Abstract
Glutamatergic neurons in ventral pallidum (VPGlu) were recently reported to mediate motivational and emotional behavior, but its role in opioid addiction still remains to be elucidated. In this study we investigated the function of VPGlu in the context-dependent heroin taking and seeking behavior in male rats under the ABA renewal paradigm. By use of cell-type-specific fiber photometry, we showed that the calcium activity of VPGlu were inhibited during heroin self-administration and context-induced relapse, but activated after extinction in a new context. The drug seeking behavior was accompanied by the decreased calcium signal of VPGlu. Chemogenetic manipulation of VPGlu bidirectionally regulated heroin taking and seeking behavior. Anterograde tracing showed that the lateral habenula, one of the epithalamic structures, was the major output region of VPGlu, and its neuronal activity was consistent with VPGlu in different phases of heroin addiction and contributed to the motivation for heroin. VPGlu axon terminals in LHb exhibited dynamic activity in different phases of heroin addiction. Activation of VPGlu-LHb circuit reduced heroin seeking behavior during context-induced relapse. Furthermore, the balance of excitation/inhibition from VP to LHb was shifted to enhanced glutamate transmission after extinction of heroin seeking motivation. Overall, the present study demonstrated that the activity of VPGlu was involved in the regulation of heroin addiction and identified the VPGlu-LHb pathway as a potential intervention to reduce heroin seeking motivation.
Similar content being viewed by others
Introduction
Opioid addiction is a severe brain disease characterized by compulsive drug taking and seeking which can be induced by drug paired context after extinction in a non-drug environment [1]. Although the vital contribution of brain reward system in drug addiction has been well demonstrated [2], the key brain region and neural circuit that modulate the context-dependent drug taking and seeking behavior in a cell-specific manner in the course of opioid addiction still remains unclear.
Ventral pallidum (VP), the major output region of basal ganglia, receives GABAergic projections from nucleus accumbens and glutamatergic innervation from prefrontal cortex, basolateral amygdala and paraventricular nucleus of thalamus and sends integrated information to downstream structures, including lateral habenula and ventral tegmental area [3]. This anatomy pattern indicates VP as a key structure involved in motivational and emotional behavior. Indeed, compelling evidence suggested that VP played a crucial role in reward seeking and taking [4,5,6]. Moreover, a substantial body of work has also studied the role of VP in addiction of alcohol and cocaine. VP mediates alcohol seeking motivation via projection to VTA, subthalamus nucleus and lateral hypothalamus [7, 8], and manipulation of different subtype of GABAergic neurons in VP regulates the relapse for alcohol [8]. A subpopulation of neurons expressing dopamine D3 receptors in VP are involved in cocaine seeking behavior during relapse [9]. These results suggest the importance of VP neurons in drug seeking motivation of non-opioid substances. However, it is well established that there is distinct neural mechanism and subcircuits between addiction of opioids and psychostimulants [10,11,12]. Although some evidence has suggested that VP may be also involved in opioid addiction [13, 14], whether and how subtype of neurons in VP and its neural circuit take part in the different phases of opioid taking and seeking behavior is still unknown.
The major type of neurons in VP is GABAergic, but the glutamatergic neurons expressing vesicular glutamate transporter 2 (VPGlu) are also identified [15]. Further studies in emotion and motivation have indicated that glutamatergic neurons in VP are involved in depressive-like behavior and place aversion [16, 17]. Activity of VPGlu is inhibited by the reward cue but activated by the negative stimuli and mediates the avoidance behavior toward threat [18]. However, on the contrary, it has also been reported that VP glutamatergic neurons participate in the salience processing and are both activated by reward and aversion [19]. These discrepant results, on the one hand, highlight the important neurological role of VPGlu, but on the other hand, make the function of VPGlu unclear. Recently, it was reported that the synaptic plasticity of VPGlu projecting to aversive targets was shifted after abstinence from cocaine addiction, indicating that VPGlu may be also involved in aberrant emotional change caused by drug addiction withdrawal [20]. Nevertheless, so far, there is little evidence supporting the role of VPGlu in modulating the pathological motivation for drug in opioid addiction. Whether VPGlu takes part in the different phases of opioid addiction and their neural circuit and adaptive change underlying the context-dependent opioid seeking motivation remain to be elucidated.
