Abstract
Rationale and objective
In humans, exposure to contexts previously associated with heroin use can provoke relapse. In rats, exposure to heroin-paired contexts after extinction of drug-reinforced responding in different contexts reinstates heroin seeking. We previously demonstrated that the projections from ventral medial prefrontal cortex (vmPFC) to nucleus accumbens (NAc) shell play a role in this reinstatement. The ventral subiculum (vSub) sends glutamate projections to NAc shell and vmPFC. Here, we determined whether these projections contribute to context-induced reinstatement.
Methods
We trained rats to self-administer heroin (0.05–0.1 mg/kg/infusion) for 3 h per day for 12 days; drug infusions were paired with a discrete tone–light cue. Lever pressing in the presence of the discrete cue was subsequently extinguished in a different context. We then tested the rats for reinstatement in the heroin- and extinction-associated contexts under extinction conditions. We combined Fos with the retrograde tracer Fluoro-Gold (FG) to determine projection-specific activation during the context-induced reinstatement tests. We also used anatomical disconnection procedures to determine whether the vSub → NAc shell and vSub → vmPFC projections are functionally involved in this reinstatement.
Results
Exposure to the heroin but not the extinction context reinstated lever pressing. Context-induced reinstatement of heroin seeking was associated with increased Fos expression in vSub neurons, including those projecting to NAc shell and vmPFC. Anatomical disconnection of the vSub → NAc shell projection, but not the vSub → vmPFC projection, decreased this reinstatement.
Conclusions
Our data indicate that the vSub → NAc shell glutamatergic projection, but not the vSub → vmPFC projection, contributes to context-induced reinstatement of heroin seeking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to environmental contexts associated with drug use in humans often provokes drug craving and relapse during abstinence (O’Brien et al. 1992; Wikler 1973). Several years ago, we adapted the ABA renewal procedure (Bouton and Bolles 1979) to study the role of drug-associated contexts in drug seeking in rats (Crombag et al. 2008; Crombag and Shaham 2002). We and others have reported that re-exposing rats to drug-associated contexts after extinction of lever pressing in different contexts reinstates seeking for heroin (Bossert et al. 2004), cocaine (Fuchs et al. 2005), alcohol (Hamlin et al. 2007; Zironi et al. 2006), nicotine (Diergaarde et al. 2008), and methamphetamine (Rubio et al. 2015).
In previous studies, we found that blockade of dopamine D1-receptors or inhibition of glutamate transmission with an mGluR2/3 agonist in nucleus accumbens (NAc) shell blocks context-induced reinstatement of heroin seeking (Bossert et al. 2006, 2007). We also showed a role of vmPFC, which sends glutamatergic projections to NAc shell (Sesack et al. 1989). We found that context-induced reinstatement is associated with increased expression of the activity marker Fos in vmPFC and that local reversible inactivation with GABAA + GABAB receptor agonists muscimol + baclofen (M + B) decreases this reinstatement. The M + B effect was mimicked by selective inactivation of context-activated (Fos-positive) vmPFC neurons with Daun02 (Bossert et al. 2011).
Based on these findings, and previous studies showing that NAc shell activity is dependent on both glutamate and dopamine D1 receptor-mediated neurotransmission (O’Donnell 2003), we next tested whether synergistic activation of D1 postsynaptic receptors and vmPFC → NAc shell glutamatergic projections mediate context-induced reinstatement. We first found that this reinstatement is associated with increased Fos expression in vmPFC → NAc shell projection neurons, as assessed by double-labeling of Fos with the retrograde tracer Fluoro-Gold (FG) (Bossert et al. 2012). Fos + FG double-labeling was observed in both the dense ipsilateral projection and the sparse contralateral projection. We then used an asymmetrical disconnection procedure (Gold 1966) and found that reversible inactivation of the vmPFC in one hemisphere with M + B combined with D1 receptor blockade in contralateral or ipsilateral NAc shell decreases this reinstatement (Bossert et al. 2012).
In the experiments involving vmPFC and its projection to NAc shell described above, heroin seeking in the context-induced reinstatement tests was attenuated, but not completely blocked (Bossert et al. 2011, 2012). In contrast, bilateral blockade of dopamine D1-receptors or inhibition of glutamate transmission in NAc shell fully blocked context-induced reinstatement (Bossert et al. 2006, 2007). A potential reason for the differences in efficacy of local NAc manipulations versus local vmPFC or vmPFC → NAc shell manipulations is that other glutamatergic projections to NAc shell (Voorn et al. 2004) play a role in context-induced reinstatement (Marchant et al. 2014). One potential candidate is the ventral subiculum (vSub). The vSub sends dense glutamatergic projections to NAc shell (Brog et al. 1993; Groenewegen et al. 1987), and these projections and tyrosine hydroxylase-labeled dopaminergic terminals converge on the same postsynaptic dendrites in NAc shell (Sesack and Pickel 1990). Additionally, we recently found that M + B reversible inactivation of vSub decreases context-induced reinstatement of heroin seeking (Bossert and Stern 2014).
Therefore, in exp. 1–2 we used the same experimental procedures we used in our previous study (Bossert et al. 2012) (Fos + FG double labeling and a disconnection procedure with unilateral M + B in the glutamatergic cell body region and unilateral D1 receptor blockade in the ipsilateral or contralateral NAc shell) to test the hypothesis that synergistic activation of NAc shell D1 receptors and vSub → NAc shell glutamatergic projection also contributes to context-induced reinstatement.
In exp. 3–4, we further studied the circuitry of context-induced reinstatement by determining the role of the vSub → vmPFC glutamatergic projection (Jay and Witter 1991; Thierry et al. 2000). We studied this projection because anatomical (French and Totterdell 2002) and electrophysiological (O’Donnell and Grace 1995) studies show that NAc shell neurons receive convergent synaptic inputs from vmPFC and vSub. Additionally, several NAc-dependent goal-directed learned behaviors are controlled by projections from these brain areas (Floresco et al. 1997; Grace et al. 2007). As in exp. 1–2, we used FG + Fos to study projection-specific activation in vSub → vmPFC neurons and a disconnection procedure to study the functional/causal role of this projection. Unlike exp. 2 (disconnection of vSub → NAc shell) where we tested projection-specific glutamate-dopamine interaction in NAc shell (see above), in exp. 4, we used a traditional M + B disconnection procedure to inactivate cell bodies in the two brain areas of interest (McFarland and Kalivas 2001) to determine the role of the vSub → vmPFC glutamatergic projection in context-induced reinstatement.
