Abstract
Rationale
Dyskinesias induced by L-3,4-dihydroxyphenylalanine, L-Dopa (LIDs), are the major complication in the pharmacological treatment of Parkinson’s disease. LIDs induce overactivity of the glutamatergic cortico-striatal projections, and drugs that reduce glutamatergic overactivity exert antidyskinetic actions. Chronic administration of immepip, agonist at histamine H3 receptors (H3R), reduces LIDs and diminishes GABA and glutamate content in striatal dialysates (Avila-Luna et al., Psychopharmacology 236: 1937-1948, 2019).
Objectives and methods
In rats unilaterally lesioned with 6-hydroxydopamine in the substantia nigra pars compacta (SNc), we examined whether the chronic administration of immepip and their withdrawal modify LIDs, the effect of L-Dopa on glutamate and GABA content, and mRNA levels of dopamine D1 receptors (D1Rs) and H3Rs in the cerebral cortex and striatum.
Results
The administration of L-Dopa for 21 days induced LIDs. This effect was accompanied by increased GABA and glutamate levels in the cerebral cortex ipsi and contralateral to the lesioned SNc, and immepip administration prevented (GABA) or reduced (glutamate) these actions. In the striatum, GABA content increased in the ipsilateral nucleus, an effect prevented by immepip. L-Dopa administration had no significant effects on striatal glutamate levels. In lesioned and L-Dopa-treated animals, D1R mRNA decreased in the ipsilateral striatum, an effect prevented by immepip administration.
Conclusions
Our results indicate that chronic H3R activation reduces LIDs and the overactivity of glutamatergic cortico-striatal projections, providing further evidence for an interaction between D1Rs and H3Rs in the cortex and striatum under normal and pathological conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease is the second most common neurodegenerative disorder worldwide, affecting approximately 1% of the population over 65 years (Bastide et al. 2015; Connolly and Lang 2014). The administration of L-3,4-dihydroxyphenylalanine (L-Dopa) remains the most effective symptomatic treatment of the disorder (Connolly and Lang 2014; LeWitt 2015; Obeso et al. 2017); however, the appearance of L-Dopa-induced dyskinesias (LIDs), characterized by abnormal involuntary movements (AIMs), hampers L-Dopa efficacy in the vast majority of patients (Ahlskog and Muenter 2001; Bastide et al. 2015; Fahn 2008; Hely et al. 2005; Huot et al. 2013; Obeso et al. 2017).
LIDs are related to alterations at the cellular and molecular levels in the basal ganglia synaptic circuits, namely increased dopamine content in the striatum, changes in the cellular distribution of dopamine D1 receptors (D1Rs), sensitization of D1R intracellular signaling, and abnormal gene expression in D1R-expressing neurons, which in turn lead to pre- and post-synaptic modifications in dopaminergic transmission in the basal ganglia (Bastide et al. 2015; Carta and Bezard 2011; De la Fuente-Fernandez et al. 2004; Porras et al. 2014; Spigolon and Fisone 2018).
The striatum is innervated by histaminergic fibers and expresses a high density of histamine H3 receptors, H3Rs (Bolam and Ellender 2016; Panula and Nuutinen 2013; Panula et al. 1989; Pillot et al. 2002), co-expressed with D1Rs in the sub-population of GABAergic medium spiny neurons (MSNs) that originate the striato-nigral, direct pathway of the basal ganglia and with D2Rs in striato-pallidal MSNs that originate the indirect pathway (González-Sepúlveda et al. 2013; Rapanelli 2017).
Several studies indicate a functional interaction between D1Rs and H3Rs in the striato-nigral MSNs. For example, H3R activation counteracts presynaptically the D1R-mediated facilitation of depolarization-evoked [3H]-GABA release in slices from rat striatum and substantia nigra pars reticulata (SNr) (Arias-Montaño et al. 2001; Garcia et al. 1997), whereas at the post-synaptic level H3R activation inhibits D1R-induced cAMP accumulation (Sánchez-Lemus and Arias-Montaño 2004) and D1R activation facilitates H3R-mediated stimulation of mitogen-activated protein kinases (MAPKs) in striatal slices (Moreno et al. 2011).
We previously reported that in rats with 6-hydroxydopamine (6-OHDA) lesion to the substantia nigra pars compacta (SNc), the concomitant administration of the selective H3R agonist immepip and L-Dopa for 14 days reduced LIDs and the L-Dopa-induced increase in GABA and glutamate levels in striatal dialysates (Avila-Luna et al. 2019). The classical model of basal ganglia function (Albin et al. 1989) indicates that overactivity of the glutamatergic cortico-striatal afferents leads to hyperactivity of the striato-nigral neurons that form the basal ganglia direct pathway (Huot et al. 2013). Accordingly, drugs that reduce glutamatergic activity, such as amantadine, MTEP (3-[(2-methyl-1,3-thia-zol-4-yl)ethynyl] pyridine), and MK-801, oppose LIDs (Bido et al. 2011; Blanchet et al. 1998; Huot et al. 2013; Jonkers et al. 2002; Lundblad et al. 2002; Lundblad et al. 2005; Mela et al. 2007; Papathanou et al. 2014).
The reduction by chronic H3R activation of LIDs could thus be associated with a counteracting effect on the overactivity of the glutamatergic cortico-striatal pathway, and may rely on the functional interaction with D1Rs, whose activation facilitates GABA and glutamate release in the striatum (Arias-Montaño et al. 2001; Ellender et al. 2011; Molina-Hernandez et al. 2001) and enhances glutamatergic transmission in MSNs (Calabresi et al. 1997; Radnikow and Misgeld 1998; Surmeier et al. 2007).
In the present study, it was hypothesized that the overactivity of the glutamatergic cortico-striatal and thalamo-cortical pathways contributes to LIDs in hemiparkinsonian rats, and that the functional interaction between H3Rs and D1Rs can therefore reduce the overactivity of the cerebral cortex and the striatum. A previous study reported that a single dose of the H3R agonist immepip did not reduce LIDs in marmosets (Papathanou et al. 2014), whereas in rats lesioned with 6-OHDA in the SNc, the chronic administration of this agonist was accompanied by a reduction of LIDs, an effect reverted by the subsequent withdrawal of immepip (Avila-Luna et al. 2019). In this study, we confirmed that the chronic administration and the subsequent withdrawal of immepip modify LIDs in rats lesioned with 6-OHDA in the SNc, and then examined the effect of the pharmacological treatments on glutamate and GABA contents in the cerebral cortex and striatum, and on striatal D1R and H3R mRNA expression.
Materials and methods
Drugs
The following drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA): L-Dopa (3,4-dihydroxy-L-phenylalanine), 6-hydroxydopamine hydrobromide, immepip dihydrobromide, benserazide hydrochloride, and apomorphine hydrochloride.
