Abstract
Introduction
Stimulant drugs are thought to alter processing of rewarding stimuli. However, the mechanisms by which they do this are not fully understood.
Method
In this study we used EEG to assess effects of single doses of methamphetamine (MA) on neural responses during anticipation and receipt of reward in healthy volunteers. Healthy young men and women (N = 28) completed three sessions in which they received placebo, a low MA dose (10 mg) or a higher MA dose (20 mg) under double blind conditions. Subjective and cardiovascular measures were obtained, and EEG was used to assess brain activity during an electrophysiological version of the Monetary Incentive Delay (eMID) task.
Results
EEG measures showed expected patterns during anticipation and receipt of reward, and MA produced its expected effects on mood and cardiovascular function. However, MA did not affect EEG responses during either anticipation or receipt of rewards.
Conclusions
These findings suggest that the effects of MA on EEG signals of reward processing are subtle, and not related to the drug’s effects on subjective feelings of well-being. The findings contribute to our understanding of the neural effects of MA during behaviors related to reward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphetamines and other stimulants are widely used for both therapeutic and non-medical purposes. Clinically, they are approved to treat attention deficit hyperactivity disorder (ADHD), sleep-disorders, obesity, and binge-eating disorder (Faraone 2018; Castells et al. 2018; Crow 2019), and they are used off-label to maintain wakefulness and counter fatigue (Maski et al. 2021) and even as antidepressants (McIntyre et al. 2017). However, stimulant drugs also have a high potential for abuse; they produce feelings of euphoria and can lead to compulsive drug-seeking (SAMHSA 2022). Both the therapeutic and non-medical use of stimulants are thought to be related to dopaminergic actions, perhaps by enhancing the processing of reward (Di Chiara and Imperato 1988).
Studying the effects of stimulant drugs on neural processing of reward can provide insights on the drugs’ actions, and how the brain processes reward. Stimulants increase synaptic levels of dopamine and norepinephrine, and to a lesser extent serotonin (Moore 1977). Their actions on reward processing and positive mood are thought to be related mainly to their effects on dopamine, but the exact mechanisms are unclear (Volkow et al. 1999; Mattay et al. 2000; Drevets et al. 2001; Zack et al. 2017). The brain circuits involved in processing of reward are complex, involving many components including baseline or tonic states, attention, and processing of salient events such as anticipated or received rewards or losses. Understanding how stimulant drugs alter neural indices of reward sensitivity, using imaging or electroencephalography, will advance our understanding of how the drugs alter motivated behaviors.
Several studies have investigated the effects of stimulant drugs on reward processing using either brain imaging techniques or electroencephalography (EEG) (Glazer et al. 2018; Helfrich and Knight 2019). In one study Knutson et al. (2004) used fMRI with the monetary incentive delay (MID) task and found that amphetamine decreased neural activity in the ventral striatum during reward anticipation. The MID task was designed to separately measure distinct brain processes during reward anticipation (e.g., “wanting”) and reward receipt (e.g., “liking”) (Knutson et al. 2004; Berridge et al. 2009; Haber and Knutson 2010; Jauhar et al. 2021), two temporally distinct stages of reward processing that display separate neurochemical, neuroanatomical, and neurophysiological correlates (Berridge et al. 2009; Schultz 2007; Liu et al. 2011). The authors suggested that blunted response to a rewarding event resulted from increased tonic dopamine levels, consistent with prior animal and human research that highlight the involvement of dopamine signaling during reward-anticipation (see Berridge 2007; Berridge et al. 2009 for review). In another study, Cavanagh et al. (2022) used EEG to examine event-related potentials (ERPs) occurring during anticipation and receipt of rewards and losses in a probabilistic learning task. They found that amphetamine increased the reward positivity peak (RewP), an EEG signal of reward processing. Crane et al. (2018) and Langenecker et al. (2020) reported indirect links between neural mechanisms of reward processing and responses to the stimulant amphetamine in healthy adults. They found a positive correlation between neural activation in reward-related areas upon receipt of a monetary reward (without drug administration) and euphorigenic effects of amphetamine, determined on a separate session.
The present study used an electrophysiological version of the MID (eMID; Broyd et al. 2012; Flores et al. 2015) to investigate the apparently conflicting previous findings of Knutson et al. (2004) and Cavanagh et al. (2022). The eMID task leverages the temporal resolution of ERP methods to decompose the neural time course of reward anticipation and receipt into separate electrophysiological components that display distinct time-courses, scalp topographies, and reflect independent psychological processes (Glazer et al. 2018; Broyd et al. 2012; Novak and Foti 2015). It provides measures of brain activation during both reward anticipation and receipt. Anticipatory Cues elicit two ERP components of interest, the Cue-N2, a frontocentral ERP reflecting early attention and perceptual processing of stimuli (Polezzi et al. 2008; Novak and Foti 2015) and the Cue-P3 (Polich 2007), a centroparietal ERP reflecting motivational salience and stimulus categorization and covaries (Polich 2007; Pfabigan et al. 2014). Reward feedback stimuli elicit four sequential ERP components, the P2 (FB-P2), Reward-Positivity (RewP), the Feedback P3 (FB-P3), and Feedback Late-Positive-Potential (FB-LPP). The RewP is of particular interest because it encodes the hedonic impact of positive (vs. negative) feedback (Holroyd et al. 2008; Proudfit 2015) and is believed to reflect reward prediction errors (Schultz et al. 1997; Walsh and Anderson 2012; Sambrook and Goslin 2015). It is dampened in individuals with affective disorders such as depression (Bowyer et al. 2019) and it is sensitive to acute effects of psychoactive drugs (Murray et al 2022; Glazer et al 2022). The FB-P3 is a centroparietal ERP that indexes the motivational salience of feedback and covaries with activation in the thalamus and hippocampus (San Martin 2012). Finally, the FB-LPP is a centroparietal ERP that reflects the affective impact of feedback and covaries with activation in the amygdala and visual cortex (Bradley et al. 2003; Pornpattananangkul and Nusslock 2015). Together, the FB-P2, RewP, FB-P3, and FB-LPP reflect the hedonic, motivational, and affective impact of feedback during reward receipt (see Glazer et al. 2018 for review).
The present study examined the effect of two doses of MA (10 and 20 mg) on neural activity during both anticipation and receipt of monetary reward in healthy adults, using ERP measures. Participants also completed self-report measures of the subjective responses to the drug, providing the opportunity to examine neural responses to monetary rewards in relation to self-reported feelings of well-being induced by the drug. Based on EEG data presented from Cavanagh et al (2022) it was hypothesized that MA would increase the cortical signal to both anticipation and receipt of reward and that these would be related to the drug-induced feelings of well-being.
