Abstract
Background
Caffeine has a well-established effect on intraocular pressure (IOP) and ocular perfusion pressure (OPP); however, the possible differences between low- and high-caffeine consumers remain unknown.
Methods
In this placebo-controlled, double-blind, and balanced crossover study, 40 healthy individuals were divided in low- (n = 21) and high (n = 19)-caffeine consumers, according to their daily caffeine consumption. All participants ingested either caffeine (4 mg/kg) or placebo, and IOP and OPP were measured after 30, 60, and 90 min of ingesting caffeine or placebo. Subjective feelings of arousal were also obtained.
Results
Caffeine induced an acute IOP rise (p < 0.001, ƞp2 = 0.408), whereas habitual caffeine demonstrated a mediating effect on the IOP changes induced by caffeine intake, with high-caffeine consumers showing a less accentuated IOP rise in comparison to low-caffeine consumers. The greatest IOP change induced by caffeine intake was reached after 90 min from capsule ingestion, being more accentuated for the low-caffeine consumers (+ 3.4 mmHg) than for the high-caffeine consumers (+ 1.2 mmHg). Consequently, the participants reported higher levels of perceived arousal after ingesting caffeine in comparison to placebo (p = 0.002, ƞp2 = 0.222); however, similar responses were given by high- and low-caffeine consumers (p = 0.256). Our data did not reveal any effect of caffeine consumption on OPP (p = 0.304).
Conclusions
These results suggest that IOP responsiveness to caffeine ingestion is subject to tolerance, which could have important implication in the management of glaucoma. This finding may be due to alterations in the adenosine receptor system caused by chronic caffeine consumption. Future studies are needed to assess if these findings are also applicable to patients with glaucoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caffeine is the most widely consumed psychoactive drug worldwide, with coffee being the primary source of caffeine intake (Barone and Roberts 1996). The consumption of caffeine has demonstrated to provoke ergogenic effects on physical and cognitive tasks (de Morree et al. 2014; Einöther and Giesbrecht 2013; Haskell et al. 2005; Smirmaul et al. 2016), as well as a wide range of physiological and behavioral benefits (Glade 2010). Also, the impact of acute and regular coffee consumption on health status has been a matter of debate and controversy in the last years (Grosso et al. 2017; Loftfield et al. 2018). Recent evidence suggests that coffee consumption has beneficial effects for a variety of chronic diseases (e.g., cancer, neurological and metabolic conditions), whereas potential adverse effects have been described for pregnancy-related outcomes (e.g., low birth weight), as well as for cardiovascular conditions, although the latter may be influenced by the confounding effect of smoking (Grosso et al. 2016, 2017).
In relation to the effects of caffeine consumption on ocular health status, there are several studies that have explored the impact of caffeine on a variety of ocular indices such as ocular blood flow (Okuno et al. 2002), retinal vessel diameter (Terai et al. 2012) choroidal thickness (Vural et al. 2014), tear secretion (Osei et al. 2014), amplitude of accommodation (Abokyi et al. 2016), or oculomotor control (Connell et al. 2017) among others. Also, it is noteworthy that numerous researches have focused their attention on the ocular variables related to the incidence and progression of glaucoma, since this disease is the leading cause of irreversible blindness worldwide (Tham et al. 2014). In this regard, intraocular pressure (IOP) and ocular perfusion pressure (OPP) are determinant factors in the management of glaucoma (De Moraes et al. 2011; Cherecheanu et al. 2013). The consumption of caffeine seems to have an acute effect on the IOP and OPP levels in clinical populations (Avisar et al. 2002; Li et al. 2011; Jiwani et al. 2012), although negligible effects have been also reported in healthy individuals (Li et al. 2011; Terai et al. 2012; Dervişoğulları et al. 2016). The long-term effects of habitual caffeine intake has demonstrated to be positively associated with elevated IOP (Chandrasekaran et al. 2005; Kang et al. 2008), suggesting that caffeine consumption may not be advisable when a stable IOP and OPP behavior is required (i.e., glaucoma patients).
A tolerance to caffeine’s effects on the central nervous system, which attenuates the cardiovascular and neuroendocrine responses, has been described for habitual caffeine consumers (Nehlig et al. 1992; Corti et al. 2002; Ferré 2008; Grosso et al. 2017). In this regard, habitual caffeine consumption has been described as a possible mediating factor on the physiological and subjective effects of caffeine consumption, although controversial results have been found in this regard (Watson et al. 2002; Childs and De Wit 2006; Kennedy and Haskell 2011). According to Corti et al. (2002), categorization of the participants depending on their caffeine consumption habits is essential when analyzing the physiological effects of caffeine intake. Recent studies on the effects of caffeine on the ocular physiology have compared the possible differences between habitual and non-habitual caffeine consumers (Ismail et al. 2018); however, the impact of caffeine consumption habits on the IOP and OPP variations caused by caffeine ingestion remains unknown. Also, the effects of caffeine vary with the weight of consumers, being the effects of an arbitrary amount of caffeine higher in an individual with lower weight (Nehlig et al. 1992), and this fact has been frequently ignored when exploring the influence of caffeine on the ocular physiology (Li et al. 2011). Importantly, some studies have used regular coffee vs. decaffeinated coffee, which may allow to know the consumed beverage to the participants, probably by differences in the taste. In these cases, the expectancy theory of placebo effects argues that participant’s beliefs about the substance consumed modulate the physiological response and subjective perceptions to caffeine (Mikalsen et al. 2001). Moreover, the factors previously mentioned need to be controlled, and may explain the different results found in the literature about the effects of caffeine consumption on the ocular physiology, and specifically on IOP and OPP.
