Abstract
Rationale
Tobacco has a higher rate of dependence than other drugs of abuse. However, the psychopharmacological effects of nicotine are incongruent with the tenacity of tobacco addiction since nicotine does not produce robust euphoria in humans or self-administration in rodents. A potential explanation is that nicotine amplifies the salience of other stimuli that have some incentive value, which could influence the initiation and persistence of smoking. However, the neural mechanisms of this process are unknown.
Objectives
One way that nicotine may amplify the salience of other stimuli is by enhancing reward prediction errors. We hypothesized that nicotine would enhance the neural response to unexpected (relative to expected) rewards compared to placebo.
Methods
Twenty-three nonsmokers underwent two fMRI scans, following nicotine (1 mg) or placebo administration, while performing an outcome expectation task. In the task, a pair of cues was associated with either a subsequent reward (the image of a $100 bill) or a nonreward (the image of a blurry rectangle). On 20% of trials, the cue was followed by an unexpected outcome.
Results
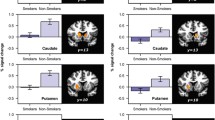
Although nicotine did not affect the magnitude of prediction errors relative to placebo, nicotine did increase BOLD activation in the anterior insula/inferior frontal gyrus and decrease activation in the caudate across all outcome types (including both rewards and nonrewards).
Conclusions
The insula and caudate could play a role in the initial effects of nicotine in nonsmokers, and these changes in baseline may be the mechanism that underlies how nicotine amplifies the salience of nondrug stimuli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco use is the leading cause of preventable disease and death in America (CDC 2011). Tobacco has a higher rate of dependence among users than other drugs of abuse, including alcohol, cocaine, and heroin (Anthony et al. 1994; Kandel et al. 1997), with estimates indicating 67.5% of individuls who have ever used tobacco will go on to develop dependence (Lopez-Quintero et al. 2011). The primary addictive component of tobacco is nicotine. Among nonsmokers, nicotine enhances cognitive performance (Rezvani and Levin 2001), which could contribute to its use and addiction liability. However, unlike other drugs of abuse, nicotine does not produce robust euphoric sensations (Dar et al. 2007) or high rates of responding during self-administration among rodents (Rose and Corrigall 1997); thus, the psychopharmacology of nicotine appears to be incongruent with the tenacity of tobacco addiction. A potential solution to this paradox is that nicotine amplifies the salience of other stimuli that have some incentive value (Bevins and Palmatier 2004; Caggiula et al. 2009; Caggiula et al. 2001). The incentive amplification hypothesis suggests that that nicotine use during an otherwise rewarding situation could influence both the initiation and persistence of smoking and may explain why both never-daily and former-daily intermittent smokers report smoking most frequently while socializing with friends and attending parties (Nguyen and Zhu 2009). The incentive amplification hypothesis addresses a potentially critical, yet often overlooked, component of nicotine’s addiction liability. While numerous studies have investigated the primary reinforcing effects of nicotine in nonsmokers, as well as the avoidance of aversive withdrawal symptoms among smokers as motivators for nicotine use (Watkins et al. 2000), only a few studies have tested the incentive amplification hypothesis in humans (Barr et al. 2008; Perkins et al. 2009; Wignall and de Wit 2011), and the neural mechanisms have not been investigated.
One way that nicotine may amplify the salience of other stimuli is by enhancing reward prediction errors. We develop expectations about reward outcomes based on past experiences, but sometimes outcomes are better or worse than expected; the difference between the actual and the expected reward is termed prediction error (PE). Perfectly predicted outcomes result in no PE, while larger errors may orient the subject’s attention to the discrepancy and trigger additional learning about stimulus-response outcomes (Schultz and Dickinson 2000). Phasic dopamine (DA) signaling in the midbrain has been shown to correlate with changes in PE (Schultz et al. 1997), and neuroimaging studies in humans have reported that activity in the mesocorticolimbic DA pathway, including the nucleus accumbens (NAcc), caudate, putamen, and prefrontal cortex (PFC), correlates with PE (Abler et al. 2006; McClure et al. 2003; O'Doherty et al. 2003; Ramnani et al. 2004). This natural learning process is vulnerable to drugs of abuse that stimulate DA signaling, which could enhance reward-related PE resulting in the overvaluation of drug- and drug-related cues, thus leading to compulsive drug use and addiction (Redish 2004; Schultz 2011). Nicotine indirectly increases DA concentrations in the NAcc and caudate (Di Chiara and Imperato 1988) and high doses of nicotine, via receptor desensitization, filter out low tonic DA activity and enhance burst firing of DA neurons in response to salient stimuli (Rice and Cragg 2004; Zhang and Sulzer 2004). By enhancing this DA signal, nicotine may exaggerate neural prediction errors and thereby amplify the salience of reward-contingent cues and facilitate associative stimulus-outcome learning (Di Chiara 2000).
