Abstract
Rationale
Understanding the behavioral and neurobiological factors that render some individuals more susceptible than others to opioid addiction will be critical in combatting the opioid crisis.
Objective
The purpose of the current study was to determine if behavioral traits associated with an increased likelihood to take and seek cocaine are the same traits that render one more susceptible to opioid-taking and opioid-seeking behavior. Individual differences in the acquisition of remifentanil self-administration and subsequent cue-induced reinstatement of remifentanil-seeking behavior were investigated using two animal models: the high-responder (HR)/low-responder (LR) and sign-tracker (ST)/goal-tracker (GT) models. Relative to LR rats, HR rats show increased novelty-induced locomotion or “sensation-seeking” behavior, and are more likely to acquire cocaine-taking behavior and do so at a faster rate. Relative to GT rats, ST rats attribute greater incentive motivational value to reward cues and are more likely to exhibit reinstatement of cocaine-seeking behavior.
Results
In contrast to previous work using cocaine, we did not observe individual differences with respect to the acquisition of remifentanil self-administration- or cue-induced reinstatement of remifentanil-seeking behavior within the context of either the HR/LR or ST/GT model. Thus, neither the sensation-seeking trait nor the propensity to attribute incentive motivational value to reward cues predicts remifentanil-taking or remifentanil-seeking behavior.
Conclusions
These findings suggest that different traits may confer the initiation of opioid- vs. cocaine-taking behavior, and the propensity to relapse to opioid- vs. cocaine-seeking. Additional studies are needed to identify which neurobehavioral constructs confer liability to opioid use and relapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the Centers for Disease Control and Prevention, in the USA, nearly 50,000 people died in 2019 due to overdoses involving opioids (Mattson et al., 2021). Approximately 21–29% of patients prescribed opioids will abuse them, and 8–12% of patients will eventually become addicted (Vowles et al., 2015). The economic burden associated with this crisis due to healthcare costs, lost job productivity, and criminal justice costs is estimated to be $78.5 billion (Florence et al., 2016). Identifying the behavioral and neurobiological factors that make some individuals more vulnerable to opioid use and abuse, and subsequently relapse, will be critical to allow implementation of more targeted prevention and intervention strategies and help put an end to the opioid epidemic.
In the laboratory setting, animal models have revealed behavioral and neurobiological factors that may render an individual more susceptible to substance use and abuse. These include the high-responder (HR)/low-responder (LR) model and the sign-tracker (ST)/goal-tracker (GT) model. With the HR/LR model, rats are placed in a novel environment and their locomotor activity is measured. Rats that display high levels of locomotor activity are designated as HRs, while rats that display low levels of activity are designated as LRs. Prior work with the HR/LR model focused on the acquisition of drug-taking behavior has shown that HRs acquire cocaine self-administration more readily than LRs (Piazza et al., 2000; Mantsch et al., 2001; Ferris et al., 2013; Marinelli & White, 2000), and these findings extend to ethanol (Nadal et al., 2002), nicotine (Suto et al., 2001), and morphine (Abrosio et al., 1995) as well. However, outbred HRs and LRs do not differ in the reinstatement of drug-seeking behavior (Sutton et al., 2000). Thus, the “sensation-seeking” trait captured in outbred rats by individual variation in novelty-induced locomotion appears to predict the initial propensity to take drugs, but not relapse propensity.
With the ST/GT model, rats receive presentation of a lever conditioned stimulus (CS) that is paired (non-contingently) with delivery of a food unconditioned stimulus (US). Over time, two different conditioned responses (CRs) emerge: sign-tracking and goal-tracking. Sign-tracking consists of approach to the CS and interaction with it, as if it were the US itself (Hearst & Jenkins, 1974). In contrast, goal-tracking consists of approach to the site of impending food delivery during CS presentation (Boakes, 1977). Both of these CRs are acquired at similar rates. However, it is argued that while GTs primarily assign predictive value to the CS, STs assign both predictive and incentive value to the CS (Flagel & Robinson, 2017). This incentive value is a crucial component of the learning process, as it transforms the CS into a “motivational magnet” (Berridge, 2004); and it is this process that is believed to make STs more vulnerable to relapse-like behavior than GTs. Indeed, in contrast to the HR/LR model, prior work using the ST/GT model has demonstrated that STs show greater levels of cue-induced and drug-induced reinstatement of cocaine-seeking behavior relative to GTs (Saunders & Robinson, 2010, 2011; Saunders et al., 2013; Kuhn et al., 2021).
The purpose of this study was to investigate individual differences in opioid-taking and opioid-seeking behaviors using both the HR/LR and ST/GT models. The µ-opioid receptor agonist, remifentanil, was used, as its fast-acting properties make it ideal for studying the effects of opioid-associated cues (half-life elimination from blood 0.3–0.7 min (Crespo et al. (2005)) on drug-seeking behavior. Remifentanil has been shown to be as potent of a reinforcer as cocaine (Wade-Galuska et al., 2007; Freeman & Woolverton, 2011; Koffarnus et al., 2012), and is readily self-administered by rodents (Panlilio & Schindler, 2000). Furthermore, individual differences have been observed using behavioral economic (Porter-Stransky et al., 2017) and Pavlovian (Yager et al., 2015) procedures with this drug as the reward. If the pattern of individual differences for remifentanil use and relapse propensity is similar to cocaine, we would expect HR rats to show a faster rate of self-administration relative to LR rats, and ST rats to show greater cue-induced reinstatement of opioid-seeking behavior relative to GTs. Contradictory results would suggest that distinct factors mediate individual differences in response to cocaine and opioids.
Methods
Two experiments were conducted to assess individual differences in opioid-taking and cue-induced opioid-seeking behavior. In Experiment 1 (Fig. 1a), we used parameters similar to those we have used in the past with cocaine (Kuhn et al., 2018) to investigate individual differences in the rate of remifentanil self-administration and the propensity for cue-induced reinstatement of drug-seeking behavior. In Experiment 2 (Fig. 1d), we altered various aspects of the experimental paradigm that are known to affect drug-taking and drug-seeking behaviors (e.g., housing conditions, drug dose), to determine if such changes would reveal individual differences that were not apparent in Experiment 1.
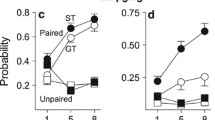
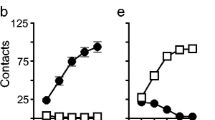
a Experimental timeline for Experiment 1. b Pavlovian conditioned approach (PavCA) index data for rats in Experiment 1 classified as sign-trackers (STs; n = 8) and goal-trackers (GTs; n = 15). c Locomotor activity scores of rats classified as high-responders (HRs; n = 11) and low-responders (LRs; n = 12). d Experimental timeline for Experiment 2. e PavCA index data for rats in Experiment 2 classified as STs (n = 13) and GTs (n = 9). f Locomotor activity scores of rats classified as HRs (n = 11) and LRs (n = 11)
Experiment 1
Subjects
The subjects were 60 male Sprague–Dawley rats from Charles River Laboratories (n = 30; 16 from Barrier R04 and 14 from Barrier C72) and Taconic Biosciences (n = 30; Barrier IBU16). Rats were obtained from different vendors to increase the likelihood of acquiring an even distribution of STs and GTs (Fitzpatrick et al., 2013). Rats weighed 225–275 g upon arrival and were allowed to acclimate to the vivarium for 1 week prior to behavioral testing. Rats were initially pair-housed in a climate-controlled vivarium with a 12-h light:dark cycle. Rats had ad libitum access to food and water throughout the experiment. All procedures conformed to the The Guide for the Care and Use of Laboratory Animals and were approved by the University of Michigan Institutional Animal Care and Use Committee.
