Abstract
An automatic phenolic compounds analyzer is presented. The system performs online magnetic-stirring-assisted dispersive liquid–liquid microextraction before multisyringe chromatography (MSC) using a monolithic Chromolith Flash RP-18e column. The extraction behavior of the following phenolic pollutants: phenol, 2-nitrophenol, 4-nitrophenol, 2-chlorophenol, 2,4-diclorophenol, and 2,4,6-trichlorophenol, has been studied. A critical comparison of extractants (tributyl phosphate, acetonitrile, hexane, and 1-chlorobutane) and disperser solvents (acetone, acetonitrile, ethanol, methanol, 1-propanol, and 2-propanol) was made. Tributyl phosphate and acetonitrile were chosen as the extractant and the disperser solvent, respectively, since these showed the best performance. Phenols were online back-extracted into NaOH and neutralized before multi-isocratic chromatographic separation. The proposed analyzer can be applied for wide linear working ranges, i.e., between 40 and 20,000 μg L-1. The precision of the developed system has been proved, with maximum values for the intraday and interday precision of 4.4 % and 5.2 %, respectively, expressed as relative standard deviation, and high preconcentration factors (9.3–10.5) for most of the compounds studied. The method developed was successfully applied to natural water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds can be found in the environment directly from anthropogenic sources and from the transformation of synthetic or natural chemicals [1, 2]. Phenol is widely used as a disinfectant for medical and industrial applications [3]. Its principal sources are discharges from different industries. It is also used in the production of explosives, pesticides, and dyes [2]. The presence of chlorophenols in the environment is mainly related to the use of organic compounds such as pesticides and growth regulators, whose biodegradation leads to the formation of phenol, 2-chlorophenol (2-CP), and 2,4-dichlorophenol (2,4-DCP) [2]. 2-CP and 2,4-DCP are also used as herbicides [4]. Nitrophenols, particularly 2-nitrophenol (2-NP) and 4-nitrophenol (4-NP), can be formed in the reaction of phenol with nitrite ions in water under UV (sunlight) irradiation [5] and also during the production of pesticides, polymers, and drugs [6] in addition to other industrial activities. Given their toxicological [7–9] and organoleptic [10] effects, the United States Environmental Protection Agency has listed some phenolic compounds as priority pollutants [11]. Thus, the monitoring of phenolic pollutants is essential to notice and control illegal discharges from industry or contamination by pesticides.
Phenolic compounds have been determined using different strategies, mainly exploiting separation techniques, e.g., gas chromatography and liquid chromatography [12, 13]. Liquid chromatography is one of the most used techniques since derivatization of analytes is avoided [13, 14]. Moreover, the advent of monolithic columns made possible the development of low-pressure chromatographic techniques such as sequential injection chromatography [15] and multisyringe chromatography (MSC) [16] as a result of the implementation of monolithic columns in sequential injection analysis (SIA) and multisyringe flow injection analysis (MSFIA) [17] systems, respectively. These techniques are low-cost alternatives to high-performance liquid chromatography (HPLC) providing a reduction of sample and organic solvent consumption, which leads to reduction of waste production [18]. Moreover, multi-isocratic chromatography can be easily implemented using MSC. Furthermore, the use of a multisyringe burette allows precolumn and postcolumn operations permitting the performance of the whole analytical protocol in a fully automated way within a single instrumental assembly.
Usually sample pretreatment is required before chromatographic separation, especially when dealing with environmental or biological samples, in order to attain analyte enrichment and matrix removal. Solid-phase extraction is the technique most commonly used for this purpose [14, 19]. However, recently there have been interesting developments in liquid–liquid extraction (LLE), such as the advent of dispersive liquid–liquid microextraction (DLLME) [20, 21] and the development of lab-in-syringe systems [22], which allow automatic LLE inside the syringe, saving time, reagents, and solvents, and consequently reducing the generation of residues. DLLME is based on the use of a ternary component (disperser solvent) miscible in both water and the extractant. The disperser is mixed with the immiscible extraction solvent and is injected into the aqueous sample, producing a cloudy solution of small droplets of the extractant, enhancing the contact surface area between the phases. DLLME has been automated by exploiting flow techniques. For example, Anthemidis and Ioannou [23] developed an SIA–DLLME system for the determination of lead and copper by exploiting flame atomic absorption spectrometry. DLLME has also been automated using MSFIA, e.g., for the determination of benzo[a]pyrene [24], chromium [25], and total phenol index [26]. The automation of DLLME improves its reproducibility and accuracy, since flow techniques allow the precise handling of small volumes. Furthermore, both DLLME and flow techniques reduce sample and reagent consumption, attaining improved environmentally friendly systems.
