Abstract
Background
Caffeine is widely consumed, and its effect on intraocular pressure (IOP) has been reported in conflicting data. The aim of this meta-analysis was to quantitatively summarize the effect of caffeine on IOP in normal individuals and in patients with glaucoma or ocular hypertension (OHT).
Methods
A comprehensive literature search was performed using the Cochrane Collaboration methodology to identify pertinent randomized controlled trials (RCTs) from the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed and EMBASE. A systematic review and meta-analysis was performed. IOP at 0.5 hour (h), 1 h and 1.5 h after caffeine ingestion was the main outcome measurement.
Results
Six RCTs (two parallel-designed and four crossover-designed) evaluating 144 participants fulfilled inclusion criteria. The risk of bias for these studies was uncertain. Among the participants, 103 were normal individuals and 41 were patients with glaucoma or OHT. In normal individuals, the IOPs measured at 0.5 h, 1 h and 1.5 h post-intervention were not affected by ingestion of caffeine. The weighted mean difference (WMD) with 95% confidence intervals (95%CI) for each measurement point were −0.740 (–2.454, 0.974), 0.522 (–0.568, 1.613) and 0.580 (–1.524, 2.684). However, in patients with glaucoma or OHT, IOP increased at each measurement point, with the WMD and 95%CI being 0.347 (0.078, 0.616), 2.395 (1.741,3.049) and 1.998 (1.522,2.474) respectively. No publication bias was detected by either Begg’s or Egger’s test.
Conclusion
Available evidences showed that caffeine had different effects on IOP in different groups of individuals. For normal individuals, IOP was not changed by ingestion of caffeine, while for patients with glaucoma or OHT, IOP increased significantly. More high-quality RCTs are warranted to confirm this. The mechanisms underlying this phenomenon and the clinical significance are to be explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caffeine is widely consumed all over the world in people’s daily life. It is a bitter, white crystalline xanthine alkaloid named trimethylxanthine1,3,5, naturally occurs as part of chemical mixtures in beans , leaves and fruits of some plants, and is most commonly consumed by humans in various foods and drinks such as coffee, tea ,cola and chocolates [1]. Approximately 80% of adults drink coffee or tea daily in the United States, and the average consumption of caffeine in the US and Canada is about 2.4 mg/kg/day for grownups and 1.1 mg/kg/day for children 5–18 years old [2].
Caffeine primarily acts as a stimulant with complex effects for many systems in the human body, which causes it to be extensively investigated as a risk factor for human disease [2]. As caffeine has been found to cause a transient rise of intraocular pressure (IOP) [3–6], it sounds reasonable that glaucoma patients should not take caffeine. However, conflicting data has also been reported [7, 8]. It is well-known that elevated IOP is an important risk factor for the exacerbation of open-angle glaucoma (OAG) [9–11] and for the progression to glaucoma in ocular hypertension (OHT) [12, 13]. Reduction of IOP has been identified as an effective way to prevent visual field damage [11, 14]. To recommend whether or not to avoid caffeine would thus have not only quite a wide sociological effect, but it is also an important issue in preventing glaucoma which is the second leading cause of blindness worldwide [15, 16].
The aim of this meta-analysis is to explore the effect of caffeine on IOP in normal individuals and patients with glaucoma or ocular hypertension, using the available evidence. To the best of our knowledge, no quantitative summary on this issue has been published before.
Materials and methods
Search strategy and literature screening
The literature included in this meta-analysis was identified using EMBASE , PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) (up to July 31 2009). The following search strategy was used: ("Coffee"[Mesh] OR "Caffeine"[Mesh] OR "Chicory"[Mesh] OR "Coffea"[Mesh]) AND ("Intraocular Pressure"[Mesh] OR "Ocular Hypertension"[Mesh]). Key words were searched both as free text words and EMTREE terms in EMBASE or MeSH terms in PubMed whenever possible. The search in EMBASE was limited to clinical trial. In PubMed, initial search results were combined with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE [17]. All references cited in these studies were surveyed to identify additional work outside of the electronic databases. Hand searching was also applied to the index of major ophthalmology journals. If duplicated articles with the same author and the same case series were found, the study with the most individuals investigated was included.