Pavlovian conditioned approach
Rats were handled briefly by experimenters for 2 days prior to behavioral testing, and given 45-mg banana-flavored pellets in their home cage (approximately 50 pellets per cage; Bioserv; Product# F0059). Pavlovian conditioned approach (PavCA) training occurred in standard behavioral chambers from Med Associates (20.5 cm × 24.1 cm × 29.2 cm; MED Associates, St. Albans, VT) that were encased in sound-attenuating boxes with ventilation fans that provided air circulation and background noise. A recessed food magazine was located in the center of one wall of the chamber 6 cm above the grid floor. Additionally, a retractable lever was located to the right or left of the food magazine (counterbalanced to control for side bias; 6 cm above the grid floor). The lever was illuminated with an LED backlight upon presentation. On the opposite wall, a house light was located 1 cm from the top of the chamber, and used to illuminate the test chamber throughout each session.
Rats were first given 2 pre-training sessions in which one pellet was delivered randomly into the food magazine on a variable interval 30-s schedule (range 0 – 60 s; 25 pellets total per session). During these sessions, the lever was retracted. Following pre-training, rats underwent 5 sessions of PavCA training. Each session consisted of 25 trials during which an illuminated lever conditioned stimulus (CS) was presented for 8 s and, upon retraction, was immediately followed by delivery of a food pellet US. Trials were presented on a variable interval 90-s schedule (range 30–150 s).
The following measures were used to calculate the PavCA index and classify each rat as a ST or GT (Meyer et al., 2012): total number of magazine entries during CS presentations, latency to the first magazine entry during CS presentations, total number of lever contacts, and latency to the first lever contact. These measures were used to calculate the Response Bias [(lever presses – food cup entries)/(lever presses + food cup entries)], Probability Difference (p|lever press – p|food cup entry), and Latency Score [(average response time to lever press – average response time to enter the food cup)/duration of CS presentation]. These three measures were then averaged to compute the PavCA index for each day. Data from sessions 4 and 5 were averaged to calculate the PavCA index, which ranged from − 1.0 to 1.0. Rats with scores of − 1.0 to − 0.5 were classified as GTs (n = 25), while rats with scores of 0.5 to 1.0 were classified as STs (n = 15). Rats with scores between − 0.5 and 0.5 were classified as intermediate responders (INs; n = 20) and, for the purposes of the current study, were excluded from subsequent procedures and data analyses.
Locomotor testing
The day after PavCA training, rats were placed in a novel environment for 30 min and their locomotor activity was measured utilizing similar procedures as those previously described by Stead et al. (2006). Briefly, the novel environment consisted of a plastic cage (33.02 cm × 68.58 cm × 60.96 cm tall) with a metal grid floor and 0.25-in. corn cob bedding underneath. Photocells were located along the sides of the shells that the cages were located in, and breaks of these photocells generated either lateral movements (lower panel) or rearing events (upper panel). A computer recorded the number of lateral movements and rearing events during the session. These values were summed to create a locomotor activity score. Rats were classified as high responders (HRs) if their score was above the median and low responders (LRs) if their score was at the median or below.
Surgical procedure
The day after locomotor activity testing, rats underwent surgery for the insertion of indwelling jugular catheters. Rats were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and then administered carprofen (5 mg/kg, s.c.) for its analgesic properties. Rats were then catheterized with catheters made in-house as described by Krueger et al. (2021). After catheterizations were complete, rats were given sterile saline (0.9%, 3 ml, s.c.) to replenish fluids lost during surgery. In addition, rats were given infusions of the anticoagulant, heparin (100 units/ml, 0.05 ml, i.v.), and the antibiotic gentamicin sulfate (1 mg/ml, 0.05 ml, i.v.). These heparin/gentamicin infusions were administered every day after surgery throughout the behavioral training procedures in order to prevent infection and maintain catheter patency. Following surgery, rats were single-housed for the duration of the experiment and were allowed to recover for 10 days before behavioral testing began. Three rats (1 ST and 2 GTs) died during surgery and their data were removed from prior portions of the experiment.
Food self-administration
To promote the acquisition of operant responding, rats underwent 4 sessions of food self-administration prior to opioid self-administration. During these sessions, rats were placed in the same Med Associates, Inc. behavioral chambers as described above, and the same food pellets used during PavCA were used for operant response training. However, the lever was removed and two nose ports were inserted on either side of the food magazine. To prevent side bias, the port designated “active” was placed on the opposite side of the chamber from where the lever was previously located. Pokes into the “active” port resulted in the delivery of a food pellet, illumination of a cue light CS located within the port for 20 s, and termination of the house light for 20 s on a fixed-ratio 1 (FR 1) schedule. This 20-s period represented a timeout period where pokes into the active port had no consequences. Pokes in the “inactive” port were without consequence throughout the session. Sessions lasted for 30 min or were terminated once a rat received 25 pellets.
Opioid self-administration
Following food self-administration, rats went through 15 sessions of drug self-administration. The food magazine was removed from the test chambers for the rest of the experiment. During drug self-administration, pokes into the active port (FR1 schedule) resulted in a 4-s infusion of remifentanil (3.2 or 1.6 µg/kg, i.v.). Similar to food self-administration, a cue light located within the port illuminated simultaneously along with the infusion for 4 s. The house light turned off for 20 s to signal a timeout period during which pokes into the active port did not result in remifentanil infusions. Pokes into the inactive port were always without consequence. Rats were allowed to self-administer remifentanil according to specific infusion criteria (IC), which controlled for the total amount of drug and drug-cue pairings that each rat was able to receive (Saunders & Robinson, 2010, 2011; Kuhn et al., 2018). The IC represents the maximum number of infusions rats could receive in one session. Rats had to complete at least two consecutive sessions meeting the IC in order to continue participating in the study. Rats that failed to do so were excluded from the study. Sessions lasted for a maximum of 3 h or until they met the IC. Rats were exposed to 3 sessions at IC5, 4 sessions at IC10, and then 3 sessions at IC20, using a dosage of 3.2 µg/kg per infusion. Rats were then exposed to 5 sessions at IC45, with a remifentanil dosage of 1.6 µg/kg per infusion. The dosage was decreased at IC45 to increase motivation for responding and the likelihood of reaching the infusion criterion in a timely manner. The behavioral outcome measures that were analyzed included the number of nose pokes into both ports, number of infusions, and rate of infusion (i.e., number of infusions received per minute). Thirteen rats (6 STs and 7 GTs) were eliminated from the study due to loss of catheter patency. In addition, another rat (a GT) was excluded as its back port became externalized during self-administration. Thus, we had a total of 15 GTs and 8 STs that completed self-administration training and were included in the data analyses.