Materials and methods
Subjects
We used male Sprague–Dawley rats (Charles River, total n = 173), weighing 250–350 g prior to surgery. We maintained the rats under a reverse 12:12 h light/dark cycle (lights off at 8:00 a.m.) with food and water freely available. We housed two rats per cage prior to surgery and then individually after surgery. We performed the experiments in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition), under the protocols approved by the Animal Care and Use Committee. We excluded a total of 41 rats due to failure of catheter patency (n = 4), misplaced cannula placements (n = 4) or FG tracer deposit (n = 4), lost head cap (n = 3), sickness (n = 14), or failure to meet an extinction criterion of a mean of less than or equal to 30 responses per 3 h over 3 days after 22 extinction sessions (n = 12). Exclusion of 20 to 25 % of rats is typical in our extinction-reinstatement experiments that involve intracranial and intravenous surgeries and prolonged self-administration and extinction training.
Iontophoresis of retrograde tracer and intravenous surgery (exp. 1 and 3)
We based the FG injection procedure on previous work from our laboratories (Bossert et al. 2012; Yamaguchi et al. 2011). We anesthetized the rats with sodium pentobarbital and chloral hydrate (60 and 25 mg/kg, i.p.) and delivered FG (1 % in cacodylate buffer, pH 7.5) (Schmued and Fallon 1986) iontophoretically unilaterally into medial NAc shell (AP +1.7 mm, ML ±2.3 mm [10° angle], DV −7.5 mm) or vmPFC (AP +3.0 mm, ML ±1.5 mm [10° angle], DV −5.3 mm) through a stereotaxically positioned glass micropipette. For NAc shell, the inner tip diameter of the micropipette was 50 μm and we applied 4 μA current in 5 s on/off pulses for 20 min. For vmPFC, the inner tip diameter of the micropipette was 60–65 μm and we applied 5-μA current in 5-s on/off pulses for 25 min.
The micropipette was left in place for an additional 5 min to prevent backflow of tracer up the injection track and we alternated iontophoresis into the left or right hemisphere. We then inserted silastic catheters into the jugular vein as described previously (Lu et al. 2004) by attaching the catheters to a modified 22-gauge cannula and mounted them to the rat’s skull with dental cement. We injected the rats with buprenorphine (0.1 mg/kg s.c.) to relieve pain and allowed them to recover 6–8 days before heroin self-administration training. During the recovery and training phase, we flushed the catheters every day with gentamicin (Butler Schein; 5 mg/ml) dissolved in sterile saline. In exp. 1 and 3, we perfused the rats after anesthesia as described in the “Double labeling Fos-FG immunohistochemistry” section below.
Intracranial and intravenous surgery (exp. 2 and 4)
We anesthetized the rats with sodium pentobarbital and chloral hydrate (60 and 25 mg/kg, i.p.) or isoflurane (5 % induction; 2–3 % maintenance) and implanted permanent guide cannulae (23-gauge, Plastics One, Roanoke, VA) unilaterally 1 mm above NAc shell or vmPFC and 1 mm above the vSub in the ipsilateral or contralateral hemisphere. The stereotaxic coordinates (Paxinos and Watson 2008) are based on our and others’ previous work (Bossert and Stern 2014; Bossert et al. 2012; Mendoza et al. 2015). The coordinates (nosebar set at −3.3 mm) for the different brain areas were for contralateral vmPFC: AP +3.0 mm, ML ±1.5 mm (10° angle), and DV −4.3 mm; for ipsilateral vmPFC: AP +3.0 mm, ML ±0.6 mm, and DV −4.1 mm; for contralateral NAc shell: AP +1.6 mm, ML ±3.0 mm (20° angle), and DV −6.5 mm; for ipsilateral NAc shell: AP +1.6 mm, ML ±1.6 mm (20° angle), and DV −7.2 mm (note that because of stereotaxic constraints, cannula for ipsilateral NAc shell were inserted at a 20° angle from the contralateral hemisphere through the midline (Bossert et al. 2012; Ikemoto et al. 2005); and for vSub: AP −6.0 mm, ML ±5.3 mm (4° angle), and DV −7.5 mm.
Following cannula implantation, we inserted silastic catheters into the jugular vein as described above or attached them to a modified 22-gauge cannula cemented to polypropylene mesh (Small Parts) and fixed the mesh to the mid-scapular region. We gave buprenorphine (0.1 mg/kg, s.c.) or ketoprofen (2.5 mg/kg, s.c., Butler Schein) after surgery and the following day (ketoprofen) to relieve pain and decrease inflammation and allowed rats to recover for 6–8 days before heroin self-administration training. During the recovery and training phases, we flushed the catheters every day with gentamicin (Butler Schein, 5 mg/ml) and sterile saline. At the end of exp. 2 and 4, we anesthetized the rats, removed their brains, and stored the brains in 10 % formalin before sectioning. Using a cryostat (Leica Microsystems), we sectioned brains in the coronal plane (50 μm), mounted them on gelatin-coated slides, stained them with cresyl violet, and verified cannula placement under a light microscope.
Intracranial injections
We dissolved SCH 23390 hydrochloride (Tocris) and muscimol + baclofen (M + B; Tocris) in sterile saline and injected the drugs 5–10 min before the test sessions. The doses of SCH 23390 (concentration, 0.6 μg/0.5 μl/side) and M + B (0.03 nmol + 0.3 nmol/0.5 μl/side) are based on our previous studies (Bossert and Stern 2014; Bossert et al. 2009, 2011, 2012). We connected the syringe pump (Harvard Apparatus) to 10-μl Hamilton syringes and attached the Hamilton syringes to the 30-gauge injectors via polyethylene-50 tubing. We injected vehicle (saline) or SCH 23390 or M + B over 1 min (injectors extended 1 mm below the tips of the guide cannulae) and left the injectors in place for an additional minute to allow diffusion.
