Abstract
Rationale
The sensorimotor cortex and the striatum are interconnected by the corticostriatal pathway, suggesting that cortical injury alters the striatal function, which may be modulated by dopamine.
Objectives
We studied whether the activation of dopamine D1 receptors (D1Rs) modulates the γ-aminobutyric acid (GABA) and glutamate levels in the striatum of recovered rats at 192 h after cortical injury.
Methods
The D1R agonist SKF-38393 (0, 2, 3, or 4 mg/kg) was administered at 24, 48, 96, and 192 h post-injury, and then rats were decapitated to determine GABA and glutamate levels and the levels of D1R mRNA on both sides of the striatum.
Results
GABAergic imbalance in the striatum contralateral to the injury site was normalized by the administration of the D1R agonist, but this treatment did not produce a significant effect on glutamate levels, suggesting that glutamate was metabolized into GABA. The administration of SKF-38393 (2 mg/kg) decreased the levels of D1R mRNA in the striatum contralateral to the injury, and this effect was blocked by the coadministration of the D1R antagonist SCH-23390 (2 mg/kg). In the striatum ipsilateral to the injury, the D1R agonist increased the D1R mRNA levels, an effect that was blocked by SCH-23390.
Conclusion
The reversal of the GABAergic imbalance in the striatum contralateral to the cortical injury can be modulated by extrastriatal D1R activation, and the D1R agonist-induced increases in the D1R mRNA levels in the striatum ipsilateral to the injury suggest that the striatum may be necessary to achieve functional recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the structures that is typically injured in traumatic brain injury is the cerebral cortex, which is anatomically connected with other brain regions, such as the striatum, thalamus, pons, and cerebellum, by afferent and efferent connections, such as the cortico-basal ganglia-thalamo-cortical loop or the cerebellar-thalamo-cortical pathways (Bostan and Strick 2010; Daskalakis et al. 2004; Mendoza and Merchant 2014). Therefore, cortical injury results in the disruption of the connectivity between one or more brain structures (Hayes et al. 2016), including those brain regions that remain intact and are rich in dopaminergic afferents, such as the striatum, nucleus accumbens, thalamus, hippocampus, and amygdala (Bales et al. 2009).
The cerebral cortex, basal ganglia, and cerebellum are brain structures involved in skilled movements and play important roles in motor control and learning (Doya 2000; Kantak et al. 2012), which are functions that can be critically modulated by dopamine (DA) (Chagniel et al. 2012). In the rehabilitation of patients with cortical injury, the administration of drugs that modulate dopaminergic transmission has led to favorable results, such as enhanced cognitive recovery, executive function, attentional function, speed of processing, and memory (Bales et al. 2009).
In the basal ganglia, the dorsal striatum is innervated by dopaminergic afferents from the substantia nigra pars compacta (SNc) (Bjorklund and Dunnett 2007) and receives glutamatergic afferent input from the cerebral cortex and thalamus (Silberberg and Bolam 2015). Both sets of afferents are necessary for modulating the activity of GABAergic medium spiny neurons (MSNs), which comprise over 90% of the striatal neuronal population (Silberberg and Bolam 2015; Wickens and Wilson 1998); their function is also modulated by DA (Gerfen and Surmeier 2011). The DA receptor family includes D1-like (D1 and D5) and D2-like (D2, D3, and D4) subtypes (Bunzow et al. 1988; Missale et al. 1998; Sunahara et al. 1991; Van Tol et al. 1991). A subpopulation of MSNs, the striato-nigral neurons, possesses high levels of D1-like receptors, predominantly the D1 subtype (D1Rs) (Bergson et al. 1995; Le Moine and Bloch 1995; Surmeier et al. 1996); however, D2-like receptors (D2Rs) are selectively expressed in a second subpopulation, the striato-pallidal MSNs (Le Moine and Bloch 1995; Valjent et al. 2009). The activation of pre- and post-synaptic D1Rs and D2Rs is associated with the modulation of corticostriatal glutamatergic transmission and synaptic plasticity in corticostriatal synapses (Hsu et al. 1995; Kerkerian et al. 1987; López de Maturana and Sánchez-Pernaute 2010). Functionally, it was demonstrated that striatal D1Rs and D2Rs but not extrastriatal DA receptors are responsible for dopaminergic motor stimulation (Wang and Zhou 2017). Our recent research shows that D1R activation maintained motor coordination and balance in both normal and injured rats (Avila-Luna et al. 2018a; Avila-Luna et al. 2018b).
At a local level, severe cortical injury includes dendritic, somal, and axonal injury. The consequences of this damage can be observed in other brain structures, as indicated by changes in the concentrations of neurotransmitters, including monoamines, such as DA, norepinephrine, and serotonin (Bueno-Nava et al. 2010; Bueno-Nava et al. 2008; Krobert et al. 1994; Shin et al. 2011), and amino acids, such as glutamate and γ-aminobutyric acid (GABA) (Amorini et al. 2017; Guerriero et al. 2015). In the acute post-injury period, the extracellular concentrations of cortical GABA and glutamate are elevated in both animals and humans (Guerriero et al. 2015). Abnormally elevated glutamate levels following cortical injury that persist over time (up to 4 days) have been associated with a high mortality rate (23.6%) and poor functional recovery (Chamoun et al. 2010). However, in the period following cortical injury, transcranial magnetic stimulation studies have shown that changes in glutamate/GABA balance (Guerriero et al. 2015), such as the increase of GABAergic neurotransmission, are associated with alterations in motor learning (De Beaumont et al. 2012) and working memory (Hoskison et al. 2009). Therefore, experimentally restoring GABA levels to normal concentrations with drugs could provide valuable information for understanding of the mechanisms that lead to functional recovery after injury.
In this study, we investigated whether D1R activation modulates GABA and glutamate levels in the striatum of rats that recovered from motor deficits 192 h after cortical injury and whether this dopaminergic modulation is associated with striatal D1Rs.
