Abstract
The nature of the halogen bond has been under debate over the last decade. Herein, the nature of the halogen bond was reinvestigated using point-of-charge (PoC) approach in which a point of negative or positive charge was used to mimic a Lewis base or acid, respectively. Halogen bond strength was estimated in terms of stabilization energy of the halo molecule in the presence of PoC. Open-ended questions regarding halogen interaction via σ-hole were discussed. A number of fundamental physical terms including σ-node, −σ-hole and +σ-hole interactions were introduced to describe the unconventional behavior of the halogen’s interactions. Several conflicts in the published results and explanations on the halogen bonding were highlighted and clarified. Based on PoC results, it may be claimed that: (i) halogen bond is mainly an electrostatic interaction and (ii) the polarization of the halogen is the key for understanding the reason behind the formation of halogen···Lewis acid/base interaction at halogen···Lewis acid/base angle of 180°. A–X···PoC angle and solvent effects on the molecular stabilization energy were estimated. Furthermore, electron correlation contribution to molecular stabilization energy was evaluated. Natural bonding orbital calculations were performed on the studied halo molecules. Finally, halogen bond test (σn-hole test) was proposed as a theoretical calculation to examine the ability of a halo molecule to form a halogen bond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Noncovalent interaction is a broad concept considering several types of interactions. The importance of investigation of noncovalent interactions is returned to their pivotal role in different fields related to biochemistry [1,2,3,4] and crystal engineering [5]. The interpretation of the role and nature of noncovalent interactions is always a major challenge [6]. Among noncovalent interactions, perhaps halogen bond is by far a well-known interaction which is considered to be an analog to the hydrogen bond [7, 8]. The halogen bond interaction is based on the occurrence of σ-hole which is a region of lower electronic density on the extension of an A–X covalent bond [9,10,11,12,13]. The latter term “σ-hole” is formed due to the anisotropic charge distribution around the halogen atom [14]. The magnitude of the σ-hole is considered to be responsible for the directionality and stability of the halogen bond [9, 15]. Other forces may contribute to the halogen bond strength such as dispersion interactions [3, 16]. Prior studies have been carried out to reveal the role and nature of the halogen bond in drug discovery [16,17,18]. It has been reported that halogen bond strength in a halo molecule···Lewis base system depends on three types of interactions: (i) attractive electrostatic interaction between the positive σ-hole and the negative Lewis base, (ii) repulsive electrostatic interaction between the negative halogen atom and the negative Lewis base and (iii) van der Waals interaction between the halogen atom and the Lewis base [14, 16, 19]. The controversy about the dominant nature of halogen bonding has raged unabated over the last decade. Moreover, a non-negligible amount of debates in the published results and explanations of the halogen bonding were observed. For instance, the ability of chloromethane and fluorine-containing molecules to form a halogen bond was subjected to considerable discussions [18, 20]. Furthermore, much uncertainty still exists about the relation between the halogen bond strength and the electronegativity of A group in the A–X···Lewis base system [21]. This overall discrepancy could be attributed to the interference of other interactions between the studied halogen-containing molecule and the Lewis base, rather than halogen bonding [16]. On the other hand, some previous study has investigated the electrostatic polarization effect on the σ-hole size [22,23,24,25,26,27]. The importance of polarization in dihalogen molecules and carbon tetrahalides with halide anions complexes was reported [28, 29]. In the current work, the noncovalent interactions rather than the electrostatic interaction between the halo molecule and the Lewis base is ruled out via the insertion of the point-of-charge (PoC) approach. Remarkably, the PoC approach was designed to mimic Lewis base and acid. In order to provide a descriptive characterization of halogen bonding, new physical terms including σ-node, −σ-hole interaction and +σ-hole interaction will be introduced for the first time. Furthermore, halogen bond test (σn-hole test) will be proposed to examine the ability of a halo molecule to form a halogen bond with a Lewis base. The PoC results will serve as a base for future studies on halogen bonding and other noncovalent interactions.