In this work a DLLME–MSC system for determination of six phenolic pollutants is presented. Several extractants and disperser solvents are studied. Multi-isocratic chromatography was implemented, and the potential of the proposed system as a powerful tool for screening of phenolic pollutants in environmental samples is studied.
Materials and methods
Reagents, samples and solutions
All solutions were prepared with distilled water (resistivity greater than 1.8 × 105 Ω cm) from a Milli-Q system (Millipore, Bedford, MA, USA). HPLC-grade acetonitrile (ACN; Scharlau, Barcelona, Spain) was used to prepare the mobile phases. Acetic acid (Sigma-Aldrich, Madrid, Spain), sodium chloride, sodium dihydrogen phosphate, and trifluoroacetic acid (Scharlau) were of analytical reagent grade. The other reagents used were tributyl phosphate (TBP), hexane, acetone, ethanol, 1-propanol, and 2-propanol from Scharlau and 1-chlorobutane and ammonium sulfate from Sigma-Aldrich.
The phenolic compounds analyzed, i.e., phenol, 2-NP, 4-NP, 2-CP, 2,4-DCP, and 2,4,6-trichlorophenol (2,4,6-TCP), were purchased from Sigma-Aldrich. Stock solutions of each phenolic compound were prepared by accurately weighing the appropriate mass of the phenolic compound and by dissolving it in HPLC-grade methanol (Scharlau) in order to obtain a final concentration of 1,000 mg L-1. Working standard solutions containing either mixtures or individual compounds were prepared by dilution of the stock solutions in water.
The water samples analyzed were tap water from the laboratory, commercial mineral water, well water from a well in Son Reus, Palma (Spain, geographic coordinates, X 473247, Y 4388551), and a leachate sample from a demolition waste treatment plant in Santa Margalida (Spain, geographic coordinates, X 512401, Y 4398015). The pH was adjusted to 2 using 0.1 M HCl.
Magnetic-stirring-assisted DLLME–MSC system and software
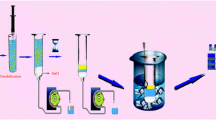
The basic element of an MSFIA system is a multisyringe burette allowing the simultaneous movement of four syringes. Each syringe has a three-way solenoid valve (N-Research, Caldwell, NJ, USA) placed at the head which allows the injection of the reagents into the system only when required and their return to their reservoirs when they are not required. The proposed magnetic-stirring-assisted (MSA) DLLME–MSC system (Fig. 1c) comprises a multisyringe burette module (CRISON, Alella, Spain) placed upside down and equipped with two 5-mL glass syringes (S1 and S4; Hamilton, Bonaduz, Switzerland). This way the organic droplet of the extractant, which is less dense than water, stays at the top and the aqueous phase (Fig. 1a) can be discarded before back-extraction with NaOH. To enhance the extraction efficiency, an MSA system (Sciware Systems, Bunyola, Spain) was mounted on S1 (Fig. 1b), where the extraction and back-extraction occur. The MSA system allows homogeneous and rapid mixing of phases. It consists of a small magnetic bar (10-mm length, 3-mm diameter) placed inside the syringe, an external stirring support placed around the syringe’s body, a motor connected to the external agitation support by a rubber band, which forces the rotation of the external agitation support, and a circuit that controls both the starting/stopping and the motor revolutions through one of the multisyringe outputs. The external stirring support is a ring (14-mm inner diameter, 30-mm outer diameter) with two small magnets facing each other creating a rotating magnetic field around the body of the syringe. When the motor is switched on, the ring starts to rotate and with it the magnetic bar inside the syringe, mixing the phases. A schematic depiction of the MSA system is shown in Fig. 1.