Forced abstinence
Following the completion of self-administration, rats completed a 14-day period of forced abstinence. During this time, rats remained undisturbed in their home cages within the vivarium. This time period of forced abstinence was chosen as it has been shown to elicit robust cue-induced reinstatement (Grimm et al., 2001).
Extinction
Once forced abstinence was complete, rats were exposed to extinction training. During these sessions, rats were placed back into the behavioral chambers in which pokes into the active port did not result in remifentanil infusions or cue presentations. In addition, the house light remained on at all times. Pokes into the inactive port continued to have no consequence. Each session lasted 2 h, and training continued until all rats made fewer than 15 pokes into the active port for 2 consecutive days. One rat (a GT) failed to meet extinction criteria and was excluded from subsequent behavioral procedures and removed from statistical analyses.
Cue reinstatement
After extinction training, rats were tested for cue-induced reinstatement of opioid-seeking behavior. During this test session, the cue light was presented at the start of the session for 20 s. Subsequent pokes into the active port resulted in a 4-s presentation of the cue light (as in self-administration). However, no remifentanil infusions were delivered. Pokes into the inactive port were without consequences. The house light remained on during the entire 60-min session.
Data analysis
PavCA data was analyzed using linear mixed models (LMMs) with factors of phenotype (ST vs. GT) and session (1–5). Locomotor response to novelty was analyzed using an unpaired t-test. Self-administration data was analyzed using LMMs with factors of phenotype (ST vs. GT or HR vs. LR), port (active vs. inactive), and IC (5–45), and the average nose pokes across sessions for each IC as the dependent variable. Extinction data was analyzed using LMMs with factors of phenotype, port, and session (1–8), and the average nose pokes for each session as the dependent variable. Cue-induced reinstatement data was analyzed with a mixed ANOVA with factors of phenotype and port, and nose pokes as the dependent variable. In addition, the cue-induced reinstatement test data was compared to the last hour of the last day of extinction using a repeated-measures ANOVA with factors of phenotype and phase (extinction vs. reinstatement), and the difference between nose pokes into the active vs. inactive port as the dependent variable. This measure allowed us to directly compare responding during extinction vs reinstatement as it controls for pokes into the inactive nose port while also measuring the ability of the cue to reinstate pokes into the active nose port. Significant interactions were followed up with Bonferroni-corrected pairwise comparisons. Finally, to determine if our behavioral results could be reduced to fewer dimensions, we performed a principal component analysis using 4 variables: “sensation seeking” (locomotor response to novelty), “sign-tracking propensity” (PavCA index as used to classify STs and GTs), “acquisition of drug-taking” (rate of infusion averaged over ICs 5 and 10), and “reinstatement propensity” (the difference between active and inactive nose pokes during the cue-induced reinstatement test). All rats that completed the study were included in the principal component analysis. Statistical outliers were determined using the boxplot method (Tukey, 1977). The significance level was p < 0.05 for all tests.
Experiment 2
Subjects
The subjects were 90 male Sprague–Dawley rats from Charles River Laboratories (n = 60; 30 from Barrier R04 and 30 from Barrier C72) and Taconic Biosciences (n = 30; Barrier IBU16). Subjects weighed 225–275 g on arrival. In contrast to Experiment 1, rats remained pair-housed for the duration of the experiment following surgery. A “buddy barrier” (modified from Boggiano et al., 2008; see also Krueger et al., 2021) was used for the first 5 days following surgery to keep rats in limited tactile contact with one another during recovery. In addition, catheters were constructed using stainless steel tubing for the base and surrounding cap of the catheter to prevent cagemates from damaging the catheter once the barrier was removed.
Pavlovian conditioned approach, locomotor testing, and surgical procedures
Rats were exposed to PavCA training, locomotor testing, and received indwelling jugular catheters as described above for Experiment 1. At the end of PavCA training, there were 33 STs and 19 GTs. Thirty-eight rats were classified as INs and were excluded from subsequent behavioral procedures and data analyses. Two rats (both STs) died during surgery, while another rat (ST) did not respond to anesthesia prior to surgery and was therefore removed from the study.
Food self-administration
Following 10 days of recovery from surgery, rats were exposed to 2 days of food self-administration, as described for Experiment 1.
Opioid self-administration
As in Experiment 1, pokes into the active port resulted in a 4-s infusion of remifentanil (i.v.) along with simultaneous presentation of the cue light for 4 s. In addition, the house light turned off for 20 s to signal a timeout period during which remifentanil was not available and pokes into the active port were without consequence. Pokes into the inactive port were always without consequence. Self-administration procedures were conducted with infusion criteria, similar to Experiment 1. However, rather than reducing the dosage administered during IC45 (from 3.2 to 1.6 µg/kg), 3.2 µg/kg was used throughout the entire self-administration schedule (IC 5–45). Fifteen STs and nine GTs were eliminated from the study due to lost catheter patency. In addition, 3 rats (2 STs and 1 GT) had to be excluded due to externalization of the back port of the catheter. Thus, a total of 13 STs and 9 GTs completed self-administration training, and only these rats are included in the data analyses.
Extinction
In contrast to Experiment 1, rats were not placed under a period of forced abstinence. Following self-administration, rats were immediately run through extinction training during which pokes into the active port no longer resulted in remifentanil infusions or cue presentations. The house light remained on throughout the entire 2-h session. Extinction training lasted until all rats made fewer than 15 pokes into the active port for 2 consecutive days. Two rats (one ST and one GT) failed to meet extinction criteria and were excluded from subsequent data analyses.
Cue reinstatement
Following extinction training, rats were tested for cue-induced reinstatement of opioid-seeking behavior using the same procedures as those in Experiment 1.
Data analysis
Data were analyzed in the same way as Experiment 1. The significance level was p < 0.05 for all tests.
Results
Experiment 1
PavCA
By the end of PavCA training, there were 8 STs and 15 GTs (Fig. 1b). As expected, STs and GTs showed significant differences in the PavCA index (effect of Phenotype (F1,38 = 359.48, p < 0.001)). In addition, there was a significant effect of Session (F4,53 = 9.94, p < 0.001) and a Phenotype × Session interaction (F4,53 = 67.38, p < 0.001) for PavCA index. A detailed analysis of each measure used to calculate the PavCA index is included in the Supplemental material (Figure S1). The distribution of the PavCA index between vendors and experiments is illustrated in Figure S2.
Locomotor response to novelty
Based on the median split, there were 11 HRs and 12 LRs (Fig. 1c). As expected, HRs showed greater locomotor activity than LRs in response to the novel environment (t(21) = 5.158, p < 0.001). The distribution of locomotor scores between vendors and experiments is illustrated in Figure S2.