Subjects
Adult male Wistar rats (280–310 g), provided by Unidad de Producción y Experimentación de Animales de Laboratorio (UPEAL, Cinvestav), were acclimatized to the laboratory conditions and maintained on a 12/12 h light/dark cycle. All experimental procedures were approved by the Animal Care Committee of the National Institute of Rehabilitation (protocol 57/16), and performed in accordance with the recommendations of the Guide for the Care and Use of Experimental Animals (Olfert et al. 1993). The bioethical and statistical criteria established by Festing (Festing 1994) were followed to use the minimum number of animals required.
6-OHDA lesion
Rats were anesthetized with a ketamine-xylazine mixture (80/10 mg/kg; i.p.) and placed in a stereotaxic frame (Stoelting Corp., Wood Dale, IL, USA). The skull was exposed, and a trephine hole of ~1 mm diameter was trepanned in the bone at the following coordinates (Paxinos and Watson 1998): anteroposterior (AP), −5 mm from bregma; lateral (L), −2 mm from midline; vertical (V), 7.2 mm below the duramadre. For SNc lesion, 6-OHDA (8 μg/μL saline solution containing 0.1% ascorbic acid) was injected unilaterally at a flow rate of 0.4 μL/min for 5 min using an injection pump (CMA/400, CMA/Microdialysis, Kista, Sweden), and the needle (30 gauge) was left in place for 5 min before withdrawn. After 2 weeks, the contralateral rotation induced by the systemic administration of apomorphine hydrochloride (0.5 mg/kg, s.c.) was evaluated and only rats with six or more contralateral turns per min were included in the study.
Experimental design
Fifty-six hemiparkinsonian rats were divided into the following four groups (14 animals per group): (a) the vehicle group (vehicle) received a daily intraperitoneal (i.p.) injection of saline solution for 21 days; (b) the L-Dopa alone group (L-Dopa) received a daily dose of benserazide (15 mg/kg, i.p.) followed by L-Dopa (6.25 mg/kg, i.p.) 20 min later, for 21 days; (c) the chronic immepip group (L-Dopa+immepip) received a daily dose of L-Dopa plus immepip for 21 days; (d) the immepip withdrawal group (immepip withdrawal) received a daily dose of L-Dopa plus immepip for 14 days, and on day 15 immepip was withdrawn and thereafter animals received a daily dose of L-Dopa alone for 7 days. For the groups of chronic immepip and immepip withdrawal (groups c and d), the injection of L-Dopa was preceded (20 min) by the administration of benserazide.
Behavioral analysis
Fifty-six animals were videotaped during the tests (14 animals per group), and an investigator blind to the treatment reviewed the videotapes. AIMs were evaluated using a scale previously validated (Andersson et al. 1999; Avila-Luna et al. 2019; Cenci et al. 1998; Johansson et al. 2001; Lundblad et al. 2002; Papathanou et al. 2014) and classified into four subtypes: axial, limb, orolingual, and locomotive. Each AIM subtype was scored from 0 to 4 according to the following criteria: 0 = absent; 1 = present less than half of the observation time; 2 = present more than half of the observation time; 3 = present all the time, but suppressible by threatening stimuli; 4 = present all the time and not suppressible. AIMs were scored daily for 1 min every 20 min from 20 to 120 min after drug or vehicle administration. In each session, the AIM scores obtained in the 6 observation recordings were summed (maximum score 24), expressed per AIM subtype, and the number of total AIMS was counted and then summed per session. Locomotive dyskinesias were excluded from the analysis because their interpretation is controversial (Cenci et al. 1998).
Analysis of GABA and glutamate levels
GABA and glutamate levels were determined by high-performance liquid chromatography (HPLC) on day 21 in 28 animals (7 per group) as previously reported (Avila-Luna et al. 2019; Gálvez-Rosas et al. 2019). Briefly, animals were decapitated, the brain was rapidly removed and placed on an ice-cold plate to dissect the dorsal striatum (neostriatum) and a portion of the cerebral cortex. The dorsal striatum was used because of its profuse innervation by dopaminergic axons originated in SNc (Björklund and Dunnett 2007), whereas the dissected sections of the brain cortex were those with strong projections to the dorsal striatum, namely the primary and secondary motor cortices and the primary somatosensory cortex in their hindlimb and forelimb regions (Reep et al. 2003; Reep and Corwin 1999; Wu et al. 2009). The regions were separated into ipsilateral and contralateral to the lesioned SNc, and the tissues were homogenized in 40 volumes of a methanol/H2O solution (85/15%, v/v). The homogenates were centrifuged at 14,000 × g (15 min, 4 °C) and the supernatants were collected and stored at −70 °C until assayed. The SNc was examined in coronal slices to corroborate the location of the lesion by inspection with a light microscope.
High-performance liquid chromatography (HPLC)
HPLC was performed with a fluorescence detector (Linear, model Fluor LC305, Hercules, CA, USA) coupled to a binary pump (Alltech, model 626, Grace Discovery Science, Deerfield, IL, USA). Analytes were separated through an Adsorbosphere OPA HS column (100 × 4.6 mm, 5 μm particle size; Alltech). The mobile phase was sodium acetate buffer (50 mM, pH 3.2) containing 1.5% tetrahydrofuran and HPLC-grade methanol (20 min, 10–65% linear gradient). For precolumn derivatization, samples (100 μL) were mixed with 100 μL ortho-phthalaldehyde reagent (5 mg ortho-phthalaldehyde dissolved in 625 μL methanol, 5.6 mL borate buffer, pH 9.5, and 25 μL mercaptoethanol). GABA and glutamate contents were calculated by interpolation of the sample chromatograms to the chromatograms from 5 standards with known concentrations (see Figure S1).
Determination of D1R and H3R mRNA levels
The analysis of mRNA expression was performed on brain samples from 35 animals (7 per group, including 7 uninjured control rats) according to Gálvez-Rosas et al. (2019). Briefly, rats were decapitated 30 min after the last drug dose, the brain was removed and placed on an ice-cold plate to dissect the left and right striata, and the SNc was examined to corroborate the location of the lesion by inspection with a light microscope. The striatal tissue was placed in RNAlater solution (Ambion, Carlsbad, CA, USA) to preserve RNA integrity, and then stored at −70°C.
For RNA isolation, the tissue was homogenized in 1 mL Trizol reagent (Ambion, Carlsbad, CA, USA) and centrifuged (14,000 rpm, 20 min, 4 °C). RNA integrity and concentration were determined by absorbance at 260/280 nm (>1.8), using a Nanodrop lite (Thermo Fisher, Waltham, MA, USA). RNA quality was determined electrophoretically on a 1.5% agarose gel using green fluorescence (SmartGlow Pre Stain).
One μg of total RNA was reverse-transcribed to cDNA using the SuperScript III Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Then, cDNA was amplified following the procedure indicated by the manufacturer, using the TaqMan Gene Expression Assay and sequence-specific primers for the amplification of D1R (Rn03062203_s1, Applied Biosystem, Foster City, CA, USA) and H3R (Rn00585276_m1, Applied Biosystem). Real-time RT-PCR was performed with the StepOnePlus Real-Time System (Applied Biosystems). Relative expression values were calculated using the ΔΔCT method, normalized to the values of the internal control β-actin, and reported as relative intensities in arbitrary units.
