Abstract
A fully automated method for the determination of chromate is described. It is based on the selective reaction of Cr(VI) with diphenylcarbazide in acidic media to form a colored complex of Cr(III) with the oxidation product diphenylcarbazone. The reaction was performed within the syringe of an automatic burette containing a magnetic stirrer for homogenization of the sample and the required reagents. In-syringe stirring was made possible using a specially designed driving device placed around the syringe barrel to achieve a rotating magnetic field in the syringe, forcing the stirrer to spin. In a second step, the reaction mixture in the syringe was neutralized to allow in-syringe magnetic-stirring-assisted dispersive liquid–liquid microextraction of the complex into 125 μL of n-hexanol. After phase separation by droplet flotation over 30 s, the organic phase was propelled into a coupled spectrophotometric detection cell. The entire multistep procedure including in-system standard preparation was done within 270 s. The method was used for the analysis of natural waters, achieving average analyte recovery of 103 %, a limit of detection of 0.26 μg L-1, and a repeatability of less than 4 % relative standard deviation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liquid–liquid extraction (LLE) was one of the earliest and is one of most used sample pretreatment techniques for analyte preconcentration and sample matrix elimination. However, conventional LLE generally involves a tedious procedure with a multitude of sample handling steps, increasing the risk of contamination or loss of analyte. Beside, LLE also requires large amounts of sample and generally hazardous organic solvents [1–3]. Therefore, many efforts have been focused on the automation of LLE processes as well as on the development of liquid–liquid microextraction techniques [1, 2].
The recently introduced dispersive liquid–liquid microextraction (DLLME) technique [4] has attracted much attention and has become very popular [5–8]. Further developments of DLLME proposed the use of short-chain lipophilic alcohols as extraction solvents [7, 9–11] as a more environmentally friendly alternative to typically used halogenated solvents.
Most DLLME methods are based on the use of a dispersion solvent such as a hydrophilic alcohol, which is miscible in both phases, i.e., the aqueous phase and the organic phase. The dispersion solvent has the function to disperse the organic phase into very fine droplets in the aqueous phase. This increases the contact surface between the two phases greatly and hence improves the extraction efficiency [1, 5]. However, the use of the additional solvent has some drawbacks, such as the increase of the analyte solubility in the aqueous phase with the consequent change of the partition coefficient and the decrease of the extraction efficiency [12]. Moreover, the use of a dispersion solvent reduces the available volume for the sample and then the preconcentration factor. Also, the method requires additional optimization involving finding the appropriate dispersion solvent and mixing ratio with the extraction solvent.
Alternative methods have been developed where the dispersion of the extraction solvent is achieved by kinetic energy instead of a dispersion solvent, leading among other methods to ultrasonic-assisted [13, 14], air-assisted [12], vortex-assisted [15], and magnetic-stirring-assisted (MSA) DLLME) [16].
Recently, there has been great interest in the development of DLLME automation [8]. In this work, we report a new and automated application of MSA-DLLME using a sequential injection analysis (SIA) system.
SIA, or more generally, flow techniques (FT), are very useful tools for DLLME automation [2, 8]. Some of the demonstrated advantages of FT-based automation are the possibility to use manifold detection techniques and reactor devices in the analyzer systems, high signal reproducibility and sample throughput, and self-cleaning of the tubing system used for solution handling [2, 9].
Hardly any methods based on FT automation have been described for DLLME assisted by mechanical dispersion of the extraction solvent rather than by the use of a dispersion solvent. Just two recent works can be found: the first based on air-assisted DLLME [17]; the second based on MSA-DLLME [18]. This second approach is the basis and the inspiration for the present work.
In this work, a simple analytical system based on the typical SIA configuration was used for fully batchwise automation of MSA-DLLME. The novelty of this configuration is based on the placement of a magnetic stirring bar inside the syringe pump, giving rise to “in-syringe” MSA-DLLME. In this way, a sealed but adaptable reaction vessel is obtained. The computer-controlled rotation of the stirring allows the homogeneous mixture of the sample and reagents. It also disperses the extraction solvent inside the aqueous phase. After extraction, the organic phase, less dense than water, floats, coalesces, and is then propelled to the detection flow cell.