Methods
Design
The study used a double-blind, within-participant design to examine effects of MA (10 and 20 mg oral) and placebo on neural responses to anticipation and receipt of reward in adult volunteers. MA tablets (5 mg, total dose 10 or 20 mg; Desoxyn, Lundbeck) were placed in an opaque size 00 capsule with dextrose filler, and placebo capsules contained only dextrose. Healthy men and women participated in three laboratory-based EEG sessions during which they completed the eMID. They completed self-report questionnaires of subjective effects (e.g., liking the drug effect) at regular intervals during the sessions. EEG measures included ERPs related to anticipation and reward. The study was approved by the local institutional review board.
Participants
Healthy men and women (N = 29) aged 18–35 years were recruited from the university and surrounding community. After initial eligibility was ascertained in a telephone interview (age, current drug use, relevant medical conditions), appropriate candidates attended an in-person interview with a physical examination, EKG and a brief clinical psychiatric interview (American Psychiatric Association 2013). Inclusion criteria were at least a high school education, fluency in English, body mass index between 19 and 26, and good physical health. Exclusion criteria were serious psychiatric disorders including psychosis, severe PTSD or depression, any regular prescription medication, history of cardiac disease, high blood pressure, consuming > 4 alcoholic or caffeinated beverages a day, or working night shifts. Women who were not on oral contraceptives were tested only during the follicular phase (1–12 days from menstruation; (White et al. 2002).
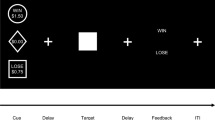
Procedure(Fig. 1)
Timeline plot showing the protocol for the Placebo, Low MA and High MA sessions. Participants received the dose conditions in randomized order, under double blind conditions. Cardiovascular and subjective measures were obtained at Time Point (TP1; baseline) and TP’s 60, 120, 180 and 240 min post capsule). The EEG task was completed 60–120 min post capsule
Participants first attended an orientation session to explain procedures and obtain consent. Participants practiced the tasks and questionnaires and were instructed to abstain from use of drugs or alcohol for 24 before and 6 h after each session. Participants were told to have a normal night’s sleep and to fast 4 h before the session. They were told that they might receive a stimulant (e.g., methamphetamine), sedative/tranquilizer (e.g., Valium), or inactive placebo in each session.
Participants attended three 4-h sessions separated by at least two days. The sessions were conducted from 9 am to 1 pm in comfortably furnished study rooms that resembled living room settings. The 1-h EEG procedure was conducted from 10–11 am in a separate room. Upon arrival at the laboratory, participants provided breath and urine samples to test for recent drug (CLIA waived Instant Drug Test Cup, San Diego, CA; amphetamine, cocaine, oxycodone, THC, PCP, MDMA, opiates, benzodiazepines, barbiturates, methadone, methamphetamine, buprenorphine), or alcohol use (Alcosensor III, Intoximeters, St. Louis, MO) and pregnancy (in females; Aimstrip, Craig Medical, Vista, CA). Participants then completed pre-capsule subjective effects questionnaires (see below) and cardiovascular measures were taken. Then they consumed a capsule containing MA (10 or 20 mg) or placebo in counterbalanced order, under double-blind conditions. Capsules contained two or four MA tablets (5 mg; Mayne Pharma, Greenville NC) with dextrose filler. Placebo capsules contained dextrose only.
Participants completed drug effect questionnaires at 60, 120, 180, and 240 min after taking the capsule, and heart rate (HR) and blood pressure (BP) were measured at the same time. At 30 min post-capsule, participants were escorted to the EEG lab where electrodes were placed (see below), and participants completed a resting state period for 10 min (not reported here), followed by the eMID task. The EEG measures (including a task not reported here) were obtained 60 min to 150 min after capsule ingestion. After EEG measures, electrodes were removed, and participants were given lunch. They remained in the laboratory and completed an End of Session Questionnaire 240 min after the capsule, before being discharged.
Self-Report Measures
-
1.
The Drug Effects Questionnaire (DEQ; Morean et al. 2013) is comprised of 100 point visual analog scales (VAS) describing their responses to the drug. Here we focused only on the question “Do you like the effects that you are feeling now?” (“Dislike” (0) to “Like very much” (100)).
-
2.
The Profile of Mood States (POMS; McNair et al. 1971) is a 72-item questionnaire used to assess momentary mood states. We focus here on the Vigor and Positive Mood subscales. The Positive Mood subscale consisted of the difference in scores on the Elation and Depression subscales. Participants indicated how they felt at that moment in relation to adjectives related to Vigor, Elation and Depression on a 5-point scale from “Not at all” (0) to “Extremely” (4).
-
3.
The Session End Questionnaire consists of questions relating to the drug received during the session. Participants indicated how pleasant they found the experience (from ‘dislike’ (0) to ‘neutral’ (50) to ‘like very much’ (100)) and what they thought they had received (i.e., sedative, stimulant, or placebo).
Cardiovascular Measures
BP and HR (beats per minute) were monitored at regular intervals using portable blood pressure cuffs (Critikon Dinamap Plus; GE Healthcare Technologies, Waukesha, WI). Mean arterial pressure (MAP) was calculated ((Systolic + 2(Diastolic))/3).
EEG Measures
Data collection
A 128 sintered Ag/AgCl active electrodes was used (ActiveTwo™ system, BioSemi B.V., Amsterdam). Electrodes were placed according to equiradial layout on a head cap with snaps. Additional electrodes were placed at reference locations of the mastoids, on the chest to capture EKG artifacts, and around the eye to exclude data contaminated by eye blink artifacts. EEG data was acquired continuously, amplified, and digitized using Biosemi ActiveView software. EEG and electrooculography (EOG) signals were processed by voltage-controlled amplifiers and digitized for storage and analysis.
Data preprocessing
Offline EEG recordings were analyzed using EEGLab (Delorme and Makeig 2004) it was first down sampled to 250 Hz. Data were visually inspected for movement and electronic artifact. PICARD Independent Components Analysis (Ablin et al. 2018) were performed to correct for EEG artifacts including blinks, horizontal and vertical eye movements, muscle movement and EKG signal. Following ICA, ICA weights were applied to clean raw data and a 0.1-30 Hz filter was applied, data were then referenced to mastoid electrodes. No participants were removed due to excessive noise, though we removed M = 9.7% total noisy trials. For the responses, we examined neural activity from 200 ms before the Cue (Reward or Neutral) to 1000 ms following the Cue. ERPs were baseline corrected using the 200 ms before the Cue presentation. For the response to receipt of reward, we examined components of the ERP following the Feedback stimuli (WIN or LOSE). Feedback ERPs were also sampled from -200 to 1000 ms and baseline corrected using 200 ms before the Feedback stimulus.