In view of the observed limitations, the present placebo-controlled, double-blind, and balanced crossover study aimed to compare the short-term effects of caffeine intake on IOP and OPP between low- (≤ one cup of coffee per day) and high (≥ two cups of coffee per day)-caffeine consumers. The caffeine dose was adjusted to participant’s weight (4 mg/kg) and the dependent variables (IOP and OPP) were assessed 30 min, 60 min, and 90 min after caffeine/placebo ingestion, as it has been commonly carried out in the related literature (Li et al. 2011). Based on the previous evidence, we hypothesized that a more stable IOP and OPP behavior after caffeine consumption could occur in high-caffeine consumers in comparison with low-caffeine consumers, as it has been demonstrated for other physiological indices (Kennedy and Haskell 2011; Grosso et al. 2017).
Methods
Participants and ethical approval
For the purposes of the study, low consumers were defined as those who reported to consume one or less cup of coffee (or other caffeinated drink) per day, whereas high consumers were defined as those who consumed two or more cups of coffee (or other caffeinated drink) per day. A total of 40 university students were recruited to participate in this study, and were divided in low consumers and high consumers according to their habitual level of caffeine intake (see Table 1 for a description of the experimental sample). The participants were screened according to the following inclusion criteria: (i) be free of any systemic or ocular disease, (ii) not taking any medication, (iii) not presenting allergy to xanthines, (iv) have a baseline IOP ≤ 21 mmHg which is considered as the upper limit for normal intraocular pressure (National Health and Medical Research Council 2010), and (v) have a blood pulse difference lower than 60 mmHg at baseline conditions, which is considered an indicator of possible cardiovascular disorders (Franklin et al. 1999). In addition, smokers were excluded from the study, since smoking causes an acute rise in blood pressure (Rysz et al. 2017). Also, all participants were asked to refrain for alcohol and caffeine-based drinks before attending to the laboratory in both experimental conditions, and to sleep at least 7 h the night prior to testing. The experimental protocol followed the guidelines of the Declaration of Helsinki, and it was approved by the University of Granada Institutional Review Board (IRB approval, 438/CEIH/2017).
Experimental design
We used a double-blind, mixed design to assess the short-term effects of caffeine consumption on IOP and OPP in low- and high-caffeine consumers. The within-participants factors were the caffeine consumption (placebo and caffeine) and point of measure (baseline, 30 min, 60, min, and 90 min), whereas the between-participants factor was the habitual caffeine intake (low consumers and high consumers). The dependent variables were IOP, OPP, blood pressure (BP), and the perceived level of activation. We also recorded the participant’s subjective level of alertness/sleepiness at the beginning of each experimental session. This placebo-controlled, double-blind study was carried out in two experimental sessions (on two different days), and both sessions were scheduled at the same time of day (± 1 h) in order to avoid circadian fluctuations in both IOP and BP (Millar-Craig et al. 1978; Agnifili et al. 2015). Both sessions were identical, and we only manipulated the caffeine ingestion by the administration of a placebo or caffeine (~ 4 mg/kg) capsule at the beginning of the experimental session. For this purpose, a pharmacist laboratory (Acofarma distribución S.A., Madrid, Spain) prepared the caffeine-containing capsules (caffeine anhydrous) and placebo capsules (corn starch); the contents of which were certified safe for human consumption. Caffeine capsules were available in steps of 20 mg (i.e., 200 mg, 220 mg, 240 mg), and they were chosen based on participant’s weight. For example, two participants whose weights were 61 kg and 79 kg consumed a caffeine capsule of 240 mg and 320 mg, respectively. The placebo capsule contained 300 mg of corn starch. Each treatment dose (placebo vs. caffeine) was administered in an identical color, size, and shape capsule, and thus, it was indistinguishable. Aiming to accomplish the double-blind procedure, the capsules were prepared and coded by a third person.