Materials and methods
Subjects
Adult male Wistar rats weighing 280–310 g were provided by the UPEAL-Bioterio/UAM-Xochimilco, acclimatized to the laboratory conditions and maintained on a 12-h/12-h light/dark cycle. The experimental procedures used in this study were conducted in accordance with the recommendations of the Guide for the Care and Use of Experimental Animals (Olfert et al. 1993). We used the minimum possible number of animals needed according to the bioethical and statistical criteria established by Festing (1994), and all procedures were approved by the Instituto Nacional de Rehabilitación Animal Care Committee.
Surgery
The animals were anesthetized with a ketamine-xylazine mixture (80–10 mg/kg; i.p.) and positioned in a stereotaxic frame (Stoelting Company, Wood Dale, IL, USA). The skull was exposed, and a trephine hole of ~ 1-mm diameter was made in the bone at the following coordinates (in mm): anteroposterior (AP), + 0.4 mm from bregma; lateral (L), − 2.3 mm from the midline (Paxinos and Watson 2007). The meninges were punctured using the tip of a syringe needle, and the procedure was concluded for rats in the sham group. In the experimental group, the rats were injured by means of tissue suction from the right primary motor cortex (M1), according to a previously reported protocol (Avila-Luna et al. 2018a). The suction was performed using a vacuum pump (Gomco, St. Louis, MO, USA) coupled to a stainless-steel syringe needle without a beveled edge (no. 18 bore), which was inserted into the electrode holder of the stereotaxic instrument, and the depth was adjusted vertically (V) to 1.3 mm (below the duramadre) to perform the aspiration. After the surgery, the rats were housed for recovery (~ 24 h) before the experiments were conducted.
Motor coordination evaluation and recovery time for the injured rats
Motor coordination was evaluated at 24, 48, 96, and 192 h post-injury using the beam-walking test previously reported by Brailowsky et al. (1986) and modified by Bueno-Nava et al. (2010), which is a method used to quantify motor coordination deficits. This method comprised the following scores: a rat with no apparent deficits received a motor score of 0; a rat that widened its base and presented four toes off the beam bilaterally received a score of 1; if the animal limped with one hind limb (hypotonus), a score of 2 was assigned; an animal with at least 3 slips and/or 4 toes off the beam (unilaterally) received a score of 3; animals with falls or more than 3 slips received a score of 4; rats that dragged a limb obtained a score of 5; and those that were unable to run received a score of 6. Finally, the scores received on the four different sections of the beam-walking test were summed (maximum score = 24). All rats were videotaped during the test, and an investigator blind to treatment conditions reviewed the videotapes.
The recovery time of the injured rats at 192 h was described and validated in a recent study (Avila-Luna et al. 2018a). After motor coordination evaluation, the recovered rats at 192 h post-injury were utilized to determine the GABA and glutamate levels and D1R mRNA expression in the striatum.
Experimental design
Experiment 1: characterization of GABA and glutamate levels in recovered rats
Forty Wistar rats were divided into the sham group and the recovered group to characterize GABA and glutamate levels in the striatum of recovered rats at 192 h post-injury. To this end, all animals were decapitated at 192 h post-injury, and to examine reproducibility, this analysis was replicated in four independent experiments.
Experiment 2: effects of different doses of the D1R agonist SKF-38393
Twenty-five Wistar rats were allocated into two groups: sham (n = 5) and injured (n = 20). In the injured group, the rats repeatedly received the systemic administration of the D1R agonist SKF-38393 (0, 2, 3, or 4 mg/kg; intraperitoneally; n = 5 per dose at 24, 48, 96, and 192 h post-injury). Thirty minutes after the administration of the D1R agonist SKF-38393, motor coordination was evaluated using the beam-walking test in all rats, including rats in the sham group. Thirty minutes after the final drug administration, all animals were decapitated at 192 h post-injury for the determination of the GABA and glutamate levels and the GABA/glutamate ratio in the dorsal striatum.
Experiment 3: the metabolism of glutamate in the striatum of recovered rats
Twelve Wistar rats were divided into the sham (n = 6) and the injured groups (n = 6) to determine of glutamine, glutamate, and GABA levels in the striatum ipsilateral and contralateral to the injury site of recovered rats at 192 h post-injury. All animals were decapitated to examine the glutamine/glutamate ratio and the GABA/glutamate ratio.
Experiment 4: effects the D1R agonist on D1R mRNA expression in the striatum
Twenty-five Wistar rats were allocated into two groups: sham (n = 5) and injured (n = 20). Twenty injured rats were allocated to the following four subgroups of five rats each: (1) injection of vehicle solution (vehicle); (2) injection of SKF-38393 alone (2 mg/kg); (3) injection of the D1R antagonist SCH-23390 alone, and (4) coadministration of SKF-38393 (2 mg/kg) with SCH-23390 (2 mg/kg) at 24, 48, 96, and 192 h post-injury. Thirty minutes after the administration of the drugs or vehicle, the motor coordination of all rats was evaluated using the beam-walking test, including the sham group. All animals were decapitated at 192 h post-injury and 35 min after the final drug administration to determine the levels of D1R mRNA expression using real-time reverse transcription polymerase chain reaction (RT-PCR) in dorsal striatum samples.
The doses of SKF-38393 and SCH-23390 were based on previous studies (Avila-Luna et al. 2018a, b).
Drugs
R(+)-SCH-23390 hydrochloride and (±)-SKF-38393 hydrochloride (Sigma-Aldrich, Mexico City, Mexico) were dissolved in deionized water and administered intraperitoneally (i.p.) at a volume of 0.1 ml/100 g.