Double labeling Fos-FG immunohistochemistry
We based our Fos, FG, and Fos-FG immunohistochemistry procedures on previous reports (Bossert et al. 2012; Miller and Marshall 2005). Ninety minutes after exposure to context A or context B, we deeply anesthetized the rats with isoflurane (∼80 s) and perfused them transcardially with 100 ml of 0.1 M sodium phosphate (PBS) followed by 400 ml of 4 % paraformaldehyde in 0.1 M PBS, pH 7.4. We removed and postfixed the brains in 4 % paraformaldehyde for 2 h before transferring them to 30 % sucrose in 0.1 M PBS, pH 7.4, for 48 h at 4 °C. We subsequently froze the brains in powdered dry ice and stored them at −80 °C until sectioning. We cut coronal sections (20 μm) containing vmPFC, NAc shell, and vSub (approximately +2.7 to +3.5, +1.2 to +2.2, and −5.4 to −6.4 mm from Bregma, respectively) using a cryostat, collected the tissue in cryoprotectant (20 % glycerol and 2 % DMSO in 0.1 M PBS, pH 7.4), and stored them at −80 °C until further processing. We rinsed free-floating sections (three times for 10 min each) in PBS, incubated them for 1 h in 3 % normal goat serum (NGS) in PBS with 0.25 % Triton X-100 (PBS-TX), and incubated them overnight at 4 °C with rabbit anti-c-Fos primary antibody (c-Fos sc-52, Lot F2330, Santa Cruz Biotechnology, diluted 1:4000 or phospho-c-Fos [Ser32] D82C12 diluted 1:8000) in 3 % NGS in PBS-TX.
We then rinsed the sections in PBS and incubated them for 2 h with biotinylated anti-rabbit IgG secondary antibody (BA-1000, Vector Laboratories) diluted 1:600 in 1 % NGS in 0.25 % PBS-TX. We rinsed the sections again in PBS and incubated them in avidin–biotin–peroxidase complex (ABC; ABC Elite kit, PK-6100, Vector Laboratories) in 0.5 % PBS-TX for 1 h. We then rinsed the sections in PBS and developed them in Vector SG (blue/gray product; Vector SG peroxidase substrate kit, Vector Laboratories) and terminated the reaction by rinsing the tissue in PBS. We then rinsed the sections several times, incubated them in 0.3 % H2O2 for 30 min, and then rinsed them before incubation for 1 h in 0.3 % PBS-TX containing 4 % bovine serum albumin (BSA) and avidin D (avidin–biotin blocking kit; Vector Laboratories). We then incubated the sections overnight at 4 °C with rabbit anti-FG primary antibody (AB153; anti-Fluorescent Gold, Millipore) diluted 1:15,000 in 4 % BSA, 0.3 % PBS-TX, and biotin. We then rinsed the sections in PBS and incubated for 1 h with biotinylated anti-rabbit IgG secondary antibody (BA-1000, Vector Laboratories) diluted 1:200 in 4 % BSA in 0.3 % PBS-TX.
We rinsed the sections again in PBS and incubated them in ABC (ABC Standard kit, PK-4000, Vector Laboratories) in PBS for 1 h. We then rinsed the sections in PBS and developed them in 3,3′-diaminobenzidine, rinsed them in PBS, mounted them onto chrome alum/gelatin-coated slides, and air dried them. We dehydrated the slides through a graded series of alcohol concentrations (30, 60, 90, 95, 100, 100 % ethanol), cleared with Citrasolv (Fisher Scientific), and cover slipped them with Permount (Fisher Scientific). Because both primary antibodies against c-Fos and FG were raised in rabbit, there is the possibility of cross-reactivity between the secondary antibody used to label FG and the bound c-Fos primary antiserum. However, our laboratory, as well as others, have previously shown evidence that this is not the case (Bossert et al. 2012; Miller and Marshall 2005).
We digitally captured brightfield images of immunoreactive (IR) cells in ventral subiculum using an EXi Aqua or Riga 2000 camera (QImaging) attached to a Zeiss Axio Imager M2 or A1. The goal was to compare the number of Fos-IR, FG-IR, and double-labeled cells in vSub in both the ipsilateral and contralateral hemispheres to the FG deposit. We identified FG-IR cells by a brown product in the cytoplasm, Fos-IR cells by a dark blue reaction product in the nuclei, and double-labeled cells by a dark blue nucleus surrounded by brown cytoplasm. For each rat, we quantified cells in two hemispheres of 2–3 sections (2–3 ipsilateral counts and 2–3 contralateral counts). We computed the mean of these counts to give a mean number of each immunoreactive cell type per area. We analyzed the images using IVision (4.5.0, Biovision Technologies) software at 10×. Image capture was performed by JMB and quantification of cells was performed blindly by SA and RS. We also captured images of Fos-IR in NAc shell and vmPFC in the hemisphere contralateral to FG injection (note that Fos was difficult to quantify in the FG-injected hemisphere). For each rat, we quantified four contralateral sections of each brain region and averaged the counts. We performed imaging and quantification as described above.
Apparatus
We trained and tested the rats in standard Med Associates self-administration chambers. Each chamber has two levers located 8-8.5 cm above the grid floor on opposing walls. Lever presses on the active retractable lever activated the infusion pump, whereas lever presses on the inactive non-retractable lever had no programmed consequences. The two contexts differed from each other in terms of their auditory, visual, tactile, and circadian [i.e., morning (session onset at 8:00 a.m.) vs afternoon (session onset at 1–2:00 p.m.) sessions] cues using procedures identical to those described in our previous studies (Bossert et al. 2004, 2012). The contexts are referred to as A and B, where A is the heroin self-administration (training) and reinstatement (testing) context, and B is the extinction context. We counterbalanced the physical environments and circadian cues of contexts A and B.
Procedures
The experiments consisted of three phases: heroin self-administration training (12 days), extinction training (16–27 days), and tests for context-induced reinstatement of heroin seeking (1 or 2 days). The experimental sequence was context A (training)—context B (extinction)—contexts A and B (testing).
Heroin self-administration training and extinction
We trained rats to self-administer heroin for 3 h/day for 12 days. We dissolved heroin (diacetylmorphine HCl; NIDA) in sterile saline. Heroin was infused at a volume of 65 μl over 2.3 s at a dose of 0.1 mg/kg/infusion (first six sessions) and 0.05 mg/kg/infusion (last six sessions). During training, the rats earned heroin infusions paired with a compound tone–light cue for 2.3 s under a fixed-ratio-1 (FR1) 2.3-s timeout reinforcement schedule. During the extinction phase (context B), responses on the previously active lever led to presentation of the tone–light cue; however, heroin was not delivered. We conducted the tests for context-induced reinstatement under extinction conditions (lever presses led to the presentation of the tone–light cue but not heroin) and began testing after a minimum of 16 daily extinction sessions when the rats met our extinction criterion of a mean of fewer than 30 presses on the previously active lever over the last three extinction sessions. We presented the discrete cue during the extinction phase because our experimental procedure is modeled after the original renewal procedure of Bouton and Bolles (1979). In this procedure, renewal is defined as a recovery of the conditioned response to the discrete cue in the original conditioning context (where the cue was previously paired with the primary reinforcer) after extinction of the response to the cue in a different context.