Two reviewers (Mao Li, Min Wang) conducted the literature search independently. Irrelevant literature was weeded out by reading titles and abstracts first. Any doubt on relevance was resolved by discussion and review of the original article.
Inclusion and exclusion criteria
For inclusion in the meta-analysis, all the following criteria had to be met: 1) the study design should be randomized controlled comparisons, including non-standard designs such as crossover trials or cluster randomized trials, 2) the studies should be carried out in vivo in human beings, and the participants could be normal individuals or patients with glaucoma or OHT, 3) ingestion of caffeine should be the only intervention, 4) the comparator should be decaffeinated materials, and 5) the outcome measurement should be IOP. Studies without controls or randomized allocations were excluded. Crossover-designed studies without enough wash-out periods were also excluded.
Assessment of risk of bias in included studies
The domain-based evaluation tool for assessing risk of bias developed by the Cochrane Collaboration [18] was used to evaluate the included references. It consists of critically assessing criteria for evaluating different aspects of trial quality. These aspects are sequence generation, allocation concealment, blinding of assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Evaluated results were “Yes” for “low risk of bias”, “No” for “high risk of bias” and “Unclear” for “uncertain risk of bias”. An overall summary assessment of the risk of the bias within and across studies was made according to the Cochrane Collaboration’s methodology [18]. Studies which were assessed as “Yes” in all main domains were considered to be at low risk of bias. Studies in which no clear judgment could be assessed in one or more domains were considered to be at least at medium risk of bias. Studies with the judgment of “No” in one or more of the key domains were considered to be at high risk of bias.
Data extraction
Data were extracted independently by two authors (Mao Li, Min Wang). Disagreement was resolved by discussion. The following data were extracted for each study: authors, year of publication, country of the study, participants, study design, sample size, the intervention, the comparator, mean IOP or mean IOP changes from baseline with standard deviation (SD) for each measurement time point.
For studies including subjects of different entity groups (normal, glaucoma or OHT), data were extracted separately for each group. For studies not presenting SD for IOP, other statistics that could be used to calculate SD were extracted [19].
Statistics
The meta-analysis was conducted using STATA 10.0 (StataCorp, College Station, TX, USA); p < 0.05 was considered statistically significant. Continuous data from the included studies were presented as mean difference (MD) with 95% confidence intervals (CI). Statistical heterogeneity was measured using the Cochran’s χ2-based Q statistic [20] (p < 0.10 was considered representative of significant statistical heterogeneity). MDs were pooled by random effect model to get the weighted mean difference (WMD) by using the DerSimonian and Laird method. The Begg's rank correlation method [21] and the Egger weighted regression method [22] were used to statistically assess publication bias (p < 0.05 was considered representative of statistically significant publication bias). Individuals who received caffeine were presented as the experimental group, and those who received comparators as the control group in this meta-analysis.
As the measurement time points varied between studies, the most common measurement points were used for the meta-analysis. For studies which reported data from both eyes, the studies were treated as cluster-randomized trials [19]. Mean IOP of the two eyes of each subject and corresponding SD were calculated, which were used as the summary measurement for the subject to avoid unit-of-analysis errors [23]. Thus, the effective sample size of each study was not the number of eyes but was the number of subjects that were randomized. For studies not presenting SD, p value, F value, t value, CI or standard error was used to impute SD according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions [23].
Explore heterogeneity
To explore the potential heterogeneity, subgroup analyses were conducted based on the characteristics that varied across the included studies. These characteristics were disease status (healthy subjects vs glaucoma or OHT subjects) and forms of caffeine delivery (beverage vs non-beverage).
