Abstract
Rationale
The endocannabinoid system (ECS) belongs to the G protein-coupled receptor family of cell membranes and is associated with neuropsychiatric conditions, and neurodegenerative diseases. Cannabinoid 2 receptors (CB2) are expressed in the central nervous system (CNS) on microglia and subgroups of neurons and are involved in various behavioural processes via immunological and neural regulation.
Objective
The objective of this paper is to summarize and explore the impact of CB2 receptors on neuronal modulation, their involvement in various neurological disorders, and their influence on mood, behavior, and cognitive function.
Results
The activation of CB2 appears to protect the brain and its functions from damage under neuroinflammatory actions, making it an attractive target in a variety of neurological conditions such as Parkinson’s disease (PD), multiple sclerosis (MS), Alzheimer’s disease (AD), and Huntington’s disease (HD). During inflammation, there is an overexpression of CB2 receptors, and CB2 agonists show a strong anti-inflammatory effect. These results have sparked interest in the CB2 receptors as a potential target for neurodegenerative and neuroinflammatory disease treatment.
Conclusion
In conclusion, CB2 receptors signalling shows promise for developing targeted interventions that could positively affect both immune and neuronal functions, ultimately influencing behavioral outcomes in both health and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabinoid receptors (CBs) are G protein-coupled receptors (GPCRs), having seven transmembrane (7-TM) spanning domains, and found in the endocannabinoid system (ECS). Cannabinoid receptors 1 (CB1) and 2 (CB2) are the two major classes of cannabinoid receptors, CB1 receptors are found prominently in various parts of the central nervous system (CNS), including cortex, hippocampus, cerebellum, and basal ganglia (Zou and Kumar 2018) and peripherally found in liver, adipose tissues, gastrointestinal tract (GIT), skeletal muscles, and reproductive organs, whereas, CB2 receptors are chiefly located in peripherally circulating immune cells, including monocytes, thymus, B cells, T cells, tonsils, macrophages, microglia, natural killer (NK) cells, and polymorphonuclear cells (Dalle et al. 2022; Adel and Alexander 2021). Preclinical and clinical research studies suggest the contribution of ECS to brain homeostatic mechanisms, including long and short-term synaptic plasticity, and behavioural aspects like learning, cognition, and reward (Luchicchi et al. 2012). Anandamide and 2-arachidonoylglycerol (2-AG), are two major members of the endocannabinoid (EC) family, derived from the pig brain and dog gut, respectively. Anandamide and 2-AG are both lipid-based compounds that bind to CB and show retrograde synaptic signalling by transmitted from the post to a pre-synaptic membrane (Devane et al. 1992; Mechoulam et al. 1995; Cheung et al. 2019). In recent years, EC signalling specificity has begun to emerge and has played a promising therapeutic role in managing several neurodegenerative diseases, including Alzheimer’s disease (AD) (Koppel and Davies 2008), Parkinson’s disease (PD) (Bie et al. 2018), multiple sclerosis (MS) (Nouh et al. 2023), and Huntington’s disease (HD) (Pazos et al. 2008). Evidence now suggests that ECS can promote the management of both motor symptoms and often seen non-motor side effects along with PD, such as pain, insomnia, and depression, are interacting with CB1 receptors. An agonist and other ECS modulators in the basal ganglia might improve motor functions, reduce dyskinesias, and modulate other neurotransmitters, including gamma-aminobutyric acid (GABA), glutamate, and opioid peptides (Pisani 2005). CB1 receptors also play a key role in regulating metabolic processes, energy balance, gastrointestinal motility, and various peripheral functions, along with modulating neurotransmitter release, altering neuronal communication, and subsequently affecting mood, memory, and pain perception (Lu and Mackie 2016). Furthermore, CB2 receptors are also crucial for regulating immunological responses, lowering inflammation, and maintaining homeostasis by regulating cytokine release, cell migration, and inflammation. Expression of CB2 receptors is most prominent during active inflammation, where reactive microglia express several receptors, such as Toll-like receptors (TLRs) (Benito et al. 2008) and purinergic P2 × 4 receptors (Sophocleous et al. 2022), with the subsequent activation of the inflammatory pathways and the release of inflammatory mediators, eventually causing neuronal damage in neurodegenerative diseases. Activating CB2 receptors on microglia helps to suppress inflammatory responses, which may protect neurons by altering the inflammatory response and decreasing the production of pro-inflammatory cytokines and chemokines. CB2 receptors activation also helps to attenuate neuroinflammation and neuronal damage through p38-MK2 pathway, extracellular signal-regulated protein kinase 1 and 2 (ERK1/2) expression, and downregulation of TLRs and P2 × 4 receptors, thus playing a protective role in neurodegenerative conditions and maintaining neural homeostasis (Ghonghadze et al. 2020). Cannabinoid ligands, their receptor and biological functions are shown in Table 1.
CB2 receptors
The CB2 receptors are found in the immune system, cardiovascular tissues, spleen, and CNS, which have protective effects against various cellular events (Bow and Rimoldi 2016). In CNS, it plays a basic defence mechanism by activating glial cells, which include perivascular microglia, parenchymal microglia, astrocytes, and oligodendrocytes, that constitute the majority of all the cells in the brain and spinal cord, whereas microglia and astrocytes are fundamental components of basic defence mechanism of the CNS. By altering the surrounding environment and synaptic structure, microglia under normal physiological conditions are essential for the development and maintenance of synaptic plasticity in neuronal cells. Synaptic development and deficits observed in a variety of neurological diseases are attributed to the production and release of various chemokines and cytokines and the microglia’s phagocytosis of synapses. The remarkable presence of CB2 receptors in microglial cells also emphasized the correlation between heightened CB2 expression and neuro-inflammation within the brain by inhibiting neuroinflammatory signalling pathways, restoring normal microglial functions, and modulating ERK1/2 activation, which indicates a robust association of CB2 receptors, specifically in microglial cells, during these inflammatory processes (Komorowska-Müller and Schmöle 2020). During brain injury or other inflammatory processes, microglia become activated, and the upregulation of CB2 receptors on these cells is part of the immune response and maintains homeostasis (Nunez et al. 2004). Homeostasis is restored by activating the negative feedback mechanism in pathological conditions and exploiting TLRs and tumour necrosis factor-alpha (TNF-α) activation to release cytokines that regulate inflammatory responses in inflammation. Overactivation of CB2 receptors exhibits neuroprotective benefits by suppressing TLRS and TNF-α, downregulating the neuroinflammatory pathways. Additionally, CB2 receptors expression is primarily dependent on the presence of active inflammation and does not appear to have any negative psychological effects or the potential to cause addiction. Research noted that CB2 expression may be intriguingly possible not only in microglia but also in some neuronal populations, highlighting the wider effects of CB2 receptors within the CNS in response to various neurodegenerative disorders and other pathological conditions (Maresz et al. 2005). During the structural elucidation, it was found that the CNR2 gene encodes CB2 receptors, which are extended by approximately 360 amino acids, which make them slightly shorter than the 473 amino acids containing the CB1 receptors shown in Fig. 1. The intracellular C-terminus portion of CB2 receptors seems to be very crucial in modulating ligand-induced receptor desensitization, and subsequently, recurrent administration of agonists causes downregulation, which could make the receptor less receptive to specific ligands (Li et al. 2023). Human CB1 and CB2 receptors share 68% within transmembrane regions, and 44% are identified throughout the entire protein. An amino acid sequence of CB2 receptors is less conserved in rats and humans as compared to CB1 receptors, and those cannabinoids that bind to CB2 receptors start a signalling cascade inside the cell. This activation has numerous secondary effects on modulating inflammatory and immunological responses, as well as other cellular functions (Svízenská et al. 2008). The CB2 receptors have been significantly associated with neurodegenerative conditions like AD, MS, and HD, indicating their substantial involvement and implications in the progression and manifestations of neurological disorders (cited Di Marzo et al. 2015). Both preclinical and clinical studies suggest that upregulation of CB2 receptors expression in striatal microglia modulates neuroinflammation and immunological responses in MS, potentially lowering disease severity. A preclinical study on the APPSw/Ind model of AD shows that activation of CB2 receptors can attenuate NMDA signalling in activated microglia, reduce the production of inflammatory mediators, and improve Aβ clearance, which potentially mitigates neuronal damage and cognitive decline. In HD, the Malonate rat model and R6/2 mouse model show a significant elevation of CB2 receptors expression in the hippocampus, striatum, and cerebellum of the brain and may play a neuroprotective role in striatal neuron degeneration by modulating neuronal inflammation and protecting against synaptic loss (Kirbet et al. 2022; Benito et al. 2008).