EEG Task
Monetary Incentive Delay: The eMID task (Broyd et al. 2012) involves 150 trials where participants first view an anticipatory cue signaling a possible win, loss, or neutral outcome. Then, they respond as quickly as possible to a white square and receive feedback stimulus (win or lose) afterward. The duration of the white square varies and is determined by the participant's reaction time. The study focused on reward processing, so only reward and neutral trials were analyzed. The adaptive algorithm controls for the probability of positive and negative feedback, ensuring that they are presented an equal number of times after reward, punishment, and neutral cues. Successful responses on reward cue trials resulted in an expected monetary gain, whereas neutral trials were not associated with monetary losses or gains, and punishment trials were not analyzed. EEG measures of reward processing (San Martin 2012; Glazer et al. 2022) were the primary outcome measures for this task, and the task took approximately 30 min to complete (full description can be found in supplementary methods).
Data Analyses:
-
1.
Subjective, behavioral, and cardiovascular measures were assessed with analysis of variance, with two within-participants factors: drug (placebo, 10, 20 mg MA) and time (pre, 60, 120, 180, and 240 min after capsule). When required, peak drug effects were calculated by subtracting the baseline values from the highest or lowest value during the session.
-
2.
The eMID EEG data were extracted during Cue (anticipation) and Feedback (receipt) phases of the trials, for both Reward Cue and Neutral Cue trials. To confirm that the task produced the expected ERPs in response to wins and losses, we first examined ERPs obtained during the placebo session. Electrodes of interest and time points were selected based on a combination of a review of reward components by Glazer et al (2018) and visual inspection of grand average ERPs
For reward anticipation we first examined the N2 average peak (Cue-N2) from 275-325ms from electrode FCz. Then P3 peak (Cue-P3) was measured from 350-450ms and late positive potential (LPP) from 450-850ms post Cue at electrode CPz. Then the amplitude of the Cue-N2 and Cue-P3 were analyzed separately using RM ANOVA with factors of Cue (Reward x Neutral) x Drug (PLC, Low MA, High MA).
For reward receipt, first, we examined the P2 (FB-P2) peak 200-220ms and RewP response occurring 250-300ms post feedback onset using the FCz electrode. Then, we examined the Feedback P3 component (FB-P3) at 350-450ms at electrode CPz. Finally, a Late positive potential (LPP) was extracted from electrode CPz between 450-850ms. Next a three-way (2x2x3); two (Cue) by two (Feedback) by three (Drug) ANOVA was used to measure drug effects on these components.
Principal Component Analysis (PCA) on Feedback-Related ERPs
Unlike cue-related ERP components, feedback elicits several positive ERP deflections that closely overlap in time (Glazer et al. 2018). For example, the RewP partially overlaps in time with the preceding FB-P2 and subsequent FB-P3, complicating their measurement. Following prior work (Sambrook and Goslin 2016; Foti et al. 2009, 2011), we performed a termporospatial principal component analysis (PCA) (Dien 2012) to separate the FB-P2, RewP, and FB-P3 during feedback processing.
Temporospatial PCA was performed using the EP Toolkit in MatLab (Dien 2010) and PCA parameters followed prior studies of feedback-related ERPs (Glazer et al. 2020). An initial temporal PCA using promax rotation extracted 19 factors and a subsequent spatial PCA using infomax rotation extracted 9 factors, yielding 171 total factors (19 temporal × 9 spatial) with a combined variance of 90%. Kaiser normalization and covariance matrix were used in both PCA operations and scree plot elbows determined the number of factors to retain (Dien et al. 2005). Factor loadings were then converted to microvolt unit covariance loadings by multiplying the correlation factor loadings by standard deviation. Principal components (PCs) with fluctuations under 1 uV were removed to isolate factors of interest. Nine total PCs remained with a combined variance of 81%.
From visual inspection, four PCs were identified roughly consistent with the polarity, latency, and scalp distribution of the FB-P2 (TF4SF1), RewP (TF5SF1), FB-P3 (TF2SF1), and FB-LPP (TF1SF1). Consistent with prior studies (Glazer et al. 2020), PCA separated the FB-P3 into an earlier, shorter FB-P3a factor (TF7SF1) and a later, longer FB-P3b factor (TF2SF1) (Polich 2007). Similarly, PCA also separated the FB-LPP into an earlier and later PC factor. However, several additional PCs also emerged, complicating interpretation (see PCA Table).Footnote 1 For completeness, both early and late PC factors for the FB-P3 and FB-LPP were included in statistical analysis. Visual inspection was also used to determine the measurement of each PC factor, which closely resembled the ERP measurement method. PC factors corresponding to the FB-P3b and Late FB-LPP ERP components were measured using the mean activity ± 50 ms and ± 150 ms around their peak latency and electrode site, respectively. All other PC factor scores were quantified as the average activity ± 25 ms around their peak latency from the electrode with peak amplitude.
PCA Table. Table showing PCA output for Factor Label, Peak Latency, Peak Polarity, Peak Electrode, Variance, Unique Variance, and measurement window (mean activity). The ERP Component column contains the corresponding ERP label for each PC factor determined from visual inspection
Factor Label | ERP Component | Peak Latency | Peak Polarity | Peak Electrode | Variance | Unique Variance | Measurement |
|---|---|---|---|---|---|---|---|
TF01SF1 | FB-LPP (Late) | 644 | Positive | C22 (FCz) | 0.25197 | 0.11879 | 500–800 ms |
TF02SF1 | FB-P3b | 372 | Positive | C11 (FCz) | 0.17800 | 0.07179 | 322–422 ms |
TF03SF1 | FB-LPP (Early) | 500 | Positive | A31 (CPz) | 0.03048 | 0.00639 | 475–525 ms |
TF04SF1 | FB-P2 | 176 | Positive | C2 (FCz) | 0.01927 | 0.01135 | 150–200 ms |
TF05SF1 | RewP | 224 | Positive | C22 (FCz) | 0.01739 | 0.00749 | 200–250 ms |
TF07SF1 | FB-P3a | 276 | Positive | A1 (Cz) | 0.01291 | 0.00708 | 250–300 ms |
TF09SF1 | FB-N1 | 136 | Negative | A1 (Cz) | 0.01106 | 0.00834 | 110–160 ms |
TF11SF1 | Unknown | 112 | Negative | A31 (CPz) | 0.00604 | 0.00426 | 85–135 ms |
TF12SF1 | FRN | 316 | Negative | C22 (FCz) | 0.00491 | 0.00416 | 290–340 ms |
Results
Sample Demographics
Participants were 14 men and 15 women, mean age of 23.7 years, most of whom had completed partial college (Table 1). Less than half had ever used a stimulant in the past, and mean lifetime uses among the 12 who did report prior use was 4.9.