In the present study, we investigated the role of VPGlu in the context-dependent heroin taking and seeking behavior by use of ABA renewal paradigm [21, 22] and illuminated the critical function of VPGlu and their neural circuit in the process of opioid addiction.
Materials and methods
Subjects
Male Sprague-Dawley Rats (Shanghai Laboratory Animal Center, Chinese Academy of Sciences) weighing 270–300 g were housed 3–4 per cage under conditions of constant temperature of 23 ± 2 °C, humidity of 50% ± 5% and 12 h light/dark cycle (lights on from 7:00 a.m. to 7:00 p.m.) with access to food and water ad libitum. Rats were housed individually after jugular catheterization surgery. One day before the daily self-administration training, food intake was restricted to 20 g to maintain the body weight. All animal care and experimental procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institute Animal Care and Use Committee at Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Surgery
Briefly, animals were anesthetized by Zoletil (Virbac, Carros, France) [50 mg/kg, intraperitoneally (i.p.)] and then placed in a stereotaxic apparatus (RWD Life Science Co. Ltd., Shenzhen, China) and the AAV vectors of 300 nL were injected into the target nuclei using a micro-infusion pump (Harvard Apparatus, Holliston, MA, USA) at a rate of 200 nL/min with the following coordinates: VP (AP: +0.12 mm, ML: ±2.00 mm, DV: −8.10 mm); LHb (AP: −3.40 mm, ML: ±0.70 mm, DV: −5.10 mm). After the injection, an additional 7 min was allowed for diffusion of the virus.
Two weeks after stereotaxic surgeries, a silicone catheter was inserted to their jugular veins. And rats were allowed for recovery in their home-cage for ~1 week. Levofloxacin [2 mg/kg, intravenously (i.v.)] and heparin (Sinopharm Group Co. Ltd., Shanghai, China) [2 mg/kg, intravenously (i.v.)] were administered every day to prevent infection and tube obstruction.
Viral vectors
rAAV-Vglut2-GCaMP6s-WPRE-hGH pA (titer: 2.94E + 12 vg/mL); rAAV-Vglut2-hM4Di-EGFP-WPREs (titer: 5.31E + 12 vg/mL); rAAV-Vglut2-EGFP-WPRE-hGH pA (titer: 3.13E + 12 vg/mL); rAAV-Vglut2-Cre-WPRE-hGH pA (titer: 5.13E + 12 vg/mL); rAAV-EF1α-DIO-hM3Dq-mCherry-WPREs (titer: 1.01E + 13 vg/mL); rAAV-EF1α-DIO-mCherry-WPRE-hGH polyA (titer: 5.13E + 12 vg/mL); rAAV-hSyn-hM3Dq-EGFP-WPRE-hGH polyA (titer: 5.45E + 12 vg/mL); rAAV-hSyn-EGFP-WPRE-hGH pA (titer: 5.09E + 12 vg/mL); rAAV-hSyn-GCaMP6m-WPRE-hGH pA (titer: 5.58E + 12 vg/mL); rAAV-Vglut2-mCherry-WPRE-hGH pA (titer: 5.40E + 12 vg/mL); rAAV-Retro-EF1α-DIO-FLP-WPRE-hGH pA (titer: 1.01E + 13 vg/mL); rAAV-hSyn-fDIO-hM3Dq-EGFP-WPREs (titer: 5.44E + 12 vg/mL); rAAV-EF1a-fDIO-EGFP-WPRE-hGH polyA (titer: 5.08E + 12 vg/mL). All obtained from BrainVTA, Wuhan, China.
Optic fiber implantation
Briefly, following the GCaMP6 virus injection, an optic fiber (200/0.37, Inper, Hangzhou, China) was immediately implanted at the target regions to record the calcium fluorescent signals of VP or LHb. Dental cement and stainless-steel screws were used to secure the optic fiber to the skull of rats and prevent it from falling out.
Heroin self-administration
Heroin was abtained from Beijing Public Security Bureau Forensic Medical Examination Center. Standard rat operant chambers (AniLab Scientific Instruments Co. Ltd., Ningbo, China) equipped with two nose pokes, a house light and a syringe pump were used. The active nose poke was accompanied with one infusion of heroin, a 20 s extinguished house light, a 20 s illuminated nose poke light, a 3 s sound cue and a 20 s time-out period, while the inactive nose poke elicited no response. Rats were conducted on a fixed-ratio 1 (FR1) schedule. Each delivery of heroin was 100 μg/kg during d 1–6 and 50 μg/kg during d 7–12. The infusion volumes were equivalent in yoked saline group. The sessions lasted either 3 h or 30 times of infusions. All responding events were recorded by the software.