Exp. 1: activation of vSub → NAc shell projections during context-induced reinstatement
We used a total of 17 rats that were injected with FG into NAc shell. We divided the rats into two groups (n = 8–9). The control group (A-B-B) underwent heroin self-administration training (3 h/day) in context A and extinction training (3 h/day) and reinstatement testing (90 min) in context B. The renewal (context-induced reinstatement) group (A-B-A) underwent heroin self-administration training in context A, extinction training in context B, and reinstatement testing in context A. The reinstatement test was 90 min, because of the known time course of Fos induction after exposure to drug or non-drug stimuli (Curran and Morgan 1995). We matched rats in the control and renewal groups for their heroin intake and number of active lever presses during training and extinction. At the end of the test session, we deeply anesthetized the rats, perfused them with PBS and 4 % paraformaldehyde, and removed their brains for subsequent immunohistochemistry.
Exp. 2: effect of disconnection of the vSub → NAc shell projection on context-induced reinstatement
We used 43 rats divided into four groups: group 1: ipsilateral vehicle NAc shell—vehicle vSub (n = 11); group 2: contralateral vehicle NAc shell—vehicle vSub (n = 8); group 3: ipsilateral SCH 23390 NAc shell—M + B vSub (n = 14); group 4: contralateral SCH 23390 NAc shell—M + B vSub (n = 10). The test sessions were 90 min and we tested each rat twice with their assigned dose (vehicle or drug): once before exposure to context A (heroin context) and once before exposure to context B (extinction context). We separated the tests by 48 h and kept the rats in the animal housing room between tests. We matched rats in the vehicle and drug groups for heroin intake and number of active lever presses during training and extinction and counterbalanced the order of testing in contexts A and B, and the implantation of cannula into either the left or right hemisphere in vSub and ipsilateral or contralateral NAc shell. After testing, we deeply anesthetized the rats and removed their brains for subsequent cannula placement verification.
Exp. 3: activation of vSub → vmPFC projections during context-induced reinstatement
We used a total of 23 rats that were injected with FG into vmPFC. We divided the rats into two groups (n = 11–12). The control group (A-B-B) underwent heroin self-administration training (3 h/day) in context A and extinction training (3 h/day) and reinstatement testing (90 min) in context B. The renewal (context-induced reinstatement) group (A-B-A) underwent heroin self-administration training in context A, extinction training in context B, and reinstatement testing in context A. We matched rats in the control and renewal groups for their heroin intake and number of active lever presses during training and extinction. At the end of the 90-min test session, we deeply anesthetized the rats, perfused them with PBS and 4 % paraformaldehyde, and removed their brains for subsequent immunohistochemistry.
Exp. 4: effect of disconnection of the vSub → vmPFC projection on context-induced reinstatement
We used 49 rats divided into five groups: group 1: ipsilateral vehicle vmPFC—vehicle vSub (n = 7); group 2: contralateral vehicle vmPFC—vehicle vSub (n = 7); group 3: ipsilateral M + B vmPFC—M + B vSub (n = 11); group 4: contralateral M + B vmPFC—M + B vSub (n = 12). Based on the results of exp. 2, we also included a fifth group of rats injected with vehicle into vmPFC and M + B into vSub (group 5, n = 12) to rule out that the inhibitory effects of the disconnection manipulations on context-induced reinstatement observed in exp. 2 are due to unilateral inactivation of vSub. The training, testing, and cannulae verification were the same as described above for exp. 2.
Statistical analyses
We analyzed the data with ANOVAs or ANCOVAs or independent t tests (Fos-IR, FG-IR, and Fos-FG-IR; two-tailed) using the statistical program SPSS (GLM procedure). We followed significant main effects and interaction effects (p < 0.05) with post hoc tests (Fisher PLSD). Because our multifactorial ANOVAs and ANCOVAs yielded multiple main and interaction effects, we only report significant effects that are critical for data interpretation. Additionally, for clarity, we indicate results of post-hoc analyses by asterisks in the figures but they are not described in the “Results” section. Finally, in exp. 2 and exp. 4, there were no significant differences between the rats injected with vehicle into the ipsilateral or contralateral hemispheres (groups 1–2). Therefore, we combined the data from these groups for each experiment to create a larger n vehicle group.
Results
Training and extinction (exp. 1–4)
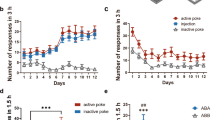
Figure 1a (left panel) shows mean ± SEM number of heroin infusions and presses on the active and inactive levers for all rats (exp. 1–4). The rats demonstrated reliable heroin self-administration, as indicated by increased number of infusions and active lever presses in response to halving the dose of heroin from 0.1 to 0.05 mg/kg/infusion on training day 7 (p < 0.01). Figure 1b (right panel) shows the mean ± SEM number of lever presses on the previously active lever and the inactive lever during the extinction phase. As expected, response rates decreased over time during the extinction phase, as evidenced by a main effect of extinction session (p < 0.01).
Heroin self-administration training and extinction of drug-reinforced responding. a Training: mean ± SEM number of infusions and active and inactive lever responses during the 12 days of heroin self-administration training for exp. 1–4. b Extinction: mean ± SEM number of presses on the active lever and inactive lever during the first 12 extinction sessions conducted in the absence of heroin in a different (extinction) context for exp. 1–4 (total n = 132)
Role of vSub → NAc shell projection in context-induced reinstatement
Exp. 1: effect of context-induced reinstatement on activation of the vSub → NAc shell projection
Exposure to the heroin context (ABA renewal group) reinstated active lever responding after extinction. The statistical analysis showed a significant interaction between group (renewal, control) and lever (active, inactive) (F (1,15) = 91.8, p < 0.01, Fig. 2a). Presses on the inactive lever were very low during testing (mean ± SEM = 3 ± 1 averaged between groups, with no significant difference between groups, data not shown). Unilateral FG injections into NAc shell resulted in reliable FG labeling in the ipsilateral vSub but almost no labeling in the contralateral vSub; these results are consistent with previous reports (Groenewegen et al. 1987; Sesack and Pickel 1990). Therefore, we only report the results for unilateral FG-IR and Fos + FG-IR quantification.