This new system was successfully used for the determination of hexavalent chromium, Cr(VI), in water samples. Although the high toxicity and carcinogenic properties of Cr(VI) [19, 20] are well known, recent studies by the Environmental Protection Agency have indicated greater human health risks from Cr(VI) than previously thought [21]. Consequently, further efforts have been focused on enhanced monitoring of Cr(VI) in drinking water [22].
The method presented automated the well-known reaction of diphenylcarbazide (DPC) oxidation and complex formation with Cr(III) and diphenylcarbazone (DPCO) as resulting products. This reaction, occurring in acidic medium, was firstly described by Cazeneuve [23] in 1900. Since then, many authors have tried to explain the mechanism being the formation of the complex and its stoichiometry [24, 25]. The complex is cationic but it can be extracted for sensitivity enhancement as an ion pair into an organic solvent [26, 27]. Doing so, we propose a sensitive, accurate, simple, and automated method for Cr(VI) quantification in natural waters. The technique in-syringe MSA-DLLME used is a valid alternative for the automation of LLE. Its potentials and limitations and its application to real samples are critically discussed in this work.
Materials and methods
Reagents and solutions
All solutions were of analytical grade and were provided by Scharlab (Barcelona, Spain). Doubly distilled water provided by a Milli-Q Direct-8 purification system (Millipore Iberica, Spain) was used throughout.
The chromium stock solution of 51.99 mg L-1 was prepared by diluting a commercial 1,000 mg L-1 Cr(VI) atomic absorption standard in Milli-Q water. Cr(VI) standard working solutions were then prepared by appropriate dilution. A DPC solution of 1 mmol L-1 was prepared weekly by dissolving 0.0121 g of DPC in 50 mL of acetone and was stored in the dark at 4 °C [28]. For acidification, 5 mol L-1 HNO3 was used. Further solutions of H2SO4, HClO4, and HCl of the same concentration were studied. n-Hexanol was used as the extraction solvent. Other extraction solvents tested were n-octanol, isoamyl alcohol, methyl isobutyl ketone, n-butanol, and ethyl acetate. Ammonium acetate (NH4Ac) buffer of 10 mol L-1 (pH 7) was prepared by dissolving 192 g of NH4Ac in 250 mL of Milli-Q water.
Standard solutions of Cd(II), Cu(II), Fe(III), Hg(II), Mo(IV), Pb(II), V(V), and Zn(II) at 1,000 mg L-1 for atomic absorption spectroscopy were used for the interference studies. Further, a stock solution of 4 mmol L-1 Cr(III) was prepared in Milli-Q water. For masking of interferences of metal cations, an EDTA solution of 5.0 g L-1 was used.
To study the influence of water hardness in the extraction process, synthetic freshwater with different hardness grades was prepared according to standard recipes [29]. “Very hard water” was prepared by dissolving 384 mg L-1 NaHCO3, 240 mg L-1 CaSO4 · 2H2O, 240 mg L-1 MgSO4, and 16 mg L-1 KCl. “Hard water,” “moderately hard water,” and “soft water” were prepared by dilution of “very hard water” in proportions of 1:2, 1:4, and 1:8, respectively.
Sample collection
Three-well water samples from different places on Mallorca and one demolition leachate sample were collected in polyethylene flasks, refrigerated at 4 °C, and immediately transported to the laboratory for analysis. A tap water sample was collected and measured immediately. The samples were measured in the proposed analyzer system and with the reference method [29] without any other previous treatment but sedimentation of coarse particles.
Detection cell and equipment
A USB2000 miniature CCD spectrophotometer from Ocean Optics (Dunedin, FL, USA) was used as a detector. A bright-white LED was used as a highly stable light source. It was directly mounted on the optical path of a 10 mm path length flow cell from Starna (Hainault, UK; type 75.1 SOG). The measurement parameters were an integration time of 5 ms and a measuring frequency of 8 Hz. Wavelength smoothing was done over nine array pixels. The difference between the values at the absorbance maximum for the reaction product (540 nm) and a reference wavelength (640 nm) was used as the analytical signal, allowing the correction of analyte-unspecific intensity variations.