Statistical analysis
Values are expressed as means ± standard error (SEM), except those corresponding to Fig. 1A, C, and E, which are presented as medians ± interquartile range. Statistical analysis of total AIMs was performed with repeated-measures ANOVA followed by Bonferroni’s post hoc test to compare the means of the drug treatment groups. Analysis of AIM scores was performed with the nonparametric Kruskal-Wallis test followed by Dunn’s test. Statistical analyses of GABA and glutamate contents and mRNA levels were performed with one-way ANOVA followed by Bonferroni’s post hoc test to compare the means between the different drug treatment groups. The statistical significance of differences was set at P < 0.05.
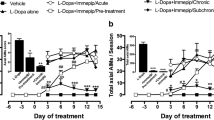
Effect of the chronic administration of H3R agonist immepip (1 mg/kg, i.p.) and their withdrawal on AIMs induced by L-Dopa (6.25 mg/kg/benserazide 15 mg/kg, i.p.): axial (A), limb (C), and orolingual (E) AIM scores; total axial (B), limb (D), and orolingual (F) scores. The chronic immepip group received a daily dose of L-Dopa + immepip (1 mg/kg, i.p.) for 21 days, and in the immepip withdrawal group, the drug was suspended from day 14 (pink line). Values are expressed as medians ± interquartile range. The statistical analysis of AIM scores was performed with the nonparametric Kruskal-Wallis test, followed by Dunn’s multiple comparisons test. The analysis of total AIMs was performed with repeated-measures ANOVA followed by Bonferroni’s post hoc test to compare the means of the drug treatment groups (14 animals per group). &,# P < 0.01, when compared with the vehicle group; * P < 0.01, when compared with the L-Dopa group. + P < 0.01, when compared with the chronic immepip group
Results
AIM expression
In accordance with previous work (Avila-Luna et al. 2019), in the L-Dopa group, dyskinesias were observed in all 14 rats, with scores in the range 1–3. In the chronic immepip, group dyskinesias were present in 3 of 14 animals, with scores 0–1, and immepip withdrawal increased the number of rats with dyskinesias to 13 of 14, with scores 1–3. In the vehicle group, dyskinetic effects were not observed in any animal.
In comparison with the respective vehicle group (Fig. 1A–F), the daily administration of L-Dopa alone for 21 days significantly increased, since day 3, the total AIM score and the scores for the three AIM subtypes analyzed (axial, limb, and orolingual). These data confirmed that the dose of L-Dopa evaluated (6.25 mg/kg) resulted in LIDs in 6-OHDA-lesioned rats with high reproducibility.
Figure 1 also shows that in comparison with the L-Dopa group, the chronic administration of immepip significantly decreased, from day 3 to day 21, the scores for total AIMs and for the three AIM subtypes analyzed. The withdrawal of immepip on days 15–21 resulted in a significant increase in the scores for total AIMs and individual AIMs on the same days, leading to a loss of the difference with the L-Dopa alone group.
Glutamate and GABA levels
Cerebral cortex
Figure 2 (panels A and B) shows that the systemic administration of L-Dopa (for 21 days) significantly increased GABA content in the ipsilateral and contralateral cerebral cortex to 61.40 ± 8.04 μg/g tissue (F3,24 = 4.69, P = 0.0102, one-way ANOVA; P = 0.0238, Tukey’s test) and 86.08 ± 12.81 μg/g tissue (F3,23 = 8.98, P = 0.0004, one-way ANOVA; P = 0.0055, Tukey’s test), respectively (values for the vehicle group: ipsilateral cortex 32.21 ± 5.83 μg/g tissue; contralateral cortex, 40.04 ± 4.35 μg/g tissue). In both hemispheres, the concomitant administration of immepip resulted in GABA levels significantly different from the corresponding L-Dopa group, but not from the control values (contralateral cortex 43.12 ± 8.76 μg/g tissue, one-way ANOVA, F3, 23 = 8.98, P = 0.0004, Tukey’s test, P = 0.0099; ipsilateral cortex, 29.66 ± 4.51 μg/g tissue, one-way ANOVA, F3, 24 = 4.69, P = 0.0102, Tukey’s test, P = 0.0128). For the immepip withdrawal group, a similar pattern was observed for the contralateral cortex (23.67 ± 6.49 μg/g tissue; F3,23 = 8.98, P = 0.0004, one-way ANOVA; P = 0.0003, Tukey’s test), whereas for the ipsilateral cortex, a marked reduction in the L-Dopa-induced increase in GABA levels was observed (43.43 ± 7.68 μg/g tissue, F3, 24 = 4.69, P = 0.0102, one-way ANOVA; P = 0.2567, Tukey’s test), although the effect did not yield statistical significance.
Effect of the systemic administration of the H3R agonist immepip (1 mg/kg, i.p.) and their withdrawal on GABA (A, B) and glutamate (C, D) levels in the cerebral cortex ipsilateral and contralateral to the lesioned SNc in rats treated with vehicle or L-Dopa. The statistical analysis was performed with one-way ANOVA followed by Tukey’s test to compare the means between the groups in each hemisphere. *P < 0.05, **P < 0.01, and ***P < 0.001. For the L-Dopa (panels A, C, and D) and immepip withdrawal (panels A and C) groups, one sample was lost during the HPLC procedure
Panels C and D of Fig. 2 show that the systemic administration of L-Dopa for 21 days to parkinsonian rats significantly increased glutamate levels to 243.26 ± 21.02 (F3, 24 = 8.26, P = 0.0006, one-way ANOVA; P = 0.0026, Tukey’s test) and 247.94 ± 22.75 μg/g tissue (F3, 23 = 7.41, P = 0.0012, one-way ANOVA; P = 0.0063, Tukey’s test) in the cerebral cortices ipsilateral and contralateral to the lesioned SNc, compared with the respective vehicle group (137.36 ± 16.61 μg/g tissue, ipsilateral cortex; 133.55 ± 25.41 μg/g tissue, contralateral cortex). The concomitant administration of immepip resulted in a tendency in both cortices for a reduction in glutamate levels compared with the L-Dopa group, but the effect was not statistically significant (Fig. 2A–B). Compared with the respective L-Dopa group, the withdrawal of immepip resulted in a significant decrease in glutamate levels in the cerebral cortices ipsilateral (127.28 ± 13.09 μg/g tissue; F3, 24 = 8.26, P = 0.0006, one-way ANOVA; P = 0.0010, Tukey’s test) and contralateral (114.87 ± 19.67 μg/g tissue; F3, 23 = 7.41, P = 0.0012, one-way ANOVA; P = 0.0022, Tukey’s test) to the lesioned SNc.
The glutamate/glutamine ratio estimates the fraction of glutamine converted to glutamate and thus evaluates glutamate synthesis. Figure 3A and B show that for the ipsilateral and contralateral cerebral cortices, in all three groups with L-Dopa administration, the glutamate/glutamine ratio increased, although the effect was statistically significant only for the chronic immepip group in both hemispheres (ipsilateral, 5.22 ± 0.96 versus 2.02 ± 0.24 for the vehicle group, one-way ANOVA, F3, 24 = 3.02, P = 0.0494, Tukey’s test, P = 0.0328; contralateral 5.07 ± 0.92 versus 2.12 ± 0.24 for the vehicle group, one-way ANOVA, F3, 25 = 3.14, P = 0.0430, Tukey’s test, with P = 0.0283).
