 ng), and then treated in the same way as previously described. The four samples derivatized with the different reagents were mixed and injected into the LC/ESI–MS/MS. The peak area ratios (derivatized TS/2H3-TS or DHEAS/2H4-DHEAS, y) were plotted versus the concentrations [TS (ng mL–1) or DHEAS (μg mL–1), x] with a 1/x weighting to construct the calibration curves.
Matrix effects
The matrix effects were assessed by the post-extraction addition experiment [22]. The matrix effects were determined as the ratios of the peak areas in the serum samples to those in the standard samples. The detailed procedure is contained in Supporting Information.
Precision and accuracy
Five repetitions of the measurements were carried out for three pooled serum samples with different androgen concentrations at one day (intra-assay) and for five consecutive days (inter-assay) to test the assay precision. The assay accuracy was evaluated based on the analytical recoveries, which were the ratios (%) of the measured concentrations to the nominal concentrations. The detailed procedures are contained in Supporting Information.
Non-derivatization method for quantification of serum TS and DHEAS
The non-derivatization LC/ESI–MS/MS method for the determination of the serum TS and DHEAS was also developed and validated in our laboratories. This method analyzed one sample during a single LC/ESI–MS/MS run. The details including the validation data (Table S3) are described in the Supporting Information.
Results and discussion
LC/ESI–MS/MS behavior of derivatized androgens
The derivatization was a key component to achieve the quantification of TS and DHEAS in four different samples during a single LC/ESI–MS/MS run [12]. A quadruplicate of the Girard reagents, i.e., GT, dGT, GP, and dGP, was used for this purpose because of their structural similarity, high reactivity to ketosteroids, and ready availability. It is well known that the 3-oxo-4-ene steroids, such as TS, are readily derivatized with the Girard reagents [17, 23]. Although the keto group of DHEAS (at the 17-position) is less reactive than that of TS (at the 3-position), our previous study verified that the derivatization using any Girard reagents almost quantitatively proceeded to DHEAS under the stated conditions [19] (Fig. 1). The derivatization allowed not only TS but also DHEAS to be sensitively detected in the positive-ion mode and all the resulting derivatives gave molecular cations ([M]+) as the base peaks. The product ions giving the greatest signal-to-noise ratios (S/N) during the SRM were [M–X]+ and [M–X–H2SO4]+ for the derivatized TSs and DHEASs, respectively, where X was the terminal charged moiety (trimethylammonium or pyridinium). Elimination of the terminal charged moieties is the well-known fragmentation for the ketosteroids derivatized with the Girard reagents [17, 19, 23, 24]. Thus, the characteristic product ions containing the steroid skeletons (i.e., analyte-specific ions) were obtained from both androgens, which enhanced the assay specificity. The SRM transitions described in Table 1 were used to quantify TS and DHEAS in the serum, and showed no overlap between the respective derivatives, including the derivatized ISs.
The derivatization of ketosteroids with Girard reagents usually produces the E- and Z-isomeric mixtures [17, 21]. For each derivatized TS, two peaks corresponding to these isomers appeared on the chromatogram and the slower eluting peak, which was the major isomer, but not identified as the E- or Z-isomer, was used for the quantification. In contrast, all the derivatized DHEASs gave single peaks under the stated LC conditions. We found that an ACQUITY UPLC BEH C18 column (1.7 μm, 100 × 2.1 mm i.d.) combined with a mobile phase of methanol–10 mM ammonium formate–formic acid (45:55:0.1, v/v/v) gave good peak shapes and satisfactory separation from coexisting substances for the short retention times (tRs) (Table 1). All the derivatized TSs and DHEASs eluted within 5 min, and therefore, a short LC/ESI–MS/MS run was consequently achieved even for the simultaneous analysis of four serum samples.
Specificity
To examine the assay specificity, the major endogenous androgens were analyzed under the stated LC/ESI–MS/MS conditions. Among the tested steroids, the derivatized androstenedione, 5α-dihydrotestosterone, and epietiocholanolone closely eluted to the derivatized TSs, and the derivatized epiandrosterone sulfate closely eluted to the derivatized DHEASs (Table S1, Supporting Information). However, only negligibly small or no peaks were detected from these derivatized steroids when the SRM transitions set for the derivatized TSs, DHEASs, and their ISs were operated. For other steroids, including DHEA, androsterone, epiandrosterone, etiocholanolone, androsterone sulfate, etiocholanolone sulfate, and epietiocholanolone sulfate, their derivatives were completely separated by LC from the derivatized TSs or DHEASs. Considering these results, we concluded that endogenous androgens do not interfere with the TS and DHEAS quantification in our LC/ESI–MS/MS platform.