The system has two switching selection valves (SV1 and SV2), two external solenoid valves (V1 and V2), a monolithic column, a debubbler (Sciware Systems), and a spectrophotometric detector. S1 is connected to an external solenoid valve (V1; MTV-3-N 1/4 UKG; Takasago, Nagoya, Japan) that drives the flow to waste (“on” position) or to the eight-port selection valve (SV1; CRISON) (“off” position), which has the extraction and back-extraction reagents located on its peripheral ports, as follows: water (port 1), cleaning solution (10:90 v/v acetone:H2O; port 2), 0.1 M NaOH (port 3), autosampler (port 4), extractant–disperser solution (port 5), and waste (port 8). The second syringe (S4) is used to perform the multi-isocratic separation by dispensing two different mobile phases through the monolithic column. As the valves of the multisyringe module can handle up to 2 bar, the valve of S4 was replaced by a Kel-F one-way connector (Sciware Systems) and an external solenoid valve (V2) (Takasago) which can withstand up to 6 bar. The “on” position of V2 connects S4 to the mobile phase 1 reservoir, and the “off” position connects it to a holding coil connected to a second, six-port selection valve (SV2; VICI Valco, Schenkon, Switzerland) whose port configuration is as follows: a neutralization tank (port 5), a Chromolith Flash RP-18e monolithic column (25 mm × 4.6-mm inner diameter) protected with a Chromolith RP-18e guard column (5 mm × 4.6-mm inner diameter) (Merck, Darmstadt, Germany) (port 3), 0.5 M acetic acid (port 2), mobile phase 2 (port 4), and waste (port 6).
The detection system was a USB2000+ spectrophotometer equipped with a 1-cm flow cell (Ocean Optics, Dunedin, FL, USA). Measurements were recorded simultaneously at 270, 290, 300, and 315 nm. The manifold was constructed with polytetrafluoroethylene tubing (0.8-mm inner diameter).
Instrumental control, data acquisition, and data processing were done with AutoAnalysis 5.0 (Sciware Systems). The distinctive feature of this software based on dynamic link libraries at 32 bits is the possibility of using a single and versatile application without further modification for whatever instrumentation and detection system needed. It involves a basic protocol which allows the implementation of specific and individual dynamic link libraries, addressing the configuration of the assembled flow analyzer.
MSA-DLLME–MSC procedure and calculations
The analytical procedure is detailed in Table 1. DLLME is performed in S1 using a mixture of TBP and ACN (10:90 v/v) as the extractant and the disperser solvent, respectively. S1 is coupled to an external solenoid valve (V1). Its “off” position connects S1 to the central port of SV1. First, the sample is loaded into S1, followed by the mixture of TBP and ACN at a high flow rate (15 mL min-1) and with the MSA system activated. As a result, a cloudy solution is formed inside S1. Agitation is maintained for 20 s, and then it is stopped. After phase separation has been achieved, the aqueous phase is discarded to waste with S1 in the “off” position and V1 in the “on” position in order to avoid cross-contamination. To combine the DLLME with the chromatographic separation, a back-extraction with NaOH is performed. NaOH is aspirated from port 3 of SV1 with the MSA system activated. Agitation is maintained for 40 s, and then NaOH is dispensed via S1 in the “on” position to holding coil 2 and S4 pushes it to the neutralization tank in port 5 of SV2. There, NaOH is neutralized with acetic acid. After neutralization, the resulting solution is injected onto the monolithic column using S4. Thus, the sample is injected into the chromatographic column, followed by 6 mL of mobile phase 1. Then S4, holding coil 1, and holding coil 2 are filled with mobile phase 2 to dispense 6.2 mL of mobile phase 2 to elute the phenolic compounds still retained on the column. All solutions pass through the debubbler before reaching the detector. Finally, a cleaning procedure is performed. It consists in filling S1 with cleaning solution (10:90 v/v acetone:H2O) before rinsing it twice with Milli-Q water, while S4 is simultaneously rinsed with mobile phase 1.
The limit of detection (LOD) was calculated according to IUPAC [27, 28] as three times the standard deviation of ten blanks divided by the slope of each calibration curve, the limit of quantification was calculated as ten times the standard deviation of ten blanks divided by the slope of each calibration curve, and relative bias in percent were calculated by dividing the difference between the mean of the results and the reference value by the reference value [29].