Analyzer system
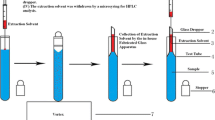
The manifold configuration of the analyzer system is given in Fig. 1. For liquid handling, a 5,000-step syringe pump (type Bu 4S) and a rotary eight-port selection valve (SV) from Crison (Alella, Barcelona, Spain) were used. PTFE tubing of 0.8-mm inner diameter was used to connect the different components of the flow system. The syringe pump was equipped with a single 5-mL glass syringe (S1) from Hamilton (Bonaduz, Switzerland, model 1005 TLL-SAL SYR). A three-way solenoid head valve (V1) allowed the connection of S1 to either the central port of the SV (on position) or a detection flow cell and further to waste (off position). Lateral ports on the SV were connected to reservoirs of water (position 1), chromate standard (2), sample (3), acid (4), DPC solution (5), extraction solvent (6), buffer (7), and air (8). A holding coil, consisting of a PTFE tube of 26-cm length and 0.8-mm inner diameter (inner volume approximately 130 μL), was used to connect the central port of the SV to S1 in the on position.
Analyzer manifold with a selection valve (SV), syringe pump with a magnetic stirring bar inside (S1), solenoid three-way head valve (V1), detection flow cell (D), and holding coil (HC). PTFE tubing (0.8-mm inner diameter) were used to connect SV with V1 (10 cm), and D with V (3 cm). The magnetic stirring bar driver is shown in deatrils at the bottom. A motor (M) was used to drive the device via a rubber band. DPC diphenylcarbazide
Stirring system
The magnetic stirring system has been presented by Horstkotte et al. [18]. It allows homogeneous and rapid mixing of the sample and reagents without the requirement of additional mixing chambers.
A diagram of the magnetic stirring system is depicted in Fig. 1. It consists of four principal parts: (1) a small magnetic stirring bar placed inside the syringe, (2) a magnetic stirring bar driver placed onto the glass barrel of the syringe, (3) a motor that forces the magnetic stirring bar driver to rotate, and (4) a homemade regulation circuit board connected to the syringe pump for revolution control. The magnetic stirring bar (10-mm length, 3-mm diameter) was provided by LINEALAB (Badalona, Spain). The top position of the piston was adjusted to leave a gap of about 0.5 mm in order to avoid any damage when emptying the syringe and to allow free rotation of the stirring bar even when the piston was in the top position. The rotation of the magnetic stirring bar driver was achieved by use of a DC motor. A rubber band was used to transmit the motor rotation to the bottom ring of the magnetic stirring bar driver.
Software
The entire instrumentation used to perform the in-syringe MSA-DLLME procedure was controlled by AutoAnalysis 5.0 (Sciware Systems SL, Bunyola, Spain). AutoAnalysis 5.0 is a versatile program that allows the control of and communication with the individual instrumental assembly components, including detectors connected to the PC, using dynamic link libraries. Detailed descriptions of this software can be found elsewhere [30, 31].
Analytical protocol
The operation protocol for MSA-DLLME is given in Table S1. It consisted of four stages, which are consecutively executed for each measurement.
In the first stage, the cleaning of the sample tube, the holding coil, the syringe, the magnetic stirring bar, and the detection flow cell is done with the solution to be measured. For this, 700 μL of sample were aspirated with activated stirring (SV in position 3 and V1 in the on position) and discharged to waste (V1 in the off position). This procedure was repeated three times.
The reaction between Cr(VI) and DPC in acidic media was done in the second stage. Reagent volumes aspirated in the given order were 160 μL of acid, 175 μL of DPC, 3.395 ml of sample, and 150 μL of air. The final aspiration of 150 μL of air was done to carry the remaining volume of the sample from the holding coil into the syringe. Then, stirring was activated for 10 s and then deactivated for the reaction time (t R) for a further 45 s. During this time, Cr(IV) is reduced to Cr(III), oxidizing at the same time a proportional quantity of DCP to DPCO with subsequent formation of the purple Cr(III)–DPCO complex.
In the third stage, MSA-DLLME was done. Here, 120 μL of n-hexanol followed by 800 μL of buffer and 150 μL of air were aspirated into the syringe. The buffer was used to adjust the pH and provided at the same time acetate as the counter ion to form a neutral extractable ion pair with the positively charged Cr(III)–DCPO complex.
Afterward, the stirring was activated for 55 s. The aspirated air inside the syringe formed a vortex, which allowed the contact of the n-hexanol with the stirring bar, leading to its disruption into fine droplets and, by this, MSA-DLLME.