2 Methods
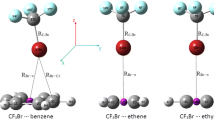
To reveal halogen bond characteristics, quantum mechanical study was carried out on a dozen of halogen-containing molecules including halobenzene, halomethane and hydrogen halide (C6H5X, CH3X and HX, where X = I, Br, Cl and F). The geometry of the studied molecules was firstly optimized at MP2 [30] level of theory using aug-cc-pVDZ basis set [31, 32] for all studied atoms with the exception of bromine and iodine atoms. Bromine and iodine atoms were treated with aug-cc-pVDZ-PP basis set [31, 33, 34]. The molecular electrostatic potential (MEP) for the studied molecules was produced at B3PW91/6-311G** [35] and then mapped on the 0.002 a.u. electron density contour. Vs,max calculation was performed to realize the numerical value of σ-hole with the help of wave functional analysis (WFA) program [36]. 2D potential energy grid (called XB grid in manuscript) was then generated for all the studied halo molecules with the integration of PoC approach. For the XB grid, a PoC with values of − 0.5 and + 0.5 a.u. was placed at a distance of 2.5 Å from the halogen atom along the x-axis and with a distance ranging from −2.5 to 2.5 Å along y- and z-axes (see Fig. 1). The grid spacing value was set to 0.1 Å. Moreover, the effect of halogen···PoC distance on halogen bond strength was investigated in the range from 2.5 to 6.0 Å along the x-axis with an A–X···PoC angle of 180°. The halogen bond strength was estimated in terms of molecular stabilization energy, given by:
The effect of A–X···PoC angle (θ) was also examined in the range from 90° to 180° with an increment value of 2.5° and estimated at A–X···PoC distance of 2.5 Å as follows:
In the halogen bond distance and angle investigations, PoC was set to a wide range of values given in Table S1. All single-point energies were calculated at MP2/aug-cc-pVDZ level of theory with the inclusion of PP functions for bromine and iodine atoms. The natural bonding orbital (NBO) calculations were also established at the same level of theory in order to study the correlation between the change (∆) in the highest occupied molecular orbital (HOMO) energy (∆EHOMO), p-orbital contribution to hybridization of A–X bond, p-electron configuration and natural charge of the halogen atom and halogen bond strength. The change (∆) was estimated as:
To account for the electron correlation contribution to halogen bond strength, single-point energies were calculated at MP2 and HF level of theory using aug-cc-pVDZ-PP. The electron correlation contribution was then estimated as follows:
Moreover, the solvent effect on the halogen bond strength \((E_{\text{solvent effect }} )\) was estimated with the inclusion of the polarizable continuum model (PCM) for water as an implicit solvent and calculated as follows:
The ability of halo molecule to form multi-halogen bonds simultaneously was also evaluated via the σn-hole test. In the latter test, two PoCs were placed at a distance of 2.5 Å from the halogen atom along the x-axis with a PoC···PoC distance ranging from 0.0 Å to 2.5 Å along y-axis (see Fig. 1).
To validate the PoC approach, all PoC-based results were compared to those of halo molecule···Lewis base/acid complexes. In the latter calculation, fluorine and lithium ions were used instead of the negative and positive PoC, respectively. All quantum calculations were performed using Gaussian 09 software [37].
3 Results
3.1 MEP and V s,max calculations
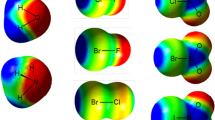
Studying the molecular electrostatic potential (MEP) of chemical systems is a preliminary approach to recognize the ability of a molecule to form a σ-hole [9]. MEP provides further how σ-hole size varies from a molecule to another based on the halogen atom and the attached group (A) in A–X. The studied molecules were firstly optimized at level of theory using aug-cc-pVDZ basis set (with PP functions for bromine and iodine atoms). For the optimized molecules, MEP was calculated at B3PW91/6-311G** level and mapped on 0.002 a.u. electron density contour (Fig. 2). The latter electron density value is recommended over the corresponding 0.001 a.u. contour for better results, as previously reported [16]. At the same level of theory, the Vs,max calculation was performed to support the MEP results with a numerical perspective using wave functional analysis (WFA) program [36] (Table S2).