Sensitivity analysis
To examine the influence of individual studies on the summary effect estimate, an influence analysis using the “metainf” command [24] in STATA was conducted, in which the meta-analysis estimate were computed omitting a study at a time. Besides, MDs were combined in a fixed-effect model by using the inverse variance method, and the results were compared with that of a random-effect model.
Outcome measures
The primary study outcome was IOP.
Results
Identification of studies
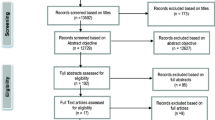
After searching all databases, 89 articles pertaining to the key words were found, among which 74 were unduplicated. By using the highly sensitive randomized trial search strategy [17], 19 randomized trials were identified. Abstracts and full texts were then reviewed further and 12 studies were weeded out. Among the 12 excluded articles, two studies were not carried out in in vivo human beings [25, 26], ingestion of caffeine was not the only intervention in three articles [27–29], outcomes in four articles were not IOP [30–33], three articles [34–36] were not RCTs despite the fact that we limited the literature search to controlled trials. One of the seven eligible studies reported only the IOP at 4 hours post-intervention [37], so six trials [3–8] were finally analyzed in the meta-analysis.
Study and participant characteristics
One hundred and forty four participants were evaluated in the six studies [3–8]. Among the participants, 103 were normal individuals and 41 were glaucoma or OHT patients. All studies reported that the experimental group and the control group were age and gender matched, but not all the studies presented data of mean age and gender ratio (Table 1). A summary description of the trials is given in Table 2. Two trials [4, 7] were parallel arm designs, and the other four [3, 5, 6, 8] were randomized crossover-designed studies. All studies except one [6] presented the exact caffeine amount delivered to the subjects. The intervention groups ingested 30 mg to 300 mg caffeine contained in beverages, capsules or tablets, while the control group took decaffeinated beverages or placebos. All the patients with glaucoma or OHT together with their controls received beverages across the studies included, while the healthy subjects and their controls were given either beverages or non-beverages (tablets or capsules). Post-intervention measurement time points varied across studies. IOPs were commonly measured 0.5 h, 1 h and 1.5 h post-intervention, and these data were combined accordingly in this meta-analysis. Applanation tonometry [3–5, 7, 8] and noncontact tonometry [6] were used to measure the IOP. Four studies [3, 5, 6, 8] reported that follow-up and data collection were carried out at approximately the same time of the day. Wash-out period for the crossover trials was over 7 days.
Risk of bias in included studies
Some of the studies did not report details on sequence generation [4, 6–8], allocation concealment [3], and blinding of assessment [3, 6, 8], and the risk of bias of these domains were judged as “unclear”, while for most studies [3, 4, 6–8], follow-up and results reporting procedures were in good quality. Since all the studies in uncertain risk of bias for one or more key domains were judged as being in uncertain risk of bias, the risk of bias for this meta-analysis was uncertain.
Effects of caffeine on IOP
The combined results of IOP for different groups at different post-intervention time points are presented in Table 3. When healthy individuals and glaucoma or OHT patients were analyzed together as an overall group, the combined results showed that IOP rose significantly in the caffeine group compared to the control group at 0.5 h, 1 h or 1.5 h post-intervention. The WMD with 95% CIs were 0.326 (0.070–0.582), 1.734 (0.951–2.517) and 1.847(1.255–2.439) accordingly. However, when analyzed separately, it was found that IOP was not elevated significantly in healthy individuals after caffeine ingestion. WMD with 95% CIs were −0.740 (−2.454, 0.974), 0.522 (−0.568, 1.613) and 0.580 (−1.524, 2.684) for 0.5 h, 1 h and 1.5 h post-intervention accordingly; while in patients with glaucoma or ocular hypertension, IOP increased at every measurement point, with WMD and 95%CIs being of 0.347 (0.078, 0.616), 2.395 (1.741, 3.049) and 1.998 (1.522, 2.474) respectively. The forms of ingestion varied between beverage and non-beverage (capsules or tablets) in healthy subjects; however, only data of 1 h post-intervention were sufficient enough to do a subgroup analysis based on the intervention forms. It was found that IOP at 1 h post-intervention did not change in healthy subjects whatever the delivery form was. WMD with 95% CIs were 1.243 (−0.621, 1.308) for beverage subgroup and 0.148 (−1.196, 0.492) for non-beverage subgroup.