Analysis of GABA and glutamate levels
After decapitation, the brains were rapidly removed and placed on an ice-cold plate to isolate and separate the striatum ipsilateral and contralateral to the injury site. Before extracting the striatum, the cortical injuries were examined by inspection with a light microscope. The tissue was homogenized in 40 volumes of 85% (v/v) methanol/H2O solution and then centrifuged at 14,000 rpm for 15 min at 4 °C. The supernatant samples were stored at − 70 °C until they were assayed with high-performance liquid chromatography (HPLC). The GABA and glutamate contents were determined according to the procedures outlined by Montes et al. (2003), using an HPLC system (Alltech, binary HPLC pump, model 626, Grace Discovery Science, Deerfield, IL, USA) coupled to a fluorescence detector (Linear, model Fluor LC305, USA). An Adsorbosphere OPA HS column (100 × 4.6 mm, 5 μm particle size; Alltech) was used. The mobile phase consisted of (A) a sodium acetate buffer (50 mM, pH 3.2) containing 1.5% tetrahydrofuran and (B) HPLC-grade methanol. A linear gradient of 15 min was used for the separation method, starting at 10% of B and ending at 65% of B. The precolumn derivatization procedure was conducted by mixing 100-μl sample and 100-μl ortho-phthalaldehyde reagent (5-mg ortho-phthalaldehyde dissolved in 625-μl methanol + 5.6-ml borate buffer pH 9.5 + 25-μl mercaptoethanol). To determine the concentrations of GABA and glutamate, the signal areas for both amino acids contained in the samples were interpolated onto the 5-point calibration curves of GABA and glutamate.
Levels of D1R mRNA expression levels
At 192 h post-injury, the recovered rats were decapitated 30 min after the last drug dose. The brains were removed and placed on an ice-cold plate to isolate and separate the striatum ipsilateral and contralateral to the injury site. Before extracting the striatum, the cortical injuries were examined by inspection with a light microscope. Striatal tissue was submerged in 100-μl RNAlater solution (Ambion, Carlsbad, CA, USA) to preserve RNA integrity and then stored at − 70 °C until RNA isolation. Striatal tissue was homogenized in the presence of 0.5-ml Trizol reagent (Ambion, Carlsbad, CA, USA) followed by treatment with a TURBO DNA-free kit (Ambion, Carlsbad, CA, USA). Then, the RNA was precipitated with sodium acetate (Sigma-Aldrich, Mexico City, Mexico). The concentration and RNA integrity were determined by absorbance at 260/280 nm (> 1.8). The ethidium bromide fluorescence of RNA separated electrophoretically on a 1.5% agarose gel was determined. A total of 1 μg of total RNA was reverse transcribed into cDNA using the SuperScript III Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and then stored at − 80 °C until use.
The cDNA samples (0.1 μg per sample) were amplified using a reaction mixture containing 10-μl TaqMan Universal PCR Master Mix (Applied Biosystems, Woolston, WA, UK) and 1-μl D1Rs sequence-specific primer (Rn03062203_s1, Applied Biosystem, Foster City, CA, USA) for the TaqMan Gene Expression Assay, following the procedure outlined in the manufacturer’s instructions. PCR was analyzed using the StepOnePlus Real-Time System (Applied Biosystems, Foster City, CA, USA). All samples were run in triplicate according to the following protocol: one cycle at 95 °C for 5 min, followed by 40 cycles of 10 s at 95 °C, and 30 s at 60 °C. The PCR products (5 μl) were electrophoresed in an agarose gel using 100-bp standards as a size reference and visualized by ethidium bromide fluorescence in an ultraviolet transilluminator. Relative expression values were calculated using the ∆∆CT method and normalized to the values of the internal control β-actin. The results are shown as relative intensities in arbitrary units.
Statistical analysis
All values are expressed as the mean ± SEM. The statistical analysis of the beam-walking test was performed using the nonparametric Kruskal-Wallis test, followed by the Mann-Whitney U test to compare the mean rank of the drug treatment groups. For two related samples, the Wilcoxon test was also applied to compare the rank of means between drug treatments. Statistical analysis of the amino acids and mRNA levels was performed using one-way ANOVA followed by Dunnett’s test to compare the means of the drug treatment groups to those of the vehicle group. Tukey’s post hoc tests were used to compare the means of the drug treatment groups compared with those of the sham group. The differences observed between the experimental conditions were considered statistically significant at P < 0.05.
Results
Experiment 1: characterization of GABA and glutamate levels in recovered rats
As shown in Table 1, the GABA and glutamate levels were individually determined in the striatum ipsilateral and contralateral to injury in the injured group. In the striatum ipsilateral to injury, there were significant increases (P < 0.05) in the GABA levels of approximately 23, 42, 49, and 43% in experiments 1, 2, 3, and 4, respectively, in the injured groups compared with those in the respective sham groups. In contrast, the glutamate levels were significantly decreased (P < 0.05) by approximately − 23, − 25, − 16, and − 22% in experiments 1, 2, 3, and 4, respectively, in the injured groups compared with those in the respective sham groups.
In the side contralateral to the injury site, there was a significant increase (P < 0.05) in the striatal GABA levels of approximately 31, 50, 41, and 52% in experiments 1, 2, 3, and 4, respectively, in the injured groups compared with that in the respective sham groups. In contrast, the glutamate levels were significantly decreased (P < 0.05) by approximately − 14, − 17, − 16, and − 10% in experiments 1, 2, 3, and 4, respectively, in the injured groups compared with those in the respective sham groups.
The striatum contralateral to the injury site also indicated a tendency toward increased glutamate levels, but the comparison between the ipsilateral and contralateral striatum did not show significant differences in either GABA or glutamate levels. Finally, these results showed an increase in GABA levels and a decrease in glutamate levels on both sides of the striatum, and these results were observed in four independent experiments with recovered rats at 192 h post-injury, which indicated the reproducibility of our analytical method for determining amino acid levels.
