Abstract
This paper reviews the role of the insula in drug craving. Evidence is presented that drug craving may be a particular instance of the anterior insula’s broader role in interoception and subjective feeling states similar, for example, to thirst and hunger. An important role for the insula in craving is supported by evidence of insular activity changing with satiety and with the top–down cognitive modulation of cravings. Cognitive processes involving the insula’s role in awareness of one’s own behaviour may also contribute to craving insofar as the avoidance of craving might require subjective awareness of the endogenous and exogenous cues that initiate it. Finally, some consideration is given to sex differences and developmental processes in craving.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Insula and subjective experiences
To understand compulsive addictive behaviour, it may be essential to understand the very strong urges that compel addicts towards use, often against their own better judgement and their stated intentions to remain drug free. As is amply demonstrated elsewhere in this special issue, the insula has a key role in producing subjective, interoceptive experiences. Thus, it is a worthy neurobiological focus for research into the powerful urges and cravings of the drug addict. The anterior insula has been implicated in a range of emotional processes including representing visceral states, particularly those that give rise to emotional experiences, in detecting discrepancies between one’s expected and actual bodily arousal levels, in empathy, in processing decision-making-related uncertainty, and in conscious awareness of one’s bodily states with implications for one’s performance on cognitive tasks (Craig 2009; Hester et al. 2005; Singer et al. 2009; Paulus and Stein 2006). Though broad, this range of functions points to an important role for the insula in feeling states. These feeling states may be central to the conscious experience of motivational states that an individual has and, in the case of addiction, may be a key neurobiological element underlying drug use urges and cravings.
Dysfunction of the insula or its connectivity to other brain structures may underlie psychopathologies characterised by aberrant processing of feelings and emotions. For example, patients with damage to the insula report a loss of suffering, despite intact perception of pain (Berthier et al. 1988). Similar effects may arise from more subtle deficits. For example, using a resting-state fMRI paradigm that is thought to identify patterns of inter-cortical communication, elevated levels of autistic traits in a normal population have been shown to be associated with negative connectivity between the anterior mid-insula and the pregenual anterior cingulate cortex (ACC) (Di Martino et al. 2009). In contrast, the especially powerful feeling state embodied within the cravings of drug users may suggest an opposite pattern. Ma and colleagues (Ma et al. 2010) recently reported increased resting-state connectivity between the left lateral orbitofrontal cortex and the left insula in heroin users relative to controls.
Neurobiology of drug craving
Given the important role that cravings are thought to play in motivating addictive behaviour (Niaura et al. 1988), minimally, in the aversive discomfort experienced by users attempting abstinence, understanding the neurobiology of this subjective experience is of clinical importance. This may be especially true given the evidence reviewed elsewhere in this issue that damage to the insula can produce an immediate loss of cravings and immediate smoking cessation (Naqvi et al. 2007). Neuroimaging studies of drug cravings are experimentally difficult given the challenges in inducing, assessing and exercising experimental control over an emotional state. This is often compounded by the alexithymia of drug users that can diminish the validity of their subjective reports. The craving experience does not easily lend itself to the “ON–OFF” activation contrast that is typically required in fMRI studies leading some investigators to employ quantitative measures such as PET or blood perfusion (Bonson et al. 2002; Brody et al. 2002; Franklin et al. 2007; Volkow et al. 2010). An important factor, when considering the literature on the neurobiology of craving, is whether an experimental manipulation succeeded in inducing an emotional craving experience. Some studies that present drug-related stimuli may have validity as tests of drug cue reactivity while not necessarily creating a drug craving response. Consequently, a drug cue might elicit, for example, a ventral striatal response reflecting the learned salience of that cue but might not induce a subjectively experienced craving response and might therefore not activate key brain areas that underlie craving. Perhaps related to the distinction between cue reactivity and craving, some studies have observed that brain responses to the initial period of exposure to drug stimuli (e.g., the first 30 s of a cocaine video) is more sensitive at discriminating users and controls (Kosten et al. 2006). One final consideration in interpreting the empirical literature is that drug availability or expectancy to consume very soon after testing can impact on drug cravings. These expectations can modulate the effects of abstinence on cravings and affect activation levels including those in the insula (Gloria et al. 2009; McBride et al. 2006; Wilson et al. 2004).