Subjective Drug Effects
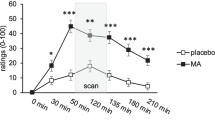
MA increased the subjective effects of Drug Liking over time (Drug x Time interaction F(10,280) = 2.84 p = 0.002, ηp2 = 0.01). Post hoc tests indicated that both doses increased scores relative to placebo, peaking at 60 min post capsule. At 120 min post capsule Liking ratings remained higher after the 20 mg dose compared to the 10 mg MA dose MA also significantly increased the subjective feelings of Vigor over time (Drug x Time interaction F(10,280) = 5.78, p < 0.001, ηp2 = 0.17), with both doses differing from placebo. The higher dose of MA also increased Positive Mood (Drug x Time interaction F(10,280) = 2.56 p = 0.006, ηp2 = 0.08).
On the Session End Questionnaire participants identified MA (20 mg) as a stimulant drug 59% of the time, MA (10 mg) 33% of the time, and PLC, 48% of the time. Participants incorrectly labeled PLC as a sedative 48% of sessions, and as a stimulant 4% (N = 1). Mean end of session liking ratings were PLC M = 51.24 (SD = 12.99), MA (20 mg) M = 61.54 (SD = 26.26) and MA (10 mg) M = 58.47 (SD = 21.96). Only MA (20 mg) differed significantly from PLC (main effect of Drug F(2,56) = 12.49, p = 0.03; post hoc 20 mg vs PLC (p < 0.01).
Cardiovascular Drug Effects
MA (both doses) significantly increased MAP between 60 and 180 min after ingestion of the capsule (F(10,280) = 14.71, p < 0.001, ηp2 = 0.34 (Fig. 2, bottom right panel). The drug did not significantly affect heart rate.
Mean (± SEM) ratings of POMS: Vigor, POMS: Positive Mood, DEQ: Liking (drug effects) and Mean Arterial Pressure (MAP) after MA (10 and 20 mg) or placebo at minutes before and after capsule. Solid green line: PLC; dashed blue line: Low MA; dotted red line: High MA). Black bar on x-axis depicts the period participants spent completing the EEG tasks. High MA increased subjective, Vigor, Positive Mood, Like drug and MAP (RMANOVA drug*time p-value < 0.05). Asterisks indicate time points at which means differed from placebo (* 20 mg vs PLC, # 10 mg vs PLC, and 20 mg vs 10 mg)
MID Task
Drug Responses to Cues
Cue-N2. MA did not affect the N2 peak amplitude to Reward Cues (F(2,78) = 0.10, p > 0.90, ηp2 = 0.09), although overall, N2 peak amplitude was significantly higher on Reward Cue trials (M = 1.18) compared to Neutral Cue trials (M = -0.10), F(1,78) = 19.81, p < 0.001, ηp2 = 0.20. There was also no significant interaction between Drug and Cue for Cue-N2 F(2,78) = 1.24, p = 0.30, ηp2 = 0.03. Cue-P3. MA did not affect the peak amplitude of P3 (F(2,78) = 0.41, p > 0.59, ηp2 = 0.01) but Reward Cues resulted in a significantly greater (M = 6.41) P3 than Neutral Cues (M = 5.19) (F(2,78) = 26.48, p < 0.001, ηp2 = 0.09) there was however no significant interaction between Drug and Cue F(2,78) = 0.53, p = 0.59, ηp2 = 0.01. Cue-LPP. There was no significant interaction between Drug and Cue for Cue-LPP F(2,78) = 0.80, p = 0.45, ηp2 = 0.02 and MA did not affect the peak amplitude of LPP (F(2,78) = 0.80, p = 0.45, ηp2 = 0.02), but Reward Cues significantly increased LPP amplitudes (M = 3.40) compared to Neutral Cues (M = 2.86) (F(2,78) = 6.54, p = 0.01, ηp2 = 0.08) (Fig. 3).
A) Mean ERP amplitudes at electrodes Fz (left) and Pz (right) following Reward and Neutral Cues, after low or high doses of MA. Sections labeled with squares indicate Cue-N2 peak (left panel), Cue-P3 peak (right panel, first box), Cue-LPP. Cue-N2 peak was significantly greater for Reward trials compared to Neutral trials (left panel) at electrode Fz but was not impacted by MA. At electrode Pz (right panel) Cue-P3 and Cue-LPP peak amplitudes were significantly greater during Reward trials compared to Neutral trials. B) Topographic maps showing the Reward-Neutral trials for Cue-P3, data is shown for PLC (left), Low MA (middle) and High MA (right) sessions
Drug Responses to Feedback: ERP Results
FB-P2. MA did not affect the FB-P2 peak amplitude (F(2,78) = 0.41, p = 0.67, ηp2 = 0.01). However, Reward Cues did increase the FB-P2 peak amplitude (M = 4.81) following receipt of Feedback (F(1,78) = 12.87, p = 0.001, ηp2 = 0.14) more than Neutral Cues (M = 5.67). Loss Feedback resulted in a larger FB-P2 peak (M = 5.01) compared to Gain feedback (M = 5.47) F(1,78) = 4.42, p = 0.04, ηp2 = 0.05. MA did not significantly interact with Feedback here either F(2,78) = 2.54, p = 0.09, ηp2 = 0.06. RewP. MA did not increase RewP response to rewarding stimuli F(2,78) = 2.59, p = 0.08, ηp2 = 0.06 nor did it interact with Feedback F(2,78) = 0.28, p = 0.76, ηp2 = 0.01. The trial Cue did however affect peak amplitude with Rewarding Cues (M = 4.54) resulting in larger RewP amplitude than Neutral Cues (M = 3.50) F(1,78) = 19.08, p < 0.001, ηp2 = 0.20. RewP amplitude also increased significantly following Gain feedback (M = 4.26) compared to Loss feedback (M = 3.78), F(1,78) = 3.75, p = 0.05, ηp2 = 0.05 (Fig. 4).