Experiment 2a: effects of different doses of the D1R agonist on the functional recovery of the injured rats
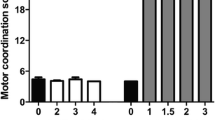
Figure 1a–c shows that the motor coordination scores obtained from the beam-walking test were significantly higher in the injured group than those in the sham group at 24, 48, and 96 h, and the motor coordination scores decreased to normal levels 192 h after the injury (Fig. 1d). These results demonstrated that both the injury model and the recovery time were consistent with our previous results (Avila-Luna et al. 2018a). Additionally, as shown in Fig. 1d, the results demonstrated that the administration of different doses of the D1R agonist SKF-38393 (2, 3, or 4 mg/kg) did not alter time to motor recovery, which was observed at 192 h post-injury.
Effects of the systemic administration of the D1R agonist SKF-38393 (0, 2, 3, and 4 mg/kg) at 24, 48, 96, and 192 h after cortical injury on motor coordination deficits. The time to recovery from the motor deficits was 192 h in the injured group. All rats were evaluated by the beam-walking test, and values are expressed as the mean ± SEM. Statistical analysis was performed using the nonparametric Kruskal-Wallis test, followed by the Mann-Whitney U test to compare the mean rank of the drug treatment groups. * P < 0.05 compared with the sham group
Experiment 2b: effects of different doses of the D1R agonist on the striatal GABA and glutamate levels
In recovered rats at 192 h post-injury, Fig. 2a–d shows the GABA and glutamate levels in the striatum ipsilateral and contralateral to the injury after the administration of different doses (0, 2, 3, and 4 mg/kg) of the D1R agonist SKF-38393 at 24, 48, 96, and 192 h post-injury. The striatum ipsilateral to the injury site showed a significant increase in GABA levels by approximately 111 to 139% (P < 0.001) in the injured group compared with those in the sham group (Fig. 2b). On the same side of the striatum, the glutamate levels were significantly decreased by approximately − 45 to − 48% (P < 0.0001) in the injured groups compared with those in the sham group. However, the administration of SKF-38393 alone (2, 3, and 4 mg/kg), compared with the respective vehicle groups, did not produce a significant alteration in the GABA and glutamate levels (Fig. 2b, d).
Effects of the systemic administration of the D1R agonist SKF-38393 (0, 2, 3, and 4 mg/kg) at 24, 48, 96, and 192 h post-injury on GABA (a, b) and glutamate (c, d) levels and on the GABA/glutamate ratio (e, f) in striatum ipsilateral and contralateral to the injury site of recovered rats at 192 h. All values are expressed as the mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s test to compare the means of the drug treatment groups with those of the vehicle group. Tukey’s post hoc tests were used to compare the means of the drug treatment groups with those of the sham group. * P < 0.05 compared with the sham group; ƒP < 0.05 compared with the vehicle group
In the striatum contralateral to the injury site, compared with the sham group, the vehicle group showed a significant increase in GABA levels by approximately 183% (P < 0.0001) (Fig. 2a). Compared with that in the sham group, the administration of 3 and 4 mg/kg SKF-38393 increased GABA levels by 61 (P = 0.034) and 79% (P = 0.022) but decreased levels by − 43 (P = 0.0021) and − 37% (P = 0.0075) relative to the vehicle group. However, the group that was administered 2-mg/kg SKF-38393 had − 57% decreased GABA levels (P = 0.0002) in comparison with the vehicle group and did not differ significantly from the sham group (Fig. 2a). On this same side of the striatum, the glutamate levels in the treated rats were significantly decreased by approximately − 26 to − 39% compared with those in the sham group. However, compared with those in the vehicle group, the administration of SKF-38393 alone (2, 3, and 4 mg/kg) did not produce a significant effect on glutamate levels (Fig. 2c).
In the striatum contralateral to the injury site, the 2 mg/kg dose showed a tendency to decrease GABA levels more than the 3 and 4 mg/kg doses, but these doses did not show any significant differences among these groups (Fig. 2a). Additionally, the tendency toward decreased glutamate levels was observed after the administration of the D1R agonist at 2 and 3 mg/kg; however, these doses did not show any differences compared with that in the vehicle group (Fig. 2c). Both decreasing trends in the GABA and glutamate levels after the administration of 2, 3, and 4 mg/kg SKF-38393 in the striatum contralateral to the injury led to an analysis of the ratio of GABA to glutamate, which showed that the systemic administration of 2, 3, and 4 mg/kg SKF-38393 significantly decreased (P < 0.05) the GABA/glutamate ratio by − 39 (0.861 ± 0.033 μM/g of fresh tissue), − 40 (0.851 ± 0.055 μM/g of fresh tissue), and − 28% (1.012 ± 0.050 μM/g of fresh tissue), respectively, compared with the administration of vehicle (1.411 ± 0.048 μM/g of fresh tissue) (Fig. 2e). These results indicated that the D1R agonist SKF-38393 decreased the rate of glutamate metabolism in the striatum contralateral to the injury.
Experiment 3: the metabolism of glutamate in the striatum of recovered rats
In the striatum ipsilateral and contralateral to the injury site, the injured group showed a significant decrease in glutamine levels by approximately − 55 (1.725 ± 0.24 μM/g of fresh tissue; P < 0.05) and − 44% (2.141 ± 0.11 μM/g of fresh tissue; P < 0.05), respectively, compared with the respective sham groups (3.88 ± 0.17 and 3.92 ± 0.39 μM/g of fresh tissue). In the injured group, the comparison between the ipsilateral and contralateral striatum did not show significant differences in the glutamine levels on either side (Fig. 3a).
Effects of cortical injury on glutamine levels (a), the glutamine/glutamate ratio (b), and the GABA/glutamate ratio (c) in the striatum ipsilateral and contralateral to the injury site of recovered rats at 192 h. All values are expressed as the mean ± SEM. Statistical analysis was performed with Student’s t test. * P < 0.05 compared with the sham group
The striatum ipsilateral to injury showed a significant decrease in the glutamine/glutamate ratio of approximately − 30% (0.496 ± 0.05 μM/g of fresh tissue; P = 0.05) in the injured group compared with those in the sham group (0.71 ± 0.05 μM/g of fresh tissue), while the contralateral striatum did not show a significant difference (Fig. 3b).