Optimization of experimental conditions
To optimize the operational conditions of the proposed system, a series of experiments were conducted using a multivariate optimization technique. Multivariate designs provide relevant knowledge of the effect of variables within the entire experimental domain selected, and the variance of the estimate of the response at every point of the domain is better than that obtained by univariate methods [30]. First, a preliminary study with wide ranges was performed to find out which variables had a significant effect on the response and to select the appropriate ranges to study. Then, a screening of the independent variables and their possible interactions was performed by a two-level full factorial design (2k). The evaluation of the screening results allows the identification of the variables with a significant influence on the analytical response and allows to discard those with negligible effects [31]. Then, a face-centered central composite design was applied in order to find the critical values of the significant variables. Three center points were included to identify any irregularities, such as the loss of linearity in the center of the interval in both studies. The statistical software program Statistica 7.0 (StatSoft, Tulsa, OK, USA) was used for the entire multivariate analysis.
Results and discussion
Study of extractants and disperser solvents
The selection of the extractant and the disperser solvent was performed off-line with aqueous solutions containing each phenolic compound at 10 mg L-1 (pH 2–3). The extractants studied were: ACN adding 10 g of ammonium sulfate to 50 mL of sample [32], 1-chlorobutane [33, 34], hexane [35], and TBP [21]. For ACN with ammonium sulfate, the absorbance of the extraction blank was too high, so this option was discarded. The results obtained with the other extractants are shown in Fig. 2a. Results are expressed as relative intensity (%), with 100 % for the solvent providing the highest absorbance. TBP showed the best performance for most of the phenolic compounds studied but for 2-NP, for which hexane was slightly better. Taking into account these results, we selected TBP as the extractant.
Then, mixtures of TBP with different disperser solvents were tested, i.e., methanol [36], ethanol [36], ACN [26, 36], acetone [36, 37], 1-propanol [26], and 2-propanol. The TBP–acetone mixture was discarded because of the high absorbance of the extraction blank. As can be seen from Fig. 2b, TBP–ACN showed the best performance in general, and especially for those phenols giving a lower absorbance signal; therefore, it was selected for further assays.
Multivariate optimization of the DLLME and back-extraction
A multivariate optimization of the parameters affecting the DLLME and the back-extraction was performed. First, a preliminary study with wide ranges was conducted to find out which variables had a significant effect on the response and to select the appropriate ranges to study. The volume of NaOH had a significant positive effect. However, because of the limitations of the system (chromatographic separation) it was fixed at 0.3 mL. Thus, the percentage of TBP, the volume of the organic phase, and the concentration of NaOH were selected as variables for the multivariate study.
A full factorial screening (2k), including three center points, with adjusted ranges was performed. The curvature and the three variables were significant; therefore, according to the results obtained (ANOVA table, Pareto chart), the studied ranges were readjusted to the following to apply a response surface face-centered central composite design: TBP percentage, 10–40 %; volume of the organic phase, 0.5–1 mL; NaOH concentration, 0.1–1 mol L-1. The desirability graph was studied to obtain the optimum values. The critical values were set to the lowest percentage of TBP studied (10 %), 0.832 mL of the organic phase, and 0.1 M NaOH.
Effect of the ionic strength
The effect of the ionic strength was studied by adding different amounts of NaCl (from 0 to 1 mol L-1) to the aqueous samples. An increase of the extraction efficiency was obtained when the NaCl concentration increased from 0 to 0.5 mol L-1, but no significant differences were observed when concentrations up to 1 mol L-1 were added. Thus, 0.5 M NaCl was selected for further assays.
Effect of agitation time
The influence of agitation time was examined. No signal improvement was observed when the DLLME agitation time was increased from 20 to 40 s. Thus, the extraction agitation time was fixed at 20 s. For back-extraction, a higher response was obtained when the agitation time was increased from 20 to 40 s, but no signal improvement was observed when it was increased to 60 s. Therefore, the back-extraction time was set at 40 s.
Chromatographic separation
Multi-isocratic chromatographic separation was performed at a flow rate of 0.6 mL min-1, the maximum flow rate showing no back-pressure problems. Different proportions of ACN in water were tested. A 16:84 (v/v) ACN:H2O mixture was selected as mobile phase 1 to elute phenol, 2-NP, 2-CP, and 4-NP. Then, a second mobile phase, 35:65 (v/v) ACN:H2O, was used to elute 2,4-DCP and 2,4,6-TCP. Two mobile phases were required to perform the separation of the phenolic compounds studied in a reasonable time.
