Abstract
Mirogabalin besylate (hereafter mirogabalin) [Tarlige®] is an orally administered gabapentinoid developed by Daiichi Sankyo for the treatment of peripheral neuropathic pain (PNP), including diabetic PNP and post-herpetic neuralgia. The drug binds to and modulates the α2δ-1 subunit of the voltage-gated calcium channels widely found in the nervous system in areas that mediate pain transmission and processing. Mirogabalin has a unique binding profile and long duration of action. The drug is approved in Japan for the treatment of PNP and is in clinical development for this indication elsewhere in Asia. Clinical development of the drug for fibromyalgia pain was discontinued in the USA and EU after the primary endpoint was not met in phase 3 trials. No recent reports of development have been identified for PNP in the USA or India or for fibromyalgia pain in Australia, India, New Zealand, Russia, Argentina, Belarus, Chile, Colombia, Israel, Mexico, Switzerland, Canada, Serbia or South Africa. This article summarizes the milestones in the development of mirogabalin leading to this first approval for PNP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Neuropathic pain can be debilitating, leading to sleep problems and a reduced quality of life [1]. It can have various underlying causes and, depending on the location of the causative damage, can be peripheral [e.g. diabetic neuropathy, post-herpetic neuralgia (PHN), cancer/chemotherapy, surgery] and/or central (e.g. stroke, traumatic brain injury) [1]. Gabapentinoids are key components of neuropathic pain management [2,3,4], providing analgesia largely by reducing dorsal horn sensitivity via mechanisms that include voltage-gated calcium channel (VGCC) blockade [5]. VGCCs are vital for the electrical activity of neurons and other excitable cells [5] and thus facilitate sensory information processing, although their activity/expression can become dysregulated and/or maladapted in various pathological conditions, possibly contributing to pain development [6]. High-voltage activated VGCCs comprise a channel-forming α1 subunit and various modulatory auxiliary subunits, including α2δ [5, 6]. By binding to α2δ-1 (one of the four known α2δ isoforms), gabapentinoids inhibit calcium-mediated neurotransmitter release in the dorsal horn that would otherwise promote neuronal excitation and sensory signalling [5, 6]. However, various other mechanisms not directly related to dorsal horn neurotransmitter release may also contribute to the analgesic effects of these drugs [5].

Key milestones in the development of mirogabalin, focussing on its use in diabetic peripheral neuropathic pain and post-herpetic neuralgia. DPNP diabetic peripheral neuropathic pain, PHN post-herpetic neuralgia
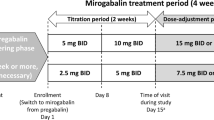
The gabapentinoid drug class includes gabapentin, pregabalin and, most recently, mirogabalin besylate (hereafter referred to as mirogabalin). In January 2019, mirogabalin tablets (Tarlige®; 2.5, 5, 10 and 15mg) were approved in Japan for the treatment of peripheral neuropathic pain (PNP) on the basis of trials conducted in patients with diabetic peripheral neuropathic pain (DPNP) or PHN [7, 8]. The initial dosage for adults is 5 mg given orally twice daily, which is then gradually increased to 15 mg twice daily; the dosage may be increased or decreased on the basis of age and symptoms [7]. Mirogabalin is in clinical development for this indication elsewhere in Asia, although no recent reports of its development for PNP have been identified in India or the USA. Clinical development of the drug for fibromyalgia pain is discontinued in the USA and EU after the primary endpoint was not met in phase 3 trials [9, 10] and there are no recent reports of its development for this indication in Australia, India, New Zealand, Russia, Argentina, Belarus, Chile, Colombia, Israel, Mexico, Switzerland, Canada, Serbia or South Africa.
1.1 Patent Information
As of January 2019, Daiichi Sankyo had been granted patent protection for mirogabalin in Japan, and many other countries including China, South Korea and Russia. Patents were issued for mirogabalin by the European Patent Office in September 2013 [11] and by the United States Patent and Trademark Office in May 2011 [12].