Activation of vSub → NAc shell projection during context-induced reinstatement. a Reinstatement test: total number of active lever presses in rats tested in the extinction (control A-B-B) or the heroin (renewal A-B-A) context. b FG-IR cells: number of FG-IR cells per mm2 in ipsilateral vSub of rats tested in the extinction or heroin context. c Fos-IR cells: number of total Fos-IR nuclei per mm2 (ipsilateral and contralateral hemispheres were averaged) in vSub of rats tested in the extinction or heroin context. d Fos + FG double-labeled cells: percentage of Fos + FG-IR cells in the ipsilateral vSub of rats tested in the extinction context or heroin context. e Representative photomicrographs of FG injection into NAc, FG labeling, and Fos + FG cells in ipsilateral vSub. *Different from the extinction context, p < 0.05, n = 8–9 per group
There was no difference between the control and renewal groups for the number of FG-labeled neurons in ipsilateral vSub (p > 0.05, Fig. 2b). Context-induced reinstatement of heroin seeking was associated with similar induction of Fos-IR nuclei in ipsilateral and contralateral vSub; therefore, we averaged the values from the two hemispheres for a total Fos-IR count. Fos-IR in vSub was higher in the renewal group than in the control group (t (15) = 6.0, p < 0.01, Fig. 2c). Fos expression in NAc shell was also higher in the renewal group than in the control group: mean ± SEM of Fos-IR counts per mm2 was 14.1 ± 2.1 and 2.4 ± 0.6, respectively (t (15) = 5.2, p < 0.05). Fos + FG double-labeled cells in vSub of the renewal group was higher than in the control group (t (15) = 3.9, p < 0.01, Fig. 2d). These findings indicate that context-induced reinstatement was associated with activation of the vSub → NAc shell projection.
Exp. 2: effect of disconnection of the vSub → NAc shell projection on context-induced reinstatement
Unilateral M + B injections into vSub and contralateral or ipsilateral SCH 23390 injections into NAc shell decreased context-induced reinstatement of heroin seeking (Fig. 3a). We analyzed the data by repeated measures ANCOVA (inactive lever presses as a covariate) using the between-subjects factors of group (vehicle [groups 1–2], ipsilateral SCH 23390 + M + B [group 3], contralateral SCH 23390 + M + B [group 4]) and the within-subjects factor of test context [heroin (A) or extinction (B)]. The analyses showed significant effects of group (F (2,38) = 8.5, p < 0.01), test context (F (1,38) = 15.1, p < 0.01), and group x test context (F (2,38) = 5.0, p < 0.05). Finally, presses on the inactive lever were very low during testing in the different groups (mean ± SEM = 6 ± 1 averaged across groups, with no significant difference between groups, data not shown).
Disconnection of vSub → NAc shell projection decreases context-induced reinstatement. a Reinstatement test: total number of active lever presses in rats tested in the extinction and heroin contexts after unilateral injections of vehicle or M + B into vSub and contralateral or ipsilateral injection of vehicle or SCH 23390 into NAc shell before exposure to the heroin context or the extinction context. b. Cannula placements and representative pictures. Approximate placements (mm from Bregma) of the injector tips for vSub and contralateral (left) or ipsilateral (right) NAc shell (Paxinos and Watson 2008) and representative photomicrographs of cannula placements are shown. Because implantation of cannula into the left or right hemisphere was counterbalanced, injector tip placements are depicted in both hemispheres (light gray circles = vehicle, black circles = drug). *Different from the vehicle condition, p < 0.01 (n = 10–19 per group)
Role of vSub → vmPFC projection in context-induced reinstatement
Exp. 3: effect of context-induced reinstatement on activation of the vSub → vmPFC projection
Exposure to the heroin context (ABA renewal group) reinstated active lever responding after extinction. The statistical analysis showed a significant interaction between group (renewal, control) and lever (active, inactive) (F (1,21) = 52.9, p < 0.01, Fig. 4a). Presses on the inactive lever were very low during testing (mean ± SEM = 2 ± 0 averaged between groups, with no significant difference between groups, data not shown). Unilateral FG injections into vmPFC resulted in reliable FG labeling in the ipsilateral vSub but almost no labeling in the contralateral vSub; these results are consistent with a previous report (Hoover and Vertes 2007). Therefore, we only report the results for unilateral FG-IR and Fos + FG-IR quantification. Three rats (2 from the control group and 1 from the renewal group) had low FG counts (<25 cells/mm2) and were omitted from the FG and Fos + FG analyses.
Activation of vSub → vmPFC projection during context-induced reinstatement. a Reinstatement test: total number of active lever presses in rats tested in the extinction (control A-B-B) or the heroin (renewal A-B-A) context. b FG-IR cells: number of FG-IR cells per mm2 in ipsilateral vSub of rats tested in the extinction or heroin context. c Fos-IR cells: number of total Fos-IR nuclei per mm2 (ipsilateral and contralateral hemispheres were averaged) in vSub of rats tested in the extinction or heroin context. (D) Fos + FG double-labeled cells: percentage of Fos + FG-IR cells in the ipsilateral vSub of rats tested in the extinction context or heroin context. e Representative photomicrographs of FG injection into vmPFC, respectively, FG labeling, and Fos + FG cells in ipsilateral vSub. *Different from the extinction context, p < 0.05 (n = 11–12 for a and c and n = 10 per group for b and d)
There was no difference between the control and renewal groups for the number of FG-labeled neurons in ipsilateral vSub (p > 0.05, Fig. 4b). Context-induced reinstatement of heroin seeking was associated with similar induction of Fos-IR nuclei in ipsilateral and contralateral vSub; therefore, we averaged the values from the two hemispheres for a total Fos-IR count. Fos-IR in vSub was higher in the renewal group than in the control group test (t (21) = 3.7, p < 0.01, Fig. 4c). Fos expression in vmPFC was also higher in the renewal group than in the control group: mean ± SEM of Fos-IR counts per mm2 was mean ± SEM was 37.7 ± 6.6 and 15.3 ± 4.2, respectively (t (21) = 2.8, p < 0.05). Fos + FG double-labeled cells in vSub of the renewal group was higher than in the control group (t (18) = 3.5, p < 0.01, Fig. 4d). These findings indicate that context-induced reinstatement was associated with activation of the vSub → vmPFC projection.