Chronic tobacco use results in neuroadaptations that underlie nicotine tolerance, dependence, and withdrawal, and smoking behavior among nicotine-dependent smokers may be largely maintained by the avoidance of withdrawal symptoms (Baker et al. 2004). Thus, the effects of nicotine in chronic smokers are not necessarily representative of the acute effects experienced by nonsmokers. In addition to avoiding tolerance and withdrawal confounds, it is important to study the effects of nicotine in nonsmokers to understand the mechanisms that underlie the acquisition of smoking behavior.
The overall goal of this study was to develop a better understanding of the acute effects of nicotine on prediction error signaling. Participants completed an outcome expectation task (OET), designed to elicit neural prediction errors, while undergoing fMRI. On each trial of the OET, one of two cues was presented followed by a reward or nonreward outcome. Participants responded to each cue via button press indicating whether they expected a reward or nonreward on that trial. Studies have shown that response times are faster on trials when there is an expectation of a reward than on trials when there is none (Murray et al. 2008; Pessiglione et al. 2006), reflecting an increased motivation to obtain rewards. Prior to each scan, participants self-administered a nicotine or placebo nasal spray. We hypothesized that compared to placebo, nicotine would reduce response time on trials with expected reward. Secondly, we hypothesized that nicotine would enhance the magnitude of positive PE-related blood oxygenation-level dependent (BOLD) signal and/or reduce the magnitude of negative PE-related BOLD signal in striatal and prefrontal cortical regions. We based this hypothesis on previous studies, in which L-DOPA, a DA precursor, enhanced PE-related BOLD activation in the striatum (Chowdhury et al. 2013; Pessiglione et al. 2006). To our knowledge, this is the first study to investigate the acute effects of nicotine in nonsmokers on PE-related BOLD activation. This research is vital to understanding how nicotine affects outcome expectation for nondrug rewards, which may contribute to its addiction liability.
Methods
Participants
Nonsmokers were recruited from the local community. Participants completed a phone interview and laboratory-based screening session to determine eligibility. To be eligible, participants had to be right-handed, aged 18–55 years, have not smoked more than 50 cigarettes or equivalent nicotine products in their lifetime, have not used any nicotine or tobacco in at least 6 months, and have an expired carbon monoxide concentration of ≤5 ppm (Vitalograph Inc., Lenexa, KS) and a urinary cotinine concentration <100 ng/ml (NicAlert, Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ). Participants were excluded if they reported significant health problems, including neurological and psychiatric disorders, or used psychoactive medication. Participants were also excluded if they tested positive for drugs (iCup, Alere Toxicology Services Portsmouth, VA), alcohol (Alco-Sensor III, Intoximeters Inc. St. Louis, MO), or pregnancy (QuickVue+, Quidel Corporation, San Diego, CA) during the screening visit, or if they had any conditions that would make MRI unsafe. During the screening session, participants were placed in a mock MRI scanner to become familiarized with the scanner environment. Smokers were also recruited as part of the parent study and those results will be presented elsewhere.
Outcome expectation task
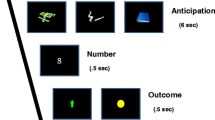
The outcome expectation task (OET) was a Pavlovian cue-outcome association task modeled after Ramnani et al. (2004). Each trial of the OET consisted of two phases: a cue phase and an outcome phase. The cues (cue A and cue B) were represented by quilt squares, and the outcomes (reward and nonreward) were represented by the image of a $100 bill and a blurry rectangle, respectively (see Fig. 1). In each trial, a single cue was presented for 2 s. Within this 2 s, the participant was asked to guess whether this cue predicted a reward or a nonreward by pressing response buttons corresponding to “$” and “0” shown on screen. The location of the “$” and “0” on screen were counterbalanced across participants. After making a response, a box outlined the selection and then the outcome was shown for 1 s. Participants were asked to make a response to ensure they were paying attention to the task and were correctly predicting outcomes based on the cue. Participant responses did not affect the outcome. If no response was made, “Missed Response” was shown during the outcome and that trial was excluded from analysis. In between the cue and outcome was a jittered delay from 0.8 to 1.6 s. Intertrial intervals ranged from 1.5 to 6 s with a positively skewed distribution.
Outcome expectation task (OET). The OET consisted of a cue phase and an outcome phase. During the cue phase, participants selected “0” or “$” to indicate whether a reward (image of a $100 bill) or nonreward (blurry rectangle) was predicted. a Example of expected reward trial. b Example of an expected nonreward trial. During the scan, 80% of outcomes were expected (i.e., expected reward or expected nonreward) and 20% of outcomes were unexpected (i.e., unexpected reward or unexpected nonreward)
During the screening session, participants completed an 80-trial training version of the OET on a computer. In this version, one cue was always rewarded and the other cue was not (cue A predicted reward for half of the participants, cue B predicted reward for the other half). Participants achieved at least 90% accuracy on this training version before continuing with the study.