2 Scientific Summary
2.1 Pharmacodynamics
Mirogabalin was shown to bind selectively and with high affinity to the α2δ-1 and α2δ-2 subunits of human VGCCs in vitro (Kd 13.5 and 22.7 nmol/L) [13]. Unlike pregabalin, mirogabalin took considerably longer to dissociate from α2δ-1 than α2δ-2 in vitro (dissociation half-life 11.1 and 2.4 h vs. 1.4 h each for pregabalin) [13], which is of interest given the respective subunits have been associated with the analgesic properties of gabapentinoids [14] and CNS adverse effects [15] in some murine studies. Mirogabalin had analgesic effects in animal models of neuropathic pain [13, 16]. The drug also dose-dependently inhibited locomotor activity and rota-rod performance in rat pharmacological safety studies, possibly reflecting the CNS effects typically associated with gabapentinoids in clinical practice [13]. Notably, mirogabalin had better safety indices than pregabalin, as calculated by the dose of drug producing 50% of the maximum CNS adverse effect versus that producing 50% of maximum analgesia in these animal models [13]. Mirogabalin also alleviated behaviours related to anxiety in a rat model of fibromyalgia [17].
2.2 Pharmacokinetics
This section focusses on data from studies in healthy volunteers and single (5–30 mg) and multiple (5–15 mg twice daily) doses of mirogabalin within the range recommended for titration/maintenance, unless specified otherwise.

Chemical structure of mirogabalin besylate
Features and properties of mirogabalin
Alternative names | DS 5565; Tarlige |
Class | γ-aminobutyric acids; analgesics; bicyclo compounds; small molecules |
Mechanism of Action | α2δ-1 protein modulator |
Route of Administration | Oral |
Pharmacodynamics | Binds selectively to the α2δ-1 and α2δ-2 subunits of human voltage-gated calcium channels but dissociates more rapidly from α2δ-2. Displays analgesic effects in animal models of neuropathic pain and alleviates anxiety-related behaviours in a rat model of fibromyalgia. |
Pharmacokinetics | Rapidly absorbed after oral administration; median time to maximum plasma concentration of 0.5–1.5 h |
Most frequent adverse events | Somnolence, dizziness and weight gain |
ATC codes | |
WHO ATC code | A10 (drugs used in diabetes); G04B (urologicals); N02 (analgesics) |
EphMRA ATC code | A10 (drugs used in diabetes); G4X (all other urological products); N2 (analgesics) |
Chemical name | [(1R,5S,6S)-6(-Aminomethyl)-3-ethylbicyclo [3.2.0] hept-3-en-6-yl] acetic acid monobenzenesulfonate |
Mirogabalin was rapidly absorbed, with a median time to maximum plasma concentration (Tmax) of 0.5–1.5 h after oral administration of single or multiple doses [18, 19]. Exposure to mirogabalin increased proportionally or almost proportionally to dose [18, 19] and steady-state plasma concentrations were achieved by day 3 [19]. Taking a single 15 mg dose of mirogabalin in a fed rather than a fasted state delayed absorption of the drug (e.g. Tmax was 0.5 h longer), but did not impact its overall exposure to any clinically relevant extent [19].
Mirogabalin had a mean apparent volume of distribution of 64–88 L after single or multiple doses [19] and was 23–26% bound to human plasma proteins in vitro [7]. Most of a 30 mg dose taken orally was excreted in the urine (≈ 97%, largely as unchanged mirogabalin), with only 1% excreted in the faeces. Urinary metabolites of mirogabalin included lactams and an N-glucuronide conjugate, with UDP-glucuronosyltransferase (UGT) producing the latter [7]. Renal excretion of mirogabalin occurs via both glomerular filtration and tubular secretion [7]. Mirogabalin had a mean terminal half-life of 2–3 h after single doses and 2–5 h after multiple doses [18, 19].