Exp. 4: effect of disconnection of the vSub → vmPFC projection on context-induced reinstatement
Unilateral M + B injection into vSub and contralateral or ipsilateral vehicle or M + B injection into vmPFC had no effect on context-induced reinstatement of heroin seeking (Fig. 5a). We analyzed the data by repeated measures ANCOVA (inactive lever presses as a covariate) using the between-subjects factors of group (vehicle [groups 1–2], ipsilateral M + B [group 3], contralateral M + B [group 4], unilateral vSub M + B [group 5]) and the within-subjects factor of test context [heroin (A) or extinction (B)]. The analysis showed a significant effect of test context (F (1,43) = 22.0, p < 0.01) but no other significant main or interaction effects [note: while previous studies have demonstrated a role of vmPFC in expression or consolidation of extinction learning (Peters et al. 2008; Quirk et al. 2000), we did not observe increased extinction responding in context B in rats injected with M + B into the vmPFC]. For clarity purposes, we do not show the data of the unilateral vSub M + B group (group 5), but active lever presses during testing in contexts A and B were similar to those of the other groups: mean ± SEM per 90 min of 38.8 ± 5.8 and 16.9 ± 4.3, respectively. Presses on the inactive lever were very low during testing in the different groups (mean ± SEM = 5 ± 1 averaged across groups, with no significant difference between groups, data not shown).
Disconnection of the vSub → vmPFC projection had no effect on context-induced reinstatement. a Reinstatement test: total number of active lever presses in rats tested in the extinction and heroin context after unilateral injections of M + B into vSub and contralateral or ipsilateral injection of M + B into vmPFC before exposure to the heroin context or the extinction context. b Cannula placements and representative pictures. Approximate placements (mm from Bregma) of the injector tips for vSub and contralateral (left) or ipsilateral (right) vmPFC (Paxinos and Watson 2008) and representative photomicrographs of cannula placements are shown (n = 11–14 per group)
Discussion
We studied the role of vSub → NAc shell and vSub → vmPFC glutamatergic projections in context-induced reinstatement of heroin seeking. We found that this reinstatement is associated with increased Fos in vSub neurons that project to either NAc shell or vmPFC, indicating that both projections are activated during the context-induced reinstatement test. However, anatomical disconnection of the vSub → NAc shell projection, but not the vSub → vmPFC projection, decreased this reinstatement. These results suggest that the vSub → NAc shell projection, but not the vSub → vmPFC projection, contributes to context-induced reinstatement. Therefore, while re-exposure to the heroin context activates both vSub projections, only the vSub → NAc shell projection is necessary for context-induced reinstatement. Our disconnection manipulation of the vSub → NAc shell projection comprised of local inactivation of vSub cell bodies with GABA receptor agonists and dopamine D1-family receptor blockade in NAc shell. Thus, we propose that an interaction between the vSub → NAc shell glutamatergic projection and local dopamine D1 postsynaptic receptors in NAc shell contributes to context-induced reinstatement of heroin seeking.
It is unlikely that the effect of the vSub → NAc shell contralateral or ipsilateral manipulations on context-induced reinstatement is due to diffusion into nearby brain areas, because we previously found that SCH 23390 injections into NAc core or M + B injections into dorsal CA1 have no effect on this reinstatement (Bossert et al. 2007; Bossert and Stern 2014). It is also unlikely that motor deficits or other non-specific behavioral effects of the drugs decreased context-induced reinstatement, because we previously found that bilateral injections of M + B into vSub or SCH 23390 into NAc shell have no effect on high-rate food-reinforced responding (Bossert et al. 2007; Bossert and Stern 2014; Marchant and Kaganovsky 2015).
The main interpretation issue in our study is how to explain the similar effects of the ipsilateral and contralateral manipulations of the vSub → NAc shell projection on context-induced reinstatement. The classic interpretation of asymmetrical disconnection findings is that a causal role of a given brain pathway in a behavior is inferred from the observation that a learned behavior is disrupted by the contralateral but not ipsilateral manipulation (Gaffan et al. 1993; Setlow et al. 2002). This interpretation is based on the assumption that learned behaviors can be maintained by an intact ipsilateral projection and that brain projections are primarily ipsilateral (Gaffan et al. 1993; Setlow et al. 2002). However, the findings from several studies on the similar effects of ipsilateral and contralateral inactivation manipulations of cortical projections to NAc and amygdala on reinstatement of drug seeking (Bossert et al. 2012; Fuchs et al. 2007; Peters et al. 2008) led us to consider alternative interpretations (Bossert et al. 2012; Crombag et al. 2008).
Perhaps the most straightforward possibility is that when the function of a given projection is critical for controlling a complex learned behavior, an intact ipsilateral projection is not sufficient to maintain the behavior (Crombag et al. 2008). In this regard, our present and previous data (Bossert et al. 2012) suggest that context-induced reinstatement may be particularly susceptible to ipsilateral disconnections. The development of optogenetic (Ma et al. 2014; Yizhar et al. 2011) and DREADD (Boender et al. 2014; Marchant et al. 2015; Nair et al. 2013) methods for projection-specific inhibition will allow us and other investigators to directly test this idea by comparing the effects of single-hemisphere projection inhibition versus dual-hemisphere projection inhibition on reinstatement of drug seeking. Another potential interpretation of our data is that unilateral inactivation of vSub in one hemisphere interferes with the normal function of vSub in the other hemisphere through commissural fibers (Kanamori 2015). However, this possibility is unlikely because in exp. 4 (see “Results”), we found that unilateral inactivation of vSub had no effect on context-induced reinstatement. Furthermore, we previously found that unilateral injections of SCH 23390 into NAc shell also had no effect on context-induced reinstatement of heroin seeking (Bossert et al. 2012).
Another interpretation of the similar effect of ipsilateral and contralateral drug injections on context-induced reinstatement is that this behavior relies on activation of both ipsilateral and contralateral vSub → NAc shell projections. However, this possibility is unlikely because unilateral FG injections into NAc shell resulted in almost no labeling in the contralateral vSub, a finding consistent with previous reports (Groenewegen et al. 1987; Sesack and Pickel 1990).