Self-administration: HRs/LRs
Both HRs (n = 11) and LRs (n = 12) acquired self-administration behavior, as evidenced by their discrimination between the active and inactive nose ports, an increase in the number of infusions, and an increase in the rate of drug-taking across sessions (Fig. 2a–c). In support, there was a significant effect of Port (F1,21 = 562.57, p < 0.001) and IC (F3,21 = 125.83, p < 0.001), as well as a significant IC × Port interaction (F3,21 = 162.00, p < 0.001) for the number of nose pokes. Responding in the active port was greater than responding in the inactive port at each IC (p’s < 0.001). However, there was not a significant effect of Phenotype (F1,21 = 0.43, p = 0.519), nor any significant interactions with Phenotype (p’s ≥ 0.277). As a function of the imposed IC, both HRs and LRs received the exact same number of infusions across training (Fig. 2b). Therefore, no statistical analyses were performed for the number of infusions. Both HRs and LRs showed a similar tendency in increasing infusion rates as training progressed (Fig. 2c). That is, there was a significant effect of IC (F3,23 = 41.65, p < 0.001), but not a significant effect of Phenotype (F1,22 = 0.02, p = 0.904), nor a significant Phenotype × IC interaction (F3,23 = 1.77, p = 0.181) for the interinfusion interval (Fig. 2c).
Self-administration of remifentanil and cue-induced reinstatement in HRs (n = 11) and LRs (n = 12) in Experiment 1. Mean ± SEM for a number of nose pokes, b number of infusions, and c interinfusion interval (in minutes) during the acquisition of drug-taking behavior. The dosage of remifentanil was 3.2 µg/kg for ICs 5–20 and 1.6 µg/kg for IC 45. Mean ± SEM for the number of nose pokes into the active or inactive ports during d extinction sessions or e the cue-induced reinstatement test. Mean ± SEM for f the difference between active and inactive nose pokes during the cue-induced reinstatement test compared to the last day of extinction. During the reinstatement test, there was a significant effect of Port (*p < 0.001). When comparing responding between the last day of extinction and reinstatement, there was a significant effect of Phase (*p = 0.002). There were no significant differences between phenotypes
Extinction: HRs/LRs
Both HRs and LRs extinguished drug-seeking behavior at a comparable rate (effect of Phenotype (F1,20 = 1.15, p = 0.296), decreasing their nose pokes into the active port over the course of training (effects of Session (F7,20 = 28.59, p < 0.001); Port (F1,20 = 59.34, p < 0.001); Session × Port interaction (F7,20 = 10.18, p < 0.001); Fig. 2d). There were no significant interactions with Phenotype (p’s ≥ 0.367). A rat that failed to meet the extinction criteria (an HR) was removed from this and subsequent statistical analyses.
Cue reinstatement: HRs/LRs
Both HRs and LRs showed greater responding in the active nose port relative to the inactive nose port during the cue-induced reinstatement test (effect of Port (F1,40 = 19.43, p < 0.001); Fig. 2e). However, there were no significant differences between phenotypes (effect of Phenotype (F1,40 = 3.26, p = 0.079); Phenotype × Port interaction (F1,40 = 2.82, p = 0.101)). Compared to the last session of extinction (analyzing the number of active – inactive nose pokes), both HRs and LRs showed greater levels of responding during the reinstatement test (effect of Phase (F1,20 = 21.47, p = 0.002); Fig. 2f), with no significant differences between phenotypes (effect of Phenotype (F1,20 = 2.82, p = 0.098)), nor a significant Phase × Phenotype interaction (F1,20 = 3.51, p = 0.076). The vendor from which the rats were obtained did not have an effect on cue-induced reinstatement behavior (see Supplemental materials for supporting statistical analyses). Notably, there was one statistical outlier (an LR) that made more nose pokes into the active port during the cue reinstatement test. Excluding this outlier did not change the pattern of results described above (see Supplemental materials); thus, for transparency, this rat is included in the primary analyses and graphical illustrations.
Given that the extinction sessions were 2 h long, the 1-h cue-induced reinstatement test was also compared to the first hour of the last extinction session. There was not a significant difference in responding between the first and second hours of the last extinction session (p = 0.107); and results were the same whether behavior during reinstatement was compared to the first or second hour of the extinction session (Figure S3; see additional analyses in Supplemental materials).
Self-administration: STs/GTs
Both STs (n = 8) and GTs (n = 15) acquired self-administration of remifentanil (Fig. 3). In support, there was a significant effect of Port (F1,21 = 489.13, p < 0.001) and IC (F3,21 = 111.84, p < 0.001), but no effect of Phenotype (F1,21 = 0.06, p = 0.807), nor a significant Phenotype × Port interaction (F1,21 = 0.21, p = 0.651). There was a significant IC × Port interaction (F3,21 = 133.69, p < 0.001), and this is reflective of the increase in responding in the active port across IC, while responding in the inactive port remained low. There was also a significant Phenotype × IC × Port interaction (F3,21 = 3.55, p = 0.032). Rats differentiated between the active and inactive nose ports at each IC (p’s < 0.001). However, post hoc comparisons between STs and GTs were not significant at any IC (p’s ≥ 0.085). As a function of the IC paradigm, both STs and GTs received the exact same number of infusions across training (Fig. 3b); and thus, no statistical analyses were performed for this measure. Both STs and GTs showed a similar tendency in increasing infusion rates as training progressed (Fig. 3c). That is, there was a significant effect of IC (F3,23 = 34.71, p < 0.001) but not a significant effect of Phenotype (F1,22 = 0.58, p = 0.453) nor a Phenotype × IC interaction (F3,23 = 1.79, p = 0.178) for the interinfusion interval (Fig. 3c).
Self-administration of remifentanil and cue-induced reinstatement in STs (n = 8) and GTs (n = 15) in Experiment 1. Mean ± SEM for a number of nose pokes, b number of infusions, and c interinfusion interval (in minutes) during the acquisition of drug-taking behavior. The dosage of remifentanil was 3.2 µg/kg for ICs 5–20 and 1.6 µg/kg for IC 45. Mean ± SEM for the number of nose pokes into the active or inactive ports during d extinction sessions or e the cue-induced reinstatement test. Mean ± SEM for f the difference between active and inactive nose pokes during the cue-induced reinstatement test compared to the last day of extinction. During the reinstatement test, there was a significant effect of Port (*p < 0.001). When comparing responding between the last day of extinction and reinstatement, there was a significant effect of Phase (*p = 0.002). There were no significant differences between phenotypes
Extinction: STs/GTs
Consistent with prior reports (Ahrens et al., 2016; Kuhn et al., 2018), both STs and GTs extinguished responding into the active nose port at similar rates (effects of Session (F7,20 = 25.28, p < 0.001); Port (F1,20 = 56.05, p < 0.001); Session × Port (F7,20 = 9.67, p < 0.001); Phenotype (F1,20 = 0.02, p = 0.897); Fig. 3d), and there were no significant interactions with Phenotype (p’s ≥ 0.118). The same rat that failed to meet extinction criteria mentioned above (a GT) was removed from this and subsequent statistical analyses.