In vivo studies in humans and animal models help to find molecular and functional alterations in the brain by positron emission tomography (PET). A unique set of pathological alterations, including inflammation, microvascular modifications, synaptic dysfunction, and neuronal death, are put on by the aberrant production of tau and amyloid-beta (Aβ) aggregates, particularly in AD models. CB2-selective PET tracers revealed a correlation between Aβ plaque formation and elevated CB2 receptors expression in the brain, which suggests that CB2 PET tracers may be used as a diagnostic tool to identify the emergence of neuroinflammation at the onset of AD. However, these models may not be optimal for assessing CB2R tracers because age-related inflammation also contributes to the relatively modest inflammation in AD animal models (Ni et al. 2019). Mitogen-activated protein kinases (MAPKs) are Ser/Thr kinases of proteins that mediate a wide range of cellular responses in response to external stimuli. Among the initially identified signal transduction pathways, MAPKs have been extensively exploited in various physiological functions. Studies on signal transmission have associated CB1/2 receptors’ function in controlling ERK1/2 to the subsequent regulation of genes that regulate transcription, govern the production of cytokines, and regulate cell differentiation. ERK1/2, or p42/44 MAPK, is activated in response to activation of CB receptors and various growth factors, such as nerve growth factor (NGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF). Mitogenic drugs rapidly increase ERK1/2 activity and are more effective in G1-to-S-phase transition in normal cells, which depends on kinases, including the activation of positive cell cycle regulators. ERK1/2 regulates the proliferation of cells by initiating the activation of c-Jun N-terminal kinases (c-JNK) and p38 mitogen-activated protein kinase (p38 MAPK) pathway and plays a critical role in normal immune and inflammatory responses. Various environmental stressors, inflammatory cytokines, oxidative stress, UV irradiation, hypoxia, ischemia, interleukin-1 (IL-1), and TNF-α, substantially activate the four p38 isoforms in mammalian cells. The generation of proinflammatory cytokines is one of the p38 isoforms primary functions that also control the expression of cytokines by modifying transcription factors like nuclear factor kappa B (NF-κB). The JNK isoforms are strongly activated in response to a variety of cellular stresses, such as heat shock, ionizing radiation, oxidative stress, DNA-damaging agents, cytokines, UV irradiation, growth factor deprivation, and to a lesser extent, serum and certain GPCR ligands. This activation pattern is similar to that of the p38 MAPKs. JNK1 and JNK2 are seen to play an important role in the control of cell proliferation (Cargnello and Roux 2011). A preclinically autoimmune encephalomyelitis mouse model was used to study the location and expression of CB2 receptors, and the study suggested that over-expression and activation of CB2 receptors can be considered as a potential therapeutic approach to limit the progress of inflammation (Fu and Taylor 2015).
Cannabinoid receptor signalling
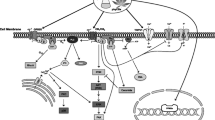
GPCRs are a major class of cell surface receptors associated with a group of G proteins that contain three subunits: α, β, and γ. Based on their α subunit, G proteins are categorized into four families: Gαi, Gαs, Gα12/13, and Gαq (Zhang et al. 2024). Cannabinoid ligands (agonists) bind to the CB2 receptors and induce an abidance change in the receptor, leading to the activation of the associated Gαi subunit, which is primarily involved in the pathway (Demuth and Molleman 2006). The Gαi subunit dissociates from the Gβγ subunits as a result of a conformational shift that occurs upon activation of CB2 receptors. While both Gαi and Gβγ can alter intracellular signalling pathways, the relationship between the Gαi subunit’s adenylate cyclase (AC) is the point of interest when it comes to CB2 receptors activation (Calandra et al. 1999). Adenosine triphosphate (ATP) is converted to cyclic adenosine monophosphate (cAMP) by the enzymatic activity of AC, which also acts as a second messenger by transmitting signals from the outside of the cell to the inside. Gαi subunits block AC activity when there is no receptor activation because its enzymatic activity can be directly inhibited by the α subunit. Nevertheless, AC is no longer blocked when the Gαi subunit is released the CB2 receptors are active, and cAMP increases as a consequence. Protein kinase A (PKA), which functions as a downstream effector in the AC pathway, is subsequently activated by elevated cAMP levels. Many target proteins are phosphorylated by the Ser/Thr kinase PKA, which sets off a series of intracellular processes. Activation of the CB2 receptors can suppress immune cell’s secretions of pro-inflammatory cytokines, and maintaining the balance of pro-inflammatory and anti-inflammatory cytokines is crucial for immune system regulation (Bayewitch et al. 1995; Opal and DePalo 2000). By inhibiting AC and reducing cAMP levels, the CB2 receptors help dampen excessive immune responses, contributing to the resolution of inflammation (Ni et al. 2019). The AC pathway is shown in Fig. 2.
MAPK pathway
The MAPK pathway is a complex signalling cascade that plays a pivotal role in transmitting signals from the cell surface to the nucleus, influencing various cellular processes (Cargnello & Roux 2011). This pathway is crucial for fundamental cellular processes such as proliferation, differentiation, apoptosis, and response to environmental stressors. Comprising a series of protein kinases, the MAPK pathway is activated by diverse signals, including growth factors, and cytokines, and it transmits these signals to the cell nucleus, orchestrating appropriate cellular responses (Bouaboula et al. 1996). The CB2 receptors undergo a conformational change that allows them to interact with G proteins, and those involved, particularly Gα subunits, initiate downstream signalling cascades, including the MAPK pathway. Tetrahydrocannabinol (THC) and other cannabinoids activate the CB2 receptors, which then trigger the MAPK pathway through a sequence of biochemical processes (Atwood and Mackie 2010). This binding activates receptor tyrosine kinases (RTK) or GPCR, initiating a series of events that culminate in the activation of MAP3K. These MAP3K, also known as Raf kinases in the case of the ERK pathway, phosphorylate and activate MAP2K, which, in turn, phosphorylate and activate the terminal MAPK. Among the MAPKs, ERK is particularly involved in regulating cell proliferation and differentiation. Once activated, ERK translocates to the nucleus and phosphorylates transcription factors, modulating gene expression and influencing cell fate decisions. On the other hand, JNK and p38 MAPK are prominent in stress response pathways as they play crucial roles in apoptosis, inflammation, and other cellular responses to various environmental stresses. MAPK and its related pathways are shown in Fig. 3 (Sánchez et al. 1998).