A potential interpretation of our data is that the similar effect of contralateral and ipsilateral manipulations is due to the disruption of communication between vSub and NAc shell through an additional brain area via both ipsilateral and contralateral projections. One possible vSub projection we tested in this study is to the vmPFC, which sends dense ipsilateral and sparse contralateral projections to NAc shell (Sesack et al. 1989; Vertes 2004). Indeed, as mentioned in the “Introduction,” we previously reported that disconnection of vmPFC → NAc shell projection decreases context-induced reinstatement of heroin seeking (Bossert et al. 2012). However, in exp. 4, we found that disconnection of the vSub → vmPFC projection has no effect on this reinstatement. Thus, it is unlikely that activity in vmPFC during the context-induced reinstatement test accounts for the similar effects of the ipsilateral and contraleral manipulations of the vSub → NAc shell projections.
Another brain area that can potentially play a role in the similar effects of the ipsilateral and contralateral vSub → NAc shell on context-induced reinstatement is the basolateral amygdala (BLA). The BLA plays an important role in context-induced reinstatement of cocaine and alcohol seeking (Chaudhri et al. 2013; Fuchs et al. 2005; Lasseter et al. 2011; McNally 2014). The BLA projects to NAc shell (Wright et al. 1996) and vSub (French et al. 2003). Additionally, anatomical (French and Totterdell 2003) and electrophysiological (Finch 1996; Mulder et al. 1998) evidence demonstrates convergence between vSub and BLA projections on individual NAc medium spiny neurons. However, the degree to which BLA activity can account for our data is unknown, because evidence for a role of BLA → NAc shell in context-induced reinstatement is mixed. Chaudhri et al. (2013) reported that reversible inactivation of this projection decreases context-induced reinstatement of alcohol seeking. In contrast, Millan and McNally (2011) reported that inactivation of this projection increases reinstatement of alcoholic beer seeking.
We speculate that ventral tegmental area (VTA) activity mediates the similar effects of the ipsilateral and contralateral vSub → NAc shell manipulations on context-induced reinstatement. The VTA sends dopaminergic (Fallon and Moore 1978; Ikemoto 2007) and glutamatergic (Yamaguchi et al. 2011; Zhang et al. 2015) projections to NAc shell, while NAc shell sends GABAergic projections to VTA (Haber et al. 2000; Oades and Halliday 1987). vSub stimulation-induced activation of NAc projection neurons inhibits tonically active GABAergic neurons in ventral pallidum, resulting in an increase in VTA dopamine cell firing (Floresco et al. 2003). Although most VTA → NAc projections are ipsilateral, some (∼8 %) of these projection neurons innervate the contralateral side (Swanson 1982); there is also evidence for sparse contralateral projections in the reciprocal NAc → VTA projection (Nauta et al. 1978; Watabe-Uchida et al. 2012). In a previous study, we found that inactivation of a small minority of context-activated (Fos-positive) vmPFC neurons by Daun02 decreased context-induced reinstatement to the same degree as inactivation of most vmPFC neurons by M + B (Bossert et al. 2011). Furthermore, we found that exposure to the heroin context activates both ipsilateral and contralateral vmPFC projections to NAc shell (Bossert et al. 2012). Taken together, these results suggest that putative context-encoding “neuronal ensembles” (Cruz et al. 2013) comprise of neurons that project both ipsilaterally and contralaterally.
The VTA also sends dopaminergic projections to vSub (Gasbarri et al. 1994). In an early study, we found that inhibition of glutamate transmission in VTA decreases context-induced reinstatement of heroin seeking (Bossert et al. 2004). Additionally, electrical or chemical stimulation of vSub increases VTA cell firing and dopamine release in NAc (Blaha et al. 1997; Legault et al. 2000; Taepavarapruk et al. 2014); the latter effect is blocked by inhibition of glutamate transmission in VTA (Floresco et al. 2001; Legault et al. 2000; Taepavarapruk et al. 2008). Furthermore, electrical stimulation of vSub reinstates cocaine and amphetamine seeking, and this effect on reinstatement is respectively blocked by inhibition of VTA glutamate transmission or blockade of NAc dopamine receptors (Taepavarapruk et al. 2014; Taepavarapruk and Phillips 2003; Vorel et al. 2001). Based on the above anatomical, physiological, and pharmacological findings, we propose that our ipsilateral manipulation of the vSub → NAc shell projection disrupted communication between vSub and NAc shell in both hemispheres by decreasing VTA dopamine cell firing and subsequent NAc shell dopamine release in VTA → NAc shell projections.
Concluding remarks
We combined a variation of an established asymmetrical disconnection procedure with retrograde tracing and Fos immunohistochemistry to demonstrate that activation of the vSub → NAc shell projection contributes to context-induced reinstatement of heroin seeking. We interpreted these data to suggest that an interaction between the vSub → NAc shell glutamatergic projection and local dopamine D1 postsynaptic receptors in NAc shell contributes to context-induced reinstatement. We also proposed two ideas that might account for the similar effects of the contralateral and ipsilateral manipulations of vSub → NAc shell projection on context-induced reinstatement. The first is that an intact ipsilateral vSub → NAc shell projection is critical for context-induced reinstatement. The second is that ipsilateral inhibition of the vSub → NAc shell projection interferes with activity in the contralateral hemispheric projection via the reciprocal connections between NAc shell and VTA. Future studies using projection-specific optogenetic and DREADD methods that overcome the interpretational issues associated with pharmacological and lesion disconnection techniques can directly test these ideas.