Cue reinstatement: STs/GTs
Both STs and GTs showed greater levels of responding in the active port relative to the inactive port during the cue-induced reinstatement test (effect of Port (F1,40 = 19.09, p < 0.001); Fig. 3e). However, there was not a significant effect of Phenotype (F1,40 = 1.32, p = 0.257) nor a Phenotype × Port interaction (F1,40 = 0.42, p = 0.521). Relative to responding during the last extinction session (using the number of active – inactive nose pokes), both STs and GTs showed elevated levels of responding during the reinstatement test (Phase (F1,20 = 20.25, p = 0.002; Fig. 3f). Again, however, there was not a significant effect of Phenotype (F1,20 = 0.50, p = 0.486), nor a significant Phase × Phenotype interaction (F1,20 = 0.41, p = 0.528). The vendor from which the rats were obtained did not have a significant effect on cue-induced reinstatement behavior (see Supplemental materials for supporting statistical analyses). One ST was identified as a statistical outlier with significantly more nose pokes into the active port relative to other STs during the reinstatement test. Excluding this outlier from the data analysis did not change the results (see Supplemental materials); thus, for transparency, this rat is included in the primary analyses and graphical illustrations.
Principal component analysis
When rats were combined into a single population, the behavioral variables of “sensation seeking” (locomotor response to novelty), “propensity to sign-track” (PavCA index), “acquisition of drug-taking” (rate of infusion averaged across IC5 and IC10), and “reinstatement propensity” (the difference between active and inactive nose pokes during the cue-induced reinstatement test) were reduced to two factors that accounted for 63% of the variance (Table 1). Factor 1 (which accounts for 32% of the variance) has strong loadings (> 0.7) from sensation-seeking and acquisition. Factor 2 (which accounts for 31% of the variance) is composed of a single variable, reinstatement propensity (loading 0.88). Thus, reinstatement propensity appears to be independent from other addiction-related behaviors.
Experiment 2
PavCA
By the end of PavCA training, there were 13 STs and 9 GTs (Fig. 1e). STs and GTs showed significant differences in the PavCA index (effect of Phenotype (F1,52 = 270.85, p < 0.001)). There was not a significant effect of Session (F4,75 = 1.97, p = 0.107), but there was a significant Phenotype × Session interaction (F4,75 = 30.99, p < 0.001) that reflects the different learning trajectories between STs and GTs. A detailed analysis of each measure used to calculate the PavCA index is included in the Supplemental material (Figure S4). The distribution of the PavCA index between vendors and experiments is illustrated in Figure S2.
Locomotor response to novelty
Based on the median split, there were 11 HRs and 11 LRs (Fig. 1f). As expected, HRs showed greater locomotor activity than LRs in response to a novel environment (t(20) = 5.349, p < 0.001). The distribution of locomotor scores between vendors and experiments is illustrated in Figure S2.
Self-administration: HRs/LRs
As in Experiment 1, rats classified as either HRs (n = 10) or LRs (n = 10) acquired self-administration behavior (Fig. 4). There was a significant effect of Port (F1,22 = 310.02, p < 0.001) and IC (F3,29 = 108.95, p < 0.001), and a significant IC × Port interaction (F3,29 = 129.78, p < 0.001) for the number of nose pokes. There was not a significant effect of Phenotype (F1,21 = 1.67, p = 0.210) nor any significant interactions with this variable (p’s ≥ 0.121). Rats made more nose pokes into the active port than the inactive port at each IC (p’s < 0.001). As a function of the imposed IC, both HRs and LRs received a similar number of infusions, but with some variability (i.e., not the exact same number of infusions during each session) that permitted statistical analyses. The number of infusions increased across IC (F3,72 = 5218.58, p < 0.001), with no effect of Phenotype (F1,72 = 0.267, p = 0.607) nor a significant Phenotype × IC interaction (F3,72 = 0.267, p = 0.849). As in Experiment 1, both HRs and LRs self-administered remifentanil at a comparable rate, with a significant decrease in the interinfusion interval with each successive IC (significant effect of IC: F3,32 = 7.97, p < 0.001), and no significant effect of Phenotype (F1,21 = 1.08, p = 0.311) nor a Phenotype × IC interaction (F3,32 = 1.61, p = 0.206; Fig. 4c).
Self-administration of remifentanil and cue-induced reinstatement in HRs (n = 11) and LRs (n = 11) in Experiment 2. Mean ± SEM for a number of nose pokes, b number of infusions, and c interinfusion interval (in minutes) during the acquisition of drug-taking behavior. The dosage of remifentanil was 3.2 µg/kg for all ICs. Mean ± SEM for the number of nose pokes into the active or inactive ports during d extinction sessions or e the cue-induced reinstatement test. Mean ± SEM for f the difference between active and inactive nose pokes during the cue-induced reinstatement test compared to the last day of extinction. During the reinstatement test, there was a significant effect of Port (*p < 0.001). When comparing responding between the last day of extinction and reinstatement, there was a significant effect of Phase (*p < 0.001). There were no significant differences between phenotypes
Extinction: HRs/LRs
Both HRs and LRs extinguished drug-seeking behavior at a comparable rate (effect of Phenotype (F1,19 = 1.66, p = 0.213)), decreasing their nose pokes into the active port over training (effect of Session (F9,58 = 8.78, p < 0.001); Port (F1,72 = 114.21, p < 0.001); Session × Port interaction (F9,53 = 5.66, p < 0.001); Fig. 4d). There were no significant interactions with Phenotype (p’s ≥ 0.550).
Cue reinstatement: HRs/LRs
Both HRs and LRs showed higher levels of responding into the active nose port relative to the inactive nose port during the cue reinstatement test (effect of Port (F1,36 = 21.51, p < 0.001); Fig. 4e). There were no significant differences between phenotypes (effect of Phenotype (F1,36 = 0.08, p = 0.778)), nor a significant Phenotype × Port interaction (F1,36 = 0.12, p = 0.731). In addition, both HRs and LRs had higher levels of responding (using the number of active – inactive nose pokes) during the reinstatement test compared to the last extinction session (effect of Phase (F1,18 = 26.80, p < 0.001); Fig. 4f), with no significant differences between phenotypes (effect of Phenotype (F1,18 = 0.20, p = 0.658)), nor a significant Phenotype × Phase interaction (F1,18 = 0.12, p = 0.738). The same pattern of results was observed when comparing the cue reinstatement test to the first hour of the last extinction session (Figure S5; see Supplemental materials for statistical analyses).
Self-administration: STs/GTs
Both STs (n = 12) and GTs (n = 8) acquired self-administration of remifentanil (Fig. 5a–c). There was a significant effect of Port (F1,22 = 279.77, p < 0.001) and IC (F3,29 = 109.00, p < 0.001), and no significant effect of Phenotype (F1,21 = 0.06, p = 0.81), nor any significant interactions with Phenotype (p’s ≥ 0.489). There was a significant IC × Port interaction (F3,29 = 130.13, p < 0.001), which reflects the increase in responding in the active port across IC. Rats responded more in the active port than the inactive port at each IC (p’s < 0.001). As a function of the IC paradigm, both STs and GTs received a similar number of infusions, but with some variability that permitted statistical analyses (i.e., not the exact same number of infusions during each session). The number of infusions increased across IC (F3,72 = 5314.83, p < 0.001), with no effect of Phenotype (F1,72 = 1.27, p = 0.264) nor a significant Phenotype × IC interaction (F3,72 = 1.27, p = 0.292). Both STs and GTs similarly decreased their interinfusion interval with each successive IC (Fig. 5c). That is, there was a significant effect of IC (F3,33 = 8.80, p < 0.001), but not a significant effect of Phenotype (F1,21 = 3.23, p = 0.087), nor a Phenotype × IC interaction (F3,33 = 1.94, p = 0.142).