MAPK pathway Abbreviations: THC (tetrahydrocannabinol), p38 or stress-activated protein kinases, RAF1(raf-1 proto-oncogene, serine/threonine kinase), JNK(jun amino-terminal kinase), MAPKs(mitogen-activated protein kinases, ERK (extracellular signal-regulated kinases, GRB2(guanine nucleotide exchange factor SOS), SRC (proto-oncogene tyrosine-protein kinase), MAPK (mitogen-activated protein kinases, EGF (epidermal growth factor), RAC1(ras-related C3 botulinum toxin substrate 1, P(phosphate) (Cabral and Griffin-Thomas 2009)
Immune modulation by CB2 and its role in immune response regulation
T cells are a vital player in adaptive immunity, and the activation of CB2 receptors has been shown to influence T cell differentiation and activation. This modulation is essential for maintaining a balanced immune response, and preventing excessive immune activation (Tóth et al. 2019). CB2 receptors help in maintaining immunological tolerance and limit overreactions by regulating T cell activity (Lunn et al. 2006). It also promotes the differentiation and function of regulatory T cells, which play a crucial role in sustaining immunological tolerance and preventing autoimmune reactions (Rodrigues et al. 2019). They impact the movement of immune cells to sites of inflammation, infection, or injury and prevent excessive immune cell infiltration, which can contribute to tissue damage and chronic inflammatory conditions (Gu et al. 2017).
EC has diverse effects on immunological regulation by apoptosis in an NF-κB-dependent manner, diminishes cellular activation, obstructs pro-inflammatory cytokine generation, and modifies the functions of T-helper1 (Th1) and T-helper2 (Th2) subsets. Therefore, it is possible to think of EC (AEA and 2-AG) and their congeners, Palmitoylethanolamide (PEA), as powerful immunomodulators (Leleu et al. 2013, Mechoulam and Parker 2013). Table 2 shows that various immune cell functions are affected by either CB1 or CB2 receptors.
Cytokine production
Activation of CB1 and CB2 receptors, either directly or indirectly, promoting various cytokines signalling pathways, is the key to determining their relation to pain (Anthony et al. 2020). Cytokines are signalling molecules in the form of proteins produced by a variety of cells in the immune system, including T and B cells, macrophages, and dendritic cells. To maintain pro- and anti-inflammatory signals, CB2 receptors are essential for controlling cytokine production, and the up-regulation of pro-inflammatory cytokines like TNF-α and interleukin-6 (IL-6), occurs with the activation of CB2 receptors. Its ability to manage inflammation is essential for avoiding a high level of immune responses that can cause tissue damage (Klein et al. 2001).
Conversely, stimulation of CB2 receptors increases the synthesis of cytokines that reduce inflammation, like interleukin-10 (IL-10). Increased production of IL-10 aids in the resolution of inflammation and is well known for its immuno-suppressive qualities. This is the primary method by which CB2 receptors carry out their immunomodulatory actions by controlling cytokine production (Klein et al. 2003). Chemokines are a type of cytokine responsible for guiding immune cells to specific locations within the body. CB2 receptors activation modulates the expression of chemokines, influencing immune cell migration and residing at the site of inflammation and infection (Basu and Dittel 2011).
Inflammation regulation
CB2 receptors play a crucial role in the immune response by regulating inflammation, and responses depend on immune cells and aspects of inflammatory responses. Activation of CB2 receptors inhibits the production of inflammatory mediators, including chemokines and prostaglandins, and reduction in inflammatory responses. Additionally, CB2 receptors affect immune cell migration to inflammatory areas and assist in regulating the degree and duration of inflammatory responses by altering chemotaxis, the direction in which immune cells migrate (Davis 2014, Turcotte et al. 2016). Cannabinoids, such as THC can downregulate the inflammatory responses by modulating the ECS. This modulation is relevant for the prevention of autoimmune diseases such as MS, systemic lupus erythematosus (SLE), diabetes mellitus type 1 (DMT1), and rheumatoid arthritis (RA) (Rodríguez Mesa et al. 2021).
CB2 receptors in neuroinflammation and neurodegeneration
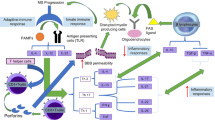
The evidence gathered here indicates that neuroinflammation causes neurodegenerative diseases, playing a crucial role in the very early development of chronic dying that neurons activate microglia, which generate several substances that cause further neuronal apoptosis (Kwon and Koh 2020). The CB2 receptors were present to be predominantly expressed in microglia and astrocytes around neuritic plaques in the postmortem brains of AD. Surrounding neuritic plaques in the postmortem brain showed that CB2 receptors were present in microglia and astrocytes. This provided the first evidence of altered expression of the CB2 receptors, termed the “peripheral” CBs, in the human brain throughout the progress of neurodegenerative diseases like AD, PD, HD, and frontotemporal dementia (Concannon and Dowd 2016). In the initial phases of neurodegenerative diseases, there is an interlink between neuroinflammation and neurodegeneration, such as the degenerating substantia nigra pars compacta (SNpc) in PD, the degenerating striatum in HD, and the demyelinated cortex in patients with MS (Calandra et al. 1999). Furthermore, there is a substantial correlation between this relationship and inflammatory diseases, such as dementia associated with Human Immunodeficiency Virus (HIV) infection of microglia and MS, as well as intense and prolonged inflammation of myelin sheaths (Heneka et al. 2015). Microglia and astrocytes are the building blocks of the first line of defence system in the CNS against neuroinflammation and are essential for producing and maintaining neural plasticity (Eyo and Dailey 2013). Activated microglia that adopt classical M1-like phenotypes primarily synthesize and release pro-inflammatory molecules including TNFα, IL-1β, IL-6, IL-12, cytokines and chemokines (Sierra et al. 2013; Block and Hong 2005). They emit pro-inflammatory cytokines, chemokines, and free radicals that damage the brain’s ability to heal itself and lead to long-term neurological deficits, oxidative stress, and chronic neuroinflammation (Shao et al. 2022). After the removal of the threat of inflammation, M1 microglia transition to an alternate activation state (M2), during which they release brain-derived neurotrophic factor (BDNF), tumor growth factor-β (TGF-β), IL-10, and other anti-inflammatory and neuroprotective substances to promote the healing process and prevent inflammation (Kettenmann et al. 2011; Tang and Le 2016). In addition to microglia, CB2 receptors activation in neurons may preserve neuronal homeostasis by modulating the production of neuronal nitric oxide synthase (NOS) (Oddi et al. 2012), excitotoxicity, and apoptosis, which in turn reduces oxidative damage. ECS activation results in the concurrent synthesis of anti-inflammatory factors and suppression of pro-inflammatory cytokines in astrocytes, which express both CB1 and CB2 receptors (Fernández-Ruiz 2007). It also lowers the expression of iNOS and reduces the release of neurotoxic substances (Molina-Holgado et al. 2003). Figure 4 shows the interaction between neuroinflammation, neurodegeneration, and the neuroprotective properties of substances mediated by CB2 receptors.