References
Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG (1997) Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci 9:902–911
Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MC, van der Plasse G, Adan RA (2014) Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS ONE 9:e95392
Bossert JM, Stern AL (2014) Role of ventral subiculum in context-induced reinstatement of heroin seeking in rats. Addict Biol 19:338–342
Bossert JM, Liu SY, Lu L, Shaham Y (2004) A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 24:10726–10730
Bossert JM, Gray SM, Lu L, Shaham Y (2006) Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology 31:2197–2209
Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27:12655–12663
Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y (2009) Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology 206:51–60
Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y (2011) Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci 14:420–422
Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y (2012) Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci 32:4982–4991
Bouton ME, Bolles RC (1979) Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process 5:368–378
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338:255–278
Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH (2013) Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci 38:2751–2761
Crombag HS, Shaham Y (2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116:169–173
Crombag H, Bossert JM, Koya E, Shaham Y (2008) Context-induced relapse to drug seeking: a review. Trans R Soc Lond B Biol Sci 363:3233–3243
Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT (2013) New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci 14:743–754
Curran T, Morgan JI (1995) Fos: an immediate-early transcription factor in neurons. J Neurobiol 26:403–412
Diergaarde L, de Vries W, Raaso H, Schoffelmeer AN, De Vries TJ (2008) Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A). Neuropharmacology 55:712–716
Fallon JH, Moore RY (1978) Catecholamine innervation of the basal forebrain IV topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol 180:545–580
Finch DM (1996) Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus 6:495–512
Floresco SB, Seamans JK, Phillips AG (1997) Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17:1880–1890
Floresco SB, Todd CL, Grace AA (2001) Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21:4915–4922
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973
French SJ, Totterdell S (2002) Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol 446:151–165
French SJ, Totterdell S (2003) Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119:19–31
French SJ, Hailstone JC, Totterdell S (2003) Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res 981:160–167
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309
Fuchs RA, Eaddy JL, Su ZI, Bell GH (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26:487–498
Gaffan D, Murray EA, Fabre-Thorpe M (1993) Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci 5:968–975
Gasbarri A, Packard MG, Campana E, Pacitti C (1994) Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull 33:445–452
Gold RM (1966) Aphagia and adipsia produced by unilateral hypothalamic lesions in rats. Am J Physiol 211:1274–1276
Grace AA, Floresco SB, Goto Y, Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neursoc 30:220–227
Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP (1987) Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23:103–120
Haber SN, Fudge JL, McFarland NR (2000) Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20:2369–2382
Hamlin AS, Newby J, McNally GP (2007) The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 146:525–536
Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78
Ikemoto S, Qin M, Liu ZH (2005) The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci 25:5061–5065
Jay TM, Witter MP (1991) Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 313:574–586
Kanamori K (2015) Disinhibition reduces extracellular glutamine and elevates extracellular glutamate in rat hippocampus in vivo. Epilepsy Res 114:32–46
Lasseter HC, Wells AM, Xie X, Fuchs RA (2011) Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 36:711–720
Legault M, Rompre PP, Wise RA (2000) Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci 20:1635–1642
Lu L, Grimm JW, Dempsey J, Shaham Y (2004) Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology 176:101–108
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schluter OM, Huang YH, Dong Y (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83:1453–1467
Marchant NJ, Kaganovsky K (2015) A critical role of nucleus accumbens dopamine D1-family receptors in renewal of alcohol seeking after punishment-imposed abstinence. Behav Neurosci 129:281–291
Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM (2014) Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res (in press).
Marchant NJ, Whitaker LR, Bossert JM, Harvey BK, Hope BT, Kaganovsky K, Adhikary S, Prisinzano TE, Vardy E, Roth BL, Shaham Y (2015) Behavioral and physiological effects of a novel kappa opioid receptor based DREADD in rats. Neuropsychopharmacology: in press.
McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663
McNally GP (2014) Extinction of drug seeking: neural circuits and approaches to augmentation. Neuropharmacology 76(Pt B):528–32
Mendoza J, Sanio C, Chaudhri N (2015) Inactivating the infralimbic but not prelimbic medial prefrontal cortex facilitates the extinction of appetitive Pavlovian conditioning in Long-Evans rats. Neurobiol Learn Mem 118:198–208
Millan EZ, McNally GP (2011) Accumbens shell AMPA receptors mediate expression of extinguished reward seeking through interactions with basolateral amygdala. Learn Mem 18:414–421
Miller CA, Marshall JF (2005) Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci 21:1385–1393
Mulder AB, Hodenpijl MG, Lopes da Silva FH (1998) Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci 18:5095–5102
Nair SG, Strand NS, Neumaier JF (2013) DREADDing the lateral habenula: a review of methodological approaches for studying lateral habenula function. Brain Res 1511:93–101
Nauta WJ, Smith GP, Faull RL, Domesick VB (1978) Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience 3:385–401
Oades RD, Halliday GM (1987) Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res 434:117–165
O’Brien CP, Childress AR, McLellan AT, Ehrman R (1992) Classical conditioning in drug-dependent humans. Ann NY Acad Sci 654:400–415
O’Donnell P (2003) Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17:429–435
O’Donnell P, Grace AA (1995) Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15:3622–3639
Paxinos G, Watson C (2008) The rat brain in stereotaxic coordinates. Sixth edition., 3 edn. Academic Press, San Diego, CA
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053
Quirk GJ, Russo GK, Barron JL, Lebron K (2000) The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20:6225–6231
Rubio FJ, Liu QR, Li X, Cruz FC, Leao RM, Warren BL, Kambhampati S, Babin KR, McPherson KB, Cimbro R, Bossert JM, Shaham Y, Hope BT (2015) Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. J Neurosci 35:5625–5639
Schmued LC, Fallon JH (1986) Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377:147–154
Sesack SR, Pickel VM (1990) In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res 527:266–279
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242
Setlow B, Holland PC, Gallagher M (2002) Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned responses. Behav Neurosci 116:267–275
Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353
Taepavarapruk P, Phillips AG (2003) Neurochemical correlates of relapse to d-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology 168:99–108
Taepavarapruk P, Howland JG, Ahn S, Phillips AG (2008) Neural circuits engaged in ventral hippocampal modulation of dopamine function in medial prefrontal cortex and ventral striatum. Brain Struct Funct 213:183–195
Taepavarapruk P, Butts KA, Phillips AG (2014) Dopamine and glutamate interaction mediates reinstatement of drug-seeking behavior by stimulation of the ventral subiculum. Int J Neuropsychopharmacol 18: in press
Thierry AM, Gioanni Y, Degenetais E, Glowinski J (2000) Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus 10:411–419
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58
Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004) Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27:468–474
Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001) Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292:1175–1178
Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873
Wikler A (1973) Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry 28:611–616
Wright CI, Beijer AV, Groenewegen HJ (1996) Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci 16:1877–1893
Yamaguchi T, Wang HL, Li X, Ng TH, Morales M (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci 31:8476–8490
Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011) Optogenetics in neural systems. Neuron 71:9–34
Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M (2015) Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci 18:386–392
Zironi I, Burattini C, Aicardi G, Janak PH (2006) Context is a trigger for relapse to alcohol. Behav Brain Res 167:150–155
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Intramural Research Program. This paper is part of a special issue entitled “Addiction research and the legacy of Steven R. Goldberg” and is dedicated to Dr. Steven Goldberg, a valued friend, colleague, and a pioneer in addiction research.
Conflict of interest
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bossert, J.M., Adhikary, S., St. Laurent, R. et al. Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology 233, 1991–2004 (2016). https://doi.org/10.1007/s00213-015-4060-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4060-5