Sensitivity analysis
No study was found to bias the meta-analysis results significantly, except that when the OHT patients’ data set of Rahamim Avisar’s study [3] was removed, IOP of glaucoma patients at 0.5 h post-intervention became insignificant (WMD 0.146,95%CI [−0.186–0.479]). The results were not affected significantly by the model used (Fig. 1).
Discussion
In the present meta-analysis, we quantitatively summarized the short-term effect of caffeine on IOP in normal individuals and patients with glaucoma or OHT. When all the subjects were analyzed together as an overall group, it showed that IOP rose significantly and persisted for a 1.5 h period after caffeine ingestion. However, the pre-specified subgroup analysis revealed that after ingestion of caffeine, IOP remained unchanged for normal individuals, but increased about 2 mmHg for patients with glaucoma or OHT. This result was not significantly biased by the delivery forms of caffeine, any individual study included, or the approach used to combine the data. It is reasonable to believe that the effects of caffeine on the overall group were biased by that on patients with glaucoma and OHT, and to draw a conclusion on the effects of caffeine on IOP without differentiating normal individuals from patients with glaucoma or OHT is inappropriate.
Caffeine has various pharmacological effects [2], and may elevate IOP by either promoting aqueous production[25] or inhibiting its drainage. In experimental animal models, caffeine acts as an adenosine receptor antagonist, which can increase intracellular cyclic AMP [2], resulting in stimulating the production of aqueous by the ciliary body and reducing the tone in smooth muscle cells of the chamber angle, leading to narrowing of the fenestrae that drainage the aqueous, and increasing resistance to the outflow of aqueous humor [4]. Ultra-structural changes in the nonpigmented ciliary epithelium which may increase aqueous transport were also observed in rats [38]. In addition, Increase in blood pressure [3, 4] due to ingestion of caffeine may also contribute to the increase in IOP.
It is interesting that different groups of subjects have different responses to caffeine, as shown in this meta-analysis. Our results corroborate the possibility that caffeine may have adverse effects for those with inherent susceptibility to glaucoma, which was raised by Kang et al. [39]. In their investigation, Kang et al. [39] found that for those with a self-reported family history of glaucoma, the risk of POAG with elevated IOP was associated with caffeine ingestion, while for those without family history, this relationship did not exist. In another cohort study [40], caffeine consumption was found to be associated with higher mean IOP in participants with open-angle glaucoma (OAG) than those without OAG. Possible explanations for the different responses to caffeine among different subjects may be that normal individuals with healthy drainage systems have much more ability to conduct aqueous than patients with glaucoma or OHT do. Another possibility is that patients with glaucoma or OHT are genetically sensitive to caffeine, in whom caffeine’s effects on IOP is magnified. It had been found that in homozygotes of CYP1A2*1F alleles, who were “slow” caffeine metabolizers, caffeine intake was associated with nonfatal myocardial infarction [41]. Glaucoma patients may also be “slow” caffeine metabolizers or genetically associated with CYP1A2*1F alleles, and thus are vulnerable to caffeine’s effect of IOP elevation. Further studies are warranted to explore the biological basis of this phenomenon.
Although the IOP increase caused by caffeine in glaucoma patients was transient and modest, this has at least three aspects of implications for the management of glaucoma patients. First, it is advisable that glaucoma patients avoid caffeine before IOP measurement during follow-ups, so that the IOP can be evaluated more accurately. Second, epidemiologic studies have confirmed that even 1 mmHg IOP variation is significant for glaucoma patients, because the risk of visual field exacerbation will be reduced [11] and the outcome will be improved 10% with the decrease of 1 mmHg IOP [12]. Thus the increasing of IOP caused by caffeine in glaucoma patients may lead to deteriorating of visual field. Third, exposure to caffeine in patients’ daily life may cause repeated or prolonged IOP elevation, which could in turn increase the risk of development of glaucomatous neuropathy [39]. Although there is no direct evidence to show that frequent caffeine ingestion would result in exacerbating of the visual field in glaucoma patients, more careful follow-ups may be necessary for glaucoma patients who frequently ingest caffeine.