Show the interaction between neuro inflammation, neuro degeneration, and the neuro protective properties of substances mediated by CB2R. The impact of ECS or CB2R agonists on various mediators of neuro inflammation and neuro degeneration is shown by purple arrows. Inducible nitric oxide synthase (iNOS); Blood brain barrier (BBB); Cannabinoid receptor 2 (CB2r); Reactive oxygen species (ROS); Reactive nitrogen species (RNS); Interferon gamma (IFNγ), lipopolysaccharide (LPS), and interleukin (IL) Tumour growth factor beta (TGFβ) and tumour necrosis factor alpha (TNFα) neurotrophic factor derived from brain tissue; neural growth factor (NGF); and neurotrophic factor derived from glial cells Nicotinamide acid dinucleotide phosphate, or NADPH; arginase 1 (Arg1)
Role of CB2 receptors in neurological disorders
Alzheimer’s disease
AD is a common neurodegenerative disorder, characterized by intracellular aggregation of tau proteins, neuritic plaques, and Aβ protein deposition. AD is also characterized by oxidative stress, excitotoxicity, neuroinflammation, intracellular accumulation of neurofibrillary tangles and extracellular Aβ-plaque deposition, and neuronal death (Selkoe and Hardy 2016). The integral membrane protein known as the amyloid precursor protein (APP) is mostly expressed in the synapse of neurons and serves as the precursor molecule whose proteolysis generates Aβ protein. A mouse model was established to evaluate APP expression and results indicate that overexpressing human APP with CB2 receptors deletion enhanced Aβ42 generation and plaque formation (Sasaguri et al. 2017). The finding indicates that cannabinoid inhibition of Aβ-generated H2O2 prevented the RS dihydro rhodamine from oxidizing into fluorescent rhodamine-123 implicated a receptor-independent pathway (Aychman et al. 2023). The function of microglia is to encircle Aβ-plaques and produced a barrier to stop their detrimental effects on neurons (Condello et al. 2015). In addition, microglia also caused neuro-inflammation by inducing the release of pro-inflammatory chemokines and cytokines, nitric oxide (NO), and free radicals when they intact with Aβ-plaque. CB2 receptors regulate inflammation and protect the brain by controlling microglia migration and infiltration into brain areas (Turcotte et al. 2016; Fernández-Ruiz al. 2008). Available evidence confirms that the astrocytes and microglia surrounding neuritic plaques have greater numbers of CB2 receptors. Through the stimulation of MAP Kinase-phosphatase (MKP), CB2 receptors activation decreases microglial migration and generates an anti-inflammatory phenotype of microglia (Romero-Sandoval et al. 2009). Researchers have used both in vitro and in vivo methods to investigate the role of CB2 receptors in the pathophysiology of AD. The majority of in vivo investigations relied on several genetic mice models of AD that replicated the primary neuropathological features of the disease. Tau neuropathology, impaired hippocampus-dependent memory, and mitochondrial dysfunction were observed in mice lacking CB2 receptors (Wang et al. 2018). In contrast, the downregulation of the transcription factor p53 and activation of the transcription factor NF-κB, which can trigger apoptosis, implicated a receptor-dependent pathway. Using the phosphoinositide 3-kinase (PI3K) inhibitor LY294002, it was possible to show that PI3K was involved in the downregulation of p53 (Jimenez-Del-Rio et al. 2008). These studies suggest that cannabis possesses a potential neuroprotective role in the pathophysiology of AD. The pharmacological findings of the CB2 receptors as a therapeutic target in AD are mentioned in Table 3.
Anti-inflammatory effects and cognitive function
Numerous neurological diseases are accompanied by chronic inflammation in the brain, and activation of CB2 receptors has been investigated as a potential target for reducing inflammation and preventing cognitive loss (Paradisi et al. 2006). The activation of CB2 receptors has been associated with decreased neuroinflammation, which could support healthy cognitive function (Saito et al. 2012). Immune response regulation in CNS is associated with anti-inflammatory effects and cognitive performance (Benito et al. 2008). Activation of CB2 receptors can limit immune cell activation and decrease the release of pro-inflammatory cytokines, potentially promoting an environment that is ideal for cognitive function (Nouh et al. 2023). Although microglial dysregulation can result in long-term inflammation and cognitive decline, microglia are crucial for maintaining brain health. It is known that stimulating CB2 receptors suppresses active microglia’s production and release of pro-inflammatory cytokines, eventually lowering neuroinflammation. CB2 receptors contribute to a suitable condition for healthy brain function by lowering inflammation in the CNS (Ashton and Glass 2007). The effects of CB2 receptors on neuroinflammation, cognitive dysfunction, and neurodegeneration are mentioned in Table 4.
Multiple sclerosis
MS is a complicated neurological disease characterized by inflammation, demyelination, and the axonal destruction of neurons. MS pathophysiology is characterized by the breakdown of the myelin sheath, which protects axons, as a result of an attack of immune system involving T and B cells, and their cytokines. The pathogenesis of MS is mostly determined by inflammation in the white and grey matter as well as a number of environmental factors, including bacteria and viruses such as the Epstein-Barr virus (EBV), human herpes virus type 6, and mycoplasma pneumonia. Moreover, greater exposure to UV radiation, nutritional deficiencies, and smoking are associated with development of MS (Ghasemi et al. 2017). CB2 receptors activation has been found to modulate immune cell activity by inhibiting the production of pro-inflammatory cytokines and restricting immune cell migration into the CNS. These anti-inflammatory effects help to mitigate the damage caused by the immune system in MS patients (Mecha et al. 2013). The dysregulation of immune system leads to inflammation, demyelination, and scar tissue development, reducing the neurological system’s ability to regulate normally. Genetic and environmental factors are thought to play a role for develop the disease and how they play important role to treat the inflammatory disease like MS. CB2 receptors have received a lot of interest for their possible anti-inflammatory benefits, especially in the setting of immune-mediated diseases like MS (Calderon et al. 2006). Immunological cells, particularly microglia, have CB2 receptors that, when engaged, can control immunological responses by lowering immune cell activation and preventing the release of pro-inflammatory cytokines. The activation of CB2 receptors is believed to have anti-inflammatory and immune system-balancing benefits. In an effort to reduce the immune system’s damage to myelin and neurons in MS, CB2 receptors activation on microglia may have a neuroprotective function (Yiangou et al. 2006).
Huntington’s disease
HD is a hereditary neurodegenerative disease characterized by progressive motor dysfunction with cognitive and behavioural disturbances. The causes of HD are genetic mutations in the Huntingtin gene (HTT gene), leading to the production of a mutant form of the Huntington protein, and abnormal proteins accumulate in neurons, especially in the basal ganglia, a region of the brain responsible for movement control and coordination. HTT has up to 35 CAG repeats in healthy subjects; however, CAG repeats over 37 are found in those with HD (Chen and Wolynes 2017). It has three HEAT domains, known as the polyproline region, the N-terminal polyQ region, nuclear export signals (NES) and nuclear localization signals (NLS), which are also present at the carboxy-terminus of HTT. An overabundance of CAG repeats can disrupt the splicing of mutant HTT (mHTT), leading to the aberrant splicing that produces the shortened transcript known as HTT exon1 (Tong et al. 2024). The key neuropathological hallmarks associated with HD are the accumulation of reactive microglia, neuroinflammation, and the progressive death of neurons in the brain and striatum. Increased concentrations of many pro-inflammatory cytokines, such as IL-6, IL-1β, TNF-α, and IL-8, found in the nerve cells and plasma of HD patients, underline the significance of neuroinflammation for the cause of the disease. CB2 receptors activation may influence oxidative stress, another factor implicated in the progression of HD (Palpagama et al. 2019). Oxidative stress develops when the production of ROS exceeds the body’s natural ability to neutralize them. The brain is particularly vulnerable to oxidative stress, and it contributes to neuronal damage in neurodegenerative disorders. The latest research indicates that an extensive variety of proteins interact with both mutant and wild-type HTT (wtHTT) for signal transduction, protein translation, chromatin organization and membrane trafficking. In addition, protein-protein interactions, including those involving HAP1, HAP40, HIP-1, syntaxin-1B, vesicle-associated membrane protein 2, SNAP25, NSF, and synapsins 1 and 2, are dependent on the polyQ sequences reliant on HTT. mHTT modifies the stability and amounts of these protein interactions, which may result in the deregulation of gene expression, intracellular signalling cascades, synaptic function, and cellular processes (Tong et al. 2024). CB2 receptors activation has been suggested to have antioxidant properties, potentially reducing oxidative damage by influencing the expression of neuronal NOS, excitotoxicity, and apoptosis (Yang et al. 2017; Tai et al. 2007). Bouchard and colleagues showed that the CB2 receptors agonist GW405833 improves motor impairments, synapse loss, neuroinflammation, and lifespan in mouse models of HD, but the CB2 receptors antagonist SR2 was able to counteract these effects (Bouchard et al. 2012). HD transgenic mice and patients had higher levels of CB2 receptors expression, while R6/2 mice with CB2 receptors deficiency had more disease symptoms, increased microglia activation, and shorter lifespans. By delaying the degeneration of striatal neurons, CB2 receptors selective agonists have been shown to reduce neuroinflammation, reduce brain oedema, and maintain motor performance in wild type mice CB2 receptors (Palazuelos et al. 2009).