The effect of adding trifluoroacetic acid was studied, and with the addition of 0.1 % trifluoroacetic acid better resolution was achieved. A chromatogram obtained with the optimum conditions detailed above is shown in Fig. 3.
Chromatogram obtained for an aqueous sample with 0.5 M NaCl, pH 2, and spiked with the six phenolic compounds studied at 2.5 mg L-1. The chromatographic separation conditions were as follows: MP1, ACN:H2O (16:84 v/v) with 0.1 % trifluoroacetic acid (TFA); MP2, ACN:H2O (35:65 v/v) with 0.1 % TFA; flow rate, 0.6 mL min-1. 1) P, 2) 4-NP, 3) 2-NP plus 2-CP, 4) 2,4-DCP, 5) 2,4,6-TCP, AU absorbance units
Quantification of phenolic compounds
As can be seen in Fig. 3, 2-CP and 2-NP were coeluted. We did not succeed in separating these two phenols with the column that our system permitted given its short length. However, both could be quantified by using one of the AutoAnalysis tools which allows to simultaneously measure up to four different wavelengths. This also allowed us to measure each phenol at the wavelength where better sensitivity is obtained. The quantification wavelengths are summarized in Table 2.
Thus, to quantify 2-CP and 2-NP, we used two wavelengths, i.e., 270 and 300 nm. At 270 nm both phenolic compounds absorb, but at 300 nm only 2-NP absorbs. The mathematical procedure followed was as follows: On the one hand, if both phenolic compounds are present in the sample, 2-NP is quantified at 300 nm. Then its concentration is interpolated in the curve obtained for 2-NP at 270 nm to reveal the area corresponding to this compound. Finally, the difference between the total area and the area associated with 2-NP at 270 nm corresponds to 2-CP. This way, both compounds are quantified. On the other hand, if no peak is observed at 300 nm, all the peak area measured at 270 nm corresponds to 2-CP, and its concentration can be calculated directly.
Figures of merit of the proposed MSA-DLLME–MSC system
To evaluate the performance of the proposed MSA-DLLME–MSC method, its figures of merit were studied, and they are listed in Table 2.
Intraday precision is expressed as the relative standard deviation (RSD) obtained when analyzing a 5 mg L-1 standard containing the six phenolic compounds studied in seven consecutive determinations on the same day. As can be seen, RSDs of 4.4 % or less were obtained. Interday precision corresponds to the RSD of a 5 mg L-1 standard containing the six phenolic compounds studied analyzed on five different days. For all the analytes, an RSD of 5.2 % or less was obtained.
For the preconcentration factor, first the theoretical preconcentration factor was calculated, assuming 100 % extraction and back-extraction efficiency, by the relation of the initial sample volume and the final NaOH volume where all the phenolic compounds are back-extracted. Thus, the theoretical preconcentration factor was 11.43. Then, 1 mL of a solution of NaOH containing each phenol at 11.43 mg L-1 was neutralized and injected into the chromatographic part of the system. Its response was compared with the signal of a solution containing each phenol at 1 mg L-1 analyzed with the proposed system. The relation of these two absorbance values gives the efficiency of extraction and back-extraction of each phenolic compound shown in Table 2 as the extraction efficiency. Then, the experimental preconcentration factor was calculated by multiplying the extraction efficiency of each phenolic compound and the theoretical preconcentration factor. As can be seen from Table 2, high preconcentration factors between 9.3 and 10.5 were achieved for most of the phenolic compounds studied, except for 2,4,6-TCP (5.6). Compared with previously reported manual methods dealing with liquid–liquid microextraction of phenolic compounds, better extraction efficiencies are achieved for most of the common phenolic compounds studied, except for 2,4,6-TCP. Fattahi et al. [38] reported extraction efficiencies of 28.7, 62, and 75.15 % for 2-CP, 2,4-DCP, and 2,4,6-TCP, respectively, Faraji et al. [39] reported extraction efficiencies of 21, 34, 63, and 86 % for phenol, 2-CP, 2,4-DCP, and 2,4,6-TCP, respectively, and Vera-Ávila et al. [36] reported extraction efficiencies of 13.1, 38.2, 76.9, and 86.2 % for phenol, 2-CP, 2,4-DCP, and 2,4,6-TCP, respectively. Furthermore, higher precision is achieved with the present method, i.e., RSDs between 6.4 and 10.4 % in comparison with RSDs of 5.2 % or less obtained with the proposed system. These methods report better LODs because of greater sample consumption and the use of more sensitive but more expensive detectors. In addition, with the proposed method, the consumption of organic solvents is reduced between three and ten times in comparison with manual methods [36, 37].