Participants completed two separate runs of the OET at each MRI scan. Each run consisted of 100 trials: 50 cue A and 50 cue B. In 80% of the trials, the cue-outcome relationship was the same as in the training version. However, in 20% of the trials, the cue-outcome relationship was inconsistent with the training version. This resulted in four outcome conditions: expected reward, expected nonreward, unexpected reward, and unexpected nonreward. The first 11 trials in the scanner were consistent with the training version. During the scanner version of the OET, participants were told that every time they saw a $100 bill, they would get points that went towards a $10 bonus each scan day. However, points would be deducted for every response they missed. At the end of the study, all participants were awarded $20 bonus.
Drug administration
Drug nasal sprays were provided by a local compounding pharmacy. The nicotine spray contained Nicotrol (Pfizer, NY, NY), and the placebo spray contained saline and a weak concentration of capsaicin oil. The nasal spray pumps and bottles (MedWestVaco, Richmond, VA) delivered 0.5 mg/spray. Participants self-administered two puffs from the spray pump, one in each nostril, delivering 1 mg of nicotine or placebo. Previous studies have reported cognitive effects at this dose without subjective effects (Marchant et al. 2010; Rusted et al. 2009). Nicotine nasal spray is rapidly absorbed and peak plasma nicotine levels occur in 10 min (Gourlay and Benowitz 1997). Nicotine and placebo spray bottles were identical and the order of administration on the scan days was double-blinded. However, due to concerns regarding tolerability, participants self-administered a dose of nicotine nasal spray at the end of the screening session.
Procedure
Participants were scheduled for two scanning sessions, between 2 and 14 days apart. The order of drug administration was counterbalanced across study days 1 and 2. Upon arrival to the scan, participants completed the Positive and Negative Affect Scale (Watson et al. 1988) and submitted a breath sample to test for alcohol. The drug spray was administered approximately 10 min before the scan began, and the OET began 30 min into the scan session. Each scan lasted 1 h. Following the MRI scan, participants had their blood pressure and heart rate measured. They also completed a side effects questionnaire, which asked about the severity of potential side effects of the nasal spray on a scale from 1 (“Not at all”) to 7 (“Extremely”). The symptoms included: nausea, vomiting, dizziness upon standing, headache, tremor or shakiness, diarrhea, sweating, heartburn, feeling faint, coughing, irritability, itching or burning in the nose, and itching or burning in the throat. At the end of each session, participants reported on whether they thought nicotine or placebo was administered that day, in order to test the drug blind.
Image acquisition
Images were acquired on a 3T General Electric MR750 scanner (Milwaukee, WI) equipped with 50 mT/m gradients. A high-resolution anatomical image was collected using a three-dimensional fast spoiled gradient recalled echo (3D-SPGR) sequence (TR = 8.156 ms, TE = 3.18 ms, field of view = 25.6 cm2, matrix = 256 × 256, flip angle = 12°, 166 slices, and slice thickness = 1 mm).
Blood oxygen level-dependent signal was measured using a gradient-recalled inward spiral pulse imaging sequence (SENSE spiral) (TR = 1500 ms, TI = 0, TE = 30 ms, flip angle = 60°, acquisition matrix = 64 × 128, field of view = 25.6 cm2, number of slices = 30, and slice thickness = 3.8 mm, resulting in 4 × 4 × 3.8 mm voxels, 430 volumes for a duration of 10 min, 51 s, per run). The first four image volumes were removed to allow for stabilization of the MR signal. An infrared camera attached to the head coil was used to monitor alertness.
Image preprocessing
Functional images were preprocessed and analyzed using the Functional MRI of the Brain (FMRIB) Software Library (FSL Version 6.00 FMRIB, Oxford, UK). Functional images were skull stripped, temporally realigned, motion corrected, smoothed with a 5 mm smoothing kernel, and high pass filtered (cutoff = 100 s). Functional images were registered to the high-resolution anatomical images then normalized to the Montreal Neurological Institute 152 template. A first-level general linear model analysis was conducted for each participant using two explanatory variables (EVs) for the decision phase—(1) rewarded cue and (2) nonrewarded cue—and four EVs for the outcome phase—(1) expected reward, (2) expected nonreward, (3) unexpected reward, and (4) unexpected nonreward. EVs were convolved with double-gamma hemodynamic response functions with the temporal derivative added. Contrasts were defined for each outcome EV. Cue phase EVs were included to control for overall BOLD variance but are not the subject of the present hypotheses and are not discussed. Motion outliers were identified and included as confound EVs in the first-level analysis. A second-level fixed-effect analysis combined the two runs of the OET for each session.