Figure 2 shows that the size of σ-hole of halogen in A–X is associated, as expected, with halogen atomic size, largest in iodo-containing molecule followed by bromine- and chlorine-containing molecules. MEPs of the studied fluorine-containing molecules showed that fluorine molecule lacks occurrence of σ-hole and, in turn, fluorine molecule is not able to form a halogen bond with a Lewis base. Moreover, WFA of the studied molecules showed that Vs,max decreased in the order I > Br > Cl to become negative in the case of fluorine-containing molecules (Table S2). Furthermore, Vs,max decreased in the order hydrogen halide > halobenzene > halomethane with Vs,max value of + 29.5, + 25.5 and + 21.6 for hydrogen iodide, iodobenzene and iodomethane, respectively. WFA results were in an excellent agreement with the corresponding MEP results. The existence of σ-hole on chloromethane molecule and its ability to form a halogen bond were under debate [16]. According to MEP and Vs,max results, chloromethane has σ-hole with Vs,max value of + 3.9 a.u. As a result, chloromethane is expected subsequently to form a weak halogen bond.
3.2 2D stabilization energy grid
The concept of the 2D stabilization energy grid (called XB grid) was introduced in the current manuscript to get a comprehensive and accurate description of the σ-hole of a covalently bonded halogen atom. In XB grid calculation, the halogen molecule was first aligned with A–X axis and then scanned with a PoC with values of − 0.5 and + 0.5 a.u. at a distance of 2.5 Å from the halogen atom (see Methods section). The generated XB grids for the investigated molecules are depicted in Fig. 3.
Following the addition of a negative PoC, significant stabilization energy was recorded in varying proportions for all studied halo molecules with an exception in the case of fluorine-containing molecules. In the latter molecules, destabilization energy was observed with a maximum molecular destabilization when the negative PoC was located at the center of fluorine atom (i.e., at an A–F···PoC angle of 180°). This absence of stabilization confirms that no σ-hole exists for the studied fluoro molecules and the electron density is localized along A–F axis.
For the chloro-, bromo- and iodo-molecules, the most favorable stabilization energy was observed in case A–X···PoC angle equals 180°. Further, the stabilization energy of the halo molecules decreased whenever PoC was moved in y- and z-directions. Comprising of the findings, the iodine-containing molecules had the most favorable stabilization energy within − 9.1 and − 12.0 kcal/mol, followed by bromine and then chlorine with stabilization energy within − 3.5 and − 6.5, and − 0.4 and − 2.9 kcal/mol, respectively (Table 1). In general, the stabilization energy was decreased in the order of hydrogen halide > halobenzene > halomethane which is consistent with MEP and Vs,max results (Fig. 2 and Table S2). It should be noted that, contrary to earlier findings [9, 38, 39], the molecular surface of chloromethane molecule contained a σ-hole which was confirmed by the detection of a stabilization energy of − 0.4 kcal/mol at A–Cl···PoC angle of 180°; however, other repulsive interaction between chloromethane and a Lewis base might lead to deformation of the halogen bond [16].
Moreover, the ability of halogen-containing molecules to interact with a Lewis acid was considered by means of stabilization energy estimation in the occurrence of a positive PoC (Fig. 3). This kind of interaction would raise open-ended questions regarding halogen interaction via σ-hole with a Lewis acid. The latter interaction at A–X···positive PoC with an angle of ≈ 180° is termed “+σ-hole interaction.” In the case of fluoro-containing molecules, the presence of a positive PoC led to a stabilization of fluoro molecules and the stability increased as the positive PoC moved toward the origin to become maximum when y- and z-coordinates equaled zero (i.e., A–F···PoC angle = 180°). This finding confirmed that the surface of fluorine atom was completely negative in nature with a maximum electron density along A–F axis. For chloro-, bromo- and iodo-molecules, molecular stabilization was observed when a positive PoC was placed around the halogen atom (i.e., around the negative belt of the halogen atom). Interestingly, considerable stabilization energy was also observed along A–X axis (i.e., at the σ-hole) in the studied halobenzene and halomethane molecules. The latter stabilization energy (+σ-hole interaction) decreased in the order chloro- > bromo- > iodo-molecule, giving values within − 1.9 and − 5.6, + 0.3 and − 4.3, and + 2.2 and − 2.6 kcal/mol, respectively. These findings were unexpected, and it might be concluded that due to the presence of the positive PoC, the halogen’s electron density migrated toward center of σ-hole.