The striatum ipsilateral and contralateral to the injury from the injured group showed a significant increase in the GABA/glutamate ratio by approximately 51 (2.431 ± 0.25 μM/g of fresh tissue; P = 0.05) and 62% (2.601 ± 0.21 μM/g of fresh tissue; P = 0.05) compared with those of the respective sham groups (1.77 ± 0.16 μM/g and 1.66 ± 0.11 of fresh tissue; Fig. 3c).
Experiment 4: effects of D1R agonist on D1R mRNA expression in the striatum
As shown in Fig. 4a–b, the effect of SKF-38393 alone and in combination with SCH-23390 on D1R mRNA levels was performed separately in the striatum ipsilateral and contralateral to injury in the injured and sham groups. In the striatum ipsilateral, the vehicle group showed a significant decrease in D1R mRNA expression by approximately − 49% (P < 0.0001) compared with the sham group. Notably, the administration of the D1R agonist SKF-38393 alone (2 mg/kg) did not alter D1R mRNA expression compared with those in the sham group but increased D1R mRNA expression by 67% (P = 0.0025) compared with those in the vehicle group. In contrast, the coadministration of SKF-38393 + SCH-23390 prevented the increase in D1R mRNA expression induced by the agonist alone and showed a significant decrease of approximately − 46% (P = 0.0003) compared with the D1R agonist alone and a decrease of approximately − 54% (P < 0.0001) compared with that in the sham group (Fig. 4b).
Effects of the systemic administration of SKF-38393 alone (2 mg/kg), SCH-23390 alone (2 mg/kg), and the coadministration of SKF-38393 and SCH-23390 (2 mg/kg for both) at 24, 48, 96, and 192 h post-injury on D1R mRNA levels in the striatum ipsilateral (b) and contralateral (a) to the injury site of recovered rats at 192 h. All values are expressed as the mean ± SEM. Statistical analysis was performed as described in Fig. 2. ** P < 0.001; *** P < 0.0001 compared with the sham group; ∋P < 0.001 compared with the group treated with SKF-38393 alone; ƒP < 0.01; ƒƒP < 0.001 compared with the vehicle group; ßP < 0.0001 compared with the group treated with SCH-23390 alone
In the striatum contralateral to the injury, the administration of the D1R agonist SKF-38393 showed a significant decrease in D1R mRNA expression by approximately − 43% (P = 0.0004) compared with the administration of vehicle and by − 48% (P < 0.0001) compared with the SCH-23390 alone. In contrast, in the group that received SKF-38393 and SCH-23390, the decrease in D1R mRNA expression induced by the agonist alone was blocked, resulting in no statistically significant difference in D1R mRNA expression compared with those in the sham and vehicle groups (Fig. 4a).
Discussion
The motor coordination deficit scores obtained from the beam-walking test were significantly higher at 24, 48, and 96 h after the injury, while normal scores were observed at 192 h. In this respect, both the injury model and the recovery time of the injured rats were consistent with our previous study (Avila-Luna et al. 2018a). The administration of the D1R agonist SKF-38393 alone (2, 3, and 4 mg/kg) did not show any differences in the beam-walking scores compared with the scores of the respective vehicle groups at 24, 48, 96, and 192 h post-injury, which confirmed that the D1R agonist SKF-38393 did not modulate the time of recovery in the injured rats (Avila-Luna et al. 2018a). The motor coordination deficits (scores of ~ 5) observed in the sham group were the result of falls in the beam-walking test that developed during training and were not the product of injury during the surgery (Avila-Luna et al. 2018a; Brailowsky et al. 1986).
Regarding the GABA and glutamate levels in the striatum, our results showed an increase in GABA levels and a decrease in glutamate levels in the striatum ipsilateral and contralateral to the injury compared with those in the respective sham groups. These results were consistent in four independent experiments examining recovered rats at 192 h post-injury (see Table 1). The glutamate and GABA imbalance that was apparent in our results was indicative of changes in the levels of neurotransmitters in the striatum of rats that had recovered from the injury-induced motor coordination deficit. In humans, previous studies have shown changes in glutamate and GABA during the first hours post-injury, in the acute period, and up to 3 years after brain injury, in the chronic period (Chamoun et al. 2010; Guerriero et al. 2015; Tremblay et al. 2014). This glutamate and GABA imbalance has been confirmed in rodents with controlled injury (Amorini et al. 2017; Cantu et al. 2015; Guerriero et al. 2015; Hinzman et al. 2010; Hinzman et al. 2012). It is important to mention that various studies associated with GABA and glutamate imbalance were locally conducted with the intraparenchymal insertion of the microdialysis membrane at the cortical injury site in both humans (Bullock et al. 1998; Chamoun et al. 2010; Guerriero et al. 2015; Vespa et al. 1998) and rodents (Folkersma et al. 2011).