However, the results of this meta-analysis should be interpreted with caution, for limitations exist in both the original studies included and the meta-analysis itself. First of all, some of the included studies did not adequately report details of randomization and blinding [3, 6–8]. Because these studies were conducted a long time ago, details of the trials could not be verified, despite the fact that we had managed to contact the authors. Had these procedures not actually been carried out, results of this meta-analysis would probably be biased. Second, factors that may interfere with IOP measurement varied across the studies included. Two [5, 7] of the six studies did not report the follow-up time point. If measuring time points had varied among follow-ups, the IOP measured might be confounded by its diurnal variation [42, 43]. Three types of tonometries with different reliability were used in the included studies. Goldmann applanation tonometry (GAT) has an intra-observer reliability of 1.7 mmHg and an inter-observer variability of 0.4 mmHg [44]. Its accuracy can be affected by several factors such as corneal biomechanics and axial length [45–48]. Whether these factors had been balanced between the cohorts were not mentioned in the parallel-designed RCTs in this meta-analysis. The Perkins applanation tonometry provides the same accuracy as GAT, while noncontact tonometry (NCT) will be less accurate [49]. These measurement errors might interfere with the validity of the results and contribute to the variety of the effects measured by different studies, although the analysis result was not biased by omitting the data measured by NCT. More large RCTs with balanced baseline characters that might interfere with IOP measurement between the experimental and control groups would be expected to provide more accurate results. Third, the conclusion regarding the caffeine effect on IOP was limited, as only one trial [3] was conducted on OHT patients, and no trials were conducted on angle-closure glaucoma patients. Effects of caffeine on IOP of these patients are to be investigated by more high quality RCTs. As the OAG prevalence increases with age [50], a large proportion of OAG patients would be much older than the patients included in this meta-analysis, whose average age was about 25 years. Effects of caffeine on IOP of elder OAG patients might be similar to that of the young patients, but more investigations on patients with a wider age range are still warranted. Fourth, due to lack of enough studies and data, we failed to do a dose–response analysis, which generally requires more than 10 studies to be included in a meta-analysis [23]. As there is a large variation in the amount of ingested caffeine in the studies included (30–300 mg), the effect of different doses of caffeine intake on IOP should be taken into consideration, and this is to be further investigated by more uniformly designed RCTs. Also, because the included studies measured IOPs at varied time points after caffeine ingestion, we could only choose the most commonly used time points to do the data synthesis.
In conclusion, available evidence suggests that caffeine has different effects on IOP in different groups of subjects. It can elevate IOP in patients with OAG or OHT in a short period after ingestion, but not in normal individuals. The biological basis for this phenomenon is to be explored. This result is to be further consolidated by RCTs uniformly designed with large enough sample size, consistent follow-up time point, the same graded dosage of caffeine and using the same reliable tonometry. If frequent exposure to caffeine may cause prolonged IOP elevation in patients with OAG or OHT, whether this elevation of IOP will lead to deteriorating of visual field damage needs to be elucidated by further studies. In addition, evidence is to be accumulated to draw a definite conclusion on the effects of caffeine on patients with ACG and OHT. Other studies that are needed may include investigations of dose–effect association and the long-term effect of caffeine on IOP.