Data analysis
Response time and accuracy in the OET was analyzed using separate 2 (drug) × 2 (cue type) analysis of variance (ANOVA) and follow-up paired comparisons. Self-reported mood, side-effect ratings, blood pressure, and heart rate were analyzed with paired-samples t tests. These behavioral measures were analyzed in SPSS (version 21.0. IBM; Chicago, IL).
Imaging data underwent an initial paired-samples t test to compare session effects (i.e., session 1 versus session 2). No session effects were found, and subsequent analyses excluded session order as a covariate. Then, a 2 (drug: nicotine or placebo) × 2 (reward: reward or nonreward) × 2 (expectation: expected or unexpected) ANOVA was conducted to measure the drug and drug interaction effects during the outcome phase. We hypothesized there would be a steeper slope between expected and unexpected outcomes following nicotine compared to placebo, i.e., a drug × expectation interaction effect.
Results of the whole-brain imaging analyses were considered significant if they passed a statistical threshold of z >3.1, with clusterwise p values <.05 using Gaussian random field theory (Worsley 2001). Figures were made using MRIcron (www.mccauslandcenter.sc.edu/mricro/).
Results
Participants
Twenty-six participants were scanned; however, three participants were removed due to poor performance in the OET (<60% correct responses). The final sample consisted of 23 participants (12 women). They were (M ± SD) 33 ± 12 years of age and had 16 ± 2 years of education. The sample consisted of nine Caucasians, nine African Americans, two Hispanics, one Asian, and two of mixed race.
Mood and side effects ratings
Side effects ratings were low overall, with an average rating of 1.3 ± 0.8. Nicotine nasal spray produced slightly higher ratings for itching or burning in the nose (3.0 vs 2.2) or throat (1.9 vs 1.2) compared to placebo, respectively (ps < 0.05). There were no other differences in side effect ratings between drug conditions. Furthermore, there were no differences in post-drug blood pressure, heart rate, or pre-drug mood ratings between drug conditions.
Drug blind
Participants were able to correctly guess which spray they had received slightly better than by chance. For both the placebo and nicotine sprays, 61% of participants guessed the correct drug.
Response time and accuracy
The analysis of response time revealed a drug × cue type interaction effect (F 1,22 = 10.3, p < 0.005). The interaction effect was due to slower response times for nonreward cues (mean = 0.686 s) than reward cues (0.668 s) during the placebo condition, and nonsignificantly faster response times for nonreward cues (0.667 s) than reward cues (0.672 s) during the nicotine condition. There were no main effects of drug or cue type on response time. Accuracy was nonsignificantly higher for the reward cue (0.95) than for the nonreward cue (0.93), and there were no significant effects of drug or cue type on accuracy.
Outcome phase fMRI
The ANOVA revealed a main effect of drug: relative to placebo, nicotine increased activation in the bilateral occipital cortex, supramarginal gyrus, anterior insula/inferior frontal gyrus, and the right middle temporal gyrus, and nicotine decreased activation in the bilateral caudate nucleus (see Table 1 and Fig. 2). To illustrate the drug effects, percent BOLD activation was extracted across both drugs and all four outcome conditions from the right anterior insula cluster and the right caudate nucleus cluster (see Fig. 3). A main effect of reward was also observed: relative to nonrewards, rewards increased activation in the bilateral occipital cortex. The main effect of expectation (unexpected > expected) produced no significant clusters; however, there were sub-threshold clusters in the bilateral supramarginal and angular gyri, dorsomedial prefrontal cortex, right anterior insula/inferior frontal gyrus, and the right dorsolateral prefrontal cortex (z > 2.3, KE > 100 voxels). The reward × expectation interaction effect (representing positive and negative PE’s) produced no significant clusters.