The dosage and administration interval of mirogabalin require adjustment in patients with renal impairment and careful administration is advised [7], as exposure to the drug increases with worsening renal function [20, 21]. By contrast, mild or moderate hepatic impairment does not impact mirogabalin exposure to any clinically relevant extent [22], and the pharmacokinetics of mirogabalin in healthy elderly subjects (aged 55–75 years) [23] are generally similar to those of the drug in healthy non-elderly subjects [7]. However, as elderly patients can have reduced renal function and may be more susceptible to falls due to dizziness or somnolence [which are potential adverse events (AEs) with mirogabalin; Sect. 2.4], careful administration is recommended, including dosage and administration interval adjustment [7].
Mirogabalin is a substrate of OAT1, OAT3, OCT2, MATE1 and MATE2K, but does not appear to inhibit these or other transporters, including BCRP, OCT1, OATP1B1, OATP1B3 or p-glycoprotein [7]. Plasma concentrations of mirogabalin may increase if the drug is coadministered with probenecid (possibly via OAT1, OAT3 and UGT inhibition) or cimetidine (possible via MATE1 and MATE2K inhibition) [7, 24], although the increase in exposure may not be clinically relevant [24]. In addition, if mirogabalin is taken with lorazepam or alcohol, the CNS depressive effects of the drugs may be enhanced [7, 25], although no pharmacokinetic interactions are apparent [25]. Key cytochrome P450 isoenzymes are not inhibited or induced by mirogabalin [7].
2.3 Therapeutic Trials
2.3.1 Diabetic Peripheral Neuropathic Pain (DPNP)
2.3.1.1 Phase 3 Trials
After 14 weeks of treatment in a phase 3 trial in patients with DPNP (REDUCER; NCT02318706), there was a significantly (p = 0.0027) greater least-squares mean (LSM) improvement from baseline in weekly average daily pain score (ADPS) with mirogabalin 30 mg/day, but not mirogabalin 20 or 15 mg/day, relative to placebo (− 1.81, − 1.47 and − 1.34 vs. − 1.31) [primary endpoint]; the mean ADPS at baseline was 5.6 in each of the treatment groups [26]. Although the proportion of patients who achieved a ≥ 30% reduction from baseline in ADPS did not significantly differ between the placebo and mirogabalin groups, the proportion of patients who achieved a ≥ 50% ADPS reduction was significantly (p = 0.0048) greater with mirogabalin 30 mg/day (but not 20 or 15 mg/day) than with placebo. The mirogabalin 30 mg/day group also had significant (p = 0.0001) improvements in sleep interference (as per average daily sleep interference score [ADSIS]) and a significantly (p < 0.02) greater proportion of patients who considered their overall health status to be ‘much improved or better’ or ‘minimally improved or better’ (on the Patient Global Impression of Change [PGIC] scale), compared with the placebo group [26]. This double-blind Asian study randomized 834 patients (603 of whom were Japanese) to mirogabalin 15 mg once daily, mirogabalin 10 mg twice daily, mirogabalin 15 mg twice daily or placebo [26]. These dosages were maintained during weeks 12–13 of the trial, subsequent to a 1- to 2-week up-titration phase, and 824 patients were included in the efficacy analysis. An 11-point numerical rating scale (NRS) [0 = no pain; 10 = worst pain imaginable] was used to evaluate the weekly average of daily pain [26]. The REDUCER study was followed by a 1-year open-label extension [27].
The analgesic benefit of mirogabalin was maintained over 52 weeks in patients with DPNP who received open-label treatment with the drug in an Asian phase 3 long-term administration study (n = 214; 77% were Japanese) [7]. The mean pain intensity score [on a 0–100 mm visual analogue scale (VAS)] was 31.1–35.7 mm across weeks 12–52 versus 42.1 mm at baseline [7].
