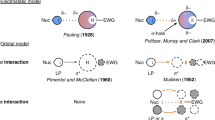
Abstract
Halogen bonding (XB) is a type of noncovalent interaction between a halogen atom X in one molecule and a negative site in another. X can be chlorine, bromine or iodine. The strength of the interaction increases in the order Cl<Br<I. After a brief review of experimental evidence relating to halogen bonding, we present an explanation for its occurrence in terms of a region of positive electrostatic potential that is present on the outermost portions of some covalently-bonded halogen atoms. The existence and magnitude of this positive region, which we call the σ-hole, depends upon the relative electron-attracting powers of X and the remainder of its molecule, as well as the degree of sp hybridization of the s unshared electrons of X. The high electronegativity of fluorine and its tendency to undergo significant sp hybridization account for its failure to halogen bond. Some computed XB interaction energies are presented and discussed. Mention is also made of the importance of halogen bonding in biological systems and processes, and in crystal engineering.

The computed B3PW91/6-31G(d,p) electrostatic potential, in kcal mol−1, on the 0.001 electrons/bohr3 surface of NC–C≡C–Cl. The chlorine atom is at the right. The color ranges are: red, more positive than 15; yellow between 7 and 15; green, between 0 and 7; blue, between −10 and 0; purple, more positive than −10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is halogen bonding?
Halogen bonding (XB) is a noncovalent interaction that is in some ways analogous to hydrogen bonding (HB). In the latter, a hydrogen atom is shared between an atom, group or molecule that “donates” and another that “accepts” it. In halogen bonding, it is a halogen atom X that is shared between a donor D and an acceptor A. Thus the two types of interaction can be depicted by:
X can be chlorine, bromine or iodine, but not, to our knowledge, fluorine. The angle D-X-A is close to 180°.
In HB and also in XB, both the donor and especially the acceptor tend to be electronegative, or electron-withdrawing. The acceptor is often a Lewis base, i.e. it has an available pair of electrons. Since hydrogens usually are considered to have partial positive charges, it is understandable that they interact attractively with electronegative atoms. But why would halogen atoms, which are generally viewed as being negative, undergo such interactions? This question shall be addressed in this paper.
Background
It has been known since the nineteenth century that Cl2, Br2 and I2 can form complexes with Lewis bases such as ammonia and methylamines [1, 2]. These were sometimes described as “charge–transfer” or “electron donor–acceptor” interactions, and Mulliken, [3] and later Flurry, [4, 5] developed theoretical formalisms for describing them. Eventually it was recognized that not only dihalogens and interhalogens but also many organic halides can form such complexes; an early review was given by Bent [6].
The analogies between such interactions and hydrogen bonding were discussed by Bent [6] and by Hassel [7]. However the first use of the term “halogen bond” that we have found was by Dumas et al. [8], in the context of experimental studies of complexes formed by CCl4, CBr4, SiCl4 and SiBr4 with tetrahydrofuran, tetrahydropyran, pyridine, anisole and di-n-butylether in organic solvents [8–10].
An important advance in understanding the noncovalent interactions of halogen atoms came through the analysis of large numbers of crystal structures from the Cambridge Structural Database, for example by Murray-Rust et al. [11–13]. They were looking for anomalously short intermolecular distances, i.e. less than the sum of the van der Waals radii of the atoms involved. Such distances were interpreted as indicating unusually strong atomic interactions. For halogens linked to carbons, certain distinct tendencies were found. Close contacts with electrophiles, such as metal ions, occurred largely at angles of 90°–120° with the C–X bond (1). With nucleophiles, however, such as oxygens and nitrogens, the angles were primarily between 160° and 180° (2). These generalizations apply for X=Cl, Br and I. (When X=F, the close contacts detected were only with electrophiles.) The near-linear interactions with nucleophiles are what has come to be known as “halogen bonding”.

In view of the analogies between halogen and hydrogen bonding, can the former compete and interfere with the latter? This question has been addressed by Sandorfy et al. in a series of studies of the infra-red spectra of solutions of various organic bases [14–16]. They found that the IR peak due to solute-solute intermolecular hydrogen bonding is considerably diminished by the introduction of a co-solute capable of significant halogen bonding. Di Paolo and Sandorfy concluded that [17] “...fluorocarbons containing higher halogens can break hydrogen bonds...probably due to competitive donor–acceptor complex formation.” They found hydrogen-bond-breaking potency to increase in the order F<Cl<Br<I.
Origins of halogen bonding
We pointed out some time ago that the basis for halogen bonding can be seen in the respective molecular electrostatic potentials [18, 19]. We shall now extend our earlier analysis, and at a higher computational level.