Instruments
A rebound tonometer (Icare Tonometer, TiolatOy, INC., Helsinki, Finland), which has been clinically validated and has showed a good level of agreement with the Goldmann tonometer (Pakrou et al. 2008), was used to assess IOP. Both eyes were measured in randomized order at the different points of measure (baseline, after 30, 60, and 90 min of capsule ingestion). For data analysis, we followed the guidelines of Armstrong (2013), who recommends to calculate the intraclass correlation coefficient between eyes, and when this value is close to 1, data from both eyes should be averaged. The intraclass correlation coefficients between eyes ranged between 0.952 and 0.992, and thus, we considered the average value from both eyes for further analyses. The participants were instructed to fixate on a distant target, and an experienced examiner took six rapidly consecutive measurements against his/her central corneal following the manufacturer’s instructions. The tonometer indicates whether differences between measurements were acceptable, we only considered values with low standard deviations (ideal measure). Central corneal thickness was also measured by ultrasound pachymetry (handheld pachymeter IOPac, Reichert Ophthalmic Instruments, Depew, NY) (see Table 1).
BP was evaluated by an RX3 wrist digital automatic blood pressure monitor (Omron, Hoofddorp, The Netherlands), which has been clinically validated (Cuckson et al. 2004), according to manufacturer’s specifications. OPP was indirectly calculated from the IOP and BP values as OPP sitting = (95/140 × MAP) − IOP (Quaranta et al. 2013), where mean arterial pressure (MAP) = diastolic BP + 1/3 (systolic BP − diastolic BP) (Costa et al. 2014).
Subjective questionnaires
The participants filled in the questionnaire Stanford Sleepiness Scale (SSS) at the beginning of both experimental sessions. This survey evaluates individuals’ self-reported activation, and it contains seven statements ranging from 1 “Feeling active, vital, alert, or wide awake” to 7 “No longer fighting sleep, sleep onset soon, having dream-like thoughts” (Hoddes et al. 1972). Additionally, we asked the participants to complete a visual analog scale in order to evaluate the subjective level of activation before the commencement of the experimental session, and after 30, 60, and 90 min after capsule ingestion. This numerical scale ranged from 1 “absolutely not activated” to 10 “extremely activated.”
Procedure
After signing the consent form, the participants were weighted, and they filled out the subjective scales (SSS and level of activation) and completed a demographic and caffeine habits questionnaire. Subsequently, we measured BP and IOP while the participants were seated with neutral neck position. At this moment, the corresponding capsule (placebo or caffeine) along with a cup of water (100 ml) was administered. Then, the level of activation, IOP and BP were assessed at the minutes 30, 60, and 90 after capsule ingestion.
Statistical analysis
Before any statistical analysis, the normal distribution of the data (Shapiro-Wilk test) and the homogeneity of variances (Levene’s test) were confirmed (p > 0.05). A mixed ANOVA with caffeine consumption (placebo and caffeine) and point of measure (baseline, 30 min, 60, min, and 90 min) as the within-participants factors, and the habitual caffeine intake (low consumers and high consumers) as the between-participants factor, was carried out for all the dependent variables (IOP, OPP, SBP, DBP, and subjective level of activation). The magnitude of the differences was reported by the partial eta squared (ƞp2) and Cohen’s effect size (ES) for Fs and t tests, respectively. Statistical significance was set at an alpha level of 0.05, and post hoc tests were corrected with Holm-Bonferroni procedure. Statistical analyses were performed using the JASP statistics package (version 0.8.1.0).
Results
Table 2 shows the descriptive values for all the variables assessed at the different points of measure. First, we checked that all participants attended to the laboratory under the same level of alertness/sleepiness by the analysis of the SSS, showing no statistical significant differences for caffeine consumption (F(1,38) < 1), habitual caffeine intake (F(1,38) = 2.211, p = 0.145), as well as the interaction caffeine consumption × habitual caffeine intake (F(1,38) < 1).
Effectiveness of the experimental manipulation on subjective perception
The perceived level of activation yielded statistical significance for caffeine consumption (F(1,38) = 10.83, p = 0.002, ƞp2 = 0.222), point of measure (F(3,114) = 5.096, p = 0.002, ƞp2 = 0.118), and the interaction caffeine consumption × point of measure (F(3,114) = 6.656, p < 0.001, ƞp2 = 0.149). However, no statistical differences were observed for habitual caffeine intake (F(1,38) = 1.331, p = 0.256), and the interactions caffeine consumption × habitual caffeine intake (F(1,38) = 1.329, p = 0.256) and point of measure × habitual caffeine intake (F(1,38) < 1). Post hoc comparisons for the point of measure revealed that the perceived level of activation after 30, 60, and 90 min after capsule ingestion was significantly higher than the baseline level of activation (corrected p values = 0.007, 0.044, and 0.044; ESs = 0.56, 0.44, and 0.43, respectively).