Given the emphasis being placed here on subjective experiences, it is important to comment on the relevance of subjective cravings to actual behaviour. The processes by which certain stimuli attain an approach, incentivised value, the distinction between wanting and (subjectively) liking an item such as a food or a drug and the distinction between those items being reinforcing as opposed to being rewarding all call into question the central relevance that subjective experience may have. Coupled with this is the evidence that limbic reactivity to drug stimuli can be induced by drug-related stimuli that are not consciously perceived (Childress et al. 2008) and theories of drug-dependence that suggest that habitual or automatic behavioural repertoires might suffice to maintain dependence (Tiffany 1990; Everitt and Robbins 2005). Moreover, brain activity measures at the onset of treatment may prove to be better predictors of relapse than subjective craving reports (Kosten et al. 2006). Naqvi and Bechara (2009) have suggested that the (subjective, conscious) motivations experienced by smokers may serve as a gatekeeper that amplifies the incentive salience of drugs and drug-related cues thereby accommodating how subjective and unconscious processes may combine to yield compulsive urges. Consistent with this, tracer studies in macaque monkeys have revealed strong afferent projections from the insula to the ventral striatum, which are thought to integrate consummatory behaviours with rewards and memory (Fudge et al. 2005). Finally, although the relationship between craving and relapse is unclear (see Table 1 in Heinz et al. 2009), in assessing the importance of the subjective experience to addictive behaviour, it is notable that current non-pharmacological therapies for addiction place emphasis on the detection of one’s (subjectively available) drug urges and conscious cognitive/behavioural methods to arrest and control them (Penberthy et al. 2010).
The extant neuroimaging literature on craving reveals widespread cortical and subcortical activation (see reviews in Heinz et al. 2009; Naqvi and Bechara 2009; Wilson et al. 2004). No doubt this reflects the numerous cognitive and emotional processes involved. These are likely to include the heightened and potentially multi-modal perceptual processing of drug cues, long-term memory recollections of previous drug experiences, working memory ruminations, arousal and so on. In addition to the insula, craving-related activations are often observed in dorsolateral prefrontal, medial prefrontal and orbitofrontal cortex, anterior and posterior cingulate cortex, ventral striatum and the amygdala. The contribution of the insula to this network may be the somatic, interoceptive processes experienced subjectively as a drug craving (Craig 2009). Consistent with this central a role in craving, insula activation in craving paradigms is a reliable observation. For example, Table 1 in Naqvi and Bechara (2009) lists 16 studies reporting insula activation during drug urges in cigarette, cocaine, alcohol and heroin users. More recently, marijuana craving has also been observed to produce insula activity (Filbey et al. 2009). Individual differences in smoking cue reactivity have also recently been linked to genetic variability (Franklin et al. 2009). Smokers with the dopamine transporter 9-repeat allele (thought to lower DAT expression and therefore prolong synaptic dopamine function) showed greater subcortical and orbitofrontal response to smoking cues. However, only those with the 10-repeat allele showed increased activity in bilateral anterior insula and a correlation between reported craving and insular activity. While the psychological interpretation of this insular activity is unclear (e.g., the authors suggest that the 10-repeat subjects, who had recently smoked and were therefore satiated, may have had a negative reaction to the smoking cues) this study, nonetheless, points towards genetic predispositions impacting on subjective and brain responses to drug cues.
Insula and non-drug cravings
The insula response when craving drugs may reflect a more general response that would also be observed for other appetitive stimuli. Insular activity has been reported in response to erotic stimuli (Gizewski et al. 2006; Safron et al. 2007) and in response to itching and scratching (Vierow et al. 2009). In a direct test of the specificity of insular activity for cocaine craving, Garavan et al. (2000) showed that insular activity of cocaine addicts when viewing a video of cocaine use was also present when those users viewed a video containing erotic content and was also present when cocaine-naïve controls viewed the erotic video. That said, the magnitude of responses to drug and non-drug stimuli in a common circuitry may be important. Heinz et al. (2009) report that although a larger ventral striatal response to alcohol-related stimuli predicted higher relapse rate in alcoholics, a larger response to non-drug, affectively positive stimuli predicted lower relapse.