The electrostatic potential V(r) that is created at a point r by a molecule’s nuclei and electrons is given rigorously by
in which Z A is the charge on nucleus A, located at R A, and ρ(r) is the molecule’s electronic density function. V(r) is a real physical property, which can be determined experimentally [20, 21] as well as computationally. Its sign in any given region depends upon whether the positive effect of the nuclei or the negative one of the electrons is dominant there.
The electrostatic potential has been found to be an effective tool for analyzing and predicting noncovalent interactions [22–25]. For this purpose, we normally compute it on the surfaces of the molecules, and denote it by V S(r). We take the surfaces to be the 0.001 electrons/bohr3 contours of the molecular electronic densities, as suggested by Bader et al. [26]. We characterize V S(r) by means of several statistically-defined quantities [23–25], including its most positive and most negative values, V S,max and V S,min.
What is particularly relevant in the present context is that hydrogen bond donating and accepting tendencies can be related quantitatively to V S,max and V S,min, respectively [27].
The electrostatic potential of a neutral free atom is positive everywhere [28, 29], the contribution of the nucleus dominating those of the dispersed electrons. However when atoms interact to form a molecule, some regions of negative V(r) and V S(r) do normally develop [21, 22, 25, 30–32], particularly near the lone pairs of electronegative atoms, i.e. N, O, F, Cl, Br, etc. Thus it is customary to expect negative V S(r) associated with halogen substituents, which makes it difficult to understand the occurrence of halogen bonding, in which they interact noncovalently with negative sites, such as the lone pairs of electronegative Lewis bases.
A reasonable explanation for this puzzling phenomenon came initially from a Hartree-Fock computational analysis of the molecular surface electrostatic potentials of six halogenated methanes, CH3X and CX4, in which X=F, Cl, and Br [18, 19]. We have now used a density functional procedure, B3PW91/6-31G(d,p), to extend this study to a more diverse group of molecules. The surface potentials for some of them are in Figs. 1, 2, 3, 4, 5, and 6.
The computed B3PW91/6-31G(d,p) electrostatic potential, in kcal mol−1, on the 0.001 electrons/bohr3 surface of CH3Cl. The chlorine atom is at the right. The color ranges are: red, more positive than 15; yellow, between 7 and 15; green, between 0 and 7; blue, between −10 and 0; purple, more negative than −10
The computed B3PW91/6-31G(d,p) electrostatic potential, in kcal mol−1, on the 0.001 electrons/bohr3 surface of CH3Br. The bromine atom is pointing out of the page. The color ranges are: red, more positive than 15; yellow, between 7 and 15; green, between 0 and 7; blue, between −10 and 0; purple, more negative than −10
The computed B3PW91/6-31G(d,p) electrostatic potential, in kcal mol−1, on the 0.001 electrons/bohr3 surface of CF3Cl. The chlorine atom is at the top. The color ranges are: red, more positive than 15; yellow, between 7 and 15; green, between 0 and 7; blue, between −10 and 0; purple, more negative than −10
The computed B3PW91/6-31G(d,p) electrostatic potential, in kcal mol−1, on the 0.001 electrons/bohr3 surface of NC–C≡C–Cl. The chlorine atom is at the right. The color ranges are: red, more positive than 15; yellow, between 7 and 15; green, between 0 and 7; blue, between −10 and 0; purple, more negative than −10
The computed B3PW91/6-31G(d,p) electrostatic potential, in kcal mol−1, on the 0.001 electrons/bohr3 surface of CF3Br. The bromine atom is pointing out of the page. The color ranges are: red, more positive than 15; yellow, between 7 and 15; green, between 0 and 7; blue, between −10 and 0; purple, more negative than −10
The computed B3PW91/6-31G(d,p) electrostatic potential, in kcal mol−1, on the 0.001 electrons/bohr3 surface of O2N–CH2CH2–Cl. The chlorine atom is at the left. The color ranges are: red, more positive than 15; yellow, between 7 and 15; green, between 0 and 7; blue, between −10 and 0; purple, more negative than −10
In CH3F, CH3Cl and CF4, the halogen surfaces are negative, as anticipated. This can be seen for CH3Cl in Fig. 1. When the halogen is bromine or iodine, however, there is a new and perhaps unexpected feature: a positive region on the outermost portion of the halogen surface, where it intersects the C–X axis. This is shown in Fig. 2 for CH3Br. Such a positive region may also appear for chlorine when electron-withdrawing substituents are introduced into the remainder of the molecule, as in CF3Cl (Fig. 3) and NC–C≡C–Cl (Fig. 4). In the case of CF3Br (Fig. 5), the presence of the fluorines strengthens the positive bromine potential already observed in CH3Br. For all of these molecules, as well as several others, the most positive halogen potentials, the V S,max, are collected in Table 1.