Afterward, the stirring was stopped, allowing the n-hexanol droplets to float and coalescence at the brim of the concave liquid meniscus formed by the aqueous phase in the syringe during 30 s.
In the forth and final stage, the measurement of the extracted complex in the organic phase was conducted. First, the air in the head of the syringe was rapidly discharged through the detection flow cell to waste. Then, the n-hexanol was slowly propelled through the detection flow cell under continuous data evaluation, followed by the fast discharge of the remaining liquid in the syringe.
For automated standard addition, variable volumes of standard (SV position 2) and Milli-Q water (SV position 1) were aspirated with the sum of both volumes, being 300 μL. Then, 3.095 mL of the sample (SV position 3) was aspirated, keeping constant the sum of the volumes of the sample, standard, and water (3.395 mL).
For the reference method [29], 90 mL of sample were acidified to pH 1 with 1 mol L-1 H2SO4. Then, 2 mL of 20.1 mmol L-1 DPC (dissolved in acetone) was added and the volume was made up to 100 mL. After color development (10 min), the absorbance was measured at 540 nm against a blank standard using a 1 cm optical path length cell.
Results and discussion
System design
The aim of this work was the development of a simple and fully automated analytical system for the determination of chromate in waters based on MSA-DLLME. In-syringe stirring makes possible homogeneous mixing of a large volume of sample with small volumes of reagents within seconds so that analytical standard protocols can be readily automated.
To save time, minimize dead volumes, and omit additional cleaning steps, the detection cell was mounted directly at the off position of V1, and a very short holding coil was used.
Batch experiments
Preliminary experiments were done in a batch in order to find an appropriate extraction solvent and ion-pair reagent. The experiments were done in glass test tubes with 5 mL of sample, 1 mL of acid, 1 mL of the DPC reagent, and 0.5 mL of extraction solvent.
Luo et al. [32] reported that alcohols and ketones allow effective extraction of the Cr(III)–DPCO ion pair with perchlorate. For the present system, solvents less dense than water were required, and hydrophobic alcohols have demonstrated their advantage for in-syringe DLLME several times [8, 10, 11]. Six alcohols and ketones were tested: n-hexanol, n-octanol, isoamyl alcohol, methyl isobutyl ketone, n-butanol, and ethyl acetate. After manual extraction, an intense color of the organic phase was observed only for n-hexanol and n-octanol, with which aqueous phase discoloration occurred. However, the color was almost imperceptible for the other solvents.
With regard to the ion-pair-forming anion, we observed that the extraction efficiency was negligible with the recommended NaClO4 [32]. The addition of 5 % (v/v) NH4OH solution improved the extractability, but the organic phase remained turbid and even changed its color from magenta to orange-red on passing pH 10 as observed previously [28]. Finally, we found that concentrated NH4Ac solution (10 mol · L-1) allowed efficient extraction into n-hexanol and yielded a transparent organic phase. This was attributed to the fact the compound provided acetate as an ion-pair-forming anion as well as the required buffer to yield the optimal pH for extraction. Further, NH4Ac is highly soluble, nontoxic, and volatile, so it can be easily purified by distillation.
Selection of the acid reagent
It is well known [26, 29] that the determination of Cr(VI) using DPC requires a strong acidic medium (pH < 2) to ensure quantitative reaction. Sulfuric acid is most commonly used for this purpose. However, the effect of three other strong acids (HCl, HNO3, and HClO4) on Cr(III)–DPC formation and extraction was studied and compared with the results obtained with H2SO4.
In the first study, the volume of buffer was kept constant while the volume of acid in the syringe was varied from 0. to 0.4 mL, i.e., 0.11–0.44 mol L-1 in the final mixture, so that the final pH of the aqueous phase decreased with increasing volume of acid. The total volume of aqueous phase was kept constant (4.425 mL) by appropriate addition of water. The results are summarized in Fig. 2A.
The highest peaks were obtained with HNO3 and HClO4 between 0.11 and 0.44 mol L-1. For HNO3, a signal maximum was found at 0.2 mol L-1. For HClO4, the signal increased with the acid concentration and reached a stable level. The results with HCl were similar to those with HNO3 but lower signals and a less distinct tendency was found. For H2SO4, the signal decreased strongly with increasing volume.