Regarding other automatic methods determining 2-NP and 4-NP [33, 34], better extraction efficiencies are achieved with the present method with a similar consumption of organic solvents: i.e., approximately 50 % in comparison with approximately 80 % with the present method.
The linear working range has been established from the limit of quantification to the highest concentration of the standard analyzed, being possible to quantify simultaneously concentrations within the range 0.33–20 mg L-1 for phenol, 0.12–20 mg L-1 for 2-NP, 0.04–20 mg L-1 for 4-NP, 0.22–20 mg L-1 for 2-CP, 0.30–20 mg L-1 for 2,4-DCP, and 0.46–20 mg L-1 for 2,4,6-TCP. The LODs achieved are similar to those obtained with previously reported automated methods. For example, Cladera et al. developed an SIA system [33] and an MSFIA system [34] for determination of nitrophenols based on LLE and spectrophotometric detection with similar LODs for 2-NP and 4-NP but lower extraction efficiencies as detailed above. Another MSFIA system exploiting nitrophenols solid-phase extraction and a detector similar to the one used in this work (USB2000, Ocean Optics) [19] obtained LODs four times higher, i.e., 0.17 and 0.04 mg L-1 for 2-NP and 4-NP, respectively. Moreover, higher accuracy is accomplished with the present method.
The injection frequency is 1.8 samples per hour. This frequency is high since it has to be taken into account that it includes the time necessary for performing the extraction and back-extraction, the neutralization, and the chromatographic separation, in addition to system cleaning and reconditioning of the monolithic column. All these steps will take several working days if the analysis is performed in a classic manual approach.
Application to water samples
To investigate the applicability of the proposed method to environmental water samples, it was applied to water samples from different sources. Addition of NaCl to adjust the ionic strength was not necessary since the salinity of the samples was sufficient. Thus, samples were acidified to pH 2 with 0.1 M HCl. The samples analyzed did not contain the phenolic compounds studied, i.e., no signal was observed when analyzing them either with the proposed system or with a commercial HPLC system (PerkinElmer) equipped with an XTerra RP18 column (150 mm × 3.9-mm inner diameter and 5-μm particle size). Samples were spiked with different amounts of phenolic compounds. The results are shown in Table 3 together with the recoveries obtained. The range of mean recoveries was 83-110 % for tap water, 89-108 % for mineral water, 89-108 % for well water, and 92-107 % for the analyzed leachate. Accuracy is normally studied as two components: trueness and precision [29]. Thus, the accuracy of the proposed method is proved given the good intraday and interday precision obtained and the low relative bias obtained (11 % or less in most samples) in the spiking-recovery test.
Moreover, to evaluate the possible matrix effect, the slopes of the calibration curves obtained with the standards and the natural water samples were statistically compared with a t test. The results showed that there were no significant differences between the slopes since all experimental t values obtained were lower than the critical t value at the 95 % confidence level. Thus, the applicability of the method to these kinds of matrices is validated.
Conclusions
In conclusion, a fast and simple method to determine six phenolic pollutants in environmental samples has been described. The low cost of the MSC system compared with an HPLC system makes this system a useful screening tool to determine phenolic compounds. In addition, the coupling of DLLME and MSC helped reduce reagent consumption and thus waste generation and the environmental impact per analysis. The use of MSC allowed the integration of the whole protocol in a single analyzer, permitting background operations and the easy implementation of multi-isocratic chromatographic separation. After a careful study of extractants and disperser solvents, TBP and ACN were selected, and we obtained high preconcentration factors and extraction efficiencies for most of the phenolic compounds studied. The coupling of DLLME and the chromatographic separation was achieved by stripping the phenolic compounds with NaOH and its neutralization. The method developed has proved to be accurate and reproducible, given the low RSDs obtained and the satisfactory recoveries achieved when applying it to environmental water samples and a leachate sample.