Self-administration of remifentanil and cue-induced reinstatement in STs (n = 13) and GTs (n = 9) in Experiment 2. Mean ± SEM for a number of nose pokes, b number of infusions, and c interinfusion interval (in minutes) during the acquisition of drug-taking behavior. The dosage of remifentanil was 3.2 µg/kg for all ICs. Mean ± SEM for the number of nose pokes into the active or inactive ports during d extinction sessions or e the cue-induced reinstatement test. Mean ± SEM for f the difference between active and inactive nose pokes during the cue-induced reinstatement test compared to the last day of extinction. During the reinstatement test, there was a significant effect of Port (*p < 0.001). When comparing responding between the last day of extinction and reinstatement, there was a significant effect of Phase (*p < 0.001). There were no significant differences between phenotypes
Extinction: STs/GTs
Over the course of extinction training, both STs and GTs decreased their levels of responding into the active nose port at similar rates (effects of Session (F9,58 = 8.09, p < 0.001); Port (F1,63 = 98.94, p < 0.001); Session × Port (F9,51 = 5.89, p < 0.001); Fig. 5d). There was not a significant effect of Phenotype (F1,19 = 0.31, p = 0.583) nor any significant interactions with Phenotype (p’s ≥ 0.057) for nose port responding during extinction.
Cue reinstatement: STs/GTs
Both STs and GTs made more nose pokes into the active port relative to the inactive port during the cue reinstatement test (effect of Port (F1,36 = 21.63, p < 0.001); Fig. 5e). However, there was not a significant effect of Phenotype (F1,36 = 0.96, p = 0.335) nor a Phenotype × Port interaction (F1,36 = 0.08, p = 0.786). In agreement, both STs and GTs showed similar levels of reinstatement (using the number of active – inactive nose pokes) relative to the last extinction session (effect of Phase (F1,18 = 26.32, p < 0.001); Fig. 5f), with no significant effect of Phenotype (F1,18 = 0.10, p = 0.762) nor a Phase × Phenotype interaction (F1,18 = 0.09, p = 0.762). Notably, responding was significantly lower during the second hour of extinction relative to the first hour (t(19) = 3.404, p = 0.003). However, the same pattern of significant results remains even when responding during the cue reinstatement test is compared to the first hour of extinction (Figure S4; see Supplemental Materials for statistical analyses). The vendor from which the rats were obtained did not have a significant effect on cue-induced reinstatement behavior (see Supplemental materials for supporting statistical analyses).
Principal component analysis
When rats were combined into a single population, the behavioral variables of “sensation seeking” (locomotor response to novelty), “propensity to sign-track” (PavCA index), “acquisition of drug-taking” (rate of infusion averaged across IC5 and IC10), and “reinstatement propensity” (the difference between active and inactive nose pokes during the cue-induced reinstatement test) were reduced to two factors that accounted for 70% of the variance (Table 1). Factor 1 (which accounts for 44% of the variance) has strong loadings (> 0.7) from sensation seeking, propensity to sign-track, and acquisition. Factor 2 (which comprises 26% of the variance) is composed of a single variable, reinstatement propensity (loading 0.87). Although the variables that comprise Factor 1 differ between Experiment 1 and Experiment 2, for both experiments Factor 2 largely represented reinstatement propensity. Together, these results suggest that the factors that contribute to cue-induced reinstatement of opioid-seeking are independent from sensation seeking, propensity to sign-track, and acquisition of drug-taking.
Discussion
The present study investigated individual differences in the acquisition of opioid-taking behavior and reinstatement of opioid-seeking behavior using two animal models: the HR/LR and ST/GT models. We found no differences between these behavioral phenotypes in either the propensity to acquire self-administration of remifentanil, a fast-acting µ-opioid receptor agonist, or in cue-induced drug-seeking behavior following the self-administration experience. Principal component analyses provided further support that the propensity for cue-induced reinstatement of opioid-seeking behavior is independent of the sensation-seeking trait (i.e., locomotor response to novelty), the propensity to attribute incentive motivational value to reward cues (i.e., PavCA phenotype), and the acquisition of drug self-administration.
The HR/LR model was introduced by Piazza et al. (1989), who showed that, relative to LR rats, HR rats exhibit a greater locomotor response to systemic amphetamine injections and are faster to acquire self-administration of amphetamine. HR rats have also been shown to more readily acquire self-administration of cocaine faster than LR rats (Piazza et al., 2000; Mantsch et al., 2001; Ferris et al., 2013); and similar results have been reported with nicotine (Suto et al., 2001) and ethanol (Nadal et al., 2002). Perhaps most relevant to the current study, Ambrosio et al. (1995) observed a positive correlation between locomotor response to a novel environment and acquisition of morphine self-administration. Thus, there is ample evidence across drug classes to suggest that the “sensation seeking” trait exhibited by HRs is associated with an increased propensity to acquire drug self-administration. In the current study, however, we did not observe such differences between HR and LR rats. These seemingly discrepant findings could be due to the self-administration paradigm that was implemented and/or the unique properties of the drug remifentanil. A recent study by O’Connor et al. (2021) demonstrated that the sensation-seeking trait is associated with addiction-related behaviors only under certain conditions of drug self-administration. The paradigm used in the current study was designed to minimize differences in the acquisition of drug-taking behavior and to control for the number of drug-cue pairings prior to the cue-induced reinstatement test (Saunders & Robinson, 2010). However, as we have previously reported (e.g., Flagel et al., 2016), this paradigm does not preclude the detection of individual differences in the rate of cocaine intake. Such differences were not apparent here, perhaps because the fast-acting properties of remifentanil reduced variability in the rate of drug-taking behavior. It is also possible that we would have detected differences in remifentanil-taking behavior between HR and LR rats, had we used more “extreme” phenotypes—either characterizing them based on a trio split rather than a median split (Kabbaj et al., 2000), or utilizing rats selectively bred based on locomotor response to a novel environment (Davis et al., 2008; Cummings et al., 2011; Flagel et al., 2016).
Prior research has demonstrated the utility of the ST/GT model for investigating individual differences in relapse propensity (Saunders & Robinson, 2010, 2011; Saunders et al., 2013; Kuhn et al., 2018). Specifically, Saunders and Robinson (2011) showed that, following relatively limited drug-taking experience, STs exhibit greater cue-induced reinstatement for a cocaine-paired cue than GTs; and this effect was recently corroborated by Kuhn et al. (2021). Here, in Experiment 1, we used a similar self-administration paradigm and reinstatement test to investigate individual differences in relapse propensity, but did not observe differences between STs and GTs with respect to cue-induced reinstatement of remifentanil-seeking behavior. In Experiment 2, we changed a number of parameters known to affect drug-taking and drug-seeking behaviors, including the housing conditions, the dose of remifentanil, and the period of forced abstinence. Yet, we still did not observe differences in remifentanil-taking or remifentanil-seeking behavior between phenotypes. These data suggest that the vulnerability factors that contribute to drug use and relapse propensity likely vary between drug classes.