Curiously, gambling craving paradigms tested in those with pathological gambling tend not to show activity in the insula. This is true despite showing robust activity in other areas typically observed during craving and cue-reactivity paradigms (Crockford et al. 2005; Ko et al. 2009; Potenza et al. 2003). Potenza (2008) directly compared the cue-reactivity of gamblers to gambling stimuli and cocaine users to cocaine stimuli and although both contrasts did reveal activation changes in the left insula, these effects appeared to be driven primarily by activation increases in the controls.
In contrast to studies on gambling, both hunger and thirst have been shown to activate the insula (Del Parigi et al. 2002; Egan et al. 2003; Tataranni et al. 1999). Using the combination of a monotonous dietary manipulation and a cue-induction technique, robust caudate, hippocampal and insular activity was observed as subjects imagined their favourite foods (Pelchat et al. 2004). Using a 5-h fasting manipulation, pictures of foods activated orbitofrontal cortex and bilateral insula with the insula activity correlating with subjective ratings of appetite (Porubska et al. 2006). The insula may also contribute to eating pathologies. Rothemund and colleagues (2007) studied the neural response of obese women to images of high- and low-calorie foods observing greater left anterior insula activity for the high-calorie foods in the obese women relative to a control group. In addition, significant correlations were observed between body mass index and right anterior insula activity. Supporting evidence comes from a brain volumetric study: In a sample of frontotemporal lobal degeneration patients, Whitwell et al. (2007) detected reduced grey matter volumes in orbitofrontal cortex and right anterior insula in those with pathological cravings for sweet foods.
This brief review suggests that the insula contributes to a broad range of urges. Although the empirical literature is small, rendering premature any speculation on why gambling urges may be an exception, the absence of insular activity in gambling craving paradigms may nonetheless suggest a difference regarding the role of insula-mediated interoception between this “behavioural addiction” and the other drug and non-drug craving phenomena. Naqvi and colleagues (this issue) place importance on the role of the insula in interoception for specifically survival-related functions such as sex, hunger and thirst. These authors also stress the importance of peripheral physiological aspects of consummatory experiences for insula activation. Either alternative could explain the absence of insula activity for gambling craving.
Converging evidence implicating the insula in craving
The challenge for a correlational method such as neuroimaging is assigning functional roles to observed activations and, given the present topic, in attributing a specific role in subjective craving to insular activations. Converging evidence from lesion studies is immensely valuable (Naqvi et al. 2007) but a number of experimental methods using neuroimaging techniques (albeit still correlational) can help in refining the insula’s role.
One such approach helps isolate the neurobiology of craving by assessing what brain regions are sensitive to the top–down modulation of craving. One of the first studies to investigate this required smokers to suppress their urge to smoke when viewing videotaped smoking cues (Brody et al. 2007). Although cigarette craving did correlate with left anterior insula activity the insula was not significantly active in response to the smoking cues nor did it show a significant decrease in activity in the suppression condition. In contrast, Volkow et al. (2010) reported that cognitive inhibition of a video-induced craving response in cocaine abusers was associated with reduced metabolism in the right posterior insula. In addition, Wang et al. (2009) reported that men, but not women, who inhibited their desire for food showed a corresponding reduction in the insula. Although the interaction between sex and hunger suppression was not significant for insula activity (it was in other pertinent areas such as the orbitofrontal cortex, amygdala, parahippocampus and putamen) these results do draw attention to potentially important differences between males and females related to cognitive control over food urges. Sex differences are relatively under-studied in drug abuse and yet there is strong human and preclinical evidence to suggest that females fare worse on very many relevant physiological, behavioural and neurobiological dimensions of addiction (Lynch et al. 2002).