Pretreatment procedure and matrix effects
The serum samples were purified by deproteinization and SPE prior to the derivatization. Because DHEAS occurs in serum at a relatively high concentration (over several hundred nanograms mL–1 for healthy male adults), saturation of the mass analyzer occurred when the amount of DHEAS injected into LC/ESI–MS/MS was beyond its capacity; the saturation of the mass analyzer narrowed the linear dynamic range, leading to a poor accuracy. To avoid this occurrence, the recovery of DHEAS during the SPE was purposely lowered by washing the cartridge with 45% methanol. On the other hand, TS (several nanograms mL–1 in serum for healthy male adults) was mostly retained on the cartridge during this washing step. The remaining TS, DHEAS, and their ISs were then recovered with methanol. Eventually, the recovery rates during the entire purification steps were 74.3 ± 2.0, 77.5 ± 3.5, 8.9 ± 0.7, and 9.1 ± 0.4% (mean ± SD, n = 3) for TS, 2H3-TS, DHEAS, and 2H4-DHEAS, respectively. Thus, the desired results were produced, and there were no significant differences in the recovery rates between the analytes and their ISs. Chromatograms obtained from a batch are shown in Fig. 2. Sixteen peaks corresponding to the derivatized TSs, DHEASs, and ISs were observed without any interfering peaks.
Chromatograms of TS, DHEAS, and their ISs in four different serum samples after derivatization using four Girard reagents. A batch consisting of four serum samples was analyzed in a single run by the quadruplex method. For the derivatized TSs, the slower eluting isomers marked with asterisks were used for the quantification. The measured concentrations were 3.98 (sample #1), 3.14 (#2), 4.66 (#3), and 3.15 ng mL–1 (#4) for TS, and 1.25 (sample #1), 3.54 (#2), 2.91 (#3), and 1.73 μg mL–1 (#4) for DHEAS
Because the serum extract per analysis multiplied fourfold in our procedure to achieve simultaneous measurements of four samples, we were concerned about the increased matrix effects (ion suppression by the serum matrix). However, no significant matrix effects were found as shown in Table 2; only approximately 5% and 10% decreases in the peak areas for the derivatized TSs and DHEASs, respectively, were found in the serum samples compared to the standard samples.
Calibration curves and lower limits of quantification
A good linearity (r2 ≥ 0.995) and reproducibility [relative standard deviations (RSDs) in slopes, ≤ 2.3%] were found in all the calibration curves, which were independently constructed using five different surrogate matrices, within the ranges of 0.40–16 ng mL–1 for TS and 0.25–10 μg mL–1 for DHEAS (Table 3).
At the lowest points on the calibration curves, the RSDs of the measured concentrations in five replicates did not exceed 20% for both TS (≤ 12.1%) and DHEAS (≤ 11.8%) (Table 3). Furthermore, the relative errors defined by the equation of [(measured concentration – nominal concentration)/nominal concentration] × 100 (%) ranged from –2.5 to 1.0% for TS and from –4.8 to –2.0% for DHEAS. Based on these results, the lower limits of quantitation (LLOQs) were the same as the lowest calibration points (0.40 ng mL–1 for TS and 0.25 μg mL–1 for DHEAS).
Precision and accuracy
The acceptable intra-assay (n = 5) and inter-assay (n = 5) RSDs (≤ 8.8% for TS and ≤ 8.5% for DHEAS), and the analytical recovery rates (96.1–108.1% for TS and 93.2–106.9% for DHEAS) were verified for every derivative of both androgens (Table S2, Supporting Information). Therefore, the proposed method was judged to be precise and accurate. Furthermore, the method yielded almost identical measured values for the same serum samples whichever reagent was used; every reagent effectively and coequally served for quantification of the serum TS and DHEAS (Table S2).
Applicability and advantage of sample-quadruplex method
Forty serum samples from apparently healthy adult males were divided at random into ten batches and then analyzed by the developed method. The measured concentrations were 2.17–6.75 ng mL–1 (3.42 ± 0.95 ng mL–1, mean ± SD) and 1.04–3.75 μg mL–1 (1.99 ± 0.65 μg mL–1) for TS and DHEAS, respectively. These values were in good agreement with the concentrations in the literatures; 2.69–8.74 ng mL–1 and 1.99–7.84 ng mL–1 for TS [25, 26] and 0.34–3.70 μg mL–1 [27] for DHEAS. These results prove that the developed method works well for clinical sample analysis. However, no patients’ samples were unfortunately available in this study, therefore, some experiments may be needed to more clearly show the clinical applicability of the method.