It is evident from Table 1 that the presence and magnitude of a positive halogen potential depends upon both the halogen and the electron-withdrawing power of the remainder of the molecule. We have never found a positive V S,max for fluorine, and for chlorine only if the rest of the molecule is sufficiently electron-attracting. Table 1 shows that this is not the case in HO–CH2CH2–Cl, but it is so when OH is replaced by NO2. However, it should be noted that VS,max is considerably less in O2N–CH2CH2–Cl (Fig. 6) than in NC–C≡C–Cl, even though the nitro group is certainly highly electron-attracting. This is because it can act only inductively in O2N–CH2CH2–Cl, whereas the cyano in NC–C≡C–Cl has both inductive and resonance possibilities:

The bromine V S,max are always stronger than the chlorine (Table 1), wherever comparisons can be made. The high V S,max of chlorine in H–C≡C–Cl may be surprising. However, there is both theoretical and experimental evidence that makes this plausible [33].
It was proposed as early as 1992 [18, 19], and again more recently, [34] that these positive outer portions of some halogen surfaces can interact with negative parts of other molecules, and thus give rise to halogen bonding. The computed data are fully consistent with the experimental observation that potency increases in the order Cl<Br<I, with fluorine being inactive.
We have suggested the term “σ-hole” to denote the positive halogen surface region, because it is centered on the C–X axis and is surrounded by negative electrostatic potential (Figs. 2, 3, 4, 5, 6) [35]. Why are there σ-holes in some instances and not in others, and what determines how strongly positive they are?
These questions were addressed in a natural bond order (NBO) analysis of the molecules CF3X, where X=F, Cl, Br and I [35]. Two factors emerged: the electronegativity of the halogen and the extent of sp hybridization of its unshared s valence electrons.
For chlorine, bromine and iodine, the formation of the F3C–X bond was found to involve essentially the half-filled p orbital of each; sp hybridization is minimal, with s-contributions of only 12% for Cl, 9% for Br and 9% for I. Similarly, the s unshared electrons of these atoms show very little p character: 12%, 8% and 8%, respectively. Thus the three unshared pairs of each of these halogens closely approximate the configuration \(s^{2} p^{2}_{x} p^{2}_{y} \), where p x and p y are perpendicular to the C–X axis. These six electrons create a belt of negative electrostatic potential around the central portion of the halogen atom. Only its outermost region, the σ-hole, retains the positive potential characteristic of the free, spherically-symmetric atom [28, 29] (in which each p orbital has just 5/3 electrons). This picture accordingly accounts for the observed preference of electrophiles to interact laterally with the halogen, and nucleophiles linearly.
How does fluorine differ from this picture? First, there is significant sp hybridization; [36, 37] the orbital involved in the F3C–F bond is 75% p, 25% s, while the unshared s electrons have 25% p character. The hybridization of the s electrons produces an influx of electronic charge into the outermost portion of the fluorine, where the σ-hole would be. Second, the high electronegativity of fluorine results in its having the major share of the F3C–F bonding electrons, 75%, compared to about 50% for the other halogens in their respective F3C–X bonds. This additional electronic charge further helps to neutralize the σ-hole.
Thus, the combined effects of sp hybridization and high electronegativity eliminate the σ-hole in the case of fluorine. Both factors diminish progressively in importance in proceeding to chlorine, bromine, and iodine. While our NBO analysis was limited to the CF3X systems, we suggest that the factors that have been identified are more general, and can account for halogen bonding capacity increasing in the order Cl<Br<I (and apparently none for F).
In CH3Cl, these factors are sufficiently significant to prevent the chlorine from having a σ-hole; however one develops as fluorines are substituted and offset the chlorine’s inherent electronegativity (Table 1). For bromine and iodine, the σ-holes that do exist in CH3Br and CH3I are enhanced by the introduction of fluorines. In Cl2 and Br2, the only issue is the degree of sp hybridization of the s unshared electrons, and this is evidently quite small since there are strong σ-holes (Table 1).
In the present context, it is relevant to mention reports of covalently-bonded halogens, in molecules RX, having shorter radii along the extended R–X axis than perpendicular to it [38–40]. This has been called “polar flattening”, and is fully consistent with the preceding discussion; the \(s^{2} p^{2}_{x} p^{2}_{y} \) configuration of unshared pairs produces a buildup of electronic charge around the central portion of the atom, the observed anisotropy.