A) ERP figures at Electrode FCz where FB-P2 (first shaded area) and RewP (second shaded area) peaks are measured separated by Neutral (left) and Reward (right) Cues. MA did not affect the amplitude of FB-P2 or RewP peaks at electrode FCz. Reward (right panel) Cues trials resulted in significantly larger peaks for both FB-P2 and RewP compared to Neutral Cues. Furthermore, for both peaks, gain feedback was also significantly higher regardless of the trial Cue compared to receiving “Loss” Feedback. B) Topographic maps showing the RewP shown as Win trials – loss trials for PLC (left), Low MA (middle) and High MA (right)
FB-P3. There was no significant interaction between Drug and Feedback for FB-P3 F(2,78) = 0.32, p = 0.73, ηp2 = 0.01 and MA did not significantly change the FB-P3 peak F(2,78) = 0.70, p = 0.50, ηp2 = 0.02. Reward Cues did result in significantly greater FB-P3 peaks (M = 7.07) compared to Neutral Cues (M = 5.06) F(1,78) = 44.94, p < 0.001, ηp2 = 0.37. Feedback also did not affect the FB-P3 amplitude F(1,78) = 0.003, p = 0.96, ηp2 < 0.01. FB-LPP. Finally, MA did not affect the amplitude of FB-LPP F(2,72) = 0.01, p = 0.99, ηp2 < 0.01. Feedback did not affect the FB = LPP amplitude F(1,72) = 1.32, p = 0.26, ηp2 = 0.02, but Neutral Cues increased FB-LPP (M = 3.05) significantly more than Reward Cues (M = 3.37) F(1,72) = 19.54, p < 0.001, ηp2 = 0.21. There was also no significant interaction between Drug and Feedback here F(2,78) = 0.95, p = 0.40, ηp2 = 0.03 (Fig. 5).
A) ERP figures for Neutral (left) and Reward (right) Cues at Electrode CPz where FB-P3 (first shaded area) and LPP (second shaded area) peaks are measured. MA did not affect the peak amplitude of either FB-P3 nor FB-LPP at electrode CPz. However, trial Cue increased the peak amplitudes significantly compared to Neutral Cued trials. FB-P3 and FB-LPP were not affected by Feedback. B) Topographic maps showing the central location for FB-P3 peak, data for Reward-minus-Neutral trials are shown for PLC (left), Low MA (middle) and High MA (right)
Drug Responses to Feedback: PCA Results
FB-P2. MA did not affect the FB-P2 (F(1,72) = 0.10, p = 0.90, ηp2 = 0.003). FB-P2 was also unaffected by the Cue (F(1,72) = 1.40, p = 0.24, ηp2 = 0.02) and the Feedback received (F(1,72) = 0.57, p = 0.45, ηp2 = 0.008). RewP. MA did not increase RewP response to reward feedback compared to PLC (F(1,72) = 0.61, p = 0.55, ηp2 = 0.02). However for the PCA findings, loss trials resulted in a greater RewP than win trials (F(1,72) = 6.95, p = 0.01, ηp2 = 0.09). Cue did not impact RewP amplitude (F(1,72) = 1.91, p = 0.17, ηp2 = 0.03). FB-P3a. MA did not affect the FB-P3a amplitude (F(1,72) = 1.22, p = 0.30, ηp2 = 0.03) however Drug did interact with trial Cue I (F(1,72) = 3.54, p = 0.03, ηp2 = 0.09), here specifically, for Low MA there was a significant difference (p = 0.001) between Reward (M = 0.44) and Neutral Cues (M = 1.27) but this was not present in the PLC or High MA groups. Cue also significantly affected FB-P3a (F(1,72) = 5.42, p = 0.02, ηp2 = 0.07) with greater peak amplitude for Reward trials overall compared to Neutral Cued trials. FB-P3b. MA did not affect the FB-P3b peak (F(1,72) = 0.63, p = 0.54, ηp2 = 0.02) however this peak was affected by both Cue and Feedback (interaction, F(1,72) = 11.77, p = 0.001, ηp2 = 0.14). Simple main effects revealed that in Reward trials, loss feedback results in a significantly greater FB-P3b than win trials (p = 0.03) however in Neutral trials it is the opposite, win trials result in a greater FB-P3b than loss trials (p = 0.006). FB-LPP (early). The early component of FB-LPP was not affected by MA (F(1,72) = 0.12, p = 0.89, ηp2 = 0.003), Cue (F(1,72) = 0.93, p = 0.34, ηp2 = 0.01) or Feedback (F(1,72) = 1.21, p = 0.27, ηp2 = 0.02).FB-LPP (late). Neither was the late FB-LPP component affected by MA (F(1,72) = 0.23, p = 0.80, ηp2 = 0.006), Cue (F(1,72) = 2.19, p = 0.14, ηp2 = 0.03) nor Feedback (F(1,72) = 0.28, p = 0.60, ηp2 = 0.004). Together, results suggest temporospatial PCA was unable to successfully separate feedback-related ERPs (Figs. 6 and 7).Footnote 2
Individual components at electrode FCz separated by Neutral and Reward Cues. Peaks are represented by Gain (solid lines) and Loss (dashed lines) feedback, for Placebo (green), Low MA (blue) and High MA (red) sessions. Panel A, shows principal component TF4SF1 (FB-P2). Panel B, shows principal component T5SF1 (RewP) and Panel C, shows principal component TF7SF1 (FB-P3a) all maximal at electrode FCz
Discussion
The current study investigated the acute effects of two oral doses (20 mg and 10 mg) of MA on cortical activity during a reward related task in healthy adults. MA as expected increased feelings of drug Liking, Vigor and Positive Mood. The eMID task resulted in the expected ERP patterns in response to anticipation of reward. However, MA did not affect the ERP signals associated with either anticipation or receipt of monetary reward. The lack of association suggests that the direct mood effects of MA and its effects on processing of discrete reward stimuli may be mediated by separate neural processes. That is, although dopamine may be involved in both subjective feelings of pleasure and processing of reward stimuli (Bressan and Crippa 2005), these data suggest that the processes are dissociable.