In the second study, the buffer volume was simultaneously increased with the acid volume in a constant ratio of 3:1 (0.68–2.71 mol L-1 NH4Ac in the final mixture) to keep the final pH constant. Consequently, for the diprotic sulfuric acid, twice the buffer volume was used. The results are summarized in Fig. 2B.
For all four acids, similar behavior is observed. The peak heights reached a stable signal level between 0.226 and 0.339 mol mL-1; only for H2SO4 did the peak height decrease with concentration higher than 0.339 mol L-1. The peak heights obtained at a concentration of 0.2 mol L-1 were as follows: 0.903 absorbance units (AU) for H2SO4; 0.882 AU for HClO4; 0.786 AU for HNO3; and 0.746 AU for HCl. However, considering the greater expense and danger of handling H2SO4 and HClO4, we finally selected HNO3 for this work. Another disadvantage of using H2SO4 was the requirement to use a higher volume of buffer to reach the final pH for extraction and, because of this, the requirement to use less sample and the loss of the method sensitivity.
Type and volume of extraction solvent
DLLME requires an extraction solvent with very low solubility in water and high dissolving power for the target complex. For in-syringe MSA-DLLME, the organic solvent must further have a density significantly lower than that of water to allow efficient phase separation by droplet flotation. Other characteristics such as low viscosity and surface tension are also desirable. These favor spontaneous droplet fusion after DLLME and reduce the sticking of the organic phase to the hydrophobic surfaces of the syringe plunger and stirring bar.
Medium-chain alcohols have been used as an alternative extraction solvent in DLLME with increasing success in this field [8, 10, 11, 18, 33–35] as they fulfill the given requirements and are considerably less toxic than classic extraction solvents such as benzene and chloroform.
As in the batch experiments, the extractability of Cr(III)–DPCO into n-hexanol, n-octanol, isoamyl alcohol, methyl isobutyl ketone, n-butanol, and ethyl acetate was tested by in-syringe MSA-DLLME. An extraction solvent volume of 0.125 mL and 3.3 mL of 52 μg L-1 Cr(VI) standard were used. Isoamyl alcohol, methyl isobutyl ketone, n-butanol and ethyl acetate yielded peak heights about three times lower than those for n-hexanol. Peaks with irregular shape, low reproducibility, and baseline alteration were observed for these organic solvents. The peak heights obtained using n-octanol were 36 % lower than those obtained using n-hexanol. Since the use of n-hexanol gave the highest sensitivity and further a reproducibility of approximately 1 % RSD and as stable baseline, it was used for further studies.
Figure 2C shows the relation between the peak height and the volume of n-hexanol for extraction. Obviously, an increase in the volume of n-hexanol implies greater dilution of the analyte in the organic phase and, hence, a lower preconcentration factor. However, for volumes lower than 0.1 mL, droplet coalescence was imperfect and segmentation of the organic phase on being dispensed to the detector caused erratic signals.
Effect of ammonium acetate concentration on the extraction process
The effect of the volume of NH4Ac buffer on the efficiency of the extraction process was studied in the range from 0.5 to 1 mL, corresponding to a final concentration of 1.1–2.3 mol L-1. To keep the reaction volume constant, a variable volume of water was added. The results are given in Fig. 2D. The peak heights increased with the volume of NH4Ac and reached a maximum at 2.03 mol L-1. Later Box–Behnken optimization showed that the use of 1.8 mol L-1 NH4Ac was optimal, allowing a larger volume of sample to be used for the procedure and because of this higher sensitivity.
Multivariate optimization
The effects of reaction time (t R), extraction time (t Ex), and the concentrations of DPC, HNO3, and NH4Ac on the signal height were studied by a multivariate experimental design for screening. A full-factorial 25 design including three central points was used. The ranges were as follows: t R from 25 to 45 s; t Ex from 30 to 50 s; volume of 1 mmol L-1 DPC from 0.1 to 0.2 mL; volume of 5 mol L-1 HNO3 from 0.2 to 0.3 mL; and volume of 10 mol L-1 NH4Ac from 0.6 to 0.9 mL.
Significant and positive effects were observed for t Ex and NH4Ac, whereas a significant and negative effect was found for the concentration of HNO3. The DPC volume and t R both showed positive but not significant effects within the experimental domain. For the DPC volume, a lower value of 0.175 μL (39.5 μmol L-1 final concentration) was then chosen to save reagent and to use more sample for the procedure.