In other brain structures, such as the striatum, increased extracellular glutamate has been observed 2 days post-injury (Hinzman et al. 2010; Hinzman et al. 2012), and this increased glutamate was ~ 80% neuronal in origin (Hinzman et al. 2012), with some glutamate possibly originating from the glutamatergic input from the cortex and/or thalamus (Silberberg and Bolam 2015). Our results showed that on both sides of the striatum, glutamate levels decreased at 192 h post-injury in the recovered rat group compared with those in the sham group. These results do not suggest that striatal afferents from the cortex and/or thalamus are inhibited at 192 h post-injury because GABA levels were increased in the striatum ipsilateral and contralateral to the injury. The striatal glutamate levels observed in the present study cannot be explained by the rapid conversion of glutamate into glutamine (Guerriero et al. 2015), because the injured rats showed a significant decrease in glutamine levels and the glutamine/glutamate ratio did not increase (Fig. 3a–b). Another potential explanation is the conversion of glutamate into GABA because glutamate is the precursor of GABA (Petroff 2002), and the ratio of GABA/glutamate may indicate the rate of glutamatergic metabolism in the striatum. In this line, our results showed an increase in the GABA/glutamate ratio compared with that in the sham groups in the striatum ipsilateral and contralateral to the injury (Fig. 3c). It was concluded that the decreased glutamate levels at 192 h post-injury in the recovered rats are associated with glutamate conversion into GABA, which is indicative of striatal GABAergic neuronal activity. As mentioned in the Introduction, the increase of GABAergic neurotransmission during the chronic period post-injury has been associated with functional alterations, such as motor learning deficits (De Beaumont et al. 2012) and working memory dysfunction (Hoskison et al. 2009). A previous report examining ischemic brain injury documented that inhibiting striatal GABAergic activity leads to functional recovery; in contrast, GABAergic activity was related to exacerbated brain injury (Jiang et al. 2017).
In this respect, we investigated whether the activation of D1Rs modulates the GABA and glutamate levels in the striatum and whether this modulation can be associated with a deficit of striatal D1Rs in recovered rats following cortical injury. D1R activation in the striatum is associated with the increased excitability of striatal medium spiny neurons (MSNs) in response to corticostriatal glutamatergic inputs (Surmeier et al. 2007). However, in our study, the systemic administration of the D1R agonist SKF-38393 at 2, 3, and 4 mg/kg decreased GABA levels and the GABA/glutamate ratio in the striatum contralateral to the injury (Fig. 2a, e), whereas the striatum ipsilateral to the injury did not show these effects. The results in the striatum contralateral to the injury may be explained by extrastriatal D1R activation. In this context, the striatum receives glutamatergic afferent inputs from the cerebral cortex and thalamus, and the cortical inputs to the striatum are highly dominant in terms of functional properties and synaptic interactions with the MSNs (Ding et al. 2008; Silberberg and Bolam 2015; Smith et al. 2004). In the cerebral cortex, autoradiographic localization studies have shown that both rodent and primate cortices contain D1Rs and D2Rs (Camps et al. 1990), but the density of D1Rs is 10–20-fold higher than that of D2Rs (Lidow et al. 1991). As previously reported (Abekawa et al. 2000), D1R activation reduces extracellular glutamate and GABA levels in the medial prefrontal cortex. Using an immunoreactivity method, it was demonstrated that D1Rs are localized in cortical GABAergic interneurons, in which D1R activation also led to the decreased activity of the corticostriatal pathway (Muly et al. 1998) (Fig. 5). This hypothesis was supported in our study by the systemic administration of SKF-38393 (2, 3, and 4 mg/kg), which decreased the GABA levels in the striatum (Figs. 2a and 5c). These data suggested that the effective dose was at 2 mg/kg, which decreased the striatal GABA levels to normal levels comparable with those in the sham group. These results are a direct indicator of reduced excitability and the modulation of a dopaminergic agonist in the striatum contralateral to the injury site (Fig. 5c). Additionally, these results explain why the systemic administration of the D1R agonist SKF-38393 decreased D1R mRNA expression on the contralateral side, which was prevented by the coadministration of the D1R antagonist SCH-23390.
Schematic representation of the cerebral cortex and the basal ganglia synaptic circuitry in normal conditions (a) and in cortical injury (b) and the proposed action of the administration of the D1R agonist SKF-38393 (c). Red lines indicate excitatory projections, and black lines indicate inhibitory projections. Dotted lines indicate depletion by injury in the cerebral cortex. The striatum communicates with neurons in the substantia nigra pars reticulata (SNr) through a direct pathway and to the external segment of the globus pallidus (GPe), which in turn projects to the subthalamic nucleus (STN) that projects to the SNr, forming the indirect pathway. Substance nigra pars compacta (SNc)
However, the GABA levels in the striatum ipsilateral to the injury site can be explained by the injury in the right M1 (Fig. 5b, c); as described above, the striatum is anatomically connected to the cortex, and it has been shown that the M1 projects to the dorsal striatum (Mathai and Smith 2011; Reep et al. 2003). These corticostriatal glutamatergic inputs are bilateral but have an ipsilateral predominance (Lei et al. 2004; Reep et al. 2003; Reiner et al. 2010; Wu et al. 2009). This may explain why the systemic administration SKF-38393 did not produce a significant effect on striatal GABA levels in the ipsilateral side to the injury site, and this result explains the trend in decreased levels of striatal glutamate on the ipsilateral side of the injured M1 in the recovered rats. One explanation is that the D1R response by the agonist was insufficient to modulate the corticostriatal pathway in the side ipsilateral to the injury (Fig. 5c). On this same side of the striatum, the administration of 2-mg/kg SKF-38393 showed a significant increase in D1R mRNA expression compared with that in the vehicle group, and the mRNA expression levels had returned to those of the sham group. This effect was prevented by the coadministration of the D1R antagonist SCH-23390. As was previously demonstrated, cortical injury can decrease DA synthesis and release in the striatum ipsilateral to an injury (Shin et al. 2011; Wagner et al. 2005), and cortical injury has been reported to decrease the activity of protein kinase A (PKA) (Shin et al. 2011) and the phosphorylation of DA and cAMP regulated phosphoprotein 32 (DARPP-32) at threonine-34 (Bales et al. 2011). Therefore, this evidence indicates that the effect of agonists on D1R mRNA may result from the activation of striatal D1Rs in an intact striatum. This is consistent with our recent research showing that a combined cortical and striatal injury (to the same side of the brain) prevented recovery at 192 h, which suggests that an intact striatum may be necessary to achieve recovery (Avila-Luna et al. 2018a).