Effect of the systemic administration of the H3R agonist immepip (1 mg/kg, i.p.) and their withdrawal on the glutamate/glutamine ratio (A, B) and the GABA/glutamate ratio (E, F) in the cerebral cortex ipsilateral and contralateral to the lesioned SNc in animals treated with vehicle or L-Dopa. The statistical analysis was performed with one-way ANOVA followed by Tukey’s test to compare the means between the groups; *P < 0.05. For panels B and C (immepip withdrawal group), one or two samples, respectively, were lost during the HPLC procedure
The GABA/glutamate ratio is an index of the fraction of glutamate converted to GABA before its release to the synaptic cleft (Gálvez-Rosas et al. 2019). In the contralateral cortical hemisphere (Fig. 3C), there was a tendency for a GABA/glutamate ratio lower than that of the corresponding control group for all three experimental groups, but none of the values yielded statistical significance. In the ipsilateral cortex (Fig. 3D), a significant effect was observed only for the immepip withdrawal group (0.35 ± 0.06; F3, 25 = 3.04, P = 0.0476, one-way ANOVA; P = 0.0295, Tukey’s test), with a ratio higher than that of the chronic immepip group (0.18 ± 0.03, Fig. 3D).
Striatum
For GABA content, no significant modifications were observed in the contralateral striatum for any of the drug treatments (Fig. 4A). For the ipsilateral striatum (Fig. 4B), L-Dopa administration increased GABA content to 143.01 ± 6.46 ng/g tissue, significantly higher than that of the vehicle (85.68 ± 7.53 μg/g, F3, 24 = 13.2, P < 0.0001, one-way ANOVA; P = 0.0002, Tukey’s test), whereas values in both the chronic immepip and immepip withdrawal groups were not different from those of the control group.
Effect of the systemic administration of the H3R agonist immepip (1 mg/kg, i.p.) and their withdrawal on GABA (A, B) and glutamate (C, D) levels, and the GABA/glutamate ratio (E, F) in the striatum ipsilateral and contralateral to the lesioned SNc in rats treated with vehicle or L-Dopa. The statistical analysis was performed with one-way ANOVA followed by Tukey’s test to compare the means between groups; *P < 0.05, **P < 0.01, and ***P < 0.001. For panels C and E (immepip withdrawal), one sample was lost during the HPLC procedure
Regarding glutamate levels, no significant modifications were observed in the contralateral striatum for any of the drug treatments (Fig. 4C). In the ipsilateral nucleus (Fig. 4D), a significant effect was observed only for the immepip withdrawal group, with reduced levels compared with the control group (189.87 ± 20.44 μg/g versus 295.05 ± 23.93 μg/g of tissue, respectively; F3, 24 = 4.84, P = 0.0090, one-way ANOVA; P = 0.0046, Tukey’s test).
For the GABA/glutamate ratio, in the striatum contralateral to the lesioned SNc, none of the drug treatments affected the ratio (Fig. 4E), whereas in the ipsilateral striatum (Fig. 4F), the ratio significantly increased in both the L-Dopa and immepip withdrawal groups, to 0.59 ± 0.03 (F3, 25 = 5.72, P = 0.0040, one-way ANOVA; P = 0.0223, Tukey’s test) and 0.59 ± 0.13 (P = 0.0216), respectively, compared with the vehicle group (0.29 ± 0.02). No change was observed in the immepip group.
Striatal D1R and H3R mRNA levels
In the striatum contralateral to the lesioned SNc, there was a tendency for reduced D1R mRNA levels in the L-Dopa group (0.62 ± 0.05; Fig. 5A), but the value did not yield statistical significance. An increase was observed in the immepip withdrawal group (1.30 ± 0.20), but this value was only significantly different from the L-Dopa alone group (F4, 30 = 2.9, P = 0.0376, one-way ANOVA; P = 0.0269, Bonferroni’s test).
Effect of the systemic administration of the H3R agonist immepip (1 mg/kg, i.p.) and their withdrawal on mRNA levels of D1Rs (A, B) and H3Rs (C, D) in the striatum ipsilateral and contralateral to the lesioned SNc in rats treated with vehicle or L-Dopa and the left and right striata of naive (control) animals. The statistical analysis was performed with one-way ANOVA followed by Bonferroni’s post hoc test to compare the means between the groups. * P < 0.05, **P < 0.01, and ***P < 0.001
As shown in Fig. 5B, for the ipsilateral striatum, a significant reduction in D1R mRNA was observed in the vehicle (0.56 ± 0.04, F4, 30 = 19, P < 0.0001, one-way ANOVA; P = 0.0114, Bonferroni’s test) and L-Dopa groups (0.58 ± 0.07, P = 0.0210), compared with control values (1.00 ± 0.04), and a significant increase was observed in the immepip group (1.40 ± 0.08) compared with the control (1.00 ± 0.04, P = 0.0181, Bonferroni’s test), vehicle (0.56 ± 0.04, P < 0.0001) and L-Dopa groups (0.58 ± 0.07, P < 0.0001). In the immepip withdrawal group (Fig. 5B), D1R mRNA was not different from the control group (1.2 ± 0.16 and 1.00 ± 0.04, respectively; P > 0.9999), but significantly different from the vehicle (0.56 ± 0.04, P = 0.0002) and L-Dopa (0.58 ± 0.07, P = 0.0004).
For H3R mRNA, no significant modifications were observed in the contralateral striatum for any of the drug treatments (F4, 30 = 1.2, P = 0.3420, one-way ANOVA; Fig. 5C). Values for the immepip withdrawal group were also significantly lower than those of the L-Dopa group (0.66 ± 0.07 and 0.97 ± 0.02, respectively; P = 0.0485). In the striatum ipsilateral to the lesioned SNc, there was a tendency for increased H3R mRNA levels in the L-Dopa and chronic immepip groups (1.30 ± 0.22 and 1.40 ± 0.13, respectively; Fig. 5D), but the values did not yield statistical significance when compared with the control and vehicle groups. There was also a tendency for reduced levels in the immepip withdrawal group (0.76 ± 0.11), but the value was only significantly different from the chronic immepip group (1.40 ± 0.13, F4, 30 = 4.7, P = 0.0047, one-way ANOVA; P = 0.0092, Bonferroni’s test).
Discussion
In line with a previous report (Avila-Luna et al. 2019), in this study, the chronic administration (14 days) of the H3R agonist immepip reduced LIDs in a model of hemiparkinsonism induced by unilateral 6-OHDA lesion to dopaminergic SNc neurons. Furthermore, glutamate and GABA levels increase in the cerebral cortex of dyskinetic rats and this effect was attenuated or prevented, respectively, by the administration of immepip, suggesting that the antidyskinetic action of H3R activation also involves effects on cortical glutamatergic and GABAergic neurons.