Then, the three significant factors and t R were optimized using a Box–Behnken experimental design. The experimental domains were adjusted under the guidance of the screening results: t R from 35 to 55 s; t Ex from 40 to 60 s; HNO3 final concentration from 0.17 to 0.28 mol L-1; NH4Ac concentration from 1.36 to 2.03 mol L-1. The peak heights were found to be optimum for 1.80 mol L-1 NH4Ac, 0.17 mol L-1 HNO3, t Ex 55 s, and t R 55 s.
As observed before, a negative coefficient was obtained for the volume of HNO3. Further, two significant and negative interactions with the HNO3 concentration were observed, i.e., with the reaction time and with the NH4Ac volume. This indicated that a small increase of the concentration of acid would improve significantly the reaction rate but would decrease the extraction efficiency (lower pH), which has to be compensated by a larger volume of NH4Ac buffer.
The optimal values found were applied in all further work. The results from both studies are given in Table S2.
Evaluation of interferences and sample matrix
It is well known that DPC and DPCO can form colored complexes with other transition metal cations, including Hg(II), Mo(VI), Cu(II), V(V), and Fe(III), which can interfere in the Cr(VI) determination [23, 25, 26, 29, 32]. However, their complexes mostly have a much lower absorptivity than the complexes with Cr(III), and at pH 1–2 the reaction for chromium is quite selective [26, 29]. However, in the present case, we were forced to apply a neutral pH for the extraction of the complex formed.
To evaluate possible interferences in the proposed automated method, nine ions were studied by adding different concentrations to blank and standard solutions of Cr(VI). The tolerance levels found, i.e., a signal deviation of less than 10 %, are summarized in Table 1. They were high enough to disregard the tested metals as an interference in most natural water samples. Moreover, the addition of 0.13 mmol L-1 EDTA was able to suppress interferences from Fe(III) and Cu(II). This allows the analysis of samples with high contents of iron and copper.
The effect of water hardness in the extraction process was also studied owing to the high contents of sulfate and carbonate in the freshwaters on Mallorca. For this, synthetic freshwaters with three different hardness levels were prepared (Table S3) as recommended elsewhere [29].
Standard addition calibration curves up to 40 μg L-1 Cr(VI) were constructed using Milli-Q water, moderately hard water, hard water, and very hard water as the matrix sample. A clear trend of increasing sensitivity with water hardness (from 12.7 to 18.7 AU L mg-1) was observed. A salting-out effect as the cause of this significant matrix effect is possible but seemed unlikely since a high concentration of buffer was used. Therefore, in-syringe standard addition was performed as the surest way to guarantee the accurateness of determination of Cr(VI) in waters of different hardness.
Calibration and analytical performance
In-syringe stirring offers the possibility of automated standard preparation for calibration or standard addition since complete homogenization of all solutions can be achieved. For evaluation, five calibration curves, two prepared manually and three prepared in-syringe, but all measured with the proposed system, were compared.
No significant differences were found between the calibration curve intercepts (less than 10 mAU) and the slopes (manual, 10.34 ± 0.06 AU L mg-1; in-syringe, 9.6 ± 0.4 AU L mg-1) for the 99 % level of confidence.
Manual standard preparation gave slightly better reproducibility and repeatability values for a 10.4 μg L-1 Cr(VI) standard with values of 0.6–4 % and 2–4 %, respectively. The limit of detection (LOD) and the limit of quantification (LOQ) were calculated as three times and ten times the standard deviation of ten blanks divided by the slope of the calibration curve. For both preparation methods, the LOD and LOQ achieved were 0.27 μg L-1 and 0.89 μg L-1, respectively.
The LOD values obtained were five times lower than the LOD found for the DPC standard method [29] (1.29 μg L-1), ten times lower than the LOD values reported for atomic absorption spectroscopy (3 μg L-1), and on the same order as that for inductively coupled plasma optical emisssion spectroscopy (0.2 μg L-1) [36]. In general, the LOD obtained with MSA-DLLME was in the same order as or even lower than those obtained with methods which use the same reaction (DPC) but different preconcentration procedures, such as solid-phase extraction (LOD 0.7 μg L-1 [37], 0.165 μg L-1 [38]) and LLME (1.25 μg L-1 [27])
Since the extraction was quantitative, the preconcentration factor can be estimated from the ratio between the volume of the sample (3.395 mL) and the volume of the extraction solvent (120 μL) to be 28.