For a better understanding of the polarization effect of negative and positive PoC on the halo molecule, MEP maps were generated for the studied halomethane molecules, as a case study, in the presence of a positive and negative PoC (Figure S1). According to the generated MEP maps of fluoromethane in the presence of positive and negative PoCs (Figure S1), no σ-hole was identified. This revealed that the fluorine surface was hard to be polarized. For chloro-, bromo- and iodomethane molecules, the presence of a negative PoC led to an increase in the σ-hole size to be maximum when PoC equaled − 1.0 a.u. This might be attributed to the inductive polarization effect of negative charge on the halogen atom. In contrast, σ-hole size gradually decreased with increasing PoC positivity due to the migration of the electron density toward the origin of σ-hole. In some cases, σ-hole disappeared due to the presence of a large positive PoC value. The latter observation varied from one halo molecule to another depending on the σ-hole size and PoC value. For instance, σ-hole in chloromethane disappeared when PoC value equaled + 0.25 a.u., while the disappearance of σ-hole in iodomethane was observed at a PoC value of + 0.5 a.u.
From the concluded data, the polarization effect played a critical role in σ-hole magnitude and subsequently affected the strength of the halogen bond as well as +σ-hole interaction. As a result, it might be claimed that in addition to the three factors affecting the halogen bond strength (see Introduction section), Lewis acid/base polarization effect on halogen bond strength must be considered.
3.3 Halogen bond strength
In the current work, halogen bond strength was assessed as a correlation with halogen bond length and angle with the integration of the PoC approach. For the investigation of the effect of halogen bond length, the stabilization energies for the studied molecules were estimated in the presence of a PoC with A–X···PoC distance ranging from 2.5 to 6.0 Å with employing large- and small-scale charges (see Methods section). The small-scale charges were considered to scan the molecular electrostatic surface of studied molecules, while the large-scale charges were integrated to account for the effect of polarization on the electron density of the halogen atom. The generated stabilization energy curves are depicted in Figures S2 and S3 for small- and large-scale PoC values, respectively.
Generally, the stabilization and destabilization effects of the PoC were gradually decreased with increasing the halogen···PoC distance, as well as with decreasing the magnitude of the PoC (Figures S2 and S3). It should be highlighted that no polarization was observed in the case of fluorine-containing molecules, which is consistent with XB grid and MEP results.
For chlorine-containing molecules, molecular destabilization was observed in the presence of a small-scale negative PoC, despite the existence of σ-hole. This finding could be attributed to the repulsion force between the negative PoC and the negative halogen belt which was higher in magnitude than the attractive force between the negative PoC and the halogen’s σ-hole. This repulsion force decreased with increasing the σ-hole size (as seen in hydrogen chloride < chlorobenzene < chloromethane) and with decreasing the magnitude of the negative PoC. This observation confirmed that: (i) the repulsive electrostatic interaction between the negative halogen atom and the negative Lewis base cannot be neglected, (ii) existence of a σ-hole is not a guarantee for the halo molecule to form a halogen bond and (iii) at long range halogen···PoC distance, the halogen’s negative belt is dominated in effect over σ-hole, resulting in a repulsive force with a Lewis base. At a short distance, the attractive force of σ-hole was dominated over the corresponding repulsive force of the halogen’s negative belt. At a definite distance termed “σ-node,” the attractive σ-hole force was equal to the repulsive halogen force, resulting in zero stabilization energy. σ-node distance varied from a molecule to another based on the σ-hole size as well as the negativity of the Lewis base. For instance, the σ-hole in hydrogen chloride was larger in magnitude than the corresponding σ-hole in chlorobenzene, resulting in σ-nodes at a distance of 2.9 Å and 2.5 Å with PoC value of − 0.1 a.u., respectively.