Main effects of drug. Nicotine increased BOLD activation in the bilateral occipital cortex (OCC), supramarginal gyrus (SMG), anterior insula/inferior frontal gyrus (INS), and right middle temporal gyrus (MTG), and nicotine decreased BOLD activation in the bilateral caudate nucleus (CN). Images cluster corrected to p < 0.05. Color bar represents z-scores
Discussion
The goal of this study was to investigate whether nicotine would enhance reward motivation and prediction errors as a possible neural mechanism underlying nicotine’s addiction liability. First, we hypothesized that nicotine would reduce response times for reward cues, reflecting an increased motivation for rewards. Instead, we found that response times were similar after nicotine and placebo, although response times were slower for nonreward cues after placebo, but slightly faster after nicotine. This suggests that nicotine diminished the motivational delay between reward and nonreward conditions shown after placebo. Alternatively, response times may have been near optimal during placebo, preventing faster responses induced by nicotine. Previous studies have shown that response times are faster during rewarded trials, but nonsmokers’ response times are not always faster following nicotine administration (Barr et al. 2008; Dawkins et al. 2006). Second, we hypothesized that there would be a greater magnitude of positivePE-related BOLD activation in the striatum and prefrontal cortex after nicotine compared to placebo. Although we did not find evidence that nicotine affected the magnitude of prediction errors, we found that nicotine lessened BOLD deactivation in the right anterior insula/inferior frontal gyrus (AI/IFG) and increased deactivation in the right caudate nucleus across all four outcome conditions of the OET. Contextual stimuli that always accompany nicotine administration, like the smell of cigarettes, are known to be important for motivating and maintaining smoking behavior among humans, suggesting there is more to tobacco addiction than nicotine alone. Extensive research using rodent models of nicotine self-administration has supported the hypothesis that nicotine acts as both a primary reinforcer and as an enhancer of the incentive motivational and reinforcing effects of accompanying stimuli (reviewed in Caggiula et al. (2009)). These accompanying stimuli become cues for nicotine and their presence strengthens subsequent nicotine/tobacco use. One particular line of research has shown that, among rodents, the codelivery of a nondrug reinforcer, such as the offset of a chamber light, is as important as nicotine itself for the acquisition of nicotine self-administration; in fact, the association between the nondrug reinforcer and nicotine produces a synergistic enhancement of nicotine self-administration (reviewed in Caggiula et al. (2009)). Similar experiments have been conducted in humans in order to investigate the effects of nicotine on nondrug reinforcer responsivity among nonsmokers (Barr et al. 2008; Perkins et al. 2009; Wignall and de Wit 2011). However, only one of these studies found increased responding for a nondrug reinforcer (Barr et al. 2008). Unlike many human behavioral experiments where individuals participate on a single occasion, rodent behaviors are studied over hundreds of trials with many, repeated exposures to nicotine. Thus, the behavioral results of these experiments may not be translatable to humans. In the current study, we did not find effects of nicotine on behavior, although we did find effects of nicotine on the BOLD response. To our knowledge, this is the first study to investigate the incentive amplification hypothesis using neuroimaging and the results of this study provide a promising new lead in the investigation of nicotine acquisition in nonsmokers. Altogether, the locations of the shifts in baseline BOLD activation may provide clues as to how nicotine influences associative learning in nonsmokers.
The insula is thought to integrate primary interoceptive representations from the body with information about emotionally salient environmental stimuli (Craig 2009), and the dorsoanterior insula plays an important role in cognitive control (Chang et al. 2013). A model of anterior insula function postulates that the insula is sensitive to salient events, and that its core function is to mark such events for additional processing and initiate appropriate control signals (Menon and Uddin 2010). A recent study, for instance, observed greater relative activation in the anterior insula in response to personally relevant, as compared to generic, smoking cues (McClernon et al. 2016). The AI/IFG are commonly coactivated during target detection and response inhibition, and the right IFG is activated when salient cues are detected (Hampshire et al. 2010). A meta-analysis of this coactivation suggested that the right AI is important for detecting behaviorally salient events, and the right IFG is important in implementing inhibitory control (Cai et al. 2014). Indeed, any stimulus that is salient/infrequent/unexpected will recruit inhibition (Aron et al. 2014) and inhibition allows time for other executive processes to guide behavior towards a more appropriate action (Smith et al. 2014).
The insula has been strongly implicated in drug use and craving due to its role in conscious awareness, emotional experience, and decision making (Garavan 2010; Naqvi and Bechara 2009). Similarly, deficits in response inhibition have been linked to drug abuse and dependence (Smith et al. 2014; Froeliger et al. 2017). Although nicotine was administered to nonsmokers in this study, the effects on the AI/IFG may provide insights into the mechanisms that underlie initial nicotine use and acquisition. We speculate that these effects could improve salience detection and response inhibition, and facilitate the executive processing of reward-related outcomes. However, neither salience detection nor response inhibition was measured in this study. Previous studies have suggested that nicotine improves nonsmokers’ performance on some measures of response inhibition (Ettinger 2009; Barr et al. 2008; Wignall and de Wit 2011), but the behavioral correlates of nicotine’s effects on the AI/IFG are unknown. Future neuroimaging studies could test the relationship between nicotine, salience/inhibition, and AI/IFG activation using go/no-go or oddball tasks.
During the OET, nicotine induced more positive activation in the inferior parietal lobule and more negative activation in the caudate nucleus. The inferior parietal lobule, consisting of the angular and supramarginal gyrus, is an associative area that receives visual, motor, and somatosensory inputs (Caspers et al. 2013). The inferior parietal lobule adjacent to the superior parietal lobule is also part of the executive control network, which includes the bilateral dorsomedial and dorsolateral prefrontal cortex (Menon and Uddin 2010; Niendam et al. 2012). The effects on this region may reflect the stimulant, cognitive-enhancing effects of nicotine on attention and working memory (Levin et al. 2006; Heishman et al. 2010). The increased deactivation in the caudate nucleus following nicotine was unexpected, since cigarette smoking has been shown to release DA in the caudate and nucleus accumbens at rest (Brody et al. 2006). However, deactivation during an active state does not necessarily contradict those results. At rest, nicotine may increase tonic DA signaling, but in our task, it may have reduced phasic DA signaling. The greater deactivation could also reflect increased efficiency or decreased inhibition from the caudate to a target brain region. Lastly, nicotine increased activation in the bilateral occipital cortex, which may be related to increased attention to visual information or enhanced reward processing. Occipital cortex activation is commonly reported in neuroimaging studies of drug cue reactivity (Hanlon et al. 2014).