2.3.1.2 Phase 2 Trial
Mirogabalin showed promise as a treatment for DPNP in a 5-week phase 2 study (NCT01496365) [28]. After 5 weeks of treatment, the LSM difference versus placebo for the change from baseline in weekly ADPS was statistically significant (p < 0.05) for mirogabalin 30, 20 and 15 mg/day (− 1.01, − 0.88 and − 0.94) but not 10 or 5 mg/day (− 0.53 and − 0.22) [primary endpoint analysis], with mirogabalin 30 mg/day meeting the criteria for a minimally meaningful effect (i.e. decrease in weekly ADPS vs. placebo was ≥ 1.0). Consistent with these findings, significantly more recipients of mirogabalin 30, 20 or 15 mg/day than of placebo achieved one or both definitions of response (i.e. ≥ 30 or ≥ 50% reduction from baseline in ADPS at week 5), whereas no significant differences in response were evident versus placebo for lower dosages of mirogabalin. The median time taken to achieve a ≥ 30% reduction from baseline in ADPS (i.e. clinically meaningful pain relief) was significantly shorter with mirogabalin 30, 20, 15 and 10 mg/day than with placebo (16, 20, 16 and 30 vs. 36 days; each p < 0.05) but could not be calculated for mirogabalin 5 mg/day (as fewer than half of the recipients achieved this degree of pain relief) [28]. Some mirogabalin dosages (30, 20 and 15 mg/day) were also associated with significant (p < 0.05 vs. placebo) improvements in sleep interference at week 5 (as per ADSIS) and, for all dosages, the proportion of patients who considered their overall health status to be ‘much improved or better’ at the end of treatment (on the PGIC scale) was significantly (p < 0.05) greater than with placebo [29]. Pregabalin 300 mg/day was the active control, but generally provided no significant benefit over placebo for efficacy measures [28, 29]. In this randomized, double-blind trial (n = 452), mirogabalin 5, 10 or 15 mg/day was taken once daily, mirogabalin 20 or 30 mg/day was taken as 10 or 15 mg twice daily and pregabalin 300 mg/day as 150 mg twice daily [28].
2.3.2 Post-Herpetic Neuralgia (PHN)
In a phase 3 trial in patients with PHN (NEUCOURSE; NCT02318719), the LSM improvement from baseline in weekly ADPS after 14 weeks of therapy (primary endpoint) was significantly (p < 0.02) greater with mirogabalin 15, 20 or 30 mg/day than with placebo (− 1.61, − 1.68 and − 1.97 vs. − 1.20) [30]. A ≥ 30% reduction from baseline in ADPS occurred in significantly (p < 0.05) more recipients of mirogabalin than of placebo, regardless of the dosage (45–50 vs. 35% of patients), and a significantly (p = 0.0336) greater proportion of patients achieved a reduction in ADPS of ≥ 50% in the mirogabalin 30 mg/day (but not 20 or 15 mg/day) group than in the placebo group (29 vs. 20%). Each mirogabalin dosage was associated with a significant (p < 0.005) improvement in sleep versus placebo (as per ADSIS). Some of the mirogabalin groups also had significantly (p < 0.05) more patients who considered their overall health status (measured on the PGIC scale) to be ‘much improved or better’ (15 mg/day) or ‘minimally improved or better’ (20 and 30 mg/day) after 14 weeks of treatment than the placebo group [30]. This Asian study randomized 765 patients (612 from Japan) and included 763 in efficacy analyses [30]. It was of similar design to REDUCER and likewise used an 11-point NRS to evaluate daily pain intensity. The mean pain score at baseline was ≈ 5.7 in each of the treatment groups [30]. NEUCOURSE was followed by a 1-year open-label extension [31].