The linearity of the working range was verified up to at least 80 μg L-1 Cr(VI). Considerably higher concentrations could be measured by in-syringe sample dilution. The time required for the entire analytical procedure was 270 s, allowing an injection frequency of up to 13 h-1.
In comparison with the method using solid-phase extraction, no time for extraction phase regeneration was required [37, 38]. Further advantageous characteristics were the use of a nontoxic organic solvent and complete automation of analysis, including in-syringe standard preparation.
Because the method required expulsion of three different phases through the detection flow cell, i.e., air, aqueous phase, and n-hexanol, the concepts of peak-shaped signals and height determination from a defined baseline had to be abandoned. Instead, the signals were approximately rectangular and the absolute absorbance was used as the analytical signal. A peak profile is depicted in Fig. S1.
Although organic solvents (hexanol) tend to stick to hydrophobic surfaces such as the piston’s head and the stirring bar (PTFE), because of the very fast rotation speed of the stirring bar and the slight solubility of hexanol in water (about 5.9 g L-1), the cleaning of the system was very efficient and no crossover contamination was observed.
Determination of chromate in natural water samples
Chromate was determined in well water, tap water, and a demolition leachate to prove the applicability of the proposed analyzer. In-syringe calibration was done for each solution to compensate for any matrix effects. To estimate the analyte recovery, samples were spiked off-line and an aliquot was measured with the proposed analyzer system as well as for comparison with a reference method [29] based on the same color reaction but omitting extraction. Reference materials were not measured since their certified value is only given for total chromium. The results and the sample’s pH and conductivity values are given in Table 2.
Two well water samples showed Cr(VI) concentrations below but close to the LOQ (0.9 μg L-1), whereas a third had a concentration two times the LOQ. Tap water and leachate showed Cr(VI) concentrations about eight times the LOQ. Standard addition tests showed that the analyte recovery was in the range between 95 % and 115 %, proving the applicability of the proposed analyzer and method for real sample analysis.
In general, the proposed method yielded similar results to those obtained with the reference method. However, for the leachate sample, the reference method was found to underestimate the analyte concentration, most likely owing to turbidity and sample hue, since no extraction step for matrix separation was used.
Conclusion
A fully automated analyzer for the determination of chromate in waters was described. The work proves that the technique applied, in-syringe MSA-DLLME, shows several advantages. Among these, straightforward automation of analytical procedures, including homogeneous mixing of large volumes of a sample with considerably smaller amounts of reagents, LLE, phase separation, and in-system standard addition calibration, should be highlighted.
The proposed method used spectrophotometric detection in a compact analyzer instrument. It was satisfactorily applied to the determination of chromate in natural waters, omitting any additional sample treatment. The analytical characteristics indicate that the instrument is well suited for routine application.