Other neuroimaging studies of the effects of nicotine in nonsmokers tend to show increased activation in regions associated with the executive control network, and decreased activation in regions associated with the default mode network (Newhouse et al. 2011). For example, one study showed that acute subcutaneous nicotine improved accuracy, reduced response time, and increased BOLD activation in the anterior cingulate, superior frontal cortex, superior parietal cortex, and caudate nucleus during a working memory task (Kumari et al. 2003). Another study reported that acute nasal nicotine decreased BOLD activation in the right inferior parietal lobule (near the temporoparietal junction) during a prospective memory task (Rusted et al. 2011). Here, the inferior parietal lobule was identified as being part of the default mode network, and by deactivating this region, nicotine may have helped reorient attention to salient visual cues (Rusted et al. 2011). Similarly, among nicotine-dependent smokers, nicotine administration reportedly induced or potentiated deactivation in regions associated with the default mode network during an attention task (Hahn et al. 2007), and a meta-analysis revealed that nicotinic acetylcholine receptor agonists decrease activation in the ventromedial prefrontal cortex and posterior cingulate cortex (part of the default mode network) and increase activation in lateral frontoparietal regions (part of the executive control network) among both smokers and nonsmokers (Sutherland et al. 2015). Altogether, the effects we found in the inferior parietal lobule align with these previous studies.
In the current study, we used nonsmokers to investigate the initial effects of nicotine and to help inform how nicotine use is acquired. However, due to the development of dependence, tolerance, and the extended associative learning taken place between smoking cues and nicotine administration, our results are unlikely to represent the effects of nicotine among daily smokers. Past research using reward processing tasks with smokers has shown that nicotine can modulate valence-related striatum activation (Rose et al. 2013; Fedota et al. 2015), although another study reported no effect of nicotine on prediction error signals in the striatum (Rose et al. 2012). Interestingly, smokers’ beliefs about the presence of nicotine in cigarettes can modulate reward prediction error signals in the striatum (Gu et al. 2015), which is counterintuitive given the role of nonnicotine cigarette factors in maintaining smoking behavior (e.g., Donny et al. 2007). More research is needed to understand how nicotine and associated smoking cues impact non-drug reward processing among smokers.
Our associative learning task was modeled after Ramnani et al. 2004, and it is also similar to the task used in Morris et al. (2012), although prediction errors were defined slightly differently across the three studies. Ramnani et al. (n = 6) reported negative PE in the anterior prefrontal cortex and positive PE in the inferior frontal sulcus; the authors also reported activation in the ventral striatum for expected rewards > expected nonrewards (Ramnani et al. 2004). Morris et al. (n = 16) reported a main effect of reward (reward > nonreward) in the insula, inferior frontal gyrus, cingulate, and midbrain but reported no main effect of expectation (unexpected > expected) (Morris et al. 2012). Morris et al. also found a reward × expectation interaction effect (unexpected reward > expected reward, expected nonreward > unexpected nonreward) in the ventral striatum. By comparison, we report a main effect of reward limited to the occipital cortex. According to the model of prediction errors described by Schultz et al. (1997), predictable outcomes offer no new information and therefore should have little or no impact on brain activation. Since the majority of our outcomes were expected, our results are consistent with this model. Additionally, we did not find the hypothesized interaction effect, although other studies have reported this pattern of BOLD in the striatum (Morris et al. 2012; Rose et al. 2012). However, a meta-analysis revealed a large extent of prediction error-related activation in the caudate nucleus, putamen, anterior insula, anterior cingulate, dorsomedial prefrontal cortex, middle frontal gyrus, and inferior parietal lobule (among other regions) (Garrison et al. 2013). These regions are consistent with our main effect trend for expectation.