Parkinson’s disease
PD is a second neurodegenerative disease, often characterized by a progressive loss of dopaminergic neurons, in SNpc of the brain. The features of PD are postural instability, stiffness, tremors, and impaired motor function, that affects approximately 1% of the elderly population. The pathophysiology of PD includes calcium dysregulation, oxidative stress, protein misfolding, mitochondrial dysfunction, and neuroinflammation, which is manifested by microglia activation and an increase in cytokine production. CB2 receptors are found in dopaminergic neurons of the nigrostriatal pathway, and because of CB2 receptors activation, antioxidant enzymes show greater effectiveness in animal models of PD. This suggests that antioxidants play a role in lowering excitotoxicity, oxidative stress, and neuroinflammation, all of which may slow down the disease’s progression (De et al. 2007). Additionally, CB2 receptors could contribute to the progression of PD by influencing neuronal signalling and function, neurotransmission, and neurological inflammation. Activation of CB2 receptors reduces the production of pro-inflammatory cytokines and gliosis, reduces the activation of astrocytes and microglia, and prevents nigro-striatal neurodegeneration. Numerous studies have demonstrated the neuroprotective qualities of CB2 receptors agonists (JWH-133, HU-308, and JWH-015) via lowering microglia activity and inflammation, inhibiting the production of pro-inflammatory cytokines, encouraging the release of anti-inflammatory cytokines, and raising glutamate uptake (Wang et al. 2002). The CB2 receptors act as neuroprotective and anti-inflammatory in PD, as mentioned in Fig. 5; Table 5.
CB2 receptors and behavior effect on mood and cognition dysfunction
Neurodegenerative diseases have been associated with immune system alterations that impact emotional behaviour, memory, learning, and cognition. CB2 receptors are found in the immune system and brain tissues, they have a variety of consequences for behaviour and cognition (Morcuende et al. 2022). CB2 receptors, have gained interest due to its potential involvement in behaviour regulation, memory, and cognition. Immune regulation, anti-inflammatory properties, memory, and cognition are all commonly associated with CB2 receptors (Zanettini et al. 2011). Researchers have detected the immune function control mechanism in microglia, astrocytes, and neurons, suggesting that it may play a role in regulating neuronal activity. Immune reactions in the CNS cause neuroinflammation, which has a significant impact on cognitive performance (Kumar 2018). Recognized for their ability to reduce inflammation, CB2 receptors are involved in controlling neuroinflammation. The anti-inflammatory properties of CB2 receptors are one of the main ways in which they affect mood (Morcuende et al. 2022). Activation of CB2 receptors reduces the release of pro-inflammatory cytokines and modulates immunological responses. By establishing an environment where inflammatory processes have less of an impact on brain circuits associated with mood (Micale et al. 2013).
Furthermore, CB2 receptors activation may mitigate its negative influence on mood-regulating areas such as the hippocampus and prefrontal cortex during periods of chronic stress or inflammation. Numerous neurotransmitter systems involved in mood and anxiety control interact with CB2 receptors. Part of the possible antidepressant effects of CB2 receptors may come from their modulation of serotonin release and reuptake. Furthermore, the GABA-argic system, which is essential for controlling anxiety, interacts with the ECS, which includes CB2 receptors. Since GABA is an inhibitory neurotransmitter, activation of the CB2 receptors may affect GABA-argic transmission and have anxiolytic effects.
Conclusion
The endocannabinoid system is intricately associated with a wide range of cells, tissues, and organs, making it a prospective target for therapeutic interventions. The CB1 and CB2 receptors are crucial to this system and have an important role in controlling neurological inflammation in both CNS and peripheral tissues, making them a potential target for treatments of autoimmune diseases and neurodegenerative diseases. The CB2 receptors are abundantly expressed in neuropsychiatric and neurodegenerative diseases, and selective CB2 receptors ligands show potential benefits in symptomatic therapy of these disorders. Further research is needed to assess the involvement of CB2 receptors in these diseases, utilizing the whole spectrum of methods accessible to investigate the CB2 receptors and their selective ligands in animal models as well as in controlled human trials. Future research should include translational and clinical characteristics, as well as in vitro and in vivo models expressing human CB2 receptors. A thorough assessment of the side effects associated with chronic CB2 receptors ligand treatment will offer deeper insights into how CB2 receptors regulate neurophysiological and behavioural functions.
Data availability
Not applicable.