To demonstrate the advantage of the sample-quadruplex method in the analysis throughput, it was compared with the non-derivatization LC/ESI–MS/MS method, which analyzed one sample during a single LC/ESI–MS/MS run. The concentrations determined by the non-derivatization method (x) and the quadruplex method (y) were almost identical for both TS and DHEAS (Wilcoxon signed-rank test, P < 0.01) and well correlated with each other (y = 0.990x + 0.038, r2 = 0.946 for TS and y = 0.987x + 0.031, r2 = 0.960 for DHEAS, Pearson’s correlation coefficient test, P < 0.01) (Fig. S1, Supporting Information). In the non-derivatization method, it took 7 min for one sample (one LC/ESI–MS/MS run); therefore, 280 min were needed after the sample purification (deproteinization and SPE) for 40 samples. On the other hand, because the sample-quadruplex method employed the derivatization, extra 45 min were required for this derivatization (operation and reaction time) prior to the LC/ESI–MS/MS analysis. However, the LC/ESI–MS/MS run time could be reduced to 60 min (6 min per run × 10 runs, 4 samples per run) by sample-multiplexing. Therefore, 105 min in total were required for the 40 samples after the SPE. This result means that the quadruplex method was able to save the post-pretreatment analysis time by more than 60% for 40 samples.
Conclusion
A derivatization-based LC/ESI–MS/MS method was developed to quantify TS and DHEAS in four different serum samples during a single run. The quadruplicate of Girard reagents was the important factor to analyze the four samples in parallel. The derivatized androgens eluted from LC with short tRs and provided intense and characteristic ions suitable for the SRM detection operating in the positive-ion mode. Control of the recovery rate of DHEAS (in μg mL–1 level) during the SPE prevented the mass analyzer from being saturated, and the quantification of DHEAS together with TS (in ng mL–1 level) was eventually achieved. The developed method significantly saved the analysis time (60% decrease compared to the non-derivatization method for 40 samples) without increased matrix effects, and decreased precision and accuracy. The method was almost unaffected by the endogenous androgens. This well-characterized method will prove to be helpful in assessing the androgen status in men, and, furthermore, in the diagnosis and treatment for androgen-related disorders.
References
M.W. O’Reilly, M. Glisic, B. Kumarendran, A. Subramanian, K.N. Manolopoulos, A.A. Tahrani, D. Keerthy, T. Muka, K.A. Toulis, W. Hanif, G.N. Thomas, O.H. Franco, W. Arlt, K. Nirantharakumar, Clin. Endocrinol. 90, 145 (2019)
C. Ohlsson, E. Barrett-Connor, S. Bhasin, E. Orwoll, F. Labrie, M.K. Karlsson, Ö. Ljunggren, L. Vandenput, D. Mellström, Å. Tivesten, J. Am. Coll. Cardiol. 58, 1674 (2011)
T.S. Tran, J.R. Center, M.J. Seibel, J.A. Eisman, M.M. Kushnir, A.L. Rockwood, T.V. Nguyen, Clin. Chem. 61, 1182 (2015)
Z. Chen, X. Shen, K. Tian, Y. Liu, S. Xiong, Q. Yu, L. Dai, Y. Shi, R. Zhang, R. Zeng, Q. Wan, C. Xiong, Y. Zhou, J. Int. Med. Res. 48, 300060520941715 (2020). https://doi.org/10.1177/0300060520941715
L. Liu, M. Wang, X. Yang, M. Bi, L. Na, Y. Niu, Y. Li, C. Sun, Clin. Chem. 59, 1338 (2013)
Y. Moriyama, H. Yasue, M. Yoshimura, Y. Mizuno, K. Nishiyama, R. Tsunoda, H. Kawano, K. Kugiyama, H. Ogawa, Y. Saito, K. Nakao, J. Clin. Endocrinol. Metab. 2000, 85 (1834)