Yet another research approach to identifying brain regions subserving craving is provided by tracking brain changes that accompany satiety. Food-specific satiety, wherein consumption of an item reduces its appeal, provides a means to reveal the reduction in the hedonic value of an item and consequently, the craving for it. Small et al. (2001) reported reduced blood flow in the insula that paralleled a reduction in the reward value of repeated consumption of chocolate and Del Parigi et al. (2002) showed insular activity to decrease once hungry participants were fed. Later, Smeets et al. (2006) studied the effects of consuming chocolate to satiety on the brain’s response to chocolate milk. Contrary to the previous studies, they found increased activity in bilateral anterior insula in response to the chocolate milk when satiated relative to when fasted; this effect may indicate an aversive response to the repeated consumption of the chocolate milk. This effect was present in males only; however, although females showed no insular effects, the difference in insular activation between the sexes was not significant. These results provide a useful reminder to be cautious in interpreting an insula response to an otherwise appetitive stimulus as necessarily reflecting a craving for it. The insula shows robust activation for aversive stimuli such as disgusting images and pain (Craig 2009) and whether the interoceptive feelings represented by insular activity are positive or negative in valence requires additional information from self-reports or other behavioural measures.
Further insight into the neurobiology of drug craving might be gained by exploiting variations in the magnitude of cravings. This could be done experimentally by, for example, manipulating levels of cue or drug exposure or by exploiting variation in craving over time. For example, Risinger et al. (2005) employed a self-administration procedure wherein cocaine addicts were able to self-administer up to six i.v. injections of cocaine within a 1-h fMRI imaging session. The addicts were also able to provide frequent subjective reports of their feelings using Likert scales throughout the imaging session. Utilising the subjective ratings of the drug addict enables researchers to track the brain regions that correspond to the powerful experiences induced by the drug self-administrations. It was found that the time-course of subjective high ratings correlated positively with right insular activity. As the time-course of subjective high across the study was inversely correlated with the time-course of subjective craving, the significant positive correlation between the insula and high might also reflect a negative relationship between insula activity and subjective craving. That said, the positive correlation between the insula and high might suggest that the interoception associated with the drug high might overwhelm any relationship between insula activity and craving. Notwithstanding the ambiguities that can arise from cravings and highs being closely associated, this strategy for tracking dynamic changes in subjective drug experience affords potential for merging subjective experience and brain function. This allows one, for example, to explore the brain state that immediately precedes the decision to self-administer.
Yet another approach that helps to identify key brain structures underlying craving is to determine what areas predict relapse. Although subjective reports of craving often prove to be poor predictors of subsequent abstinence, cognitive and neuroimaging measures have proven efficacious in predicting relapse (Grüsser et al. 2004; Kosten et al. 2006). Note that the literature is small and the tasks that produce activation are not necessarily designed to induce a craving response. For example, using a two-button prediction task, Paulus and colleagues (Paulus et al. 2005) observed right insula activity early in abstinence to be a predictor of subsequent relapse for methamphetamine users. A regression analysis, however, revealed that other prefrontal, temporal and posterior cingulate regions proved to be better predictors of outcome. Curiously, in a task that did show drug-related stimuli, Grüsser et al. (2004) found that activity in response to alcohol-related stimuli in the putamen, anterior cingulate and medial prefrontal cortex, but not the insula, predicted relapse. Here, subjective craving was also a poor predictor which may suggest that an insula-mediated craving response is not the most sensitive in predicting relapse. Kosten et al. (2006) used cocaine video clips, finding the initial responses to them to be most sensitive for discriminating cocaine users from controls. Here, no insula activity was observed so insula function was not assessed for predicting relapse. Given the important role that cognitive processes may play in avoiding relapse in drug users and gamblers (Cox et al. 2002; Waters et al. 2003 Goudriaan et al. 2008; Passetti et al. 2008), it may be the case that the best predictors of treatment outcome are those that reflect cognitive control over drug urges rather than the drug urges themselves. This is supported by a study by Brewer et al. (2008) who identified cognitive control prefrontal regions in addition to other subcortical and posterior cingulate regions as being the best predictors of treatment outcome in a treatment-receiving sample of cocaine users. Although the task, a colour-word STROOP task did produce insular activity, this activity did not predict outcome.
Differentiating between (typically, prefrontal) cognitive control regions and the insula might imply that the insula does not have an important role in cognitive processes. Contrary to this, this review addresses next the role that the insula has been shown to play in important cognitive control functions that may, in fact, also contribute to drug cravings.