Abbreviations
- ECS:
-
Endocannabinoid system
- CB:
-
Cannabinoid receptors
- GPCR:
-
G protein-coupled receptor
- 2-AG:
-
2-Arachidonoylglycerol
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- HD:
-
Huntington’s disease
- MS:
-
Multiple sclerosis
- AC:
-
Adenylate cyclase
- MAPK:
-
Mitogen-activated protein kinase
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- CNS:
-
Central nervous system
- PNS:
-
Peripheral nervous system
- BDNF:
-
Brain-derived neurotrophic factor
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- NOS:
-
Nitric oxide synthase
- ATP:
-
Adenosine triphosphate
- APP:
-
Amyloid precursor protein
- cAMP:
-
Cyclic adenosine monophosphate
- iNOS:
-
Inducible nitric oxide synthase
- PET:
-
Positron emission tomography
- GABA:
-
Gamma-aminobutyric acid
References
Adel Y, Alexander SP (2021) Neuromolecular mechanisms of cannabis action. Cannabinoids Neuropsychiatric Disorders 15–28. https://doi.org/10.1007/978-3-030-57369-0_2
Anthony AT, Rahmat S, Sangle P, Sandhu O, Khan S (2020) Cannabinoid receptors and their relationship with chronic pain: a narrative review. Cureus 12(9). https://doi.org/10.7759/cureus.10436
Ashton JC, Glass M (2007) The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol 5(2):73–80. https://doi.org/10.2174/157015907780866884
Aso E, Andrés-Benito P, Ferrer I (2016) Delineating the efficacy of a Cannabis-Based Medicine at Advanced Stages of Dementia in a murine model. J Alzheimers Dis 54(3):903–912. https://doi.org/10.3233/JAD-160533
Atwood BK, Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160(3):467–479. https://doi.org/10.1111/j.1476-5381.2010.00729.x
Aychman MM, Goldman DL, Kaplan JS (2023) Cannabidiol’s neuroprotective properties and potential treatment of traumatic brain injuries. Front Neurol 14:1087011. https://doi.org/10.3389/fneur.2023.1087011
Bachmeier C, Beaulieu-Abdelahad D, Mullan M, Paris D (2013) Role of the cannabinoid system in the transit of beta-amyloid across the blood–brain barrier. Mol Cell Neurosci 56:255–262. https://doi.org/10.1016/j.mcn.2013.06.004
Basu S, Dittel BN (2011) Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res 51(1):26–38. https://doi.org/10.1007/s12026-011-8210-5
Bayewitch M, Avidor-Reiss T, Levy R, Barg J, Mechoulam R, Vogel Z (1995) The peripheral cannabinoid receptor: adenylate cyclase inhibition and G protein coupling. FEBS Lett 375(1–2):143–147. https://doi.org/10.1016/0014-5793(95)01207-u
Benito C, Tolón RM, Pazos MR, Núñez E, Castillo AI, Romero J (2008) Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol 153(2):277–285. https://doi.org/10.1038/sj.bjp.0707505
Bie B, Wu J, Foss JF, Naguib M (2018) An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol 31(4):407–414. https://doi.org/10.1097/ACO.0000000000000616
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76(2):77–98. https://doi.org/10.1016/j.pneurobio.2005.06.004
Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrié B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P (1996) Signalling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem 237(3):704–711. https://doi.org/10.1111/j.1432-1033.1996.0704p.x
Bouchard J, Truong J, Bouchard K, Dunkelberger D, Desrayaud S, Moussaoui S, Tabrizi SJ, Stella N, Muchowski PJ (2012) Cannabinoid receptor 2 signalling in peripheral immune cells modulates disease onset and severity in mouse models of Huntington’s disease. J Neurosci 32(50):18259–18268. https://doi.org/10.1523/JNEUROSCI.4008-12.2012
Bow EW, Rimoldi JM (2016) The structure-function relationships of classical cannabinoids: CB1/CB2 modulation. Perspect Medicin Chem 8:17–39. https://doi.org/10.4137/PMC.S32171
Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A (2000) Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol 396(2–3):141–149. https://doi.org/10.1016/s0014-2999(00)00211-9
Bueb JL, Lambert DM, Tschirhart EJ (2001) Receptor-independent effects of natural cannabinoids in rat peritoneal mast cells in vitro. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1538(2–3):252–259. https://doi.org/10.1016/S0167-4889(01)00076-3
Cabral GA, Griffin-Thomas L (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11: e3. https://doi.org/10.1017/S1462399409000957
Cabral GA, Toney DM, Fischer-Stenger K, Harrison MP, Marciano-Cabral F (1995) Anandamide inhibits macrophage-mediated killing of tumor necrosis factor-sensitive cells. Life Sci 56(23–24):2065–2072. https://doi.org/10.1016/0024-3205(95)00190-H
Calandra B, Portier M, Kernéis A, Delpech M, Carillon C, Le Fur G, Ferrara P, Shire D (1999) Dual intracellular signalling pathways mediated by the human cannabinoid CB1 receptor. Eur J Pharmacol 374(3):445–455. https://doi.org/10.1016/s0014-2999(99)00349-0
Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW (2006) A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol 177(1–2):27–39. https://doi.org/10.1016/j.jneuroim.2006.05.003
Carayon P, Marchand J, Dussossoy D, Derocq JM, Jbilo O, Bord A, Bouaboula M, Galiègue S, Mondiere P, Pénarier G, Fur GL (1998) Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood J Am Soc Hematol 92(10):3605–3615. https://doi.org/10.1182/blood.V92.10.3605.422k05_3605_3615
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75(1):50–83. https://doi.org/10.1128/MMBR.00031-10
Chen M, Wolynes PG (2017) Aggregation landscapes of huntingtin exon 1 protein fragments and the critical repeat length for the onset of Huntington’s disease. Proc Natl Acad Sci U S A 114(17):4406–4411. https://doi.org/10.1073/pnas.1702237114
Cheung KAK, Peiris H, Wallace G, Holland OJ, Mitchell MD (2019) The interplay between the Endocannabinoid System, Epilepsy and cannabinoids. Int J Mol Sci 20(23):6079. https://doi.org/10.3390/ijms20236079
Concannon RM, Dowd E (2016) Central CB2 receptors in inflammation-driven neurodegeneration: dysregulation and therapeutic potential. Neural Regeneration Res 11(9):1409–1410. https://doi.org/10.4103/1673-5374.191208
Condello C, Yuan P, Schain A, Grutzendler J (2015) Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat Commun 6(1):6176. https://doi.org/10.1038/ncomms7176
Correa F, Docagne F, Mestre L, Clemente D, Hernangómez M, Loría F, Guaza CA (2009) Role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells. Biochem Pharmacol 77(1):86–100. https://doi.org/10.1016/j.bcp.2008.09.014
Correa F, Hernangómez M, Mestre L, Loría F, Spagnolo A, Docagne F, Di Marzo V, Guaza C (2010) Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB. Glia 58(2):135–147. https://doi.org/10.1002/glia.20907
Dalle S, Schouten M, Meeus G, Slagmolen L, Koppo K (2022) Molecular networks underlying cannabinoid signalling in skeletal muscle plasticity. J Cell Physiol 237(9):3517–3540. https://doi.org/10.1002/jcp.30837
Davis MP (2014) Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands. Expert Opin Investig Drugs 23(8):1123–1140. https://doi.org/10.1517/13543784.2014.918603
De Filippis D, Russo A, D’amico A, Esposito G, Concetta P, Cinelli M, Russo G, Iuvone T (2008) Cannabinoids reduce granuloma-associated angiogenesis in rats by controlling transcription and expression of mast cell protease‐5. Br J Pharmacol 154(8):1672–1679. https://doi.org/10.1038/bjp.2008.211
Demuth DG, Molleman A (2006) Cannabinoid signalling. Life Sci. 2006;78(6):549 – 63. https://doi.org/10.1016/j.lfs.2005.05.055
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A (1992) Mechoulam R. isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–1949. https://doi.org/10.1126/science.1470919
Di Marzo V, Stella N, Zimmer A (2015) Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 16(1):30–42. https://doi.org/10.1038/nrn3876
Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS (2004) Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-κB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol 173(4):2373–2382. https://doi.org/10.4049/jimmunol.173.4.2373
Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD (2005) Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation 2:29. https://doi.org/10.1186/1742-2094-2-29