In patients with PHN who received open-label mirogabalin in an Asian, phase 3, long-term administration study (n = 237), the analgesic benefit of the drug was maintained over 52 weeks [7]. The mean pain intensity score on a 0–100 mm VAS was 28.6–34.7 mm over weeks 12–52 versus 43.5 mm at baseline. Most patients (79%) were Japanese [7].
2.3.3 DPNP or PHN with Renal Impairment
Mirogabalin improved DPNP or PHN in patients with renal impairment in an open-label phase 3 trial (NCT02607280) [7]. After 14 weeks of treatment, patients with moderate or severe renal impairment had LSM changes from baseline in ADPS of − 1.79 and − 2.07 versus mean values at baseline of 5.65 and 5.97 [7]. The maintenance dosage of mirogabalin was 15 mg/day in those with moderate renal impairment [creatinine clearance (CRCL) 30–59 mL/min] and 7.5 mg/day in patients with severe renal impairment (CRCL 15–29 mL/min). The trial comprised 2 weeks of dosage titration followed by 12 weeks of maintenance and used an 11-point NRS to evaluate daily pain intensity [7].
Key clinical trials of mirogabalin (Daiichi Sankyo Inc)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Mirogabalin | DPNP or PHN, + renal impairment | 3 | Completed | Japan | NCT02607280; DS5565-A-J313 |
Mirogabalin vs. placebo | DPNP | 3 | Completed | Japan, South Korea, Taiwan, Philippines | NCT02318706; DS5565-A-J303; REDUCER |
Mirogabalin vs. placebo | PHN | 3 | Completed | Japan, South Korea, Taiwan, Malaysia, Singapore, Thailand, Philippines | NCT02318719; DS5565-A-J304; NEUCOURSE |
Mirogabalin vs. placebo vs. pregabalin | DPNP | 2 | Completed | Japan, South Korea, Taiwan | NCT01504412; DS5565-A-J202 |
Mirogabalin vs. placebo vs. pregabalin | DPNP | 2 | Completed | USA | NCT01496365; DS5565-A-U201 |
Mirogabalin vs. placebo vs. pregabalin | Fibromyalgia pain | 3 | Completed | Multinational | NCT02187471; DS5565-A-E310; EudraCT2013-005162-20 |
Mirogabalin vs. placebo vs. pregabalin | Fibromyalgia pain | 3 | Completed | Multinational | NCT02187159; DS5565-A-E311; EudraCT2013-005163-10 |
Mirogabalin vs. placebo vs. pregabalin | Fibromyalgia pain | 3 | Completed | Multinational | NCT02146430; DS5565-A-E309; EudraCT2013-005161-40 |
Mirogabalin | Fibromyalgia pain | 3 | Completed | Multinational | NCT02234583; DS5565-A-E312; EudraCT2013-005164-26 |
Mirogabalin vs. placebo | Fibromyalgia pain + CKD | 3 | Completed | Multinational | NCT02496884; DS5565-A-U307; EudraCT2014-003972-21 |
2.4 Adverse Events
Across Asian clinical trials in patients with DPNP (n = 854) or PHN (n = 553), drug-related AEs occurred in 31.3 and 43.6% of those who received mirogabalin, the most common of which included somnolence (12.5 and 19.9%), dizziness (9.0 and 11.8%) and weight gain (3.2 and 6.7%) [7].
Indeed, in the dose-ranging phase 2 US study in patients with DPNP [28], dizziness (7.6%) and somnolence (5.1%) were the most common AEs considered to be related to study drug with mirogabalin 5, 10, 15, 20 or 30 mg/day (pooled dosage data), compared with increased blood creatinine phosphokinase levels with placebo (1.9%) and somnolence, balance disorder, fatigue and peripheral oedema with pregabalin 300 mg/day (4.0–8.0%). Only one patient (a mirogabalin 15 mg/day recipient) had a serious AE considered to be related to the drug; this was acute elevation of AST, ALT and total bilirubin that resolved 6–14 days after the last dose [28]. All treatment-emergent AEs of special interest, with the exception of CNS effects (14% of mirogabalin vs. 3% of placebo and 12% of pregabalin recipients), occurred with low (≤ 5%) incidence with mirogabalin, including cardiac conduction abnormalities (0.4 vs. 0.9 and 0%), arrhythmias (1 vs. 0 and 2%), oedema (5 vs. 1 and 10%), mildly blurred vision (2 vs. 2 and 4%) and abuse potential (0.4 vs. 0 and 0%) [28].