Effectiveness of the experimental manipulation on intraocular pressure and ocular perfusion
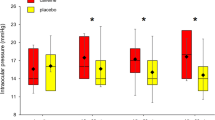
The analysis of IOP showed statistically significant differences for caffeine consumption (F(1,38) = 28.053, p < 0.001, ƞp2 = 0.408), point of measure (F(3,114) = 5.029, p = 0.003, ƞp2 = 0.110), and the interaction caffeine consumption × point of measure (F(3,114) = 28.430, p < 0.001, ƞp2 = 0.409). For its part, habitual caffeine intake (F(1,38) < 1) and the interaction caffeine consumption × habitual caffeine intake (F(1,38) = 2.680, p = 0.110) did not reach statistical significance; however, the interaction point of measure × habitual caffeine intake (F(3,114) = 2.862, p = 0.040, ƞp2 = 0.062) and the triple interaction caffeine consumption × point of measure × habitual caffeine intake (F(3,114) = 3.078, p = 0.030, ƞp2 = 0.044) exhibited statistical significance. Post hoc comparisons of the different points of measure demonstrated higher IOP values after 60 and 90 min of capsule ingestion in comparison to the baseline IOP measure (corrected p values = 0.013 and 0.011, and ESs = 0.51 and 0.53, respectively). The mean changes on IOP for the low-caffeine consumers were 2.7, 2.9, and 3.4 mmHg at 30, 60, and 90 min after caffeine consumption in comparison to baseline values, respectively. For its part, high-caffeine consumers had an IOP mean change after caffeine intake of 1.2 mmHg for all points of measure in comparison to baseline (Fig. 1).
Effect of caffeine consumption in high-and low-caffeine consumers at the different points of measure. Panel a shows the difference between the caffeine and placebo conditions, and panel b represents the magnitude of the difference (effect size) at the different points of measure after caffeine intake in comparison to the baseline IOP measurement. Error bars represent the standard error and the 90% confidence intervals in panels a and b, respectively. Asterisk denotes statistical significance (corrected p value < 0.05). All values are calculated across the participants (n = 40)
OPP data did not indicate any statistical effect either for the factor caffeine consumption (F(1,38) = 1.088, p = 0.304), point of measure (F(3,114) < 1), and habitual caffeine (F(1,38) < 1), as well as for all the possible interactions (F < 1, in all cases).
Effectiveness of the experimental manipulation on blood pressure
Systolic BP yielded statistical significance for the main factor of caffeine consumption (F(1,38) = 9.786, p = 0.003, ƞp2 = 0.203), and the interactions point of measure × habitual caffeine intake (F(3,114) = 4.782, p = 0.004, ƞp2 = 0.109) and caffeine consumption × point of measure × habitual caffeine intake (F(3,114) = 2.807, p = 0.043, ƞp2 = 0.056). Post hoc analyses for the different points of measure did not reach statistical significance for any comparison (all corrected p values > 0.05).
Lastly, caffeine consumption demonstrated a statistical significant effect on diastolic BP for caffeine consumption (F(1,38) = 10.168, p = 0.003, ƞp2 = 0.209), and the main factor of point of measure did not reach statistical significant but may represent an effect (F(3,114) = 2.573, p = 0.057, ƞp2 = 0.062). The rest of possible effects did not show any statistical difference (all p values > 0.170). The comparison between the different points of measure did not yield statistical significance (all corrected p values > 0.05).
Discussion
Our data demonstrate that a single administration of caffeine (~ 4 mg/kg) promotes an acute IOP rise. Relevantly, these changes were dependent on habitual caffeine intake, with low-caffeine consumers exhibiting a more accentuated IOP increment in comparison to high-caffeine consumers. However, the level of habitual caffeine intake did not reveal any effect on the perceived level of activation, since low- and high-caffeine consumers reported similar subjective perceptions (higher activation) after caffeine consumption. For its part, OPP did not vary after caffeine administration. This finding suggests that the acute effect of a moderate dose of caffeine on IOP is subject to tolerance as a consequence of habitual caffeine consumption, and it should be considered by ophthalmologists and optometrists, specially (i) when recommendations about caffeine habits are given to glaucoma patients or those at risk, and (ii) it would be advisable that patients, especially with glaucoma diagnosis or with ocular hypertension, avoid caffeine consumption before IOP evaluation during follow-ups, in order to obtain an accurate IOP assessment.
An appropriate experimental design was confirmed by the analysis of the SSS, which revealed that the participants attended to both experimental sessions with a comparable level of alertness/sleepiness. Also, subjective levels of activation converged with previous studies (Childs and De Wit 2006), and revealed that caffeine intake increases feelings of arousal, which cannot be attributable to participant’s beliefs since the placebo and caffeine capsules were indistinguishable (identical color, size, and shape). In agreement with the accumulated evidence about the caffeine ability to raise blood pressure as a consequence of increases in total peripheral resistance (Grosso et al. 2017), and with these effects being attenuated in habitual caffeine consumers (Echeverri et al. 2010), we found an acute effect of caffeine consumption on systolic and diastolic blood pressure, with regular caffeine intake being a modulator of this effect. Taken together, these results corroborate earlier studies, and allow us to ascertain a successful experimental design.