References
Barrico ML, Nabais C, Martins MJ, Freitas H (2006) Sources of phenolic compounds in two catchments of southern Portugal – effect of season, land use and soil type. Chemosphere 65:482–488
Michalowicz J, Duda W (2007) Phenols – sources and toxicity. Pol J Environ Stud 16:347–362
Office of Environmental Health Hazard Assessment (2000) Chronic toxicity summary. Phenol. CAS Registry Number: 108-95-2. http://oehha.ca.gov/air/chronic_rels/pdf/108952.pdf. Accessed 4 Nov 2014
Cernakowa M, Zemanovicowa A (1998) Microbial activity of soil contaminated with chlorinated phenol derivatives. Folia Microbiol 43:411–416
Patnaik P, Khoury J (2004) Reaction of phenol with nitrite ion: pathways of formation of nitrophenols in environmental waters. Water Res 38:206–210
Pocurull E, Marce R, Borrull B (1996) Determination of phenolic compounds in natural waters by liquid chromatography with ultraviolet and electrochemical detection after on-line trace enrichment. J Chromatogr A 738:1–9
Agency for Toxic Substances and Disease Registry (2008) Toxicological profile for phenol. http://www.atsdr.cdc.gov/toxprofiles/tp115.pdf. Accessed 4 Nov 2014
Agency for Toxic Substances and Disease Registry (1999) Toxicological profile for chlorophenols. http://www.atsdr.cdc.gov/toxprofiles/tp107.pdf. Accessed 4 Nov 2014
Agency for Toxic Substances and Disease Registry (1992) Toxicological profile for nitrophenols. http://www.atsdr.cdc.gov/ToxProfiles/tp50.pdf. Accessed 4 Nov 2014
Renberg L (1982) In: Bjørseth A, Angeletti G (eds) Analysis of organic micropollutants in water, 1st edn. Springer, Dordrecht
US Environmental Protection Agency (1984) EPA method 604, phenols, part VIII, 40 CFR part 136. http://www.epa.gov/region9/qa/pdfs/40cfr136_03.pdf. Accessed 4 Nov 2014
Simoes NG, Cardoso VV, Ferreira E, Benoliel MJ, Almeida CMM (2007) Experimental and statistical validation of SPME-GC–MS analysis of phenol and chlorophenols in raw and treated water. Chemosphere 68:501–510
Cledera-Castro M, Santos-Montes A, Izquierdo-Hornillos R, Gonzalo-Lumbreras R (2007) Comparison of the performance of different reversed-phase columns for liquid chromatography separation of 11 pollutant phenols. J Sep Sci 30:699–707
Oliveira HM, Segundo MA, Lima JLFC, Cerdà V (2009) Multisyringe flow injection system for solid-phase extraction coupled to liquid chromatography using monolithic column for screening of phenolic pollutants. Talanta 77:1466–1472
Šatínsky D, Solich P, Chocholous P, Karlicek R (2003) Monolithic columns—a new concept of separation in the sequential injection technique. Anal Chim Acta 499:205–214
Gonzalez HM, Alpizar JM, Cerdà V (2007) Development of a new high performance low pressure chromatographic system using a multisyringe burette coupled to a chromatographic monolithic column. Talanta 72:296–300
Cerdà V, Estela JM, Forteza R, Cladera A, Becerra E, Altimira P, Sitjar P (1999) Flow techniques in water analysis. Talanta 50:695–705
Cerdà V, Ferrer L, Avivar J, Cerdà A (2014) Flow analysis: a practical guide. Elsevier, Amsterdam
Manera M, Miró M, Estela JM, Cerdà V (2007) Multi-syringe flow injection solid-phase extraction system for on-line simultaneous spectrophotometric determination of nitro-substituted phenol isomers. Anal Chim Acta 582:41–49
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Hu X, Wu J, Feng Y (2010) Molecular complex-based dispersive liquid–liquid microextraction: Analysis of polar compounds in aqueous solution. J Chromatogr A 1217:7010–7016
Maya F, Horstkotte B, Estela JM, Cerdà V (2014) Automated in-syringe dispersive liquid-liquid microextraction. Trends Anal Chem 59:1–8
Anthemidis AN, Ioannou KIG (2009) On-line sequential injection dispersive liquid–liquid microextraction system for flame atomic absorption spectrometric determination of copper and lead in water samples. Talanta 79:86–91