In healthy recreational polydrug users, abuse potential (as measured by the maximum observed effect on the bipolar Drug Liking VAS) was not significant (vs. placebo) with mirogabalin 15 or 45 mg (i.e. up to three times a therapeutic dose), but was significant (p < 0.001) with 60 or 105 mg (i.e. four or seven times a therapeutic dose) in two small single-dose trials (n =38 and 56) [32]. The studies were of randomized, double-blind design and used diazepam or pregabalin as positive controls [32]. Suicide-related AEs appear to be rare with mirogabalin, according to data from an Asian study (0.24% of 1227 mirogabalin recipients vs. 0.14% of 721 placebo recipients) [7].
2.5 Ongoing Clinical Trials
We are not aware of any mirogabalin trials that are currently ongoing.
3 Current Status
Mirogabalin tablets received their first global approval in January 2019 for the treatment of PNP in Japan [8].
Change history
26 February 2019
The title, which currently reads:
References
Best Practice Advocacy Centre New Zealand. Managing patients with neuropathic pain. 2016. https://bpac.org.nz/bpj/2016/may/pain.aspx. Accessed 22 Jan 2019.
National Institute for Health and Care Excellence. Neuropathic pain in adults: pharmacological management in nonspecialist settings. 2013. https://www.nice.org.uk. Accessed 22 Jan 2019.
Scottish Intercollegiate Guidelines Network. Management of chronic pain. A national clinical guideline. 2013. http://www.sign.ac.uk. Accessed 22 Jan 2019.
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73.
Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth. 2018;120(6):1315–34.
Park J, Luo ZD. Calcium channel functions in pain processing. Channels. 2010;4(6):510–7.
Daiichi Sankyo Company. Tarlige® Tablets: prescribing information 2019. http://www.info.pmda.go.jp/downfiles/ph/PDF/430574_11900B0F1020_1_02.pdf Accessed 14 Jan 2019.
Daiichi Sankyo Company. Daiichi Sankyo announces marketing approval in Japan of “Tarlige® Tablets” for pain treatment [media release]. 8 Jan 2019.
Daiichi Sankyo Company. Consolidated financial results for Q4 FY2017. 2018. In: https://www.daiichisankyo.com/media_investors/investor_relations/ir_calendar/files/005385/Reference%20Data.pdf. Accessed 22 Jan 2018
Daiichi Sankyo Company. Daiichi Sankyo announces top-line results from phase 3 global clinical development program evaluating mirogabalin in pain syndromes [media release]. 30 Jun 2017.
European Patent Office. European patent specification. Application number: 08833399.2. 2008. https://patentimages.storage.googleapis.com/fb/8b/48/462137eb934a5d/EP2192109B1.pdf. Accessed 22 Jan 2019.
United States Patent and Trademark Office. Patent No.: US 7,947,738 B2. 2011. https://patentimages.storage.googleapis.com/c3/f8/b9/191405f3ffb709/US7947738.pdf. Accessed 22 Jan 2019.
Domon Y, Arakawa N, Inoue T, et al. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. J Pharmacol Exp Ther. 2018;365(3):573–82.
Field MJ, Cox PJ, Stott E, et al. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103(46):17537–42.
Ivanov SV, Ward JM, Tessarollo L, et al. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am J Pathol. 2004;165(3):1007–18.
Domon Y, Kitano Y, Makino M. Analgesic effects of the novel alpha2delta ligand mirogabalin in a rat model of spinal cord injury. Pharmazie. 2018;73(11):659–61.