It should be highlighted that an unexpected +σ-hole interaction was observed between chloro molecule and a large-scale positive PoC resulting in molecule stabilization. This raises a question of the ability of a halogen atom to act as a Lewis base and a Lewis acid with an angle of A–X···Lewis acid and ···Lewis base at 180°, respectively. For instance, the existence of + 1.0 and − 1.0 a.u. PoC at A–X···PoC distance of 3.0 Å led to a stabilization of − 5.0 and − 10.9 kcal/mol, respectively, for the chlorobenzene molecule. This observation could be explained by polarization effect of PoC on the halogen’s electron density. The same findings were observed for the bromine-containing and iodine-containing molecules.
Furthermore, a number of studies have demonstrated the correlation between the σ-hole magnitude and directionality of the halogen bond [15, 40,41,42]. It had been reported that favorable halogen bond angles were restricted between linearity (i.e., 180°) and perpendicular orientation (i.e., 90°) [43]. Based on these considerations, the molecular stabilization of halo molecules was estimated with the integration of a PoC approach with an A–X···PoC angle (θ) in the range from 90˚ to 180˚ at A–X···PoC distance of 2.5 Å (see Methods section). The correlation between A–X···PoC angle (θ) and the molecular stabilization energy \((E_{\text{angle effect}} )\) is plotted in Figures S4 and S5 with the incorporation of small- and large-scale PoC values, respectively.
Generally, for chlorine-, bromine-, and iodine-containing molecules, molecular destabilization and stabilization were observed with the integration of negative and positive PoCs, respectively (Figures S4 and S5). The latter molecular stabilization was increased by the decrease in A–X···PoC angle to a maximum at 90°. For fluorine-containing molecules, the integration of a positive PoC led to stabilization which reached maximum at 180° and a minimum at 90°. These results were in agreement with those obtained from XB grid scanning calculations (see Fig. 3). As expected, the stabilization and destabilization in the case of positive and negative PoC, respectively, were larger in the order I > Br > Cl with σ-hole size decreasing in the same order.
3.4 Natural bond orbital
Up to now, there has been no detailed investigation of the correlation between the magnitude of σ-hole and the highest occupied molecular orbital (HOMO) energy of the halo molecule. Moreover, the correlation between σ-hole size and atomic descriptors of the halogen atom such as p-electron configuration has been seldom studied. In the current study, the natural bond orbital (NBO) analysis was performed with the integration of the PoC approach to understand the latter correlations at MP2 level. PoCs with a charge of − 0.5 and + 0.5 a.u. were chosen as a case study with A–X···PoC distance ranging from 2.5 to 6.0 Å (see Fig. 4). According to the calculated change (∆) in the EHOMO energy due to the presence of PoC (Fig. 4 and Table S3), EHOMO energy was increased (i.e., more negative) with the incorporation of a positive PoC due to the reduction in σ-hole size. On the other hand, the presence of a negative PoC led to a decrease (i.e., less negative) in the EHOMO energy. The current results indicated that the EHOMO is inversely proportional to the magnitude of σ-hole. Furthermore, the change (∆) in p-orbital contribution to hybridization of A–X bond, p-electron configuration and natural charge of the halogen atom due to the presence of ± 0.5 a.u. PoC were also studied and depicted in Fig. 4. According to the results shown in Fig. 4, p-orbital contribution to A–X bond hybridization was directly correlated with the σ-hole size. In contrast, p-electron configuration of the halogen atom increased as the halogen bond strength decreased. Moreover, the natural charge of the halogen atom decreased (i.e., less positive or more negative) as the σ-hole size decreased (i.e., halogen bond strength decreased).