Of particular relevance to this study, Yager et al. (2015) observed individual differences in approach to a light cue that was paired in a Pavlovian manner with remifentanil. STs approached the remifentanil-paired cue more than GTs, and the same cue attained greater conditioned reinforcing properties for STs than GTs. Furthermore, relative to GTs, STs showed greater c-Fos expression upon presentation of the remifentanil-paired cue in areas associated with the “motive” circuit including the nucleus accumbens core and shell, the basolateral amygdala, and the paraventricular nucleus of the thalamus. In light of these results, it is especially surprising that we did not observe greater cue-induced reinstatement of drug-seeking behavior in STs compared to GTs following remifentanil self-administration. There are several factors that may have contributed to these negative results, as discussed below.
As noted above, infusion criteria (IC) were imposed during self-administration sessions in order to control for the amount of drug and drug-cue pairings received by rats; and utilizing an IC with remifentanil may have prevented us from observing individual differences in both the rate of acquisition and cue-induced reinstatement. Using a behavioral-economics procedure, Porter-Stransky et al. (2017) observed individual differences in the self-administration of remifentanil. They identified two populations of rats: high takers and low takers. High takers are rats that continue to respond for remifentanil infusions despite increasing costs to receive infusions, while low takers are rats that decrease their responding for infusions as the price increases. Relative to low takers, high takers showed a greater motivation for remifentanil as the dosage of remifentanil decreased and when infusions were accompanied by footshocks. Importantly, these individual differences were observed with unlimited access to remifentanil during training. Thus, we might have observed individual differences in drug-taking and drug-seeking had we not imposed an IC and allowed rats unlimited access to remifentanil. It is also possible that a different schedule of reinforcement, like progressive ratio, might have revealed individual differences in drug-taking and drug-seeking behavior (e.g., Piazza et al., 2000).
Although we observed cue-induced reinstatement of opioid-seeking behavior, the levels of reinstatement were considerably lower than what has been observed in previous studies using remifentanil (e.g., Porter-Stransky et al., 2017), and this was true for both experiments. Therefore, individual differences in opioid-seeking behavior may have been masked by the generally low levels of responding. Factors that might have precluded the observation of robust cue-induced reinstatement include context, sex, and the amount of drug administered. Prior work from Badiani and colleagues has shown that context plays a large role in drug preference when rats are given a choice between opioids or psychostimulants (Caprioli et al., 2009; De Luca et al., 2019). Rats that remain in their home cage for drug self-administration prefer heroin over cocaine, whereas those who self-administer in an environment distinct from their home cage prefer cocaine over heroin (Caprioli et al., 2009). Although heroin was used in these prior reports, it is possible that we did not observe robust cue-induced reinstatement (and therefore diminished our chances of observing individual differences) in the current study because we trained and tested our rats outside of their home cages, where they tend to prefer psychostimulants over opioids (De Luca et al., 2019). In relation, it should be noted that contextual cues reinstate cocaine-seeking behavior to a greater degree in GTs than STs (Saunders et al., 2014). Thus, utilizing contextual rather than discrete cues may reveal individual differences in opioid-seeking behaviors that were not apparent with the current experimental design.
Another important factor that may have decreased the likelihood of observing individual differences is the sex of our rodents. A recent study by Thorpe et al. (2020) showed that female rats acquire self-administration of remifentanil faster than male rats. In addition, Bertz et al. (2016) reported that a remifentanil-associated cue acquires conditioned reinforcing properties in a dose- and sex-dependent manner, with only female rats showing conditioned responding at high doses. Female rats have also been shown to exhibit greater reinstatement of heroin-seeking behavior following drug priming or drug-cue exposure (Smethells et al., 2020; Vazquez et al., 2020). Thus, similar to cocaine (Kippin et al., 2005; Kerstetter et al., 2008; Kawa & Robinson, 2019; Algallal et al., 2020), there are a number of studies to suggest that female rats may be more prone to opioid use and reinstatement of opioid-seeking behavior. It is possible, therefore, had we used female rats, we may have seen individual differences in cue-induced opioid-seeking behavior. Sex is a factor that will be explored in future studies.
As described above, in these experiments, we used two animal models that capture distinct traits of relevance to addiction. High-responder and low-responder rats differ in the “sensation-seeking” trait, with high-responders more likely to initiate drug-taking behavior and to take drugs at a faster rate than low-responder rats. Sign-tracker and goal-tracker rats differ in their propensity to attribute incentive motivational value to discrete reward cues and, as a function of this, are more likely to reinstate drug-seeking behavior following the presentation of a drug-paired cue. Importantly, however, neither the HR/LR nor ST/GT model appears to predict the transition to compulsive cocaine-seeking behavior or other behaviors reminiscent of the psychiatric criteria for addiction following cocaine self-administration (Belin et al., 2008; Deroche-Gamonet et al., 2004; Pohorala et al., 2021). Nonetheless, as recently discussed (Flagel et al., 2021), we believe there is great utility in identifying the traits that predict the likelihood of addiction at each point of entry, which was the aim of the current study with respect to the initiation of opioid use and subsequent opioid-seeking behavior.
In summary, the current results highlight the contrast between opioids (specifically remifentanil) and cocaine with respect to individual differences in drug-taking and drug-seeking behaviors. As most prior studies with the HR/LR and ST/GT animal models have focused on cocaine-taking and cocaine-seeking behavior, here we investigated individual differences with respect to opioid-taking and opioid-seeking behavior. Contrary to our hypotheses, we found that all rats self-administered remifentanil and exhibited cue-induced reinstatement of opioid-seeking behavior to the same degree. While these negative results may be a function of the experimental paradigm that was utilized and/or the unique properties of the drug, remifentanil, they also suggest distinct factors mediate individual differences in response to cocaine and opioids. Namely, those traits that confer the initiation of cocaine use and the propensity to reinstate cocaine-seeking behavior following abstinence appear to be distinct from those that confer the initiation of opioid use and the propensity to reinstate opioid-seeking behavior. Future studies should investigate the factors mentioned above—context, drug, sex—to determine if individual differences in opioid-taking and opioid-seeking behavior might otherwise be revealed.