D. Lee, H. Kim, S.H. Ahn, S.H. Lee, S.J. Bae, E.H. Kim, H.K. Kim, J.W. Choe, B.J. Kim, J.M. Koh, Clin. Endocrinol. 83, 173 (2015)
T. Michikawa, Y. Nishiwaki, M. Nakano, S. Iwasawa, M. Yamada, K. Asakura, N. Yoshioka, E. Kuwahara, T. Takebayashi, Am. J. Geriatr. Psychiatry. 21, 1154 (2013)
Ž Debeljak, I. Marković, J. Pavela, I. Lukić, D. Mandić, S. Mandić, V. Horvat, V. Šerić, Biochem. Med. 30, 030701 (2020). https://doi.org/10.11613/BM.2020.030701
K.-I. Ohno, T. Hasegawa, T. Tamura, H. Utsumi, K. Yamashita, Anal. Sci. 34, 1017 (2018)
H. Kishi, T. Kumazaki, S. Kitagawa, H. Ohtani, Analyst 144, 2922 (2019)
T. Higashi, S. Ogawa, J. Chromatogr. A 1634, 461679 (2020). https://doi.org/10.1016/j.chroma.2020.461679
S. Ogawa, H. Kittaka, A. Nakata, K. Komatsu, T. Sugiura, M. Satoh, F. Nomura, T. Higashi, J. Pharm. Biomed. Anal. 136, 126 (2017)
V.K. Narayana, V.M. Tomatis, T. Wang, D. Kvaskoff, F.A. Meunier, Chem. Biol. 22, 1552 (2015)
S.E. Chen, S. Zhu, J. Hu, J. Sun, Z. Zheng, X.E. Zhao, H. Liu, Anal. Chim. Acta 1124, 40 (2020)
J. Zheng, S.J. Zheng, C.B. Qi, D.M. Wu, Y.Q. Feng, Anal. Chem. 91, 11440 (2019)
M. Kamemura, M. Yokota, S. Ogawa, T. Sugiura, T. Higashi, J. Chromatogr. B 1146, 122117 (2020). https://doi.org/10.1016/j.jchromb.2020.122117
A. Jo, S. Koyagi, W. Hobo, S. Otani, S. Ogawa, T. Higashi, Anal. Sci. 36, 1099 (2020)
S. Aso, S. Ogawa, S. Nishimoto-Kusunose, M. Satoh, T. Ishige, F. Nomura, T. Higashi, Biomed. Chromatogr. 35, e5027 (2021). https://doi.org/10.1002/bmc.5027
J.D. Colletti, M.M. Redor-Goldman, A.E. Pomperada, A.K. Ghoshal, W.W. Wu, M.J. McPhaul, N.J. Clarke, Clin. Chem. 66, 1181 (2020)
T. Higashi, M. Akaishi, M. Yokota, T. Suzuki, S. Ogawa, Y. Sugiura, T. Nishikawa, K. Nishimoto, M. Suematsu, J. Chromatogr. B 1092, 106 (2018)
R. Thakare, Y.S. Chhonker, N. Gautam, J.A. Alamoudi, Y. Alnouti, J. Pharm. Biomed. Anal. 128, 426 (2016)
A.J. Frey, Q. Wang, C. Busch, D. Feldman, L. Bottalico, C.A. Mesaros, I.A. Blair, A. Vachani, N.W. Snyder, Steroids 116, 60 (2016)
P.J. Crick, J. Aponte, T.W. Bentley, I. Matthews, Y. Wang, W.J. Griffiths, Biochem. Biophys. Res. Commun. 446, 756 (2014)
G. Sun, J. Xue, L. Li, X. Li, Y. Cui, B. Qiao, D. Wei, H. Li, Mol. Med. Rep. 22, 1576 (2020)
M.M. Kushnir, A.L. Rockwood, W.L. Roberts, E.G. Pattison, A.M. Bunker, R.L. Fitzgerald, A.W. Meikle, Clin. Chem. 52, 120 (2006)
G. Eisenhofer, M. Peitzsch, D. Kaden, K. Langton, C. Pamporaki, J. Masjkur, G. Tsatsaronis, A. Mangelis, T.A. Williams, M. Reincke, J.W.M. Lenders, S.R. Bornstein, Clin. Chim. Acta 470, 115 (2017)
Acknowledgements
This study was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 20K06990.
Author information
Authors and Affiliations
Corresponding author
Additional information
Advanced Publication Released Online by J-STAGE October 1, 2021.
Supplementary Information
Below is the link to the electronic supplementary material.
44211_2022_37_MOESM1_ESM.docx
Supplementary file1 The ESI–MS/MS parameters, method validation procedures and data, and non-derivatization method are available free of charge on the Web at http://www.jasc.or.jp/analsci/. (DOCX 82 KB)
Rights and permissions
About this article
Cite this article
Fujimura, S., Ito, T., Ogawa, S. et al. An LC/MS/MS method for quantifying testosterone and dehydroepiandrosterone sulfate in four different serum samples during a single run. ANAL. SCI. 38, 167–173 (2022). https://doi.org/10.2116/analsci.21P268
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.21P268