Domon Y, Arakawa N, Murasawa H, et al. Anxiolytic effects of the novel α2δ ligand mirogabalin (DS-5565) in Sluka model, an experimental animal model of fibromyalgia [abstract no. 374]. Arthritis Rheumatol. 2016;68 (Suppl 10):476–8.
Jansen M, Warrington S, Dishy V, et al. A randomized, placebo-controlled, double-blind study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and repeated doses of mirogabalin in healthy Asian volunteers. Clin Pharmacol Drug Dev. 2018;7(6):661–9.
Brown K, Mendell J, Ohwada S, et al. Tolerability, pharmacokinetics, and pharmacodynamics of mirogabalin in healthy subjects: results from phase 1 studies. Pharmacol Res Perspect. 2018;6(5):e00418.
Kato M, Tajima N, Shimizu T, et al. Pharmacokinetics and safety of a single oral dose of mirogabalin in Japanese subjects with varying degrees of renal impairment. J Clin Pharmacol. 2018;58(1):57–63.
Yin OQ, Merante D, Truitt K, et al. Population pharmacokinetic modeling and simulation for assessing renal impairment effect on the pharmacokinetics of mirogabalin. J Clin Pharmacol. 2016;56(2):203–12.
Duchin K, Senaldi G, Warren V, et al. Open-label single-dose study to assess the effect of mild and moderate hepatic impairment on the pharmacokinetics of mirogabalin. Clin Drug Investig. 2018;38(11):1001–9.
Brown K, Kumagae Y, Ohwada S, et al. A multiple ascending-dose study to evaluate safety, tolerability, pharmacokinetics and pharmacodynamics of mirogabalin in healthy elderly subjects [abstract no. 1443]. In: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting. 2015.
Tachibana M, Yamamura N, Atiee GJ, et al. Coadministration of probenecid and cimetidine with mirogabalin in healthy subjects: a phase 1, randomized, open-label, drug-drug interaction study. Br J Clin Pharmacol. 2018;84(10):2317–24.
Jansen M, Mendell J, Currie A, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of mirogabalin when coadministered with lorazepam, zolpidem, tramadol, or ethanol: results from drug-drug interaction studies in healthy subjects. Clin Pharmacol Drug Dev. 2018;7(6):597–612.
Baba M, Matsui N, Kuroha M, et al. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. J Diabetes Investig. 2019. https://doi.org/10.1111/jdi.13013.
Daiichi Sankyo Company. Daiichi Sankyo announces positive top-line results from phase 3 clinical trial evaluating mirogabalin in diabetic peripheral neuropathic pain [media release]. 31 Aug 2017.
Vinik A, Rosenstock J, Sharma U, et al. Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study. Diabetes Care. 2014;37(12):3253–61.
Merante D, Rosenstock J, Sharma U, et al. Efficacy of mirogabalin (DS-5565) on patient-reported pain and sleep interference in patients with diabetic neuropathic pain: secondary outcomes of a phase II proof-of-concept study. Pain Med. 2017;18(11):2198–207.
Kato J, Matsui N, Kakehi Y, et al. Mirogabalin for the management of postherpetic neuralgia: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain. 2019. https://doi.org/10.1097/j.pain.0000000000001501.
Daiichi Sankyo Company. Daiichi Sankyo announces first patients in large-scale, multi-national phase 3 clinical programs for mirogabalin [media release]. 4 Feb 2015.
Mendell J, Cooperman N, Sellers E, et al. Abuse potential of mirogabalin in recreational polydrug users [abstract no. PII-148]. Clin Pharmacol Ther. 2018;103 (Suppl 1):S94.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Emma Deeks is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Mirogabalin: First Global Approval. Drugs 79, 463–468 (2019). https://doi.org/10.1007/s40265-019-01070-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01070-8