Insula, cognition and craving
There is accumulating evidence that there are differences in insular structure and function in addicts. This might be anticipated given the high concentration within the insula of neurotransmitters such as dopamine and opioids that are highly relevant to the reinforcing and subjective effects of drugs of abuse (Baumgartner 2006; Gaspar 1989; Hurd 2001). Structural changes within the insula are consistent with drug use impacting on this area. Former alcoholics show reduced right anterior insula volumes which, notably, tend to increase with length of abstinence (Makris et al. 2008b). Similar reductions in insular volume and in cortical thickness have been reported for cocaine users (Franklin et al. 2002; Makris et al. 2008a).
Cognitive neuroscience approaches to investigating addiction have sought to determine the profile of cognitive deficits that might contribute to drug dependence (Garavan and Hester 2007). Given the loss of control that is characteristic of dependence (indeed, is required for a diagnosis of dependence), much of this research has focussed on the cognitive processes involved in the risky and impulsive decision-making of users. Evidence implicates the insula in risk-taking behaviour and this is plausibly linked to deficits in subjective, bodily states that serve as somatic markers that typically guide people towards risk-averse decisions (Paulus 2007). The insula has also been implicated in monitoring behavioural performance which, again, is plausibly linked to somatic states that register when behaviour is sub-optimal. For example, while most of the research on performance monitoring (e.g., detecting errors in a cognitive task) has focussed on the role of the ACC, robust co-activation in the anterior insula is a very reliable phenomenon (Hester et al. 2004). Indeed, evidence exists that the anterior insula may have a more specific role in error processing than the ACC (Magno et al. 2006, 2009; Modirrousta et al. 2008). In the fMRI studies by Magno and colleagues, activity in the ACC was found to be maximal when participants interrupted ongoing behaviour either to avoid a loss or to obtain a bigger reward on a visual search task. Notably, the ACC activity levels in these tasks did not differ between error trials and correct trials. In contrast, error-specific activity was observed in bilateral anterior insula.
Consistent with an error-specific role for the insula, there is also evidence that this structure may play a critical role in the conscious awareness of errors (Hester et al. 2005; Klein et al. 2007; see article in this issue by Ullsperger and colleagues). For example, Hester and colleagues showed equivalent levels of ACC activity on error trials of which participants were or were not aware (awareness was indicated by participants making a different response on trials that immediately followed errors); in contrast, the insula was more active for aware errors. The conscious awareness of errors that these findings suggest are related to insula function may underlie the post-error corrective behaviour that has also been linked to this region (Ramautara et al. 2006; Paulus et al. 2008; Li et al. 2008). While evidence points to similar and dissimilar functions served by the ACC and insula (see papers by Allman and Medford in this issue), these studies suggest that distinct monitoring processes might be attributed to these two structures. The ACC process may be more general (e.g., activated on correct and error trials; Roger et al. 2010) and may not necessitate, or generate, awareness. In contrast, the pattern of results for the insula, in keeping with the studies reviewed throughout this paper, suggest a role in the subjective, conscious, feelings that might arise from making an error and that might therefore guide deliberative, conscious behavioural control.
Returning to addiction, given the evidence of insular dysfunction in addicts, compromise on the cognitive processes described above might be predicted. And indeed, drug dependent individuals do show a hypoactive brain response to their errors in both the ACC and the insula (Forman et al. 2004; Kaufman et al. 2003). Cocaine can directly impact on insular function as demonstrated by increased insular activity for performance errors in cocaine users when the task was preceded by an i.v. cocaine administration (Garavan et al. 2008). Deficits in this rudimentary cognitive function (i.e., detecting when one makes a mistake) may contribute to the cognitive deficits in users on risk-taking and learning tasks (Garavan and Stout 2005). Indeed, stimulant users are less likely to make strategic shifts in performance (e.g., win-stay, lose-shift) and this is related to activation differences in dorsolateral prefrontal cortex and left insula (Paulus et al. 2008).