Cortical glutamate and GABA levels
As mentioned in the “Introduction” section, glutamatergic overactivity is associated with LIDs (Bido et al. 2011; Blanchet et al. 1998; Huot et al. 2013; Jonkers et al. 2002; Lundblad et al. 2002; Lundblad et al. 2005; Mela et al. 2007; Papathanou et al. 2014). In this study, glutamate levels were significantly higher in both cortical hemispheres of rats with LIDs. The increase was partially, but not significantly reduced by immepip, and values were lower and not different from those of control animals after immepip withdrawal, suggesting that the immepip effect requires an extended period that could be fulfilled by the remanent effect of the drug after its withdrawal. Chronic H3R stimulation could thus reduce cortical glutamate levels. H3R activation has been shown to inhibit glutamate release in several regions of the rodent brain, including the cerebral cortex (Takei et al. 2017) and striatum (Ellender et al. 2011; Molina-Hernandez et al. 2001). Although there is no experimental evidence, our data suggest that H3R activation could also regulate glutamate synthesis in glutamatergic nerve terminals. An important consideration for glutamate content (and to a certain degree also for GABA) is that only a minor proportion of the neuronal pool is directly available for synaptic release whereas our determinations measure the total amount of these amino acids (Aas et al. 1992; Waagepetersen et al. 2007). Furthermore, we cannot discard the contribution to our results of the glial compartment, although it represents minor pools of glutamate and GABA (Gadea and López-Colomé 2001; Schousboe 1981; Waagepetersen et al. 2007).
In the cerebral cortex, 70–85% of neuronal cells are glutamatergic pyramidal neurons, whose axons project to other regions of the brain, including the striatum. Collaterals of projecting axons establish synaptic contacts with adjacent pyramidal neurons (Conti et al. 1989; DeFelipe and Fariñas 1992; Dori et al. 1989) and represent thus the main source of cortical glutamate, although neurotransmitter synthetized in the axons of cortico-cortical neurons can also contribute to cortical glutamate content (Elhanany and White 1990).
The enzyme glutaminase catalyzes the hydrolysis of glutamine to glutamate (Guerriero et al. 2015; Waagepetersen et al. 2007), and the glutamate/glutamine ratio estimates thus the fraction of glutamine converted to glutamate. Our results show a tendency in both cortical hemispheres for a higher ratio in the L-Dopa group and a significant increase in the L-Dopa/immepip group, with no difference in the immepip withdrawal group (Fig. 3A and B). These effects could be due to enhanced glutamate synthesis induced by L-Dopa administration.
In both cerebral cortices, a significant increase in GABA content was also observed in the L-Dopa group, and this effect was prevented by the co-administration of immepip and remained after the withdrawal of the H3R agonist. GABAergic interneurons are estimated in 15–30% of total cortical neurons (DeFelipe and Fariñas 1992) and GABA synthesis occurs mainly in GABAergic nerve terminals (Martin and Rimvall 1993) by the decarboxylation of glutamate to GABA by the enzyme glutamate decarboxylase, GAD (Guerriero et al. 2015; Martin and Rimvall 1993; Waagepetersen et al. 2007). The GABA/glutamate ratio is considered thus an index of the fraction of glutamate converted to GABA (Gálvez-Rosas et al. 2019) before its release to the synaptic cleft and conversion to glutamine following uptake by glial cells (Diaz-Ruiz et al. 2007). However, in this study, no changes were observed in the cortical GABA/glutamate ratio (Fig. 3), most likely reflecting the parallel modifications in the levels of both neurotransmitters (Fig. 2).
Striatal glutamate and GABA levels
For glutamate levels, the only significant change was a reduction below control values in the striatum ipsilateral to the lesioned SNc for the immepip withdrawal group. For GABA levels, a significant increase compared with control values was observed in the ipsilateral striatum for the L-DOPA, immepip and immepip withdrawal groups. These effects are in accord with in vivo studies that showed that the chronic administration of L-Dopa that results in LIDs also increases GABA levels (Avila-Luna et al. 2019; Bido et al. 2011; Mela et al. 2012) and could be underlain by increased activity of the GABAergic medium spiny neurons, the main neuronal population in the striatum (Calabresi et al. 2014), driven by enhanced cortico-striatal synaptic activity. In this regard, positron emission tomography with 2-fluorodeoxyglucose as an indicator of metabolic activity showed increased glucose uptake in the posterior putamen for the more affected hemisphere in a patient with Parkinson’s disease (Obeso et al. 2014). Accordingly, our results show that the GABA/glutamate ratio increased in the ipsilateral nucleus of the L-Dopa and the immepip withdrawal group, reflecting the significant increase in GABA content and reduction in glutamate levels, respectively. The enhanced conversion of glutamate to GABA (Petroff 2002) could also contribute to the increase in the GABA/glutamate ratio in the ipsilateral striatum found in this study.
D1R and H3R mRNA levels
Striato-nigral neurons co-express H3Rs and D1Rs (González-Sepúlveda et al. 2013) and there is evidence for functional interactions between these receptors (Arias-Montaño et al. 2001; Avila-Luna et al. 2019; Garcia et al. 1997; Moreno et al. 2011; Rapanelli 2017; Rapanelli et al. 2016; Ryu et al. 1994a). In this study, we found a reduction (−50 ± 5%) in D1R mRNA in the striatum ipsilateral to the lesioned SNc in accord with some previous studies. For example, Gerfen et al. (1990) reported a modest (⁓10%) but significant reduction in D1R mRNA expression, evaluated by in situ hybridization, in rat striato-nigral neurons following 6-OHDA lesion to the SNc; also by employing in situ hybridization, Capper-Loup et al. (2013) found a decrease (20–25% reduction) in the ipsilateral striatum after 6-OHDA injection into the median forebrain bundle. Quantitative RT-PCR analysis also indicates reduced D1R mRNA levels in the rat striatum following 6-OHDA injection into the same nucleus (50–60% reduction; Rui et al. 2013) or the median forebrain bundle (−27%, Zheng et al. 2018).
Noteworthy, in the study by Gerfen et al. (1990), the decrease in mRNA was reversed by the intermittent (once daily, i.p.) but not by the continuous (i.p. osmotic pump) administration of the D1R agonist SKF-38393 (12.5 mg/kg for 21 days), whereas in our study, the administration of L-Dopa, whose conversion to dopamine leads to the activation of D1Rs and D2Rs, failed to modify the lesion effect. This discrepancy could be due to the extended half-life of L-Dopa (6.5 h) upon administration in combination with carbidopa (Dankyi et al. 2020). Furthermore, Ryu et al. (1996) reported that specific [3H]-SCH23390 binding to D1Rs increased by 24% in the striatum ipsilateral to the 6-OHDA-lesioned SNc, although D1R activation with SKF-38393 (10 mg/kg, i.p., 10 days) had no effect on receptor density. The reduction in D1R mRNA (this study; Capper-Loup et al. 2013; Gerfen et al. 1990; Rui et al. 2013) may therefore represent a mechanism to compensate for the sensitization of D1R-triggered signaling pathways that follows dopaminergic denervation (Aubert et al. 2005; Berthet et al. 2009; Blanchet et al. 2012; Morin et al. 2014; Mosharov et al. 2015; Papathanou et al. 2011; Spigolon and Fisone 2018).