References
Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE (2016) Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav Brain Res 296:418–430
Algallal H, Allain F, Ndiaye NA, Samaha AN (2020) Sex differences in cocaine self-administration behavior under long access versus intermittent access conditions. Addict Biol 25:e12809
Ambrosio E, Goldberg SR, Elmer GI (1995) Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol 6:229–237
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355
Berridge KC (2004) Motivation concepts in behavioral neuroscience. Physiol Behav 81:179–209
Bertz JW, Jackson EL, Barron DR, Woods JH (2016) Effects of sex and remifentanil dose on rats’ acquisition of responding for a remifentanil-conditioned reinforcer. Behav Pharmacol 27:137–147
Boakes R (1977) Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H (eds) Operant-Pavlovian interactions. Lawrence Erlbaum Associates, Hillsdale, NJ, pp 67–97
Boggiano MM, Cavigelli SA, Dorsey JR, Kelley CEP, Ragan CM, Chandler-Laney PC (2008) Effect of a cage divider permitting social stimuli on stress and food intake in rats. Physiol Behav 95:222–228
Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A (2009) Ambience and drug choice: cocaine- and heroin- taking as a function of environmental context in humans and rats. Biol Psychiatry 65:893–899
Crespo JA, Sturm K, Saria A, Zernig G (2005) Simultaneous intra-accumbens remifentanil and dopamine kinetics suggest that neither determines within-session operant responding. Psychopharmacology 183:201–209
Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB (2011) Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. https://doi.org/10.1186/2042-6410-2-3
Davis BA, Clinton SM, Akil H, Becker JB (2008) The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav 90:331–338
De Luca MT, Montanari C, Meringolo M, Contu L, Celentano M, Badiani A (2019) Heroin versus cocaine: opposite choice as a function of context but not of drug history in the rat. Psychopharmacology 236:787–798
Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305(5686):1014–7
Ferris MJ, Calipari ES, Melchior JR, Roberts DCS, España RA, Jones SR (2013) Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci 38:2628–2636
Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, Saunders BT, Parker CC, Gonzales NM, Aryee E, Flagel SB, Palmer AA, Robinson TE, Morrow JD (2013) Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS One 8:e75042
Flagel SB, Robinson TE (2017) Neurobiological basis of individual variation in stimulus-reward learning. Curr Opin Behav Sci 13:178–185
Flagel SB, Robinson TE, Sarter M (2021) Comment on Pohorala et al: Sign-tracking as a predictor of addiction vulnerability. Psychopharmacology 238(9):2661–2664
Flagel SB, Chaudhury S, Waselus M, Kelly R, Sewani S, Clinton SM, Thompson RC, Watson SJ Jr, Akil H (2016) Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. PNAS 113:E2861–E2870
Florence CS, Zhou C, Luo F, Xu L (2016) The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care 54:901–906
Freeman KB, Woolverton WL (2011) Self-administration of cocaine and remifentanil by monkeys: choice between single drugs and mixtures. Psychopharmacology 215:281–290
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142
Hearst E, Jenkins H (1974) Sign-tracking: the stimulus-reinforcer relation and directed action. Monograph of the Psychonomic Society, Austin
Kabbaj M, Devine DP, Savage VR, Akil H (2000) Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci 20:6983–6988
Kawa AB, Robinson TE (2019) Sex differences in incentive-sensitization produced by intermittent access cocaine self-administration. Psychopharmacology 236:625–639
Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE (2008) Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology 198:63–75
Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology 182:245–252
Koffarnus MN, Hall A, Winger G (2012) Individual differences in rhesus monkeys’ demand for drugs of abuse. Addict Biol 17:887–896
Krueger LD, Chang SE, Motoc M, Chojecki M, Freeman ZT, Flagel SB (in press) Impact of social housing on long-term patency of jugular catheters in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci. https://doi.org/10.30802/AALAS-JAALAS-20-000071
Kuhn BN, Campus P, Klumpner MS, Chang SE, Iglesias AG, Solberg Woods L, Flagel SB (2021) Inhibition of a cortico-thalamic circuit attenuates cue-induced reinstatement of drug-seeking behavior in “relapse prone” rats. Psychopharmacology. https://doi.org/10.1007/s00213-021-05894-9
Kuhn BN, Klumpner MS, Covelo IR, Campus P, Flagel SB (2018) Transient inactivation of the paraventricular nucleus of the thalamus enhances cue-induced reinstatement in goal-trackers, but not sign-trackers. Psychopharmacology 235:999–1014
Mantsch JR, Ho A, Schlussman SD, Kreek MJ (2001) Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology 157:31–39
Marinelli M, White FJ (2000) Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci 20:8876–8885
Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL (2021) Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep 70:202–207
Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE (2012) Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One 7:e38987
Nadal R, Armario A, Janak PH (2002) Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology 162:333–338
O’Connor SL, Aston-Jones G, James MH (2021) The sensation-seeking trait confers a dormant susceptibility to addiction that is revealed by intermittent cocaine self-administration in rats. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2021.108566
Panlilio LV, Schindler CW (2000) Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology 150:61–66
Piazza PV, Deminière JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245:1511–1513
Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M (2000) Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20:4226–4232
Pohorala V, Enkel T, Bartsch D, Spanagel R, Bernardi RE (2021) Sign- and goal-tracking score does not correlate with addiction-like behavior following prolonged cocaine self-administration. Psychopharmacology 238:2335–2346
Porter-Stransky KA, Bentzley BS, Aston-Jones G (2017) Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol 22:303–317
Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE (2014) A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology 39:2816–2823
Saunders BT, Robinson TE (2010) A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry 67:730–736
Saunders BT, Robinson TE (2011) Individual variation in the motivational properties of cocaine. Neuropsychopharmacology 36:1668–1676
Saunders BT, Yager LM, Robinson TE (2013) Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci 33:13989–14000
Smethells JR, Greer A, Dougen B, Carroll ME (2020) Effects of voluntary exercise and sex on multiply-triggered heroin reinstatement in male and female rats. Psychopharmacology 237:453–463
Stead JDH, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H (2006) Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet 36:697–712
Suto N, Austin JD, Vezina P (2001) Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology 158:175–180
Sutton MA, Karanian DA, Self DW (2000) Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology 22:626–641
Thorpe D, Lacy RT, Strickland JC (2020) Acquisition of remifentanil self-administration: enhanced in female rats but no effect of adolescent stress exposure. Pharmacol Biochem Behav 199:173038. https://doi.org/10.1016/j.pbb.2020.173038
Tukey JW (1977) Exploratory data analysis. Addison-Wesley, Reading, MA
Vazquez M, Frazier JH, Reichel CM, Peters J (2020) Acute ovarian hormone treatment in freely cycling female rats regulates distinct apsects of heroin seeking. Learn Mem 27:6–11
Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN (2015) Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156:569–576
Wade-Galuska T, Winger G, Woods JH (2007) A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacology 194:563–572
Yager LM, Pitchers KK, Flagel SB, Robinson TE (2015) Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology 40:1269–1277
Acknowledgements
We would like to thank Dr. Paolo Campus for assistance with figures, Allison Johnson and Hannah Davies for assistance with jugular catheterization surgeries, and Maurice Chojecki for technical support. We would also like to thank Drs. Terry Robinson and Sara Westbrook for helpful comments on previous versions of this manuscript.
Funding
This work was supported by the National Institute on Drug Abuse branch of the National Institutes of Health (R01-DA038599-04S2) awarded to Dr. Shelly B Flagel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, S.E., Krueger, L.D. & Flagel, S.B. Investigating individual differences in opioid-taking and opioid-seeking behavior in male rats. Psychopharmacology 239, 1065–1080 (2022). https://doi.org/10.1007/s00213-021-06023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-06023-2