There are numerous studies which have addressed the short-term effects of caffeine intake on IOP (see Li et al. 2011 for a detailed review). Available evidence reflected that this effect is highly dependent on group’s characteristics, showing no IOP changes in normal individuals and significant increases in glaucoma patients or those with ocular hypertension. The main novelty of this study is the tolerance effect found on the IOP response to a single dose of caffeine. Some studies have reported tolerance to the physiological effects of caffeine; however, the human central nervous system does not seem to develop easily a tolerance to the stimulant effects of caffeine (Nehlig et al. 1992). There is evidence that habitual caffeine consumers show an acute increase in the activity of the sympathetic nervous system after caffeine intake that is not linked to blood pressure rise, whereas caffeine promotes a comparable increase in sympathetic nerve activity and blood pressure in non-habitual caffeine consumers (Corti et al. 2002). From the present results and the previous evidence, it is reasonable to argue that the potential detrimental effects of caffeine consumption could be less apparent in habitual consumers, and thus, caffeine restriction may not be medically necessary in these individuals (Corti et al. 2002; Kennedy and Haskell 2011; Grosso et al. 2017). In addition, the antioxidant compounds found in coffee have been proposed as possible protectors of blood pressure changes caused by caffeine consumption, which may partially explain the reduced responsiveness to caffeine consumption found in habitual caffeine consumers (D’Elia et al. 2017). Of note, there was a relative IOP increase of 21.7% after 90 min of caffeine ingestion for the low-caffeine consumers, this percentage of change is similar to lowering effects of commonly used glaucoma drugs (IOP decrease around 25% (van der Valk et al. 2005)). Thus, the acute impact of caffeine intake on IOP may be of clinical relevance, especially in non-habitual caffeine consumers. Moreover, based on the current finding and the accumulated evidence on the area of cardiovascular health, caffeine consumption habits need to be taken into account when analyzing the impact of caffeine on the ocular health, especially in glaucoma.
Caffeine increases blood vessel resistance and decreases blood flow in the human optical nerve head (Okuno et al. 2002), showing in non-habitual caffeine consumers a weaker blood vessel resistance and a higher significant decrease blood flow when compared to the habitual consumers (Ismail et al. 2018), most likely as a result of the adaptation to long-term caffeine consumption. Although the relationship between OPP and blood flow is complex (Schmidl et al. 2011), it is generally accepted that a low OPP may lead to reduced ocular blood flow and in turn could contribute to glaucomatous optic neuropathy (Leske 2009). Therefore, it is reasonable for us to think that IOP is influenced by habitual caffeine consumption, as it was found for ocular blood flow (Ismail et al. 2018). In the present study, the acute effect of caffeine on OPP was assessed; however, we failed to find any difference between low- or high-caffeine consumers. The similar tendency to increase from IOP and blood pressure permitted to maintain a stable OPP, and thus, it remained unaltered after caffeine consumption. These results agree with those reported by Okuno et al. (2002), although the results comparison from both studies must be cautiously interpreted since they used a caffeine dose of 100 mg, whereas our participants ingested a caffeine capsule of 4 mg/kg. On the other hand, our data are contrary to those found by Terai et al. (2012), who found an OPP increase after 1 h of ingesting 200 mg of caffeine; however, it should be noted that all participants included had an average daily caffeine consumption lower than one cup whereas the average caffeine intake of our experimental sample was 1.70 ± 1.44, and thus, it may explain the differences observed between both studies. The lack of homogeneity in the caffeine dose and participant’s caffeine habits found in the related studies did not allow to reveal solid conclusion in relation to the acute effects of caffeine intake on OPP. Future studies should test these mediating factors (i.e., caffeine dose, caffeine habits, placebo effect) in their experimental designs.
A plausible physiological explanation
Several mechanisms may be responsible for the IOP changes induced by caffeine; however, there is no clear evidence whether it is due to greater aqueous humor production or poorer drainage (Li et al. 2011). Potential mechanisms may be (i) caffeine may block the enzyme phosphodiesterase (Nurminen et al. 1999), which may increase aqueous humor formation (Jiwani et al. 2012), (ii) the inhibition of adenosine receptors caused by caffeine can increase intracellular cyclic AMP, which may stimulate the production of aqueous humor by the ciliary body, as well as to reduce the chamber angle, and leading to greater levels of IOP (Ajayi and Ukwade 2001), and (iii) the increase in blood pressure induced by caffeine’s adenosine receptor blockade could enhance the hydrostatic pressure for aqueous humor formation, being linked the blood pressure and IOP elevations (Nurminen et al. 1999; Li et al. 2011). Importantly, caffeine’s tolerance seems to be mediated by a reduction on the number and sensitivity of adenosine receptors, and it may be responsible for reduced physiological responses in habitual caffeine consumers (Ferré 2008). Additionally, the antioxidant compounds contained in coffee have been proposed as a possible preventive method of the effects of caffeine in raising blood pressure (Grosso et al. 2017), and thus, could also affect IOP.