i HOMO energy difference (∆EHOMO) for, ii ∆p-orbital contribution to hybridization of A–X bond in, iii ∆p-orbital configuration of the halogen atom in, and (iv) the natural charge of the halogen atom in the studied molecules in the presence of ± 0.5 a.u. PoC at A–X···PoC distance ranging from 2.5 to 6.0 Å
3.5 Electron correlation effect
For better understanding of the halogen bond nature, the electron correlation contribution to the halogen bond was investigated with the help of the PoC approach. Molecular stabilization energy of the studied molecules was estimated at second-order Møller–Plesset (MP2) and Hartree–Fock (HF) levels of theory with the same basis set used. The electron correlation contribution (Eelectron correlation) was then calculated as the difference between the calculated MP2 and HF stabilization energies (see Eq. 4). The correlation between the A–X···PoC distance and Eelectron correlation is depicted in Fig. 5. As could be seen from Fig. 5, the electron correlation contribution was favorable in the presence of a negative PoC and unfavorable in the presence of a positive PoC. Interestingly, it was observed that Eelectron correlation decreased in the order F > Cl > Br > I, which reflected the negativity of the halogen atom, as well as the σ-hole size.
3.6 Effect of solvent
Investigation of solvent effect on the halogen bond strength is of utmost importance. Studies revealed that the solvent led to a decrease in the halogen bond strength and the halogen bond equilibrium distances [44]. In contrast, other studies alluded to an increase in the halogen bond strength due to the polarization effect of the solvent [45]. According to this controversy, the effect of solvent was reinvestigated in the current study. For all studied molecules, the solvent effect (\(E_{\text{solvent effect}}\)) was evaluated as given in Eq. 5 with the integration of PoC approach and polarizable continuum model (PCM) for water. PoCs with a charge of − 0.5 and + 0.5 a.u. were used as a case study. The correlation between the solvent effect (\(E_{\text{solvent effect}}\)) and A–X···PoC distance was estimated and depicted in Fig. 6. According to results presented in Fig. 6, the presence of a negatively charged PoC resulted in molecular destabilization for the studied halo molecules except in the cases of iodobenzene, bromobenzene and hydrogen iodide molecules where the solvent led to an increase in the σ-hole size resulting in molecular stabilization. In contrast, the presence of positively charged PoC led to a considerably higher molecular stabilization. The latter effect of solvent was confirmed by Vs,max calculation for all studied molecules with A–X···PoC distance of 2.5 Å in solvent phase and compared to the corresponding values in gas phase (Table S4). According to Vs,max results, the magnitude of σ-hole was decreased in most of the studied molecules. In the case of chlorobenzene, iodomethane and hydrogen bromide, a slight increase in σ-hole positivity was noticed which was compatible with results given in Fig. 6.
3.7 Halogen bond test (σ n-hole test)
In this study, halogen bond test (σn-hole test) was incorporated to examine the ability of a halo molecule to form halogen bond. It has been previously reported that halogen may form more than one halogen bond simultaneously [46]. Therefore, σn-hole test with n order of 2 was performed for halomethane molecule as a case study with the integration of two PoCs with values of − 0.1, − 0.25 and − 0.50 a.u. The σ2-hole test results are given in Fig. 7. According to the σ2-hole test results, molecular destabilization was observed in the case of fluoromethane due to the hardness nature of the fluorine atom. In contrast, molecular stabilization was observed in the case of chloro-, bromo- and iodomethane, and the stabilization energy was increased with increasing the PoC value as a result of the polarization effect. The latter molecular stabilization reflects the ability of chloro-, bromo- and iodomethane to form simultaneously more than one halogen bonding. Overall, these results showed that the increase in molecular stabilization to be reached at maximum value was associated with the proximity of PoCs to each other, which correspond to A–X···PoC angle of 180°. Moreover, the ability of a halo molecule to form multi-halogen bonding increases with the increase in σ-hole size in the order I > Br > Cl.