References
Wikipedia c (2009) Caffeine. Wikipedia, The Free Encyclopedia. available from http://en.wikipedia.org/w/index.php?title=Caffeine&oldid=320136971 Accessed July 31,2009
Benowitz NL (1990) Clinical pharmacology of caffeine. Annu Rev Med 41:277–288
Avisar R, Avisar E, Weinberger D (2002) Effect of coffee consumption on intraocular pressure. Ann Pharmacother 36:992–995
Ajayi OB, Ukwade MT (2001) Caffeine and intraocular pressure in a Nigerian population. J Glaucoma 10:25–31
Higginbotham EJ, Kilimanjaro HA, Wilensky JT, Batenhorst RL, Hermann D (1989) The effect of caffeine on intraocular pressure in glaucoma patients. Ophthalmology 96:624–626
Okimi PH, Sportsman S, Pickard MR, Fritsche MB (1991) Effects of caffeinated coffee on intraocular pressure. Appl Nurs Res 4:72–76
Ozkan B, Yuksel N, Anik Y, Altintas O, Demirci A, Caglar Y (2008) The effect of caffeine on retrobulbar hemodynamics. Curr Eye Res 33:804–809
Okuno T, Sugiyama T, Tominaga M, Kojima S, Ikeda T (2002) Effects of caffeine on microcirculation of the human ocular fundus. Jpn J Ophthalmol 46:170–176
Nemesure B, Honkanen R, Hennis A, Wu SY, Leske MC (2007) Incident open-angle glaucoma and intraocular pressure. Ophthalmology 114:1810–1815
Leske MC, Connell AM, Wu SY, Nemesure B, Li X, Schachat A, Hennis A (2001) Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch Ophthalmol 119:89–95
Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E (2003) Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol 121:48–56
Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Kass MA (2002) The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 120:714–720, discussion 829-730
Higginbotham EJ, Gordon MO, Beiser JA, Drake MV, Bennett GR, Wilson MR, Kass MA (2004) The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol 122:813–820
AGIS Investigators (2000) The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 130:429–440
Spaeth GL (2002) Re: Ajayi OB, Ukwade NT. Caffeine and intraocular pressure in Nigerian population. (J Glaucoma 2001;10:25-31). J Glaucoma 11:76
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267
Lefebvre C, Manheimer M, Glanville J (2009) Chapter 6:Searching for studies. In: Higgins J, Green S (eds) Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration. Available from www.cochranehandbook.org. Accessed July 31,2009
Higgins JPT, Altman DG (2008) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG (eds) Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration.Available from www.cochrane-handbook.org. Accessed July 31,2009
Higgins JPT, Deeks JJ, Altman DG (2009) Chapter 16: Special topics in statistics. In: Higgins J, Green S (eds) Cochrane handbook for systematic reviews of interventions. The Ccochrane Collaboration.Available from www.cochranehandbook.org. Accessed July 31,2009
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Deeks JJ, Higgins JPT, Altman DG (2008.) Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions version 501 (updated September 2008). The Cochrane Collaboration. Available from www.cochrane-handbook.org. Accessed July 31,2009
Sterne JAC, Bradburn MJ, Egger M (2001) 18 Meta-analysis in StataTM. In: Egger M, Smith GD, Altman DG (eds) Systematic reviews in health care:meta-analysis in context. BMJ Publishing Group, London, pp 347–369
Kurata K, Fujimoto H, Tsukuda R, Suzuki T, Ando T, Tokuriki M (1998) Aqueous humor dynamics in beagle dogs with caffeine-induced ocular hypertension. J Vet Med Sci 60:737–739
Genee E (1973) Effect of vasoactive drugs on intra-ocular pressure. Klin Monbl Augenheilkd 162:637–642
Opremcak EM, Weber PA (1985) Interaction of timolol and caffeine on intraocular pressure. J Ocul Pharmacol 1:227–234
Strempel I (1979) Timolol: long-term results, tolerance test and early-morning measurements (author's transl). Klin Monbl Augenheilkd 175:619–626
Graeber W (1968) On the effect of caffeine on intraocular pressure in surgically or conservatively managed simple chronic glaucoma. Klin Monbl Augenheilkd 152:357–365