In conclusion, rats that had recovered motor function at 192 h after cortical injury showed an increase in GABA levels and a decrease in glutamate levels on both sides of the striatum compared with the levels of the respective sham groups. The GABAergic imbalance was normalized by D1R stimulation with the agonist SKF-38393 in the striatum contralateral to the cortical injury, but this effect was not observed on the side ipsilateral to the injury. Our results showed that decreases in the glutamate levels in the recovered rats at 192 h post-injury are associated with glutamate conversion into GABA and prevented the D1R agonist effect in the contralateral striatum. In the striatum contralateral to the injury, the decrease in GABA levels following the administration of the D1R agonist at 2 mg/kg correlated with the decrease in D1R mRNA, an effect that was prevented by the coadministration of the D1R antagonist SCH-23390. In the striatum ipsilateral to the injury, the administration of the D1R agonist increased D1R mRNA to the normal levels similar to the sham group, an effect that was prevented by the D1R antagonist. Finally, our results showed that the reversal of the GABAergic imbalance can be modulated by dopaminergic drugs and that the striatum may be necessary to achieve functional recovery. It is possible that the potential utility of these dopaminergic effects after cortical injury in TBI models may be highly relevant in the corticostriatal circuit and to our understanding of pharmacological mechanisms.
Abbreviations
- D1R:
-
dopamine D1 receptor
- D2R:
-
dopamine D2 receptor
- DA:
-
dopamine
- GABA:
-
γ-aminobutyric acid
- MSNs:
-
medium spiny neurons
- TBI:
-
traumatic brain injury
- SNc:
-
substantia nigra pars compacta
- M1:
-
primary motor cortex
- HPLC:
-
high-performance liquid chromatography
- PKA:
-
protein kinase A
- DARPP-32:
-
DA- and cAMP-regulated phosphoprotein 32
References
Abekawa T, Ohmori T, Ito K, Koyama T (2000) D1 dopamine receptor activation reduces extracellular glutamate and GABA concentrations in the medial prefrontal cortex. Brain Res 867:250–254
Amorini AM, Lazzarino G, Di Pietro V, Signoretti S, Belli A, Tavazzi B (2017) Severity of experimental traumatic brain injury modulates changes in concentrations of cerebral free amino acids. J Cell Mol Med 21:530–542
Avila-Luna A, Galvez-Rosas A, Alfaro-Rodriguez A, Reyes-Legorreta C, Garza-Montano P, Gonzalez-Pina R, Bueno-Nava A (2018a) Dopamine D1 receptor activation maintains motor coordination in injured rats but does not accelerate the recovery of the motor coordination deficit. Behav Brain Res 336:145–150
Avila-Luna A, Galvez-Rosas A, Durand-Rivera A, Ramos-Languren LE, Rios C, Arias-Montano JA, Bueno-Nava A (2018b) Dopamine D1 receptor activation maintains motor coordination and balance in rats. Metab Brain Dis 33:99–105
Bales JW, Wagner AK, Kline AE, Dixon CE (2009) Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci Biobehav Rev 33:981–1003
Bales JW, Yan HQ, Ma X, Li Y, Samarasinghe R, Dixon CE (2011) The dopamine and cAMP regulated phosphoprotein, 32 kDa (DARPP-32) signaling pathway: a novel therapeutic target in traumatic brain injury. Exp Neurol 229:300–307
Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS (1995) Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci 15:7821–7836
Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202
Bostan AC, Strick PL (2010) The cerebellum and basal ganglia are interconnected. Neuropsychol Rev 20:261–270
Brailowsky S, Knight RT, Blood K, Scabini D (1986) Gamma-aminobutyric acid-induced potentiation of cortical hemiplegia. Brain Res 362:322–330
Bueno-Nava A, Montes S, DelaGarza-Montano P, Alfaro-Rodriguez A, Ortiz A, Gonzalez-Pina R (2008) Reversal of noradrenergic depletion and lipid peroxidation in the pons after brain injury correlates with motor function recovery in rats. Neurosci Lett 443:32–36
Bueno-Nava A, Gonzalez-Pina R, Alfaro-Rodriguez A, Nekrassov-Protasova V, Durand-Rivera A, Montes S, Ayala-Guerrero F (2010) Recovery of motor deficit, cerebellar serotonin and lipid peroxidation levels in the cortex of injured rats. Neurochem Res 35:1538–1545
Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, Marmarou A, Young HF (1998) Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg 89:507–518
Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O (1988) Cloning and expression of a rat D2 dopamine receptor cDNA. Nature 336:783–787
Camps M, Kelly PH, Palacios JM (1990) Autoradiographic localization of dopamine D1 and D2 receptors in the brain of several mammalian species. J Neural Transm Gen Sect 80:105–127
Cantu D, Walker K, Andresen L, Taylor-Weiner A, Hampton D, Tesco G, Dulla CG (2015) Traumatic brain injury increases cortical glutamate network activity by compromising GABAergic control. Cereb Cortex 25:2306–2320
Chagniel L, Robitaille C, Lacharite-Mueller C, Bureau G, Cyr M (2012) Partial dopamine depletion in MPTP-treated mice differentially altered motor skill learning and action control. Behav Brain Res 228:9–15
Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C (2010) Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 113:564–570
Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R (2004) Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 557:689–700
De Beaumont L, Tremblay S, Poirier J, Lassonde M, Theoret H (2012) Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb Cortex 22:112–121
Ding J, Peterson JD, Surmeier DJ (2008) Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci 28:6483–6492
Doya K (2000) Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10:732–739
Festing MF (1994) Reduction of animal use: experimental design and quality of experiments. Lab Anim 28:212–221
Folkersma H, Foster Dingley JC, van Berckel BN, Rozemuller A, Boellaard R, Huisman MC, Lammertsma AA, Vandertop WP, Molthoff CF (2011) Increased cerebral (R)-[11C]PK11195 uptake and glutamate release in a rat model of traumatic brain injury: a longitudinal pilot study. J Neuroinflammation 8:67
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34(34):441–466