Esposito G, Scuderi C, Savani C, Steardo L Jr, De Filippis D, Cottone P, Iuvone T, Cuomo V, Steardo L (2006) Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol 151(8):1272–1279. https://doi.org/10.1038/sj.bjp.0707337
Esposito G, Iuvone T, Savani C, Scuderi C, De Filippis D, Papa M, Di Marzo V, Steardo L (2007) Opposing control of cannabinoid receptor stimulation on amyloid-β-induced reactive gliosis: in vitro and in vivo evidence. J Pharmacol Exp Ther 322(3):1144–1152. https://doi.org/10.1124/jpet.107.121566
Eubanks LM, Rogers CJ, Beuscher AE 4th, Koob GF, Olson AJ, Dickerson TJ, Janda KD (2006) A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol Pharm 3(6):773–777. https://doi.org/10.1021/mp060066m
Eyo UB, Dailey ME (2013) Microglia: key elements in neural development, plasticity, and pathology. J Neuroimmune Pharmacol 8(3):494–509. https://doi.org/10.1007/s11481-013-9434-z
Fernández-Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M (2007) Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci 28(1):39–45. https://doi.org/10.1016/j.tips.2006.11.001
Fernández-Ruiz J, Pazos MR, García-Arencibia M, Sagredo O, Ramos JA (2008) Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol Cell Endocrinol 286(1–2 Suppl 1):S91–S96. https://doi.org/10.1016/j.mce.2008.01.001
Fu W, Taylor BK (2015) Activation of cannabinoid CB2 receptors reduces hyperalgesia in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Neurosci Lett 595:1–6. https://doi.org/10.1016/j.neulet.2015.04.002
Ghasemi N, Razavi S, Nikzad E (2017) Multiple sclerosis: Pathogenesis, symptoms, diagnoses and Cell-based therapy. Cell J 19(1):1–10. https://doi.org/10.22074/cellj.2016.4867
Ghonghadze M, Pachkoria K, Okujava M, Antelava N, Gongadze N (2020) Endocannabinoids receptors mediated central and peripheral effects (review). Georgian Med News 298:137–143
Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J, García C (2016) Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 64:200–208. https://doi.org/10.1016/j.pnpbp.2015.03.017
Gu Y, Wang Z, Shi J (2017) Titanium particle-induced osteogenic inhibition and bone destruction are mediated by the GSK-3β/β-catenin signal pathway. Cell Death Dis 8:e2878. https://doi.org/10.1038/cddis.2017.275
Heneka MT, Golenbock DT, Latz E (2015) Innate immunity in Alzheimer’s disease. Nat Immunol 16(3):229–236. https://doi.org/10.1038/ni.3102
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, Alzheimer, van Duijn CM, Breteler MM, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, CHARGE consortium, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alpérovitch A, Lathrop M, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Björnsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossù P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J (2011) Nat Genet 43(5):429–435 Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. https://doi.org/10.1038/ng.803
Jimenez-Del-Rio M, Daza-Restrepo A, Velez-Pardo (2008) The cannabinoid CP55,940 prolongs survival and improves locomotor activity in Drosophila melanogaster against paraquat: implications in Parkinson’s disease. Neurosci Res 61(4):404–411. https://doi.org/10.1016/j.neures.2008.04.011
Kaminski NE, Koh WS, Yang KH, Lee M, Kessler FK (1994) Suppression of the humoral immune response by cannabinoids is partially mediated through inhibition of adenylate cyclase by a pertussis toxin-sensitive G-protein coupled mechanism. Biochem Pharmacol 48(10):1899–1908. https://doi.org/10.1016/0006-2952(94)90588-6
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91(2):461–553. https://doi.org/10.1152/physrev.00011.2010
Kishimotos, Gokoh M (2005) 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces rapid actin polymerization in HL-60 cells differentiated into macrophage-like cells April 2005. Biochem J 386(Pt3):583–589. https://doi.org/10.1042/BJ20041163
Klegeris A, Bissonnette CJ, McGeer PL (2003) Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol 139(4):775–786. https://doi.org/10.1038/sj.bjp.0705304
Klein TW, Newton CA, Friedman H (2001) Cannabinoids and the immune system. Pain Res Manag 6(2):95–101. https://doi.org/10.1155/2001/326867
Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H (2003) The cannabinoid system and immune modulation. J Leukoc Biol 74(4):486–496. https://doi.org/10.1189/jlb.0303101
Köfalvi A, Lemos C, Martín-Moreno AM, Pinheiro BS, García-García L, Pozo MA, Valério-Fernandes Â, Beleza RO, Agostinho P, Rodrigues RJ, Pasquaré SJ (2016) Stimulation of brain glucose uptake by cannabinoid CB2 receptors and its therapeutic potential in Alzheimer’s disease. Neuropharmacology 110:519–529. https://doi.org/10.1016/j.neuropharm.2016.03.015
Komorowska-Müller JA, Schmöle AC (2020) CB2 receptor in Microglia: the Guardian of Self-Control. Int J Mol Sci 22(1):19. https://doi.org/10.3390/ijms22010019
Koppel J, Davies P (2008) Targeting the Endocannabinoid System in Alzheimer’s Disease. J Alzheimers Dis 15(3):495–504. https://doi.org/10.3233/jad-2008-15315
Kumar A (2018) Neuroinflammation and cognition. Front Aging Neurosci 10:413. https://doi.org/10.3389/fnagi.2018.00413
Kurihara R, Tohyama Y, Matsusaka S, Naruse H, Kinoshita E, Tsujioka T, Katsumata Y, Yamamura H (2006) Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J Biol Chem 281(18):12908–12918. https://doi.org/10.1074/jbc.M510871200
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9(1):42. https://doi.org/10.1186/s40035-020-00221-2
Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J (2005) Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis 19(1–2):96–107. https://doi.org/10.1016/j.nbd.2004.11.009
Leleu-Chavain N, Desreumaux P, Chavatte P, Millet R (2013) Therapeutical potential of CB₂ receptors in immune-related diseases. Curr Mol Pharmacol 6(3):183–203. https://doi.org/10.2174/1874467207666140219122337
Li X, Chang H, Bouma J (2023) Structural basis of selective cannabinoid CB2 receptor activation. Nat Commun 14, 1447 (2023). https://doi.org/10.1038/s41467-023-37112-9
Lu HC, Mackie K (2016) An introduction to the endogenous cannabinoid system. Biol Psychiatry 79(7):516–525. https://doi.org/10.1016/j.biopsych.2015.07.028
Luchicchi A, Pistis M (2012) Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol Neurobiol 46(2):374–392. https://doi.org/10.1007/s12035-012-8299-0
Lunn CA, Reich EP, Bober L (2006) Targeting the CB2 receptor for immune modulation. Expert Opin Ther Targets 10(5):653–663. https://doi.org/10.1517/14728222.10.5.653
Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN (2005) Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95(2):437–445. https://doi.org/10.1111/j.1471-4159.2005.03380
Matias I, Pochard P, Orlando P, Salzet M, Pestel J, Di Marzo V (2002) Presence and regulation of the endocannabinoid system in human dendritic cells. Eur J Biochem 269(15):3771–3778. https://doi.org/10.1046/j.1432-1033.2002.03078.x
Mecha M, Feliú A, Iñigo PM, Mestre L, Carrillo-Salinas FJ, Guaza C (2013) Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis 59:141–150. https://doi.org/10.1016/j.nbd.2013.06.016
Mechoulam R, Parker LA (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47. https://doi.org/10.1146/annurev-psych-113011-143739
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1):83–90. https://doi.org/10.1016/0006-2952(95)00109-d
Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F (2013) Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther 138(1):18–37. https://doi.org/10.1016/j.pharmthera.2012.12.002
Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, Rothwell NJ (2003) Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci 23(16):6470–6474. https://doi.org/10.1523/JNEUROSCI.23-16-06470.2003
Morcuende A, García-Gutiérrez MS, Tambaro S, Nieto E, Manzanares J, Femenia T (2022) Immunomodulatory Role of CB2 receptors in Emotional and Cognitive disorders. Front Psychiatry 13:866052. https://doi.org/10.3389/fpsyt.2022.866052
Ni R, Mu L, Ametamey S (2019) Positron emission tomography of type 2 cannabinoid receptors for detecting inflammation in the central nervous system. Acta Pharmacol Sin 40(3):351–357. https://doi.org/10.1038/s41401-018-0035-5