Alekseev BN, Ziangirova GG, Pisetskaia SF, Malakhova LA (1983) [M. M. Krasnov's caffeine therapy in treating ciliochoroidal detachment (an experimental-clinical study)]. Vestn Oftalmol: 11–14
Lotfi K, Grunwald JE (1991) The effect of caffeine on the human macular circulation. Investig Ophthalmol Vis Sci 32:3028–3032
Hinzpeter B, Diestelhorst M (1992) [1, 3, 7-trimethylxanthine. Effects on circadian aqueous humor dynamics in probands]. Ophthalmologe 89:465–467
Mozaffarieh M, Flammer J (2007) Is there more to glaucoma treatment than lowering IOP? Surv Ophthalmol 52(Suppl 2):S174–S179
Mozaffarieh M, Grieshaber MC, Orgul S, Flammer J (2008) The potential value of natural antioxidative treatment in glaucoma. Surv Ophthalmol 53:479–505
Mozaffarieh M, Flammer J (2007) A novel perspective on natural therapeutic approaches in glaucoma therapy. Expert Opin Emerg Drugs 12:195–198
Johnson S (2001) The multifaceted and widespread pathology of magnesium deficiency. Med Hypotheses 56:163–170
Adams BA, Brubaker RF (1990) Caffeine has no clinically significant effect on aqueous humor flow in the normal human eye. Ophthalmology 97:1030–1031
Kurata K, Maeda M, Nishida E, Tsukuda R, Suzuki T, Ando T, Tokuriki M (1997) Relationship between caffeine-induced ocular hypertension and ultrastructure changes of non-pigmented ciliary epithelial cells in rats. J Toxicol Sci 22:447–454
Kang JH, Willett WC, Rosner BA, Hankinson SE, Pasquale LR (2008) Caffeine consumption and the risk of primary open-angle glaucoma: a prospective cohort study. Invest Ophthalmol Vis Sci 49:1924–1931
Chandrasekaran S, Rochtchina E, Mitchell P (2005) Effects of caffeine on intraocular pressure: the Blue Mountains Eye Study. J Glaucoma 14:504–507
Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H (2006) Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 295:1135–1141
Drance SM (1963) Diurnal variation of intraocular pressure in treated glaucoma. Significance in patients with chronic simple glaucoma. Arch Ophthalmol 70:302–311
De Venecia G, Davis MD (1963) Diurnal variation of intraocular pressure in the normal eye. Arch Ophthalmol 69:752–757
Kotecha A, White ET, Shewry JM, Garway-Heath DF (2005) The relative effects of corneal thickness and age on Goldmann applanation tonometry and dynamic contour tonometry. Br J Ophthalmol 89:1572–1575
Orssengo GJ, Pye DC (1999) Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bull Math Biol 61:551–572
Tonnu PA, Ho T, Newson T, El Sheikh A, Sharma K, White E, Bunce C, Garway-Heath D (2005) The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br J Ophthalmol 89:851–854
Stodtmeister R (2002) Central corneal thickness on GAT (Goldman applanation tonometry accuracy). J Glaucoma 11:543
Lleo A, Marcos A, Calatayud M, Alonso L, Rahhal SM, Sanchis-Gimeno JA (2003) The relationship between central corneal thickness and Goldmann applanation tonometry. Clin Exp Optom 86:104–108
Recep OF, Hasiripi H, Cagil N, Sarikatipoglu H (2001) Relation between corneal thickness and intraocular pressure measurement by noncontact and applanation tonometry. J Cataract Refract Surg 27:1787–1791
Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D (2006) Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Investig Ophthalmol Vis Sci 47:4254–4261
Author information
Authors and Affiliations
Corresponding author
Additional information
No authors have any financial/conflicting interests to disclose. There is no funding or financial support.The authors have full control of all primary data, and we agree to allow Graefe's Archive for Clinical and Experimental Ophthalmology to review our data upon request.
Rights and permissions
About this article
Cite this article
Li, M., Wang, M., Guo, W. et al. The effect of caffeine on intraocular pressure: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol 249, 435–442 (2011). https://doi.org/10.1007/s00417-010-1455-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1455-1