Limitations and future research
The present study incorporates preliminary evidence on the mediating role of habitual caffeine consumption on IOP changes induced by acute caffeine intake. However, there are several factors that warrant some caution in the interpretation of the present results. The main limitation of this study is that we only included young healthy individuals, and the inclusion of clinical populations, especially glaucoma patients, would be of interest since they may show a different response to caffeine consumption in comparison to our experimental sample. A recent study of De Moraes et al. (2018) demonstrated that even transient IOP fluctuations during the day, as it may be caused by caffeine ingestion, have a negative impact on the visual fields of glaucoma patients, and thus, the relevance of the IOP behavior in glaucoma patients is of great relevance. Caffeine’s plasma half-life lasts approximately 3 to 6 h, depending on doses, habitual consumption, and individual differences in the metabolism (Mort and Kruse 2008); therefore, futures studies should evaluate the IOP behavior after 90-min caffeine intake. Also, the amount of caffeine administered was chosen based on the estimations of mean daily caffeine intake for US consumers (Barone and Roberts 1996); however, the impact of caffeine on the ocular physiology may have a dose-response effect, as it has been showed for other physiological variables (Quinlan et al. 2000; Chen and Parrish 2009). It is our hope that future studies will test the association between caffeine dose and ocular physiological variations. Different physiological responses, including the ocular physiology, have been showed depending on the age and ethnicity of individuals, and thus, further studies should include other age and ethnics groups in their experimental designs (Chan et al. 2016). Additionally, the relatively small sample size used in this study could mask small effects, and thus, the present outcomes should be cautiously interpreted in this regard. Finally, the exact mechanism of the IOP variations (increased production of aqueous humor, reduced drainage, or a combination of both) induced by caffeine is not yet fully understood, and the incorporation of new advances in ocular imaging techniques may help to deepen our understanding in the mechanisms of how IOP is altered by caffeine.
Conclusions
In this placebo-controlled, double-blind study, we found that the effects of caffeine intake (4 mg/kg) on IOP are dependent on the habitual caffeine consumption in healthy individuals, with low consumers showing a more abrupt IOP increase after ingesting caffeine in comparison to high-caffeine consumers. Nevertheless, OPP remains unaltered after consuming caffeine. This preliminary evidence that the effects of caffeine consumption on the IOP response are subject to tolerance, and it needs to be considered for eye care specialists in the management and prevention of glaucoma. Adaptations of the adenosine receptor system caused by chronic caffeine consumption are discussed as a potential explanation of this finding.
References
Abokyi S, Owusu-Mensah J, Osei KA (2016) Caffeine intake is associated with pupil dilation and enhanced accommodation. Eye 31:615–619. https://doi.org/10.1038/eye.2016.288
Agnifili L, Mastropasqua R, Frezzotti P, Fasanella V, Motolese I, Pedrotti E, Iorio AD, Mattei PA, Motolese E, Mastropasqua L (2015) Circadian intraocular pressure patterns in healthy subjects , primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol 93:14–21. https://doi.org/10.1111/aos.12408
Ajayi OB, Ukwade MT (2001) Caffeine and intraocular pressure in a Nigerian population. J Glaucoma 10:25–31. https://doi.org/10.1097/00061198-200102000-00006
Armstrong RA (2013) Statistical guidelines for the analysis of data obtained from one or both eyes. 7–14. https://doi.org/10.1111/opo.12009
Avisar R, Avisar E, Weinberger D (2002) Effect of coffee consumption on intraocular pressure. Ann Pharmacother 36:992–995. https://doi.org/10.1345/aph.1A279
Barone JJ, Roberts HR (1996) Caffeine Consumption. Fd Chem Toxic 34:119–129. https://doi.org/10.1016/0278-6915(95)00093-3
Chan MPY, Grossi CM, Khawaja AP, Yip JL, Khaw KT, Patel PJ, Khaw PT, Morgan JE, Vernon SA, Foster PJ, UK Biobank Eye and Vision Consortium (2016) Associations with intraocular pressure in a large cohort: results from the UK Biobank. Ophthalmology 123:771–782. https://doi.org/10.1016/j.ophtha.2015.11.031
Chandrasekaran S, Rochtchina E, Mitchell P (2005) Effects of caffeine on intraocular pressure: the blue mountains eye study. J Glaucoma 14:504–507
Chen Y, Parrish TB (2009) Caffeine dose effect on activation-induced BOLD and CBF responses. Neuroimage 46:577–583. https://doi.org/10.1016/j.neuroimage.2009.03.012
Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L (2013) Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol 13:36–42. https://doi.org/10.1016/j.coph.2012.09.003
Childs E, De Wit H (2006) Subjective, behavioral, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology 185:514–523. https://doi.org/10.1007/s00213-006-0341-3
Connell CJW, Thompson B, Turuwhenua J, Hess RF, Gant N (2017) Caffeine increases the velocity of rapid eye movements in unfatigued humans. Psychopharmacology 234:2311–2323. https://doi.org/10.1007/s00213-017-4638-1
Corti R, Binggeli C, Sudano I, Spieker L, Hänseler E, Ruschitzka F, Chaplin WF, Lüscher TF, Noll G (2002) Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content role of habitual versus nonhabitual drinking. Circulation 106:2935–2940. https://doi.org/10.1161/01.CIR.0000046228.97025.3A
Costa VP, Harris A, Anderson D, Stodtmeister R, Cremasco F, Kergoat H, Lovasik J, Stalmans I, Zeitz O, Lanzl I, Gugleta K, Schmetterer L (2014) Ocular perfusion pressure in glaucoma. Acta Ophthalmol 92:252–266. https://doi.org/10.1111/aos.12298
Cuckson AC, Moran P, Seed P, Reinders A, Shennan AH (2004) Clinical evaluation of an automated oscillometric blood pressure wrist device. Blood Press Monit 9:31–37
D’Elia L, la Fata E, Galletti F, Scalfi L, Strazzullo P (2017) Coffee consumption and risk of hypertension: a dose–response meta-analysis of prospective studies. Eur J Nutr 0:1–10. https://doi.org/10.1007/s00394-017-1591-z
De Moraes CGV, Juthani VJ, Liebmann JM et al (2011) Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol 129:562–568. https://doi.org/10.1001/archophthalmol.2011.72
De Moraes CG, Mansouri K, Liebmann JM, Ritch R (2018) Association between 24-hour intraocular pressure monitored with contact lens sensor and visual field progression in older adults with glaucoma. JAMA Ophthalmol 10022:1–7. https://doi.org/10.1001/jamaophthalmol.2018.1746
de Morree HM, Klein C, Marcora SM (2014) Cortical substrates of the effects of caffeine and time-on-task on perception of effort. J Appl Physiol 117:1514–1523. https://doi.org/10.1152/japplphysiol.00898.2013
Dervişoğulları MS, Totan Y, Yüce A, Kulak AE (2016) Acute effects of caffeine on choroidal thickness and ocular pulse amplitude. Cutan Ocul Toxicol 35:281–286. https://doi.org/10.3109/15569527.2015.1104330
Echeverri D, Montes FR, Cabrera M, Galán A, Prieto A (2010) Caffeine’s vascular mechanisms of action. Int J Vasc Med 2010:1–10. https://doi.org/10.1155/2010/834060
Einöther SJL, Giesbrecht T (2013) Caffeine as an attention enhancer: reviewing existing assumptions. Psychopharmacology 225:251–274. https://doi.org/10.1007/s00213-012-2917-4
Ferré S (2008) An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem 105:1067–1079. https://doi.org/10.1111/j.1471-4159.2007.05196.x
Franklin SS, Khan SA, Wong ND, Larson MG, Levy D (1999) Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation 100:354–360. https://doi.org/10.1161/01.cir.100.4.354
Glade MJ (2010) Caffeine-not just a stimulant. Nutrition 26:932–938. https://doi.org/10.1016/j.nut.2010.08.004
Grosso G, Micek A, Godos J, Sciacca S, Pajak A, Martínez-González MA, Giovannucci EL, Galvano F (2016) Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis. Eur J Epidemiol 31:1191–1205. https://doi.org/10.1007/s10654-016-0202-2
Grosso G, Godos J, Galvano F, Giovannucci EL (2017) Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr 37:131–156. https://doi.org/10.1146/annurev-nutr-071816-064941
Haskell CF, Kennedy DO, Wesnes KA, Scholey AB (2005) Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacology 179:813–825. https://doi.org/10.1007/s00213-004-2104-3
Hoddes E, Zarcone V, Dement W (1972) Development and use of Stanford Sleepiness Scale (SSS). Psychophysiology 9:150
Ismail A, Bhatti MS, Faye I, Lu CK, Laude A, Tang TB (2018) Pulse waveform analysis on temporal changes in ocular blood flow due to caffeine intake: a comparative study between habitual and non-habitual groups. Graefes Arch Clin Exp Ophthalmol 256:1711–1721. https://doi.org/10.1007/s00417-018-4030-9
Jiwani AZ, Rhee DJ, Brauner SC, Gardiner MF, Chen TC, Shen LQ, Chen SH, Grosskreutz CL, Chang KK, Kloek CE, Greenstein SH, Borboli-Gerogiannis S, Pasquale DL, Chaudhry S, Loomis S, Wiggs JL, Pasquale LR, Turalba AV (2012) Effects of caffeinated coffee consumption on intraocular pressure, ocular perfusion pressure, and ocular pulse amplitude: a randomized controlled trial. Eye 26:1122–1130. https://doi.org/10.1038/eye.2012.113
Kang J, Willet W, Rosner B et al (2008) Caffeine consumption and the risk of primary open - angle glaucoma: a prospective cohort study. Invest Ophthalmol Vis Sci 49:1924–1931. https://doi.org/10.1038/jid.2014.371
Kennedy DO, Haskell CF (2011) Cerebral blood flow and behavioural effects of caffeine in habitual and non-habitual consumers of caffeine: a near infrared spectroscopy study. Biol Psychol 86:298–306. https://doi.org/10.1016/j.biopsycho.2010.12.010