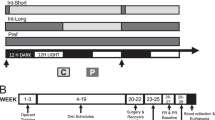
Notably, cocaine addicts and heavy cannabis users have been shown to have a poorer subjective awareness of performing errors on the task designed specifically to assess error awareness (Hester et al. 2007, 2009). This task requires subjects to indicate, by button press, when they make an error-of-commission on a Go/NoGo task that contains two rules that dictate when to inhibit responding. The awareness of errors is distinct from overall performance on the task (i.e., the proportion of errors of which one is aware is not correlated with how many errors one makes) suggesting a deficit that is separate from the poor inhibitory control already associated with drug use (Verdejo-García et al. 2008). Neuroimaging data, currently only available on the cannabis users, links this awareness deficit to the functioning of the ACC and the insula (Fig. 1; Hester et al. 2009). These deficits in subjective awareness of behaviour are notable given (1) the evidence of volumetric differences in the insula in drug users and (2) evidence that greater anterior insular volumes are linked to better subjective awareness of inner bodily states (Critchley et al. 2004).
a The Error Awareness Task (Hester et al. 2005) is a Go/NoGo task in which participants must inhibit responses when either a stimulus is repeated (Repeat NoGo) or when the stimulus contains an incongruity between the word name and its font colour (Stroop NoGo). Maintaining two NoGo rules leads to commission errors, some of which go unnoticed by participants who indicate their awareness of an error by making their Go response on a different button on trials that follow errors. b Heavy cannabis users did not differ in inhibitory performance but they had poorer awareness of their errors. c For controls, unawareness of errors was associated with reduced activity in the insula while for the cannabis users, it was associated with reduced activity in both insula and ACC
These cognitive deficits in monitoring and being consciously aware of one’s behaviour may have direct relevance to drug cravings. Compromised monitoring may result in drug users being poor to realise when they are exposing themselves to exogenous and endogenous craving triggers. For example, deficient monitoring might lead the user to high-risk situations containing drug availability or drug cues that might initiate craving. With regard to endogenous triggers, compromised monitoring may also affect the user’s insight into their own drug urges or stress-levels. A recent study has linked an electrophysiological marker of error processing (the error-related negativity) to success over 2 weeks in monitoring and regulating one’s stress levels (Compton et al. 2008). Not until the user can detect that the craving response (or its antecedents) has begun can he or she attempt to control it.
Conclusion
The focus of this review has been on the role of the insula in drug craving. It is essential to note that no study has shown isolated insular activity and there is considerable evidence that other regions (e.g., ventral striatum, posterior cingulate cortex, dorsolateral prefrontal, orbitofrontal and occipital cortex) are also consistently activated in cue-reactivity and drug-craving paradigms. There is, for example, substantial evidence linking the posterior cingulate to cue reactivity and craving and the orbitofrontal cortex to the motivation to consume. The challenge remains to determine the specific functions of these structures in drug-taking behaviour: one working hypothesis is that regions such as the ventral striatum, orbitofrontal cortex and anterior insula may all contribute to the motivation to consume drugs with the insula providing the conscious awareness of the aversive bodily feeling states that constitute the craving response.
As the understanding of the neurobiology of craving improves, one can then start to address questions concerning individual differences in craving. The evidence of craving being modulated by genes (Franklin et al. 2009) indicates a role for genetic predispositions. A number of studies, primarily those addressing food cravings, have shown interesting sex differences but little is known about how sex differences mediate the neurobiology of craving. Women are more likely to experience specific food cravings (Pelchat 1997; Weingarten and Elston 1991) and it is possible that sex differences in cravings may be related to obesity and eating disorders. Similarly, little is known about developmental processes. Tapert et al. (2003) studied cue-reactivity in adolescents with alcohol use disorders observing that drug cue responses were observed in frontal and limbic areas but not in the insula. However, bilateral insula responses to alcohol words were observed in heavy drinking young (18–25 years) adult females (Tapert et al. 2004). There is evidence of neurodevelopmental interactions between sex and drug use with larger prefrontal volumes in males with alcohol use disorder relative to male controls and smaller prefrontal volumes in females with alcohol use disorder relative to female controls (Medina et al. 2008). It will be important to understand if differences in drug cravings contribute to these sex and developmental differences. Finally, research efforts on understanding the mechanisms by which craving can be controlled, including how important modulation of the insula is to this control, is of significant clinical relevance.