As to the effect of the H3R agonist immepip, its concomitant administration with L-Dopa not only prevented the reduction in D1R mRNA in the striatum ipsilateral to the lesioned SNc, but increased mRNA levels above control values, an effect partially restored after drug withdrawal. These results suggest that after dopaminergic denervation, chronic H3R activation leads to up-regulation of D1R expression.
The 5′-flanking sequences of the D1R gene contain high G+C content, potential cyclic AMP and glucocorticoid response element sequences (Wu et al. 2018; Zhou et al. 1992), and predicted binding sites for transcription factors such as SP1, AP1, AP2 (Healy and O’Rourke 1997; Zhou et al. 1992), Pax-6, RFX1 24, Elk-1, Ik-1, c-Rel, and USF (Wu et al. 2018). H3Rs couple to Gαi/o proteins and via their Gβγ subunits activate the MAPK signaling pathway (Nieto-Alamilla et al. 2016) leading to the phosphorylation of a number of transcription factors including c-Fos, c-Jun (part of AP1), Elk-1, and Pax-6 (Yoon and Seger 2006). Systemic administration of the H3R agonist RAMH induces MAPK activation in mouse D1R-expressing striatal neurons (Rapanelli et al. 2016), and H3R/D1R heterodimerization allows for H3R agonists to activate MAPK signaling in rat striatal slices (Moreno et al. 2011). Thus, H3R activation could lead to MAPK-mediated up-regulation of D1R gene expression as observed in this study. Noteworthy, immepip withdrawal had no effect on D1R mRNA compared with the chronic immepip group despite increasing the scores for individual and total LIDs.
Regarding striatal H3R expression, quantitative autoradiography studies showed a modest (10–16%) but significant increase in the binding of the H3R agonist [3H]-N-α-methyl histamine ([3H]-NAMH) in the ipsilateral striatum 31 days (Ryu et al. 1994b; Ryu et al. 1996) or 5 and 8 weeks (Anichtchik et al. 2000) after 6-OHDA injection into the SNc, and in the study by Ryu et al. (1996), the effect was reverted by the systemic administration of the D1R agonist SKF-38393. Moreover, in the study by Anichtchik et al. (2000), in situ hybridization also revealed an increase in H3R mRNA in the ipsilateral striatum, statistically significant at 8 weeks, but not 5 weeks, after SNc lesion. These results indicated that via D1R activation, dopamine controls H3R expression in striatal neurons. However, in this study for both hemispheres, neither SNc lesion nor L-Dopa administration affected striatal H3R mRNA, which increased in the denervated striatum following H3R activation (Fig. 5). Differences in methodological approaches, namely autoradiography (Anichtchik et al. 2000; Ryu et al. 1994b; Ryu et al. 1996), in situ mRNA hybridization (Anichtchik et al. 2000) or qRT-PCR (this study), and the period post-lesion (21 days, Ryu et al. 1994b, 1996); 5 and 8 weeks (Anichtchik et al. 2000) or 3 weeks (this study), could account for the discrepancies.
The increase in H3R mRNA in response to immepip administration and the reversal by the withdrawal of the H3R agonist found in this study suggest that H3Rs control their own expression in the denervated striatum. However, the lack of effect in the contralateral nucleus is intriguing and requires further investigation. To the best of our knowledge, there is no information on the transcriptional regulation of the H3R; however, it can be suggested that receptor expression is regulated by the PLC/PKC, MAPK, and PI3K signaling pathways, known to be activated by H3R stimulation (Nieto-Alamilla et al. 2016).
Finally, the discussion focusses on the effects of the dopaminergic lesion and drug administration on D1R-expressing medium spiny neurons. Although H3Rs are also expressed by striatal GABAergic and cholinergic interneurons, these cells represent < 5% of striatal neurons (Kreitzer and Malenka 2008) making unlikely a significant contribution to the actions reported. D2R-containing medium spiny neurons also express H3Rs (Ellenbroek and Ghiabi 2014; González-Sepúlveda et al. 2013) and we cannot therefore discard that drug effects are also exerted on these neuronal cells. Further research is required to discriminate the actions on specific striatal neuronal populations.
Conclusion
In a rat model of hemiparkisonism and L-Dopa treatment, the antidyskinetic effect of the chronic administration of the H3R agonist immepip is accompanied by a reduction in the L-Dopa-induced increase in GABA and glutamate levels in the cerebral cortex and GABA content in the striatum. Immepip administration also reduced the decrease in striatal D1R mRNA levels induced by dopaminergic denervation and increases H3R mRNA levels.
These findings support that H3R activation counteracts the disturbances in the glutamatergic cortico-striatal system that participate in L-Dopa-induced dyskinesias and could contribute to the understanding of the pathophysiology of disorders affecting the basal ganglia and the design of novel pharmacological treatments.
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- AIMs:
-
Abnormal involuntary movements
- D1Rs:
-
Dopamine D1 receptors
- H3Rs:
-
Histamine H3 receptors
- L-Dopa :
-
L-3,4-dihydroxyphenylalanine
- LIDs:
-
L-Dopa-induced dyskinesias
- MSN:
-
Medium spiny neurons
- SNc:
-
Substantia nigra pars compacta
- SNr:
-
Substantia nigra pars reticulata
References
Aas JE, Laake JH, Brodal P, Ottersen OP (1992) Immunocytochemical evidence for in vitro release of glutamate and GABA from separate nerve terminal populations in the rat pontine nuclei. Exp Brain Res 89:540–548
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Albin RL, Young AB, Penney JB (1989) The functional-anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Andersson M, Hilbertson A, Cenci MA (1999) Striatal fosB expression is causally linked with L-Dopa-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis 6:461–474
Anichtchik OV, Huotari M, Peitsaro N, Haycock JW, Männistö PT, Panula P (2000) Modulation of histamine H3 receptors in the brain of 6-hydroxydopamine-lesioned rats. Eur J Neurosci 12:3823–3832
Arias-Montaño JA, Floran B, Garcia M, Aceves J, Young JM (2001) Histamine H(3) receptor-mediated inhibition of depolarization-induced, dopamine D(1) receptor-dependent release of (3)H-gamma-aminobutryic acid from rat striatal slices. Br J Pharmacol 133:165–171
Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E (2005) Increased D-1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57:17–26
Avila-Luna A, Rios C, Galvez-Rosas A, Montes S, Arias-Montano JA, Bueno-Nava A (2019) Chronic administration of the histamine H-3 receptor agonist immepip decreases L-Dopa-induced dyskinesias in 6-hydroxydopamine-lesioned rats. Psychopharmacol 236:1937–1948
Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, Francardo V, Alcacer C, Ding YM, Brambilla R, Fisone G, Stoessl AJ, Bourdenx M, Engeln M, Navailles S, De Deurwaerdere P, Ko WKD, Simola N, Morelli M et al (2015) Pathophysiology of L-Dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 132:96–168
Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B (2009) Pharmacological analysis demonstrates dramatic alteration of D-1 dopamine receptor neuronal distribution in the rat analog of L-Dopa-induced dyskinesia. J Neurosci 29:4829–4835
Bido S, Marti M, Morari M (2011) Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J Neurochem 118:1043–1055
Björklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202
Blanchet PJ, Konitsiotis S, Chase TN (1998) Amantadine reduces levodopa-induced dyskinesias in parkinsonian monkeys. Mov Disord 13:798–802
Blanchet PJ, Parent M-T, Rompre PH, Levesque D (2012) Relevance of animal models to human tardive dyskinesia. Behav Brain Funct 8:1–9
Bolam JP, Ellender TJ (2016) Histamine and the striatum. Neuropharmacol 106:74–84
Calabresi P, Pisani A, Centonze D, Bernardi G (1997) Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev 21:519–523
Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17:1022–1030
Capper-Loup C, Frey CM, Rebell D, Kaelin-Lang A (2013) Adaptive gene expression changes on the healthy side of parkinsonian rats. Neurosci 233:157–165
Carta M, Bezard E (2011) Contribution of pre-synaptic mechanisms to L-Dopa-induced dyskinesia. Neurosci 198:245–251
Cenci MA, Lee CS, Bjorklund A (1998) L-Dopa-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10:2694–2706
Connolly BS, Lang AE (2014) Pharmacological treatment of parkinson disease a review. JAMA 311:1670–1683
Conti F, Defelipe J, Fariñas I, Manzoni T (1989) Glutamate-positive neurons and axon terminals in cat sensory cortex: a correlative light and electron microscopic study. J Comp Neurol 290:141–153
Dankyi BO, Amponsah SK, Allotey-Babington GL, Adams I, Goode NA, Nettey H (2020) Chitosan-coated hydroxypropylmethyl cellulose microparticles of levodopa (and Carbidopa): in vitro and rat model kinetic characteristics. Curr Ther Res 93:100612
De la Fuente-Fernandez R, Sossi V, Huang ZG, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ (2004) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain 127:2747–2754
DeFelipe J, Fariñas I (1992) The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol 39:563–607
Diaz-Ruiz A, Salgado-Ceballos H, Montes S, Maldonado V, Tristan L, Alcaraz-Zubeldia M, Ríos C (2007) Acute alterations of glutamate, glutamine, GABA, and other amino acids after spinal cord contusion in rats. Neurochem Res 32:57–63
Dori I, Petrou M, Parnavelas JG (1989) Excitatory transmitter amino acid-containing neurons in the rat visual cortex: a light and electron microscopic immunocytochemical study. J Comp Neurol 290:169–184
Elhanany E, White EL (1990) Intrinsic circuitry: synapses involving the local axon collaterals of corticocortical projection neurons in the mouse primary somatosensory cortex. J Comp Neurol 291:43–54
Ellenbroek BA, Ghiabi B (2014) The other side of the histamine H3 receptor. Trends Neurosci 37:191–199
Ellender TJ, Huerta-Ocampo I, Deisseroth K, Capogna M, Bolam JP (2011) Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J Neurosci 31:15340–15351
Fahn S (2008) The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord 23:S497–S508
Festing MF (1994) Reduction of animal use: experimental design and quality of experiments. Lab Anim 28:212–221
Gadea A, López-Colomé AM (2001) Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J Neurosci Res 63:461–468
Gálvez-Rosas A, Avila-Luna A, Valdés-Flores M, Montes S, Bueno-Nava A (2019) GABAergic imbalance is normalized by dopamine D1 receptor activation in the striatum contralateral to the cortical injury in motor deficit-recovered rats. Psychopharmacol 236:2211–2222
Garcia M, Floran B, Arias-Montaño JA, Young JM, Aceves J (1997) Histamine H3 receptor activation selectively inhibits dopamine D1 receptor-dependent [3H] GABA release from depolarization-stimulated slices of rat substantia nigra pars reticulata. Neurosci 80:241–249
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
González-Sepúlveda M, Rosell S, Hoffmann HM, Castillo-Ruiz MM, Mignon V, Moreno-Delgado D, Michel V, Díaz J, Sabriá J, Ortiz J (2013) Cellular distribution of the histamine H3 receptor in the basal ganglia: functional modulation of dopamine and glutamate neurotransmission. BG 3:109–121
Guerriero RM, Giza CC, Rotenberg A (2015) Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 15: 1-11
Healy DP, O’Rourke DA (1997) Regulation of dopamine-1A (D1A) receptor gene transcription. Clin Exp Hypertens 19:1–13
Hely MA, Morris JGL, Reid WGJ, Trafficante R (2005) Sydney multicenter study of Parkinson’s disease: non-L-Dopa-responsive problems dominate at 15 years. Mov Disord 20:190–199
Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM (2013) The pharmacology of L-Dopa-induced dyskinesia in Parkinson’s disease. Pharmacol Rev 65:171–222
Johansson PA, Andersson M, Andersson KE, Cenci MA (2001) Alterations in cortical and basal ganglia levels of opioid receptor binding in a rat model of L-Dopa-induced dyskinesia. Neurobiol Dis 8:220–239
Jonkers N, Sarre S, Ebinger G, Michotte Y (2002) MK801 suppresses the L-Dopa-induced increase of glutamate in striatum of hemi-Parkinson rats. Brain Res 926:149–155
Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60:543–554
LeWitt PA (2015) Levodopa therapy for Parkinson’s disease: pharmacokinetics and pharmacodynamics. Mov Disord 30:64–72
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Lundblad M, Usiello A, Carta M, Håkansson K, Fisone G, Cenci MA (2005) Pharmacological validation of a mouse model of L-DOPA-induced dyskinesia. Exp Neurol 194:66–75
Martin DL, Rimvall K (1993) Regulation of γ-aminobutyric acid synthesis in the brain. J Neurochem 60:395–407
Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA (2007) Antagonism of metabotropic glutamate receptor type 5 attenuates L-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem 101:483–497
Mela F, Marti M, Bido S, Cenci MA, Morari M (2012) In vivo evidence for a differential contribution of striatal and nigral D1 and D2 receptors to L-DOPA induced dyskinesia and the accompanying surge of nigral amino acid levels. Neurobiol Dis 45:573–582
Molina-Hernandez A, Nunez A, Sierra JJ, Arias-Montano JA (2001) Histamine H-3 receptor activation inhibits glutamate release from rat striatal synaptosomes. Neuropharmacol 41:928–934
Moreno E, Hoffmann H, Gonzalez-Sepulveda M, Navarro G, Casado V, Cortes A, Mallol J, Vignes M, McCormick PJ, Canela EI, Lluis C, Moratalla R, Ferre S, Ortiz J, Franco R (2011) Dopamine D-1-histamine H-3 receptor heteromers provide a selective Link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J Biol Chem 286:5846–5854