The strengths of this study include the use of a placebo control and nicotine nasal spray, which has a similar rate of nicotine absorption as cigarettes. However, this study is not without limitations. Nicotine concentrations decrease rapidly following nasal spray administration, and we estimate that the nicotine concentrations during the OET (approximately 40–60 min post-administration) were about 3–4 ng/ml (Gourlay and Benowitz 1997). Although we found drug effects in the BOLD data, these concentrations may have been too low to affect response time. Second, participants guessed the nicotine/placebo conditions slightly better than chance (i.e., >50%), which may have impacted the results. In addition, cigarettes contain hundreds of chemicals, in addition to nicotine, that could also affect learning and memory. Since this study only administered nicotine, caution should be used in generalizing these findings to the initial effects of cigarette smoking. Furthermore, the type of outcome phase that we modeled (e.g., expected reward) was based on the cue that was presented, not the response made by each participant. We modeled the task this way because participants were explicitly told to make decisions based on the outcomes they learned during task training and errors during the fMRI scan were low (<10%); some of which may have been commission errors. However, we did not investigate how participants modified their response during the fMRI scan once they learned their predictions were not perfect. A more precise measure of prediction errors would account for participants’ trial-by-trial expectations of outcomes. Lastly, the hypothetical dopaminergic mechanisms of prediction errors cannot be directly studied with fMRI, since the BOLD signal does not differentiate between neurotransmitters.
In summary, nicotine has a higher rate of addiction than would be expected given its acute effects and a potential explanation is that nicotine amplifies the salience of other (nondrug) reinforcers, which would promote associative learning between nicotine use and other stimuli. This study investigated the neural mechanisms that may underlie nicotine’s addiction liability. Our results show that acute nicotine produces more positive activation in the anterior insula, inferior frontal gyrus, inferior parietal lobule, and visual cortex during cue-anticipated outcomes, regardless of whether the outcomes were rewarding or expected. We speculate that nicotine may amplify the salience of external events by enhancing interoceptive awareness subserved by the insula, inhibition subserved by the inferior frontal gyrus, and visual attention subserved by the occipital cortex. These mechanisms could promote continued nicotine use and contribute to its addiction liability. This initial study can guide future investigations of tobacco acquisition; in particular, future research should assess whether nicotine’s effects on neural reward processing in nonsmokers relate to individual differences that increase the risk of developing tobacco addiction.
References
Abler B, Walter H, Erk S, Kammerer H, Spitzer M (2006) Prediction error as a linear function of reward probability is coded in human nucleus accumbens. NeuroImage 31:790–795
Anthony JC, Warner LA, Kessler RC (1994) Comparative epidemiology of dependence on tobacco, alchohol, controlled substances, and inhalants: basic findings from the national comorbidity survey. Exp Clin Psychopharm 2:244–268
Aron AR, Robbins TW, Poldrack RA (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18:177–185
Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004) Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 111:33–51
Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE (2008) A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiat 63:1061–1065
Bevins RA, Palmatier MI (2004) Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev 3:143–158
Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG (2006) Cigarette smoking saturates brain alpha(4)beta(2) nicotinic acetylcholine receptors. Arch Gen Psychiat 63:907–915
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Be 70:515–530
Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009) The role of nicotine in smoking: a dual-reinforcement model. Neb Symp Motiv 55:91–109
Cai WD, Ryali S, Chen TW, Li CSR, Menon V (2014) Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci 34:14652–14667
Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, Zilles K (2013) Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex 23:615–628
CDC (2011) Tobacco use: targeting the nation’s leading killer at a glance. Center for Disease Control and Prevention, Atlanta
Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2013) Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 23:739–749
Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Duzel E, Dolan RJ (2013) Dopamine restores reward prediction errors in old age. Nat Neurosci 16:648–653
Craig AD (2009) How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70
Dar R, Kaplan R, Shaham L, Frenk H (2007) Euphoriant effects of nicotine in smokers: fact or artifact? Psychopharmacology 191:203–210
Dawkins L, Powell JH, West R, Powell J, Pickering A (2006) A double-blind placebo controlled experimental study of nicotine: I - effects on incentive motivation. Psychopharmacology 189:355–367
Di Chiara G (2000) Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 393:295–314
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278
Donny EC, Houtsmuller E, Stitzer ML (2007) Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction 102:324–334
Ettinger U, Williams SCR, Patel D et al (2009) Effects of acute nicotine on brain function in healthy smokers and non-smokers: estimation of inter-individual response heterogeneity. Neuroimage 45:549–561
Fedota JR, Sutherland MT, Salmeron BJ, Ross TJ, Hong LE, Stein EA (2015) Reward anticipation is differentially modulated by varenicline and nicotine in smokers. Neuropsychopharmacol 40:2038–2046
Froeliger, B., McConnell, P.A., Bell, S., Sweitzer, M., Kozink, R.V., Eichberg, C., Hallyburton, M., Kaiser, N., Gray, K. M., McClernon, F. J. (2017). A corticothalamic pathway mediates inhibitory control and smoking relapse vulnerability. JAMA Psychiat (in press)