Computational treatments of halogen bonding interactions
There have been several detailed computational analyses of halogen-bonded systems [40–42], which have examined the roles of electrostatics, dispersion, exchange–repulsion, charge–transfer, highest-occupied and lowest-unoccupied orbitals, etc. Some of the calculated interaction energies are listed in Table 2. In most instances, the interaction distance is also included.
The data in Table 2 bring out certain points, all of them in agreement with the discussion in Section 3:
-
(1)
The effects of increasing the electron-withdrawing powers of the R portions of the molecules RX can be seen in the series CHxFyI · NH3 and CHxFyCl NH3. As expected, increasing the number of fluorines leads to a more negative interaction energy and smaller separation.
-
(2)
The series CF3X · NH3, where X=Cl, Br and I, shows the strength of the halogen bonding to increase in the order Cl<Br<I.
-
(3)
CH3Cl does not form a stable complex with NH3, nor (as shown by Lommerse et al. [40]) with CH2O or NC–C≡C–Cl.
We are not aware of any experimental interaction energies for the systems in Table 2; however measured values for complexes 5 and 6, −5.0±0.1 kcal mol−1 [43] and −7.4 kcal mol−1 [44], respectively, are quite similar to the −6.4 kcal mol−1 calculated for CF3I · NH3 and −5.64 kcal mol−1 for CF3I · N(CH3)3 (Table 2).

Importance of halogen bonding
The significance of halogen bonding in biological systems and processes has recently been surveyed by Auffinger et al. [34] and by Metrangolo et al. [45]. It occurs, for example, in ligand binding, recognition, conformational equilibria and molecular folding. For instance, a key factor in the recognition of thyroid hormones by their protein receptors is believed to be numerous short I┄O contacts with carbonyl groups of amino acid residues. [34, 46] Some inhibitors have been found to function by the formation of halogen-bonded complexes; this emphasizes the potential of exploiting such interactions in drug design. An example is 4, 5, 6, 7-tetrabromobenzotriazole, which efficiently displaces charged ATP from its binding site on phospho-CDK2-cyclin A, primarily by means of Br–O interactions. [47] Halogen bonding has also been implicated in the actions of some anesthetics. [45]
Another important and intriguing application of halogen bonding is in crystal engineering. [45] This involves formulating cocrystals with specific desired features, such as the spatial orientations and/or separations of the components. Thus by combining α,ω-diiodoperfluoroalkanes, I-(CF2)n-I with 1,4-dicyanobutane, NC-(CH2)4-CN, and 1,6-dicyanohexane, NC-(CH2)6-CN, it is possible to produce chains with hydrocarbon and perfluorocarbon segments of variable lengths: [48]

In 7, n is 2, 4, 6 or 8 and m is 4 or 6. Such approaches have been used, for example, to obtain materials with nonlinear optical activity, [49] or increased superconducting capacity [50].
Conclusions
Despite all of the work that has been mentioned and cited, it seems fair to say that halogen bonding is still relatively little recognized as a widely-occurring type of noncovalent interaction, certainly much less than hydrogen bonding. The concept that a covalently-bonded halogen atom could be at least weakly attracted to a negative site on another molecule may still seem unlikely to many researchers. Nevertheless, it is now well-established that this does occur, and we have sought in this paper to make this interaction plausible. Furthermore, as has been discussed at much greater length elsewhere, [34, 45] exploiting halogen bonding offers remarkable possibilities for designing and developing a whole array of compounds and materials, with applications ranging from drugs to electronics. To quote Metrangolo et al. [51], “The XB concept is still in its infancy.” It should have an important future.