Guerriero RM, Giza CC, Rotenberg A (2015) Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 15:27
Hayes JP, Bigler ED, Verfaellie M (2016) Traumatic brain injury as a disorder of brain connectivity. J Int Neuropsychol Soc 22:120–137
Hinzman JM, Thomas TC, Burmeister JJ, Quintero JE, Huettl P, Pomerleau F, Gerhardt GA, Lifshitz J (2010) Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: an enzyme-based microelectrode Array study. J Neurotrauma 27:889–899
Hinzman JM, Thomas TC, Quintero JE, Gerhardt GA, Lifshitz J (2012) Disruptions in the regulation of extracellular glutamate by neurons and glia in the rat striatum two days after diffuse brain injury. J Neurotrauma 29:1197–1208
Hoskison MM, Moore AN, Hu B, Orsi S, Koboric N, Dash PK (2009) Persistent working memory dysfunction following traumatic brain injury: evidence for a time-dependent mechanism. Neuroscience 159:483–491
Hsu KS, Huang CC, Yang CH, Gean PW (1995) Presynaptic D2 dopaminergic receptors mediate inhibition of excitatory synaptic transmission in rat neostriatum. Brain Res 690:264–268
Jiang L, Li WL, Mamtilahun M, Song YY, Ma YY, Qu MJ, Lu YF, He XS, Zheng JY, Fu ZJ, Zhang ZJ, Yang GY, Wang YT (2017) Optogenetic inhibition of striatal GABAergic neuronal activity improves outcomes after ischemic brain injury. Stroke 48:3375–3383
Kantak SS, Stinear JW, Buch ER, Cohen LG (2012) Rewiring the brain: potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil Neural Repair 26:282–292
Kerkerian L, Dusticier N, Nieoullon A (1987) Modulatory effect of dopamine on high-affinity glutamate uptake in the rat striatum. J Neurochem 48:1301–1306
Krobert KA, Sutton RL, Feeney DM (1994) Spontaneous and amphetamine-evoked release of cerebellar noradrenaline after sensorimotor cortex contusion: an in vivo microdialysis study in the awake rat. J Neurochem 62:2233–2240
Le Moine C, Bloch B (1995) D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol 355:418–426
Lei WL, Jiao Y, Del Mar N, Reiner A (2004) Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci 24:8289–8299
Lidow MS, Goldmanrakic PS, Gallager DW, Rakic P (1991) Distribution of dopaminergic receptors in the primate cerebral-cortex—quantitative autoradiographic analysis using 3H raclopride, 3H spiperone and 3H SCH23390. Neuroscience 40:657–671
López de Maturana R, Sánchez-Pernaute R (2010) Regulation of corticostriatal synaptic plasticity by G protein-coupled receptors. Cns Neurol Disord-Drug Targets 9:601–615
Mathai A, Smith Y (2011) The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Front Syst Neurosci 5:1–10
Mendoza G, Merchant H (2014) Motor system evolution and the emergence of high cognitive functions. Prog Neurobiol 122:73–93
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225
Montes S, Alcaraz-Zubeldia M, Muriel P, Rios C (2003) Role of manganese accumulation in increased brain glutamine of the cirrhotic rat. Neurochem Res 28:911–917
Muly EC, Szigeti K, Goldman-Rakic PS (1998) D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci 18:10553–10565
Olfert E, Cross B, Mc William A (1993) Guide for the care and use of experimental animals. Can Counc Anim Care 1:211
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, London
Petroff OAC (2002) GABA and glutamate in the human brain. Neuroscientist 8:562–573
Reep RL, Cheatwood JL, Corwin JV (2003) The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J Comp Neurol 467:271–292
Reiner A, Hart NM, Lei WL, Deng YP (2010) Corticostriatal projection neurons - dichotomous types and dichotomous functions. Front Neuroanat 4:1–15
Shin SS, Bray ER, Zhang CQ, Dixon CE (2011) Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res 1369:208–215
Silberberg G, Bolam JP (2015) Local and afferent synaptic pathways in the striatal microcircuitry. Curr Opin Neurobiol 33:182–187
Smith Y, Raju DV, Pare JF, Sidibe M (2004) The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci 27:520–527
Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB (1991) Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350:614–619
Surmeier DJ, Song WJ, Yan Z (1996) Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16:6579–6591
Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30:228–235
Tremblay S, Beaule V, Proulx S, Marjanska M, Doyon J, Lassonde M, Theoret H (2014) Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin Neurophysiol 125:1371–1379
Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault J-A (2009) Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci 32:538–547
Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O (1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350:610–614
Vespa P, Prins M, Ronne-Engstrom E, Caron M, Shalmon E, Hovda DA, Martin NA, Becker DP (1998) Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg 89:971–982
Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE (2005) Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem 95:457–465
Wang YH, Zhou FM (2017) Striatal but not extrastriatal dopamine receptors are critical to dopaminergic motor stimulation. Front Pharmacol 8:1–13
Wickens JR, Wilson CJ (1998) Regulation of action-potential firing in spiny neurons of the rat neostriatum in vivo. J Neurophysiol 79:2358–2364
Wu JH, Corwin JV, Reep RL (2009) Organization of the corticostriatal projection from rat medial agranular cortex to far dorsolateral striatum. Brain Res 1280:69–76
Acknowledgments
We thank MVZ Hugo Lecona Butrón for the support with the housing, care, maintenance, and monitoring the health of the experimental animals in the INR-LGII. We thank MVZ Javier Pérez Gallaga and M en C René Valdez Mijares for technical support.
Funding
This work was supported by INR-LGII, CONACyT (grant 288512 to A. Avila-Luna).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gálvez-Rosas, A., Avila-Luna, A., Valdés-Flores, M. et al. GABAergic imbalance is normalized by dopamine D1 receptor activation in the striatum contralateral to the cortical injury in motor deficit-recovered rats. Psychopharmacology 236, 2211–2222 (2019). https://doi.org/10.1007/s00213-019-05215-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05215-1