References
Pena-Pereira F, Lavilla I, Bendicho C (2009) Spectrochim Acta B At Spectrosc 64(1):1–15
Silvestre CIC, Santos JLM, Lima JLFC, Zagatto EAG (2009) Anal Chim Acta 652(1–2):54–65
Chen W, Zhong GP, Zhou ZD, Wu P, Hou XD (2005) Anal Sci 21(10):1189–1193
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghaee E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116(1–2):1–9
Rezaee M, Yamini Y, Faraji M (2010) J Chromatogr A 1217(16):2342–2357
Kocúrová L, Balogh IS, Šandrejová J, Andruch V (2012) Microchem J 102:11–17
Anthemidis AN, Ioannou K-IG (2011) Talanta 84(5):1215–1220
Maya F, Estela JM, Cerdá V (2012) Anal Bioanal Chem 402:1383–1388
Anthemidis AN, Ioannou K-IG (2009) Talanta 80(2):413–421
Suárez R, Horstkotte B, Duarte CM, Cerdà V (2012) Anal Chem 84:9462–9469
Horstkotte B, Alexovič M, Maya F, Duarte CM, Andruch V, Cerdá V (2012) Talanta 99:349–356
Farajzadeh MA, Mogaddam MRA (2012) Anal Chim Acta 728:31–38
Saleh A, Yamini Y, Faraji M, Rezaee M, Ghambarian M (2009) J Chromatogr A 1216(39):6673–6679
Yan H, Wang H, Qin X, Liu B, Du J (2011) J Pharm Biomed Anal 54(1):53–57
Zhang Y, Lee HK (2012) J Chromatogr A 1249:25–31
Zhang P-P, Shi Z-G, Yu Q-W, Feng Y-Q (2011) Talanta 83(5):1711–1715
Skrlikova J, Andruch V, Sklenarova H, Chocholous P, Solich P, Balogh IS (2010) Anal Methods (2):1134–1139
Horstkotte B, Suarez R, Solich P, Cerdà V (2013) Anal Chim Acta. http://dx.doi.org/10.1016/j.aca.2013.05.049
World Health Organization (1990) World Health Organization (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva. ISBN: 978 92 4 154815 1
Gómez V, Callao MP (2006) Trends Anal Chem 25(10):1006–1015
Silva PS (2011) Guidance for public water systems on enhanced monitoring for chromium-6 (hexavalent chromium) in drinking water. Environmental Protection Agency, Washington
World Heath Organization (2011) Guidelines for drinking-water quality, vol WA 675, 4th edn. World Heath Organization, Geneva
Cazeneuve MP (1900) Bull Soc Chim Fr 23:701–706
Babko AK, Paulii LA (1950) Zh Anal Khim 5:201
Bose M (1954) Anal Chim Acta 10:201–209
Pflaum RT, Howick LC (1956) J Am Chem Soc 78:4862–4866
Majidi B, Shemirani F (2012) Microchim Acta 176(1–2):143–151. doi:10.1007/s00604-011-0711-3
Salinas-Hernández P, Rojas-Hernández A, Ramı́rez-Silva MT (2003) Spectrochim Acta A Mol Biomol Spectrosc 59(11):2667–2675
Franson MAH, Clesceri LS, Greenberg AE, Rhodes Trussell R (eds) (1992) Métodos normalizados para el análisis de aguas potables y residuales APHA, AWWA, WPCF
Becerra E, Cladera A, Cerda V (1999) Lab Robot Autom 11(3):131–140
Cerdà A, Cerdà V (2009) An introduction to flow analysis. Sciware Systems S.L., Barcelona
Luo Y, Nakano S, Holman DA, Ruzicka J, Christian GD (1997) Talanta 44(9):1563–1571
Leong M-I, Huang S-D (2008) J Chromatogr A 1211(1–2):8–12
Herrera-Herrera AV, Asensio-Ramos M, Hernández-Borges J, Rodríguez-Delgado MÁ (2010) Trends Anal Chem 29(7):728–751
Dadfarnia S, Haji Shabani AM (2010) Anal Chim Acta 658(2):107–119
Thompson M, Ellison SLR, Wood R (2002) Pure Appl Chem 74(5):835–855
Castilleja-Rivera WL, Hinojosa-Reyes L, Guzmán-Mar JL, Hernández-Ramírez A, Ruíz-Ruíz E, Cerdà V (2012) Talanta 99:730–736
Ma J, Yang B, Byrne RH (2012) J Hazard Mater 219–220:247–252
Acknowledgments
The authors acknowledge the financial support from the Spanish Ministry of Science and Innovation through the project CTQ2010-15541 and from the Conselleria d’Economia, Hacienda, e Innovació of the Government of the Balearic Islands through the allowance to competitive groups (43/2011) through Feder Funds. C.H. is very grateful to the Conselleria d´Educació, Cultura I Universitat, and the European Social Fund for funding her PhD grant. B.H. was supported by a postdoctoral fellowship of the project CZ.1.07/2.3.00/30.0022 supported by the Education for Competitiveness Operational Program and co-financed by the European Social Fund and the state budget of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 283 kb)
Rights and permissions
About this article
Cite this article
Henríquez, C., Horstkotte, B., Solich, P. et al. In-syringe magnetic-stirring-assisted liquid–liquid microextraction for the spectrophotometric determination of Cr(VI) in waters. Anal Bioanal Chem 405, 6761–6769 (2013). https://doi.org/10.1007/s00216-013-7111-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7111-y