3.8 Real complex
Using PoC approach as a contemporary approach to reveal halogen bond properties gave promising results which resolved the debated interpretations of halogen bond nature. As well, PoC approach raised the important implications of the polarization effect on the halogen bond interaction. Undoubtedly, further investigations were needed to confirm and validate the PoC results. Therefore, potential energy curves of A–X···fluorine and ···lithium ions were generated and compared to the corresponding stabilization curves with negative and positive values of PoC, respectively. The interaction and stabilization energies of A–X···ion and ···PoC, respectively, are depicted in Fig. 8. Comparison of the calculated energies revealed that interaction and stabilization curves were very similar with a slight difference at a short A–X···ion and ···PoC distances due to the van der Waals effect which is not counted in the case of PoC approach. The latter effect is larger in the order I > Br > Cl > F. The current data highlighted and evaluated the effectiveness of PoC model and proved that PoC approach can be used as an alternative to the Lewis acid/base molecules to investigate noncovalent interactions.
4 Conclusions
Characterization of the halogen bonding was reinvestigated from the quantum mechanical perspective using the point-of-charge (PoC) approach. The following conclusions can be drawn from the present study: (i) The existence of a σ-hole is not a guarantee for the halo molecule to form a halogen bond with a Lewis base, (ii) the halo molecule has the ability to interact with both Lewis base and acid at A–X···angle of ≈ 180°, termed “−σ-hole interaction” and “+σ-hole interaction,” respectively, (iii) increasing negative PoC charge leads to an increase in σ-hole size and, in turn, the halogen bond strength in contrast with increasing positive PoC charge, (iv) polarization plays a significant role in σ-hole magnitude and, in turn, halogen bond strength, (v) the repulsive electrostatic interaction between the negative halogen atom and the negative Lewis base cannot be neglected, (vi) at a certain distance between the halogen atom and the Lewis base/acid, the attractive σ-hole’s force is equal to the repulsive negative belt’s force, resulting in zero stabilization energy known as “σ-node” distance, (vii) the EHOMO is inversely proportional to the magnitude of σ-hole, (viii) the natural charge of the halogen atom decreases (i.e., less positive or more negative) as the σ-hole size decreases, (ix) p-electron configuration of the halogen atom increases as the halogen bond strength decreases, (x) p-orbital contribution to A–X bond hybridization is directly correlated with the σ-hole size, (xi) in the presence of a negative PoC, the electron correlation contribution is favorable, while the latter contribution is unfavorable in the presence of a positive PoC, (xii) in aqueous phase, the negatively charged PoC leads to a molecular destabilization for most halo molecules, while positively charged PoC leads to a noticeable molecular stabilization, (xiii) halomethane molecules as a case study have the ability to form simultaneously more than one halogen bonding, and (xiv) finally, halogen bond is considered as an electrostatic interaction and supported by other interactions such as van der Waals. These findings of the current manuscript contribute in several ways to our understanding of the halogen bond nature and provide a basis for further research.