Nouh RA, Kamal A, Abdelnaser A (2023) Cannabinoids and multiple sclerosis: a critical analysis of therapeutic potentials and safety concerns. Pharmaceutics 15(4):1151. https://doi.org/10.3390/pharmaceutics15041151
Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, González S, Tolón RM, Romero J (2004) Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse 53(4):208–213. https://doi.org/10.1002/syn.20050
Oddi S, Latini L, Viscomi MT, Bisicchia E, Molinari M, Maccarrone M (2012) Distinct regulation of nNOS and iNOS by CB2 receptor in remote delayed neurodegeneration. J Mol Med (Berl) 90(4):371–387. https://doi.org/10.1007/s00109-011-0846-z
Opal SM, DePalo VA (2000) Anti-inflammatory cytokines. Chest 117(4):1162–1172. https://doi.org/10.1378/chest.117.4.1162
Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, Fernández-Ruiz J, Guzmán M, Galve-Roperh I (2009) Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 132(Pt 11):3152–3164. https://doi.org/10.1093/brain/awp239
Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A (2019) The role of microglia and astrocytes in Huntington’s Disease. Front Mol Neurosci 12:258. https://doi.org/10.3389/fnmol.2019.00258
Paradisi A, Oddi S, Maccarrone M (2006) The endocannabinoid system in ageing: a new target for drug development. Curr Drug Targets 7(11):1539–1552. https://doi.org/10.2174/1389450110607011539
Pazos M, Sagredo O, Fernandez-Ruiz J (2008) The endocannabinoid system in Huntington’s disease. Curr Pharm Design 14(23):2317–2325. https://doi.org/10.2174/138161208785740108
Pisani A (2005) High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Annals Neurology: Official J Am Neurol Association Child Neurol Soc 57(5):777–779. https://doi.org/10.1002/ana.20462
Raborn ES, Cabral GA, Griffin L, Dennis J, Marciano-Cabral F (2008) CB2 receptors in the brain: role in central immune function. Br J Pharmacol 153(2):240–251. https://doi.org/10.1038/sj.bjp.0707617
Rodrigues RS, Lourenço DM, Paulo SL, Mateus JM, Ferreira MF, Mouro FM, Moreira JB, Ribeiro FF, Sebastião AM, Xapelli S (2019) Cannabinoid actions on neural stem cells: implications for pathophysiology. Molecules 24(7):1350. https://doi.org/10.3390/molecules24071350
Rodríguez Mesa XM, Moreno Vergara AF, Contreras Bolaños LA, Guevara Moriones N, Mejía Piñeros AL, Santander González SP (2021) Therapeutic prospects of cannabinoids in the Immunomodulation of Prevalent Autoimmune diseases. Cannabis Cannabinoid Res 6(3):196–210. https://doi.org/10.1089/can.2020.0183
Romero-Sandoval EA, Horvath R, Landry RP, DeLeo JA (2009) Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain 5:25. https://doi.org/10.1186/1744-8069-5-25
Saito VM, Rezende RM, Teixeira AL (2012) Cannabinoid modulation of neuroinflammatory disorders. Curr Neuropharmacol 10(2):159–166. https://doi.org/10.2174/157015912800604515
Sánchez C, Galve-Roperh I, Canova C, Brachet P, Guzmán M (1998) ∆9-Tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett 436(1):6–10. https://doi.org/10.1016/S0014-5793(98)01085-0
Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, Hardy J, Vassar R, Winblad B, Saido TC (2017) APP mouse models for Alzheimer’s disease preclinical studies. EMBO J 36(17):2473–2487. https://doi.org/10.15252/embj.201797397
Schwarz H, Blanco FJ, Lotz M (1994) Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol 55(1):107–115. https://doi.org/10.1016/0165-5728(94)90152-X
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6):595–608. https://doi.org/10.15252/emmm.201606210
Shao F, Wang X, Wu H, Wu Q, Zhang J (2022) Microglia and Neuroinflammation: crucial pathological mechanisms in traumatic Brain Injury-Induced Neurodegeneration. Front Aging Neurosci 14:825086. https://doi.org/10.3389/fnagi.2022.825086
Sierra A, Abiega O, Shahraz A, Neumann H (2013) Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. https://doi.org/10.3389/fncel.2013.00006
Sophocleous RA, Ooi L, Sluyter R (2022) The P2X4 receptor: cellular and molecular characteristics of a promising neuroinflammatory target. Int J Mol Sci 23(10):5739. https://doi.org/10.3390/ijms23105739
Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, Ogawa H (2009) Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 119(1):28–36. https://doi.org/10.1161/CIRCULATIONAHA.108.811992
Svízenská I, Dubový P, Sulcová A (2008) Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures–a short review. Pharmacol Biochem Behav 90(4):501–511. https://doi.org/10.1016/j.pbb.2008.05.010
Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P (2007) Imaging microglial activation in Huntington’s disease. Brain Res Bull 72(2–3):148–151. https://doi.org/10.1016/j.brainresbull.2006.10.029
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Tong H, Yang T, Xu S, Li X, Liu L, Zhou G, Yang S, Yin S, Li XJ, Li S (2024) Huntington’s disease: complex pathogenesis and therapeutic strategies. Int J Mol Sci 25(7):3845. https://doi.org/10.3390/ijms25073845
Tóth KF, Ádám D, Bíró T, Oláh A (2019) Cannabinoid signalling in the skin: therapeutic potential of the Cannabinoid System. Molecules 24(5):918. https://doi.org/10.3390/molecules24050918
Turcotte C, Blanchet MR, Laviolette M, Flamand N (2016) The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci 73(23):4449–4470. https://doi.org/10.1007/s00018-016-2300-4
Wang M, Liu H, Ma Z (2002) Roles of the Cannabinoid System in the basal ganglia in Parkinson’s Disease. Front Cell Neurosci 16:832854. https://doi.org/10.3389/fncel.2022.832854
Wang L, Liu BJ, Cao Y, Xu WQ, Sun DS, Li MZ, Shi FX, Li M, Tian Q, Wang JZ, Zhou XW (2018) Deletion of Type-2 cannabinoid receptor induces Alzheimer’s Disease-Like Tau Pathology and Memory Impairment through AMPK/GSK3β Pathway. Mol Neurobiol 55(6):4731–4744. https://doi.org/10.1007/s12035-017-0676-2
Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M (2013) Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol Aging 34(3):791–804. https://doi.org/10.1016/j.neurobiolaging.2012.06.011
Yang HM, Yang S, Huang SS, Tang BS, Guo JF (2017) Microglial activation in the Pathogenesis of Huntington’s Disease. Front Aging Neurosci 9:193. https://doi.org/10.3389/fnagi.2017.00193
Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P (2006) COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol 6:12. https://doi.org/10.1186/1471-2377-6-12
Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S (2011) Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci 5:57. https://doi.org/10.3389/fnbeh.2011.00057
Zhang M, Chen T, Lu X (2024) G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery. Sig Transduct Target Ther 9, 88 (2024). https://doi.org/10.1038/s41392-024-01803-6
Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, Dubinett SM (2000) Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol 165(1):373–380. https://doi.org/10.4049/jimmunol.165.1.373
Zou S, Kumar U (2018) Cannabinoid receptors and the endocannabinoid system: signalling and function in the central nervous system. Int J Mol Sci 19(3):833. https://doi.org/10.3390/ijms19030833
Acknowledgements
Special thanks to Shri. Parveen Garg, Chairman, ISFCP, for providing an excellent research platform. This work wouldn’t have been possible without their collective influence.
Funding
There is no funding agency in this article.
Author information
Authors and Affiliations
Contributions
KB and PP: Writing – review & editing the manuscript. KRA: Conceptualization, review, editing, visualization.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors assert that they do not have any known financial interests or personal relationships that could be perceived as influencing the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bala, K., Porel, P. & Aran, K.R. Emerging roles of cannabinoid receptor CB2 receptor in the central nervous system: therapeutic target for CNS disorders. Psychopharmacology (2024). https://doi.org/10.1007/s00213-024-06683-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00213-024-06683-w