References
Baumgartner U (2006) High opiate receptor binding potential in the human lateral pain system. Neuroimage 30:692–699
Berthier M, Starkstein S, Leiguarda R (1988) Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol 24:41–49
Bonson K, Grant S, Contoreggi C, Links J, Metcalfe JW, Weyl HL, Kurian V et al (2002) Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26:376–386
Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN (2008) Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry 64:998–1004
Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG et al (2002) Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59:1162–1172
Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS (2007) Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry 62:642–651
Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J et al (2008) Prelude to passion: limbic activation by ‘unseen’ drug and sexual cues. PLoS ONE 3:e1506
Compton RJ, Robinson MD, Ode S, Quandt LC, Fineman SL, Carp J (2008) Error-monitoring ability predicts daily stress regulation. Psychol Sci 19:702–708
Cox WM, Hogan LM, Kristian MR, Race JH (2002) Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend 68:237–243
Craig AD (2009) How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70
Critchley H, Wiens S, Rotshtein P, Ohman A, Dolan R (2004) Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195
Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N (2005) Cue-induced brain activity in pathological gamblers. Biol Psychiatry 58:787–795
Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E, Tataranni PA (2002) Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci 967:389–397
Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP (2009) Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry 166:891–899
Egan G, Silk T, Zamarripa F, Williams J, Federico P, Cunnington R, Carabott L, Blair-West J, Shade R, McKinley M, Farrell M, Lancaster J, Jackson G, Fox P, Denton D (2003) Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci USA 100:15241–15246
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8(11):1481–1489 (erratum in Nat Neurosci 2006 9(7):979)
Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE (2009) Marijuana craving in the brain. Proc Natl Acad Sci USA 106:13016–13021
Forman SD et al (2004) Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry 55:531–537
Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR (2002) Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 51:134–142
Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR (2007) Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology 32:2301–2309
Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR (2009) DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology 34:717–728
Fudge J, Breitbart M, Danish M, Pannoni V (2005) Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol 490(2):101–118
Garavan H, Hester R (2007) The role of cognitive control in cocaine dependence. Neuropsychol Rev 17:337–345
Garavan H, Stout JC (2005) Neurocognitive insights into substance abuse. Trends Cogn Sci 9:195–201
Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798
Garavan H, Kaufman JN, Hester R (2008) Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci 363:3267–3276
Gaspar P (1989) Catecholamine innervation of the human cerebralcortex as revealed by comparative immunohistochemistry of tyrosinehydroxylase and dopamine-b-hydroxylase. J Comp Neurol 279:249–271
Gizewski ER, Krause E, Karama S, Baars A, Senf W, Forsting M (2006) There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli: a fMRI study. Exp Brain Res 174:101–108
Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, Curtin JJ, Davidson RJ, Baker TB (2009) An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology 46:681–693
Goudriaan AE, Oosterlaan J, De Beurs E et al (2008) The role of self-reported impulsivity and reward sensitivity versus neurocognitive measures of disinhibition and decision-making in the prediction of relapse in pathological gamblers. Psychol Med 38:41–50
Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A (2004) Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175:30–296
Heinz A, Beck A, Grusser SM et al (2009) Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol 14:108–118
Hester R, Fassbender C, Garavan H (2004) Individual differences in error processing: a review and meta-analysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex 14:966–973
Hester R, Shpaner M, Molholm S, Foxe JJ, Garavan H (2005) Neural correlates of error detection with and without awareness. Neuroimage 27:602–608
Hester R, Simões-Franklin C, Garavan H (2007) Post-error behaviour in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology 32:1974–1984
Hester R, Nestor L, Garavan H (2009) Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 34:2450–2458
Hurd YL (2001) D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat 22:127–137
Kaufman J, Ross TJ, Stein EA, Garavan H (2003) Cingulate hypoactivity in cocaine users during a GO/NOGO task as revealed by event-related fMRI. J Neurosci 23:7839–7843
Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M (2007) Neural correlates of error awareness. Neuroimage 34:1774–1781
Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, Yen CF, Chen CS (2009) Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res 43:739–747
Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE (2006) Cue-induced brain activity changes and relapse in cocaine dependent patients. Neuropsychopharmacology 31:644–650
Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R (2008) Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci 20:1021–1029
Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164:121–137
Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, Xu H-S, Fu X-M, Hu X, Zhang D-R (2010) Addiction related alteration in resting-state brain connectivity. Neuroimage 49:738–744
Magno E, Foxe JJ, Molholm S, Robertson I, Garavan H (2006) The anterior cingulate and error avoidance. J Neurosci 26:4769–4773
Magno E, Simoes-Franklin C, Robertson IH, Garavan H (2009) The role of the dorsal anterior cingulate in evaluating behavior for achieving gains and avoiding losses. J Cogn Neurosci 21:2328–2342
Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Kim BW, Blood AJ, Evins AE, Seidman LJ, Iosifescu DV, Lee S, Baxter C, Perlis RH, Smoller JW, Fava M, Breiter HC (2008a) Cortical thickness abnormalities in cocaine addiction—a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron 60:174–188
Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ (2008b) Decreased volume of the brain reward system in alcoholism. Biol Psychiatry 64:192–202
McBride D, Barrett SP, Kelly JT, Aw A, Dagher A (2006) Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology 31:2728–2738
Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF (2008) Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res 32:386–394
Modirrousta M, Fellows LK (2008) Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci 28:14000–14005
Naqvi NH, Bechara A (2009) The hidden island of addiction: the insula. Trends Neurosci 32:56–67
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534
Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB (1988) Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol 97:133–152
Passetti F, Clark L, Mehta MA, Joyce E, King M (2008) Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend 94:82–91
Paulus MP (2007) Decision-making dysfunctions in psychiatry-altered homeostatic processing? Science 318:602–606
Paulus MP, Stein MB (2006) An insular view of anxiety. Biol Psychiatry 60:383–387
Paulus MP, Tapert SF, Schuckit MA (2005) Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry 62:761–768
Paulus MP, Lovero KL, Wittmann M, Leland DS (2008) Reduced behavioral and neural activation in stimulant users to different error rates during decision making. Biol Psychiatry 63:1054–1060
Pelchat ML (1997) Food cravings in young and elderly adults. Appetite 28:103–113
Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD (2004) Images of desire: food-craving activation during fMRI. Neuroimage 23:1486–1493
Penberthy JK, Ait-Daoud N, Vaughan M, Fanning T (2010) Review of treatments for cocaine dependence. Curr Drug Abuse Rev 3:49–62
Porubska K, Veit R, Preissl H et al (2006) Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage 32:1273–1280
Potenza MN (2008) The neurobiology of pathological gambling and drug. Philos Trans R Soc Lond B Biol Sci 363:3181–3189
Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK et al (2003) Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Arch Gen Psychiatry 60:828–836
Ramautara JR, Slagter HA, Kok K, Ridderinkhof R (2006) Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Res 1105:143–154
Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA (2005) Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage 26:1097–1108
Roger C, Bénar CG, Vidal F, Hasbroucq T, Burle B (2010) Rostral Cingulate Zone and correct response monitoring: ICA and source localization evidences for the unicity of correct- and error-negativities. Neuroimage 51:391–403
Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF (2007) Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37:410–421
Safron A, Barch B, Bailey JM, Gitelman DR, Parrish TB, Reber PJ (2007) Neural correlates of sexual arousal in homosexual and heterosexual men. Behav Neurosci 121:237–248
Singer T, Critchley HD, Preuschoff K (2009) A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340
Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M (2001) Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124:1720–1733
Smeets PAM, de Graaf C, Stafleu A et al (2006) Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 83:1297–1305
Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA (2003) Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry 60:727–735
Tapert SF, Brown GG, Baratta MV, Brown SA (2004) fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav 29:33–50
Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E (1999) Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96:4569–4574
Tiffany ST (1990) A cognitive model of drug urges and drug-use behavior—role of automatic and nonautomatic processes. Psychol Rev 97:147–168
Verdejo-García A, Lawrence AJ, Clark L (2008) Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32:777–810
Vierow V, Fukuoka M, Ikoma A, Do¨rfler\ A, Handwerker HO, Forster C (2009) Cerebral representation of the relief of itch by scratching. J Neurophysiol 102:3216–3224
Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM (2010) Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage 49:2536–2543
Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS (2009) Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA 106:1249–1254
Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH (2003) Attentional bias predicts outcome in smoking cessation. Health Psychol 22:378–387
Weingarten HP, Elston D (1991) Food cravings in a college population. Appetite 17:167–175
Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, Warren JD (2007) VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage 35:207–213
Wilson SJ, Sayette MA, Fiez JA (2004) Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci 7:211–214