Contrary to expectations, MA did not affect the ERP responses receipt of reward. PCA results suggest MA may influence the FB-P3a, but the current study was unable to temporally separate this component from the preceding RewP. One previous study (Cavanagh et al. 2022) reported that d-amphetamine increased ERP signals of reward processing using a different task, a probabilistic learning task. In that task, participants responded to one of two images displayed on the screen, and received probabilistic feedback of ‘correct’ or ‘incorrect.’ They found that d-amphetamine (10 and 20 mg) increased the RewP ERP for reward signals. The apparently different outcomes could be related to differences in the tasks (i.e., probabilistic learning vs adjusting task to equate performance), EEG processing, differences in the drugs or their time course, or differences in the subject samples. The eMID requires no reward learning as it uses an adaptive algorithm, so it is not possible to perform better under the influence of a drug. With the present results, this suggests amphetamine may only influence reward learning when participants can learn to increase their performance. The present results are, however, consistent with findings reported by Knutson et al. (2004) from an fMRI study in which amphetamine did not alter brain activation during receipt of reward on the MID task (although, as noted below, it did affect activation during anticipation). The conditions under which stimulant drugs alter brain signals during receipt of reward remain to be determined.
MA also did not affect ERP responses to cues signaling anticipation of reward. Although we observed the expected increase in signal in response to an anticipatory reward cue during placebo sessions (Pornpattananangkul and Nusslock 2015; Glazer et al. 2018; Schutte et al. 2020), MA did not alter this response. This was surprising, considering the Knutson et al. (2004) report that d-amphetamine decreased neural responses to reward cues using the MID task. The apparent discrepancy between our results and those of Knutson may be related to several factors, such as differences in sensitivity, variability or anatomical sources of the EEG and fMRI signals, differences in subject samples, or other methodological variables. It is notable, however, that the present study included more participants than the Knutson study (29 vs 8). In addition, the proportion of win and loss trials differed between the two studies (66/30 Win/Loss vs 50/50 Win/Loss). Considering that we did detect a robust ERP in response to the anticipatory cue during placebo sessions, we conclude that these doses of MA do not reliably dampen neural responses predicting reward.
An important question in the present study was whether the effects of MA on ERP responses were correlated with its effects on subjective feelings of wellbeing. Both measures (neural responses to discrete reward signals and self-reported euphoria) have been linked to increased dopamine activity, suggesting they may be correlated. Although conclusion on this question is limited by the absence of an effect of MA on neural processing of reward, the lack of association reported suggests that neural reward signals are mediated by different processes than states of wellbeing. Knutson et al. (2004) examined the relationship between subjective arousal in relation to wins or losses (feelings of excitement) and brain activity. They reported that increased ratings of excitement with wins were associated with increased activity in the NAcc with wins. Cavanagh et al (2022) did not measure the mood-altering effects of amphetamine in their study with a probabilistic learning task. However, they raise the possibility that mood states (including those induced by a drug) might in themselves modulate the neural signals that contribute to learning (Berridge and Waterhouse 2003). The lack of correlation between effects of a drug on ERP and mood was also reported in another study using a different drug, LSD (Glazer et al 2022). Future studies are needed to determine how neural responses to reward signals are, or are not, related to drug-induced subjective states.
The study had several limitations. The sample was relatively small, which limited the power to detect subtle effects or analyze individual differences (e.g., sex differences). Limited power may also contribute to the PCA results that failed to successfully separate feedback-related ERPs elicited close together in time. The subject sample was homogenous, which limits the generalizability of the findings to a more heterogenous population (e. g., those with psychiatric symptoms, ADHD, or different ages). The doses of MA were relatively low, and it is possible that higher doses would produce more pronounced effects on neural signals related to reward. Other limitations include the task used to detect changes in the neural reward signal. Although the eMID is sensitive to trait differences in psychiatric symptomatology (Nusslock and Alloy 2017), it may be less sensitive to momentary changes induced by a drug. Other aspects of the task may also have contributed: participants in the present study did not receive real monetary rewards, and trials were adjusted to ensure equal numbers of wins and losses, features that might have decreased the value of the rewards.
Using temporally sensitive EEG measures of brain activity in healthy adults, this study showed that MA did not affect the cortical responses related to reward processing during either anticipation or receipt of monetary reward. Yet, the drug produced its expected effects on feelings of well-being and positive mood, another indicator of dopaminergic activity. The present findings suggest that the mechanisms underlying the modulatory effects of MA on mood may be unrelated to its effects on neural responses to monetary rewards. How either of these effects influence either the therapeutic effects of the drug or its abuse potential is not fully known. This remains an interesting question for future studies. It will also be of interest to investigate other cognitive measures of reward processing as well as other indices of neural function. A better understanding of the neural effects of stimulant drugs will improve our understanding of the therapeutic effects as well as their potential to be abused.
Data availability
Data is available raw or processed, upon request to the corresponding author.
Notes
Two major issues with results suggest the PCA was not successfully able to separate the RewP from the preceding FB-P2 and subsequent FB-P3. First, although TF4SF1 is consistent with FB-P2, the next adjacent factor, TF5SF1, does not resemble the RewP. For example, TF4SF1 peaks at 176 ms around FCz and shows no difference between Reward Win and Lose conditions, consistent with the FB-P2. But the next adjacent factor, TF5SF1, does not align with RewP effects in ERP waveform, peaks too early (224 ms at FCz), and is greatest for Reward Lose (vs. Win). These results suggest that while TF4SF1 likely reflects the FB-P2, TF5SF1 may not reflect the RewP but rather some combination of overlapping FB-P2 and RewP activity. In support, one recent PCA study reported the RewP PC factor was not significantly modulated by outcome valence (Clayson et al. 2019), suggesting temporospatial PCA may be unable to adequately separate feedback-related ERPs in some cases.
Second, two positive PCs emerged in the FB-P3 time window: TF7SF1 and TF2SF1. Numerous studies have identified two P3-related ERP components: an earlier, frontal P3a reflecting early attention and a later, parietal P3b reflecting stimulus categorization (Polich 2007). One previous reward processing study used PCA to separate the FB-P3 into the FB-P3a and FB-P3b after feedback and produced PC factor distributions strikingly similar to the present results (Glazer et al. 2020). Consistent with this prior study, the current results suggest TF2SF1 corresponds to the FB-P3b. However, unlike this previous study, the current results suggest PCA was unable to successfully separate the RewP from the FB-P3a. While TF7SF1 may reflect the FB-P3a, it rather resembles the RewP in peak latency and electrode (276 ms at Cz), is greatest for Reward Win (vs. Lose), and lines up with expected RewP effects in the ERP waveform. These results suggest it is likely that TF5SF1 and TF7SF1 PC factors contain overlapping activity corresponding to the RewP and FB-P3a, respectively.