References
Guthrie F (1863) J Chem Soc 16:239–244
Remsen I, Norris JF (1896) Am Chem J 18:90–96
Mulliken RS (1952) J Am Chem Soc 74:811–824
Flurry RL Jr (1969) J Phys Chem 69:1927–1933
Flurry RL Jr (1969) J Phys Chem 73:2111–2117
Bent HA (1968) Chem Rev 68:587–648
Hassel O (1970) Science 170:497–502
Dumas J-M, Peurichard H, Gomel MJ (1978) Chem Res (S) 54–57
Dumas J-M, Geron C, Peurichard H, Gomel M (1976) Bull Soc Chim Fr 720–722
Dumas J-M, Kern M, Janier-Dubry JL (1976) Bull Soc Chim Fr 1785–1787
Murray-Rust P, Motherwell WDS (1979) J Am Chem Soc 101:4374–4376
Murray-Rust P, Stallings WC, Monti CT, Preston RK, Glusker JP (1983) J Am Chem Soc 105:3206–3214
Ramasubbu N, Parthasarathy R, Murray-Rust P (1986) J Am Chem Soc 108:4308–4314
Bernard-Houplain M-C, Sandorfy C (1973) Can J Chem 51:1075–1083
Bernard-Houplain M-C, Sandorfy C (1973) Can J Chem 3640–3647
Di Paolo T, Sandorfy C (1974) Chem Phys Lett 26:466–469
Di Paolo T, Sandorfy C (1974) Can J Chem 52:3612–3622
Brinck T, Murray JS, Politzer P (1992) Int J Quantum Chem, Quantum Biol Symp 19:57–64
Murray JS, Paulsen K, Politzer P (1994) Proc Indian Acad Sci, Chem Sci 106:267–275
Stewart RF (1972) J Chem Phys 57:1664–1668
Politzer P, Truhlar DG (eds) (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Politzer P, Laurence PR, Jayasuriya K (1985) Environ Health Perspect 61:191–202
Murray JS, Politzer P (1998) J Mol Struct, Theochem 425:107–114
Politzer P, Murray JS (1999) Trends Chem Phys 7:157–165
Politzer P, Murray JS, Peralta-Inga Z (2001) Int J Quantum Chem 85:676–684
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) J Am Chem Soc 109:7968–7979
Hagelin H, Brinck T, Berthelot M, Murray JS, Politzer P (1995) Can J Chem 73:483–488
Weinstein H, Politzer P, Srebrenik S (1975) Theor Chim Acta 38:159–163
Politzer P, Murray JS (2002) Theor Chem Acc 108:134–142
Scrocco E, Tomasi J (1973) Top Curr Chem 42:95–170
Politzer P, Daiker KC (1981) In: Deb BM (ed) The force concept in chemistry (Ch 6). Van Nostrand, New York
Politzer P, Murray JS (1991) In: Lipkowitz KB, Boyd DB (eds) Reviews in computational chemistry Ch 7 Vol 2. VCH, New York
Politzer P, Harris RR (1970) J Am Chem Soc 92:6451–6454 (and references cited)
Auffinger P, Hays FA, Westhof E, Shing Ho P (2004) Proc Nat Acad Sci 101:16789–16794
Clark T, Hennemann M, Murray JS, Politzer P (2006) J Mol Model DOI 10.1007/s00894-006-0130-2
Kutzelnigg W (1984) Angew Chem 96:262–269
Kutzelnigg W (1984) Angew Chem, Int Ed Engl 23:272–275
Nyburg SC, Wong-Ng W (1979) Proc Roy Soc (London) A 367:29–45
Price SL, Stone AJ, Lucas J, Rowland RS, Thornley AE (1994) J Am Chem Soc 116:4910–4918
Lommerse JPM, Stone AJ, Taylor R, Allen FH (1996) J Am Chem Soc 118:3108–3116
Valerio G, Raos G, Meille SV, Metrangolo P, Resnati G (2000) J Phys Chem, A 104:1617–1620
Romaniello P, Lelj F (2002) J Phys Chem, A 106:9114–9119
Larsen DW, Allred AL (1965) J Phys Chem 69:2400–2401
Corradi E, Meille SV, Messina MT, Metrangolo P, Resnati G (2000) Angew Chem, Int Ed Engl 39:1782–1786
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Acc Chem Res 38:386–395
Cody V, Murray-Rust P (1984) J Mol Struct 112:189–199
De Moliner E, Brown NR, Johnson LN (2003) Eur J Biochem 270:3174–3181
Metrangolo P, Pilati T, Resnati G, Stevenazzi A (2003) Curr Opin Colloid Interface Sci 8:215–222
Thallapally PK, Desiraju GR, Bagien-Bencher M, Masse R, Bourgogne C, Nicoud JF (2002) Chem Commun 1052–1053
Imakubo T, Tajima N, Tamura M, Kato R, Nishio Y, Kajita K (2003) Synth Met 133–134:181–183. DOI 10.1007/s00894-006-0130-2
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Acc Chem Res 38:393–394
Acknowledgment
We would like to express our gratitude to Professor Jaroslav Burda, who very kindly provided facilities at the Charles University in Prague so that we could prepare this paper while most of us were evacuees from Hurricane Katrina, which hit New Orleans on August 29, 2005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proceedings of “Modeling Interactions in Biomolecules II”, Prague, September 5th–9th, 2005.
Rights and permissions
About this article
Cite this article
Politzer, P., Lane, P., Concha, M.C. et al. An overview of halogen bonding. J Mol Model 13, 305–311 (2007). https://doi.org/10.1007/s00894-006-0154-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-006-0154-7