Leske MC (2009) Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol 20:73–78. https://doi.org/10.1097/ICU.0b013e32831eef82
Li M, Wang M, Guo W, Wang J, Sun X (2011) The effect of caffeine on intraocular pressure: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol 249:435–442. https://doi.org/10.1007/s00417-010-1455-1
Loftfield E, Cornelis M, Caporaso N et al (2018) Association of coffee drinking with mortality by genetic variation in caffeine metabolism: findings from the UK Biobank. JAMA Intern Med 178:1086–1097. https://doi.org/10.1001/jamainternmed.2018.2425
Mikalsen A, Bertelsen B, Flaten MA (2001) Effects of caffeine, caffeine-associated stimuli, and caffeine-related information on physiological and psychological arousal. Psychopharmacology 157:373–380. https://doi.org/10.1007/s002130100841
Millar-Craig MW, Bishop CN, Raftery EB (1978) Circadian variation of blood-pressure. Lancet 311:795–797. https://doi.org/10.1016/S0140-6736(78)92998-7
Mort JR, Kruse HR (2008) Timing of blood pressure measurement related to caffeine consumption. Ann Pharmacother 42:105–110. https://doi.org/10.1345/aph.1K337
National Health and Medical Research Council (2010) Guidelines for the screening, prognosis, diagnosis, management and prevention of glaucoma. Commonwealth of Australia, Canberra. http://www.nhmrc.gov.au/publications/synopses/cp113syn.htm. Accessed June 2011
Nehlig A, Daval JL, Debry G (1992) Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Rev 17:139–170. https://doi.org/10.1016/0165-0173(92)90012-B
Nurminen ML, Niittynen L, Korpela R, Vapaatalo H (1999) Coffee, caffeine and blood pressure: a critical review. Eur J Clin Nutr 53:831–839. https://doi.org/10.1038/sj.ejcn.1600899
Okuno T, Sugiyama T, Tominaga M, Kojima S, Ikeda T (2002) Effects of caffeine on microcirculation of the human ocular fundus. Jpn J Ophthalmol 46:170–176. https://doi.org/10.1016/S0021-5155(01)00498-1
Osei KA, Ovenseri-Ogbomo G, Kyei S, Ntodie M (2014) The effect of caffeine on tear secretion. Optom Vis Sci 91:171–177. https://doi.org/10.1097/OPX.0000000000000129
Pakrou N, Gray T, Mills R, Landers J, Craig J (2008) Clinical comparison of the Icare tonometer and Goldmann applanation tonometry. J Glaucoma 17:43–47. https://doi.org/10.1097/IJG.0b013e318133fb32
Quaranta L, Katsanos A, Russo A, Riva I (2013) 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Surv Ophthalmol 58:26–41. https://doi.org/10.1016/j.survophthal.2012.05.003
Quinlan PT, Lane J, Moore KL, Aspen J, Rycroft JA, O’Brien DC (2000) The acute physiological and mood effects of tea and coffee. Pharmacol Biochem Behav 66:19–28. https://doi.org/10.1016/S0091-3057(00)00192-1
Rysz J, Franczyk B, Banach M, Gluba-Brzozka A (2017) Hypertension - current natural strategies to lower blood pressure. Curr Pharm Des 23:2453–2461. https://doi.org/10.2174/1381612823666170215144649
Schmidl D, Garhofer G, Schmetterer L (2011) The complex interaction between ocular perfusion pressure and ocular blood flow - relevance for glaucoma. Exp Eye Res 93:141–155. https://doi.org/10.1016/j.exer.2010.09.002
Smirmaul BPC, de Moraes AC, Angius L, Marcora SM (2016) Effects of caffeine on neuromuscular fatigue and performance during high-intensity cycling exercise in moderate hypoxia. Eur J Appl Physiol 117:1–12. https://doi.org/10.1007/s00421-016-3496-6
Terai N, Spoerl E, Pillunat LE, Stodtmeister R (2012) The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Ophthalmol 90:1–5. https://doi.org/10.1111/j.1755-3768.2012.02486.x
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH (2005) Intraocular pressure–lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology 112(7):1177–1185
Vural AD, Kara N, Sayin N, Pirhan D, Ersan HBA (2014) Choroidal thickness changes after a single administration of coffee in healthy subjects. Retina 34:1223–1228. https://doi.org/10.1097/IAE.0000000000000043
Watson J, Deary I, Kerr D (2002) Central and peripheral effects of sustained caffeine use: tolerance is incomplete. Br J Clin Pharmacol 54:400–406. https://doi.org/10.1046/j.1365-2125.2002.01681.x
Acknowledgments
The authors thank to all the participants who selflessly collaborated in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocol followed the guidelines of the Declaration of Helsinki, and it was approved by the University of Granada Institutional Review Board (IRB approval, 438/CEIH/2017).
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Vera, J., Redondo, B., Molina, R. et al. Effects of caffeine on intraocular pressure are subject to tolerance: a comparative study between low and high caffeine consumers. Psychopharmacology 236, 811–819 (2019). https://doi.org/10.1007/s00213-018-5114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5114-2