Results suggest it is likely that substantial temporal overlap remained after PCA among TF4SF1, TF5SF1, and TF7SF1, presumably reflecting the FB-P2, RewP, and FB-P3a. Further complicating interpretation, one negative PC emerged (TF12SF1) consistent with the peak latency, scalp topography, and polarity of the feedback-related negativity (FRN). While the RewP reflects a positive ERP deflection after better-than-expected feedback, a negative ERP deflection called the FRN may index worse-than-expected feedback in the same time window (Proudfit 2015, Holroyd et al. 2008, 2011). Although most reward processing studies using PCA do not report a negative deflection consistent with the FRN, some previous studies have done so and are consistent with the present results (Valadez et al. 2016, Valadez 2019). The present results suggest temporospatial PCA of feedback-related ERPs may produce more complex PC distributions than previously reported. Future studies should consider comprehensive reporting of all PCs in the FB-P2/RewP/FB-P3 time window to better interpret and understand the rapid cascade of feedback-related ERPs.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. https://doi.org/10.1176/appi.books.9780890425596
Ablin P, Cardoso J-F, Gramfort A (2018) Faster independent component analysis by preconditioning with Hessian approximations. IEEE Trans Signal Process 66:4040–4049
Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42(1):33–84. https://doi.org/10.1016/s0165-0173(03)00143-7
Berridge KC, Robinson TE, Aldridge JW (2009) Dissecting components of reward: “liking”, “wanting”, and learning. Curr Opin Pharmacol 9(1):65–73. https://doi.org/10.1016/j.coph.2008.12.014
Bowyer CB, Joyner KJ, Yancey JR, Venables NC, Hajcak G, Patrick CJ (2019) Toward a neurobehavioral trait conceptualization of depression proneness. Psychophysiology 56(7):e13367. https://doi.org/10.1111/psyp.13367
Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P (2003) Activation of the visual cortex in motivated attention. Behav Neurosci 117(2):369–380. https://doi.org/10.1037/0735-7044.117.2.369
Bressan RA, Crippa JA (2005) The role of dopamine in reward and pleasure behaviour–review of data from preclinical research. Acta Psychiatr Scand Suppl 427:14–21. https://doi.org/10.1111/j.1600-0447.2005.00540.x
Broyd SJ, Richards HJ, Helps SK, Chronaki G, Bamford S, Sonuga-Barke EJ (2012) An electrophysiological monetary incentive delay (e-MID) task: a way to decompose the different components of neural response to positive and negative monetary reinforcement. J Neurosci Methods 209(1):40–49. https://doi.org/10.1016/j.jneumeth.2012.05.015
Castells X, Blanco-Silvente L, Cunill R (2018) Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 8(8):CD007813. https://doi.org/10.1002/14651858.CD007813.pub3
Cavanagh JF, Olguin SL, Talledo JA, Kotz JE, Roberts BZ, Nungaray JA, Sprock J, Gregg D, Bhakta SG, Light GA, Swerdlow NR, Young JW, Brigman JL (2022) Amphetamine alters an EEG marker of reward processing in humans and mice. Psychopharmacology (Berl) 239(3):923–933. https://doi.org/10.1007/s00213-022-06082-z
Clayson PE, Wynn JK, Infantolino ZP, Hajcak G, Green MF, Horan WP (2019) Reward processing in certain versus uncertain contexts in schizophrenia: An event-related potential (ERP) study. J Abnorm Psychol 128(8):867–880. https://doi.org/10.1037/abn0000469
Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL (2018) Neural activation to monetary reward is associated with amphetamine reward sensitivity. Neuropsychopharmacology 43(8):1738–1744. https://doi.org/10.1038/s41386-018-0042-8
Crow SJ (2019) Pharmacologic Treatment of Eating Disorders. Psychiatr Clin North Am 42(2):253–262. https://doi.org/10.1016/j.psc.2019.01.007
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134(1):9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85(14):5274–5278. https://doi.org/10.1073/pnas.85.14.5274
Dien J (2010) The ERP PCA Toolkit: an open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods 187(1):138–145. https://doi.org/10.1016/j.jneumeth.2009.12.009
Dien J (2012) Applying principal components analysis to event-related potentials: a tutorial. Dev Neuropsychol 37(6):497–517. https://doi.org/10.1080/87565641.2012.697503
Dien J, Beal DJ, Berg P (2005) Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clin Neurophysiol 116(8):1808–1825. https://doi.org/10.1016/j.clinph.2004.11.025
Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA (2001) Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 49(2):81–96. https://doi.org/10.1016/s0006-3223(00)01038-6
Faraone SV (2018) The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev 87:255–270. https://doi.org/10.1016/j.neubiorev.2018.02.001
Flores A, Münte TF, Doñamayor N (2015) Event-related EEG responses to anticipation and delivery of monetary and social reward. Biol Psychol 109:10–19. https://doi.org/10.1016/j.biopsycho.2015.04.005
Foti D, Hajcak G, Dien J (2009) Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology 46(3):521–530. https://doi.org/10.1111/j.1469-8986.2009.00796.x
Foti D, Weinberg A, Dien J, Hajcak G (2011) Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp 32(12):2207–16. https://doi.org/10.1002/hbm.21182
Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal VA, Nusslock R (2018) Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. Int J Psychophysiol Part B 32:184–202. https://doi.org/10.1016/j.ijpsych
Glazer J, King A, Yoon C, Liberzon I, Kitayama S (2020) DRD4 polymorphisms modulate reward positivity and P3a in a gambling task: Exploring a genetic basis for cultural learning. Psychophysiology 57(10):e13623. https://doi.org/10.1111/psyp.13623
Glazer J, Murray CH, Nusslock R, Lee R, de Wit H (2022) Low doses of lysergic acid diethylamide (LSD) increase reward-related brain activity. Neuropsychopharmacology. https://doi.org/10.1038/s41386-022-01479-y
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1):4–26. https://doi.org/10.1038/npp.2009.129
Helfrich RF, Knight RT (2019) Cognitive neurophysiology: Event-related potentials. Handb Clin Neurol 160:543–558. https://doi.org/10.1016/B978-0-444-64032-1.00036-9
Holroyd CB, Pakzad-Vaezi KL, Krigolson OE (2008) The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology 45(5):688–697
Holroyd CB, Krigolson OE, Lee S (2011) Reward positivity elicited by predictive cues. NeuroReport 22(5):249–252. https://doi.org/10.1097/WNR.0b013e328345441d
Jauhar S, Fortea L, Solanes A, Albajes-Eizagirre A, McKenna PJ, Radua J (2021) Brain activations associated with anticipation and delivery of monetary reward: a systematic review and meta-analysis of fMRI studies. PLoS One 16(8):e0255292. https://doi.org/10.1371/journal.pone.0255292
Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR (2004) Amphetamine modulates human incentive processing. Neuron 43(2):261–269. https://doi.org/10.1016/j.neuron.2004.06.030
Langenecker SA et al (2020) Anticipation of monetary reward in amygdala, insula, caudate are predictors of pleasure sensitivity to D-amphetamine administration. Drug Alcohol Depend 206:107725. https://doi.org/10.1016/j.drugalcdep.2019.107725
Liu X, Hairston J, Schrier M, Fan J (2011) Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35(5):1219–36. https://doi.org/10.1016/j.neubiorev.2010.12.012
Maski K, Trotti LM, Kotagal S, Robert Auger R, Swick TJ, Rowley JA, Hashmi SD, Watson NF (2021) Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 17(9):1895–1945. https://doi.org/10.5664/jcsm.9326
Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR (2000) Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 12(3):268–275. https://doi.org/10.1006/nimg.2000.0610
McIntyre RS, Lee Y, Zhou AJ, Rosenblat JD, Peters EM, Lam RW, Kennedy SH, Rong C, Jerrell JM (2017) The Efficacy of Psychostimulants in Major Depressive Episodes: A Systematic Review and Meta-Analysis. J Clin Psychopharmacol 37(4):412–418. https://doi.org/10.1097/JCP.0000000000000723
Moore KE (1977) The actions of amphetamine on neurotransmitters: a brief review. Biol Psychiatry 12(3):451–462
Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS (2013) The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 227(1):177–92. https://doi.org/10.1007/s00213-012-2954-z
Murray, CH, JE Glazer, R Lee, R Nusslock, H de Wit (2022) THC reduces reward-related brain activity in healthy adults. Psychopharmacology. https://doi.org/10.1007/s00213-022-06164-y
McNair D, Lorr M, Doppleman L (1971) POMS manual for the profile of mood states. Educational and Industrial Testing Service, San Diego
Novak KD, Foti D (2015) Teasing apart the anticipatory and consummatory processing of monetary incentives: An event-related potential study of reward dynamics. Psychophysiology 52(11):1470–1482. https://doi.org/10.1111/psyp.12504
Nusslock R, Alloy LB (2017) Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord 216:3–16. https://doi.org/10.1016/j.jad.2017.02.001
Pfabigan DM, Seidel EM, Sladky R, Hahn A, Paul K, Grahl A, Lamm C (2014) P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: an EEG and fMRI experiment. NeuroImage 96:12–21
Polich J (2007) Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118(10):2128–2148
Polezzi D, Lotto L, Daum I, Sartori G, Rumiati R (2008) Predicting outcomes of decisions in the brain. Behav Brain Res 187(1):116–122
Pornpattananangkul N, Nusslock R (2015) Motivated to win: Relationship between anticipatory and outcome reward-related neural activity. Brain Cogn 100:21–40
Proudfit GH (2015) The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology 52:449–459
Sambrook TD, Goslin J (2015) A neural reward prediction error revealed by a meta-analysis of ERPs using great grand averages. Psychol Bull 141(1):213–235. https://doi.org/10.1037/bul0000006
Sambrook TD, Goslin J (2016) Principal components analysis of reward prediction errors in a reinforcement learning task. Neuroimage 124(Pt A):276–286. https://doi.org/10.1016/j.neuroimage.2015.07.032
San Martín R (2012) Event-related potential studies of outcome processing and feedback-guided learning. Front Human Neurosci 6. https://doi.org/10.3389/fnhum.2012.00304
Schultz W (2007) Multiple dopamine functions at different time courses. Annu Rev Neurosci 30:259–288. https://doi.org/10.1146/annurev.neuro.28.061604.135722
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599
Schutte I, Deschamps PKH, van Harten PN, Kenemans JL (2020) Dopaminergic and noradrenergic manipulation of anticipatory reward and probability event-related potentials. Psychopharmacology (Berl) 237(7):2019–2030. https://doi.org/10.1007/s00213-020-05515-x
Substance Abuse and Mental Health Services Administration (2022) Key substance use and mental health indicators in the United States: Results from the 2021 National Survey on Drug Use and Health (HHS Publication No. PEP22-07-01-005, NSDUH Series H-57). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/report/2021-nsduh-annual-national-report
Valadez EA (2019) Electrophysiological indicators of feedback processing related to obsessive-compulsive symptoms [Doctoral dissertation]. University of Delaware. https://udspace.udel.edu/items/f22ca054-c3c6-45c3-9e6c-92765d2e8bb2
Valadez EA, Farrell KN, Infantolino ZP, Roberts K, Simons RF (2016) Principal component analysis of mock-gambling task ERPs revealed negativity specific to nonrewards. Psychophysiology 53(Suppl 1):S71. https://doi.org/10.1111/psyp.12673
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR (1999) Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291(1):409–415
Walsh MM, Anderson JR (2012) Learning from experience: event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neurosci Biobehav Rev 36(8):1870–84. https://doi.org/10.1016/j.neubiorev.2012.05.008
White TL, Justice AJ, de Wit H (2002) Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 73(4):729–741. https://doi.org/10.1016/s0091-3057(02)00818-3
Zack MH, Lobo DS, Biback C, Fang T, Smart K, Tatone D, Kalia A, Digiacomo D, Kennedy JL (2017) Parallel role for the dopamine D1 receptor in gambling and amphetamine reinforcement in healthy volunteers. J Psychopharmacol 31(1):31–42. https://doi.org/10.1177/0269881116665329
Funding
This research was supported by National Institute on Drug Abuse grant DA02812.
Author information
Authors and Affiliations
Contributions
CJH – analysis, interpretation, writing.
JG – analysis, interpretation, writing.
RL – design, interpretation.
RN –analysis, interpretation.
HdW – design, analysis, interpretation, writing.
Corresponding author
Ethics declarations
Competing interests
HdW serves on the Board of Directors of PharmAla Biotech and has served as Scientific Advisor to Awakn Life Sciences, Gilgamesh Pharmaceuticals and Mind Foundation. These roles are unrelated to the research reported here. CJH, JEG, IT, MK, and RL have no conflicts of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haggarty, C.J., Glazer, J.E., Nusslock, R. et al. Lack of effect of methamphetamine on reward-related brain activity in healthy adults. Psychopharmacology 241, 181–193 (2024). https://doi.org/10.1007/s00213-023-06475-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06475-8