Morin N, Jourdain VA, Di Paolo T (2014) Modeling dyskinesia in animal models of Parkinson disease. Exp Neurol 256:105–116
Mosharov EV, Borgkvist A, Sulzer D (2015) Presynaptic effects of levodopa and their possible role in dyskinesia. Mov Disord 30:45–53
Nieto-Alamilla G, Márquez-Gómez R, García-Gálvez A-M, Morales-Figueroa G-E, Arias-Montaño J-A (2016) The histamine H3 receptor: structure, pharmacology, and function. Mol Pharmacol 90:649–673
Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ (2014) The expanding universe of disorders of the basal ganglia. Lancet 384:523–531
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, Burn D, Halliday GM, Bezard E, Przedborski S, Lehericy S, Brooks DJ, Rothwell JC, Hallett M, DeLong MR, Marras C, Tanner CM, Ross GW, Langston JW et al (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord 32:1264–1310
Olfert E, Cross B, Mc William A (1993) Guide for the care and use of experimental animals. Can Council Anim Care 1:211
Panula P, Nuutinen S (2013) The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci 14:472–487
Panula P, Pirvola U, Auvinen S, Airaksinen MS (1989) Histamine-immunoreactive nerve-fibers in the rat-brain. Neuroscience 28:585–610
Papathanou M, Rose S, McCreary A, Jenner P (2011) Induction and expression of abnormal involuntary movements is related to the duration of dopaminergic stimulation in 6-OHDA-lesioned rats. Eur J Neurosci 33:2247–2254
Papathanou M, Jenner P, Iravani M, Jackson M, Stockwell K, Strang I, Zeng B-Y, McCreary AC, Rose S (2014) The H-3 receptor agonist immepip does not affect L-Dopa-induced abnormal involuntary movements in 6-OHDA-lesioned rats. Eur J Pharmacol 741:304–310
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn, Academic Press, San Diego
Petroff OAC (2002) GABA and glutamate in the human brain. Neuroscientist 8:562–573
Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM (2002) A detailed mapping of the histamine H-3 receptor and its gene transcripts in rat brain. Neuroscience 114:173–193
Porras G, De Deurwaerdere P, Li Q, Marti M, Morgenstern R, Sohr R, Bezard E, Morari M, Meissner WG (2014) L-Dopa-induced dyskinesia: beyond an excessive dopamine tone in the striatum. Sci Rep 4(1):3730
Radnikow G, Misgeld U (1998) Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci 18:2009–2016
Rapanelli M (2017) The magnificent two: histamine and the H3 receptor as key modulators of striatal circuitry. Prog Neuropsychopharmacol Biol Psychiatry 73:36–40
Rapanelli M, Frick LR, Horn KD, Schwarcz RC, Pogorelov V, Nairn AC, Pittenger C (2016) The histamine H3 receptor differentially modulates mitogen-activated protein kinase (MAPK) and Akt signaling in striatonigral and striatopallidal neurons. J Biol Chem 291:21042–21052
Reep RL, Corwin JV (1999) Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Res 841:43–52
Reep RL, Cheatwood JL, Corwin JV (2003) The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J Comp Neurol 467:271–292
Rui G, Guangjian Z, Yong W, Jie F, Yanchao C, Xi J, Fen L (2013) High frequency electro-acupuncture enhances striatum DAT and D1 receptor expression, but decreases D2 receptor level in 6-OHDA lesioned rats. Behav Brain Res 237:263–269
Ryu JH, Yanai K, Iwata R, Ido T, Watanabe T (1994a) Heterogeneous distributions of histamine H3, dopamine D1 and D2 receptors in rat-brain. Neuroreport 5:621–624
Ryu JH, Yanai K, Watanabe T (1994b) Marked increase in histamine H3 receptors in the striatum and substantia nigra after 6-hydroxydopamine-induced denervation of dopaminergic neurons: an autoradiographic study. Neurosci Lett 178:19–22
Ryu JH, Yanai K, Zhao X-L, Watanabe T (1996) The effect of dopamine D1 receptor stimulation on the up-regulation of histamine H3-receptors following destruction of the ascending dopaminergic neurones. Br J Pharmacol 118:585–592
Sánchez-Lemus E, Arias-Montaño JA (2004) Histamine H-3 receptor activation inhibits dopamine D-1 receptor-induced cAMP accumulation in rat striatal slices. Neurosci Lett 364:179–184
Schousboe A (1981) Transport and metabolism of glutamate and GABA in neurons are glial cells. Int Rev Neurobiol 22:1–45
Spigolon G, Fisone G (2018) Signal transduction in L-Dopa-induced dyskinesia: from receptor sensitization to abnormal gene expression. J Neural Transm 125:1171–1186
Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30:228–235
Takei H, Yamamoto K, Bae Y-C, Shirakawa T, Kobayashi M (2017) Histamine H3 heteroreceptors suppress glutamatergic and GABAergic synaptic transmission in the rat insular cortex. Front Neural Circuits 11:1–18
Waagepetersen HS, Sonnewald U, Schousboe A (2007) Glutamine, glutamate, and GABA: metabolic aspects. In: Lajtha A, Oja SS, Schousboe A, Saransaari P (eds) Handbook of Neurochemistry and Molecular Neurobiology: Amino Acids and Peptides in the Nervous System. Springer, US, Boston, MA, pp 1–21
Wu JH, Corwin JV, Reep RL (2009) Organization of the corticostriatal projection from rat medial agranular cortex to far dorsolateral striatum. Brain Res 1280:69–76
Wu X, Xu F-l, Wang B-j, Yao J (2018) Analysis of the promoter region of human dopamine receptor D1. J Mol Neurosci 65:438–443
Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24:21–44
Zheng X, Wu J, Zhu Y, Chen S, Chen Z, Chen T, Huang Z, Wei J, Li Y, Lei W (2018) A Comparative study for striatal-direct and -indirect pathway neurons to DA depletion-induced lesion in a PD rat model. Neurochem Int 118:14–22
Zhou Q-Y, Li C, Civelli R (1992) Characterization of gene organization and promoter region of the rat dopamine D1 receptor gene. J Neurochem 59:1875–1883
Acknowledgements
We thank MVZ Hugo Lecona Butrón for support with the housing, care, maintenance, and monitoring of the health of the experimental animals in INR-LGII. We also thank MVZ Javier Pérez Gallaga and Biol. Mónica Guadalupe Santamaria Olmedo for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Chronic administration of the H3R agonist immepip decreases L-Dopa-induced dyskinesias
• L-Dopa administration increases cortical GABA and glutamate levels
• Chronic immepip normalizes cortical GABA and glutamate levels
• Chronic H3R activation reduces cortico-striatal glutamatergic overactivity
Supplementary information
ESM 1
Figure S1. Representative chromatograms for glutamate, glutamine, and GABA. Panel A shows standards (10 μM). Panel B shows a striatal sample.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Avila-Luna, A., Gálvez-Rosas, A., Aguirre-Pérez, A. et al. Chronic H3R activation reduces L-Dopa-induced dyskinesia, normalizes cortical GABA and glutamate levels, and increases striatal dopamine D1R mRNA expression in 6-hydroxydopamine-lesioned male rats. Psychopharmacology 240, 1221–1234 (2023). https://doi.org/10.1007/s00213-023-06339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06339-1