References
Frieden E (1975) J Chem Educ 52:754–761
Gromiha MM, Saraboji K, Ahmad S, Ponnuswamy MN, Suwa M (2004) Biophys Chem 107:263–272
Auffinger P, Hays FA, Westhof E, Ho PS (2004) Proc Natl Acad Sci USA 101:16789–16794
Hanshaw RG, Stahelin RV, Smith BD (2008) Chem Eur J 14:1690–1697
Braga D, Brammer L, Champness NR (2005) CrystEngComm 7:1–19
Müller-Dethlefs K, Hobza P (2000) Chem Rev 100:143–168
Scholfield MR, Zanden CMV, Carter M, Ho PS (2013) Protein Sci 22:139–152
Zheng Y-Z, Deng G, Zhou Y, Sun H-Y, Yu Z-W (2015) ChemPhysChem 16:2594–2601
Clark T, Hennemann M, Murray J, Politzer P (2007) J Mol Model 13:291–296
Politzer P, Murray JS, Clark T (2010) PCCP 12:7748–7757
Politzer P, Murray JS, Clark T (2015) In: Metrangolo P, Resnati G (eds) Halogen bonding I: impact on materials chemistry and life sciences. Springer International Publishing, Cham, pp 19–42
Murray JS, Resnati G, Politzer P (2017) Faraday Discuss 203:113–130
Politzer P, Murray J (2017) Crystals 7:212–225
Riley KE, Hobza P (2013) PCCP 15:17742–17751
Kolar M, Hostas J, Hobza P (2014) PCCP 16:9987–9996
Ibrahim MAA (2012) J Mol Model 18:4625–4638
Xu Z, Yang Z, Liu Y, Lu Y, Chen K, Zhu W (2014) J Chem Inf Model 54:69–78
Ibrahim MAA, Hasb AAM, Mekhemer GAH (2018) Theor Chem Acc 137:38–47
Oliveira V, Kraka E, Cremer D (2016) PCCP 18:33031–33046
Eskandari K, Lesani M (2015) Chem Eur J 21:4739–4746
Huber SM, Jimenez-Izal E, Ugalde JM, Infante I (2012) Chem Commun 48:7708–7710
Hennemann M, Murray JS, Politzer P, Riley KE, Clark T (2012) J Mol Model 18:2461–2469
Murray JS, Macaveiu L, Politzer P (2014) J Comput Sci 5:590–596
Politzer P, Murray JS, Clark T (2015) J Mol Model 21:52–61
Clark T, Politzer P, Murray JS (2015) Wiley Interdiscip Rev Comput Mol Sci 5:169–177
Ibrahim MAA, Moussa NAM, Safy MEA (2018) J Mol Model 24:219–231
Ibrahim MAA, Safy MEA (2018) Phosphorus Sulfur Silicon Relat Elem. 10.1080/10426507.2018.1528255
Clark T, Murray JS, Politzer P (2018) ChemPhysChem. https://doi.org/10.1002/cphc.201800750
Clark T, Heßelmann A (2018) PCCP 20:22849–22855
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Dunning TH Jr (1989) J Chem Phys 90:1007–1023
Woon DE Jr, Dunning TH (1995) J Chem Phys 103:4572–4585
Peterson KA (2003) J Chem Phys 119:11099–11112
Peterson KA (2003) J Chem Phys 119:11113–11123
Becke AD (1988) Phys Rev A 38:3098–3100
Bulat F, Toro-Labbé A, Brinck T, Murray J, Politzer P (2010) J Mol Model 16:1679–1691
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven TJA, Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision E.01. Gaussian Inc., Wallingford, CT
Politzer P, Lane P, Concha M, Ma Y, Murray J (2007) J Mol Model 13:305–311
Murray J, Concha M, Lane P, Hobza P, Politzer P (2008) J Mol Model 14:699–704
Riley KE, Hobza P (2011) Cryst Growth Des 11:4272–4278
Wilcken R, Zimmermann M, Lange A, Zahn S, Boeckler F (2012) J Comput Aided Mol Des 26:935–945
Grant HJ, Legon AC (2015) PCCP 17:858–867
Huber SM, Scanlon JD, Jimenez-Izal E, Ugalde JM, Infante I (2013) PCCP 15:10350–10357
Forni A, Rendine S, Pieraccini S, Sironi M (2012) J Mol Graph Model 38:31–39
Carlsson A-CC, Veiga AX, Erdélyi M (2015) In: Metrangolo P, Resnati G (eds) Halogen bonding II: impact on materials chemistry and life sciences. Springer International Publishing, Cham, pp 49–76
Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G, Terraneo G (2016) Chem Rev 116:2478–2601
Acknowledgements
The current research was financially supported by the Science and Technology Development Fund, STDF, Egypt, Grant No. 5480.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial and nonfinancial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ibrahim, M.A.A., Hasb, A.A.M. Polarization plays the key role in halogen bonding: a point-of-charge-based quantum mechanical study. Theor Chem Acc 138, 2 (2019). https://doi.org/10.1007/s00214-018-2388-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2388-8