Maya F, Estela JM, Cerdà V (2012) Completely automated in-syringe dispersive liquid-liquid microextraction using solvents lighter than water. Anal Bioanal Chem 402:1383–1388
Henriquez C, Horstkotte B, Solich P, Cerdà V (2013) In-syringe magnetic-stirring-assisted liquid–liquid microextraction for the spectrophotometric determination of Cr(VI) in waters. Anal Bioanal Chem 405:6761–6769
Horstkotte B, Maya F, Duarte CM, Cerdà V (2012) Determination of ppb-level phenol index using in-syringe dispersive liquid-liquid microextraction and liquid waveguide capillary cell spectrophotometry. Microchim Acta 179:91–98
Long GL, Winefordner JD (1983) Limit of detection. A closer look at the IUPAC definition. Anal Chem 55:712–724
Miller JC, Miller JN (1993) Statistics for analytical chemistry. Horwood, Chichester
Magnusson B, Örnemark U (eds) (2014) Eurachem guide: the fitness for purpose of analytical methods—a laboratory guide to method validation and related topics, 2nd edn. https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf
Leardi R (2009) Experimental design in chemistry: a tutorial. Anal Chim Acta 652:161–172
Lundstedt T, Seifert E, Abramo L, Thelin B, Nyström A, Pettersen J, Bermang R (1998) Experimental design and optimization. Chemom Intell Lab Syst 42:3–40
Podolina EA, Rudakov OB, Khorokhordina EA, Kharitonova LA (2008) Use of acetonitrile for the extraction of dihydric phenols from salt aqueous solutions followed by HPLC determination. J Anal Chem 63:468–471
Cladera A, Miró M, Estela JM, Cerdà V (2000) Multicomponent sequential injection analysis determination of nitro-phenols in water by on-line liquid-liquid extraction and preconcentration. Anal Chim Acta 421:155–166
Miró M, Cladera A, Estela JM, Cerdà V (2001) Dual wetting-film multi-syringe flow injection analysis extraction. Application to the simultaneous determination of nitrophenols. Anal Chim Acta 438:103–116
González-Barreiro C, Cancho-Grande B, Araujo-Nespereira P, Cid-Fernández JA, Simal-Gándara J (2009) Occurrence of soluble organic compounds in thermal waters by ion trap mass detection. Chemosphere 75:34–47
Vera-Avila LE, Rojo-Portillo T, Covarrubias-Herrera R, Peña-Alvarez A (2013) Capabilities and limitations of dispersive liquid-liquid microextraction with solidification of floating organic drop for the extraction of organic pollutants from water samples. Anal Chim Acta 805:60–69
Zhou C, Tong S, Chang Y, Jia Q, Zhou W (2012) Ionic liquid-based dispersive liquid-liquid microextraction with back-extraction coupled with capillary electrophoresis to determine phenolic compounds. Electrophoresis 33:1331–1338
Fattahi N, Assadi Y, Hosseini MRM, Jahromi EZ (2007) Determination of chlorophenols in water samples using simultaneous dispersive liquid-liquid microextraction and derivatization followed by gas chromatography-electron-capture detection. J Chromatogr A 1157:23–29
Faraji H, Tehrani MS, Husain SW (2009) Pre-concentration of phenolic compounds in water samples by novel liquid-liquid microextraction and determination by gas chromatography-mass spectrometry. J Chromatogr A 1216:8569–8574
Acknowledgments
The authors acknowledge financial support from the Spanish Ministry of Economy and Competitiveness (MINECO) through project CTQ2013-47461-R cofinanced by FEDER funds. The Conselleria d’Economia, Hisenda, I Innovació of the Government of the Balearic Islands is acknowledged for a grant to competitive groups (43/2011), also cofinanced by FEDER funds. J.A. acknowledges the Torres Quevedo Program of MINECO cofinanced by European Funds for financial support through PTQ-2012-05755.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González, A., Avivar, J. & Cerdà, V. Determination of priority phenolic pollutants exploiting an in-syringe dispersive liquid–liquid microextraction–multisyringe chromatography system. Anal Bioanal Chem 407, 2013–2022 (2015). https://doi.org/10.1007/s00216-015-8464-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8464-1