Garavan H (2010) Insula and drug cravings. Brain Struct Funct 214:593–601
Garrison J, Erdeniz B, Done J (2013) Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 37:1297–1310
Gourlay SG, Benowitz NL (1997) Arteriovenus differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin Pharmacol Ther 62:453–463
Gu X, Lohrenz T, Salas R, Baldwin PR, Soltani A, Kirk U, Cinciripini PM, Montague PR (2015) Belief about nicotine selectively modulates value and reward prediction error signals in smokers. PNAS 112:2539–2544
Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA (2007) Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci 27:3477–3489
Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010) The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage 50:1313–1319
Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM (2014) Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depen 143:206–212
Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology 210:453–469
Kandel D, Chen K, Warner LA, Kessler RC, Grant B (1997) Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the US population. Drug Alcohol Depen 44:11–29
Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SCR, Simmons A, Sharma T (2003) Cognitive effects of nicotine in humans: an fMRI study. NeuroImage 19:1002–1013
Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology 184:523–539
Lopez-Quintero C, de los Cobos JP, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C (2011) Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depen 115:120–130
Marchant NL, King SL, Tabet N, Rusted JM (2010) Positive effects of cholinergic stimulation favor young APOE epsilon 4 carriers. Neuropsychopharmacol 35:1090–1096
McClernon FJ, Conklin CA, Kozink RV et al (2016) Hippocampal and insular response to smoking-related environments: neuroimaging evidence for drug-context effects in nicotine dependence. Neuropsychopharmacology 41:877–885
McClure SM, Berns GS, Montague PR (2003) Temporal prediction errors in a passive learning task activate human striatum. Neuron 38:339–346
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667
Morris RW, Vercammen A, Lenroot R, Moore L, Langton J, Short B, Kulkarni J, Curtis J, O'Donnell M, Weickert CS, Weickert TW (2012) Disambiguating ventral striatum fMRI-related bold signal during reward prediction in schizophrenia. Mol Psychiatr 17:280–289
Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC (2008) Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatr 13:267–276
Naqvi NH, Bechara A (2009) The hidden island of addiction: the insula. Trends Neurosci 32:56–67
Newhouse PA, Potter AS, Dumas JA, Thiel CM (2011) Functional brain imaging of nicotinic effects on higher cognitive processes. Biochem Pharmacol 82:943–951
Nguyen QB, Zhu SH (2009) Intermittent smokers who used to smoke daily: a preliminary study on smoking situations. Nicotine Tob Res 11:164–170
Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Ne 12:241–268
O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ (2003) Temporal difference models and reward-related learning in the human brain. Neuron 38:329–337
Perkins KA, Grottenthaler A, Wilson AS (2009) Lack of reinforcement enhancing effects of nicotine in non-dependent smokers. Psychopharmacology 205:635–645
Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006) Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442:1042–1045
Ramnani N, Elliott R, Athwal BS, Passinghm RE (2004) Prediction error for free monetary reward in the human prefrontal cortex. NeuroImage 23:777–786
Redish AD (2004) Addiction as a computational process gone awry. Science 306:1944–1947
Rezvani AH, Levin ED (2001) Cognitive effects of nicotine. Biol Psychiat 49:258–267
Rice ME, Cragg SJ (2004) Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci 7:583–584
Rose JE, Corrigall WA (1997) Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology 130:28–40
Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis M, Stein EA (2012) Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol Psychiat 71:206–213
Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis M, Stein EA (2013) Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biol Psychiatry 73:280–288
Rusted J, Sawyer R, Jones C, Trawley S, Marchant N (2009) Positive effects of nicotine on cognition: the deployment of attention for prospective memory. Psychopharmacology 202:93–102
Rusted J, Ruest T, Gray MA (2011) Acute effects of nicotine administration during prospective memory, an event related fMRI study. Neuropsychologia 49:2362–2368
Schultz W (2011) Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron 69:603–617
Schultz W, Dickinson A (2000) Neuronal coding of prediction errors. Annu Rev Neurosci 23:473–500
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599
Smith JL, Mattick RP, Jamadar SD, Iredale JM (2014) Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend 145:1–33
Sutherland MT, Ray KL, Riedel MC, Yanes JA, Stein EA, Laird AR (2015) Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol Psychiatry 78:711–720
Watkins SS, Koob GF, Markou A (2000) Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2:19–37
Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect—the Panas scales. J Pers Soc Psychol 54:1063–1070
Wignall ND, de Wit H (2011) Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Exp Clin Psychopharm 19:183–191
Worsley KJ (2001) Statistical analysis of activation images. Ch 14. In: Jezzard P, Matthews PM, Smith SM (eds) Functional MRI: an introduction to methods. University Press, Oxford
Zhang H, Sulzer D (2004) Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci 7:581–582
Acknowledgments
This work was supported by NIDA K01 DA033347 (MAA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Participants provided informed consent, and this protocol was approved by the Duke University’s Institutional Review Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Addicott, M.A., Oliver, J.A. & Joseph McClernon, F. Nicotine increases anterior insula activation to expected and unexpected outcomes among nonsmokers. Psychopharmacology 234, 1145–1154 (2017). https://doi.org/10.1007/s00213-017-4550-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4550-8