For self-administration test (SA test) involving chemogenetic manipulation, the training was performed as described above. The test was conducted on d 10 and 11 in a randomized counter-balanced design within-subjects, with half of rats in each group injected with clozapine (C6305, Sigma-Aldrich Corp., St. Louis, MO, USA) (0.1 mg/kg, i.p.) [8] and others with vehicle (i.p.) 30 min before test.
Extinction and context-induced relapse
The extinction and relapse test were conducted using ABA renewal paradigm established by Yavin Shaham in 2002 [22] with minor modification. Two different operant chambers (A and B) were distinct in wall pattern, floor texture and the color of house light. Following heroin self-administration in context A, rats were introduced to another operant chambers (context B) for extinction, where both two nose pokes had no drug infusions but were recorded. Extinction sessions lasted 3 h per day for 12 d or until the number of active pokes was stable for 3 consecutive days. One day after the completion of extinction, a 90 min context-induced relapse test (CIR test) was conducted in context A but the active nose poke triggered no delivery of heroin.
For CIR test involving chemogenetic manipulation, the self-administration and extinction training was performed as described above. Clozapine (0.1 mg/kg, i.p.) was injected into all groups of rats 30 min prior to the start of CIR test.
Fiber photometry
For fiber photometry recordings, the behavioral procedures were performed as described above. Baseline of calcium signal was recorded when rats were in their home cage before sessions, and the calcium signal during different phases of heroin addiction was recorded on d 10 or 11 of self-administration session, the last day of extinction session and in the initial 30 min of CIR test.
Data of calcium signal were collected by a multichannel fiber photometry recording system (RWD Life Science Co. Ltd., Shenzhen, China) with a 410 nm and a 470 nm laser source in a power between 20 and 40 μW.
The fiber photometry data obtained during the different sessions were analyzed and plotted with analysis software provided by RWD. For comparing the change of calcium signal around the active nose poke, the area under curve (AUC) of ΔF/F, calculated by (F-F0)/F0, was compared between pre (−2 s to 0 s) and post (0 s to 2 s) active nose poke. For comparing event frequency and averaged amplitude, a value of 4 times the mean of absolute deviation (4×MAD) of the baseline signal was used as a threshold for detecting events in both baseline and other sessions. For comparing signal intensity between groups of different sessions, z-score values calculated by (ΔF/F-mean△F/F)/std and the corresponding area under curve (AUC) were used. And the data were presented with histograms or heatmaps for better visualization.
Histology and immunofluorescence
Rats were deeply anesthetized with Zoletil (50 mg/kg, i.p.) and perfused transcardially with ice-cold saline followed by 4% PFA (4% paraformaldehyde in PBS). The brain was immersed into 4% PFA overnight at 4 °C and then dehydrated by 30% sucrose. Coronal sections (40 μm) were sliced with a freezing microtome (Leica, Germany). For immunofluorescence staining, each slice selected was blocked in 3% normal goat serum in PBST (0.3% Triton X-100 + PBS) for 1 h at room temperature and followed by incubation with primary antibodies overnight at 4 °C. Slices were then washed in PBS, incubated with fluorescent secondary antibodies for 2 h at room temperature. The images were acquired using LSM 710 laser-scanning confocal microscope. Primary antibodies used were: rabbit anti-c-fos (1:1000, 226003, Synaptic systems, Goettingen, Germany), mouse anti-substance P (1:1000, ab14184, Abcam, Cambridge, UK), guinea pig anti-Vglut2 (1:500, AB2251, Merck Millipore Ltd., MA, USA), mouse anti-Vgat (1:1000, 131011, Synaptic systems, Goettingen, Germany). Secondary antibodies were: goat anti-rabbit (1:1000, Alexa Fluor 488, A11008), goat anti-rabbit (1:1000, Alexa Fluor 594, A11012), goat anti-rabbit (1:1000, Alexa Fluor 647, A32733), goat anti-mouse (1:1000, Alexa Fluor 594, A11005), goat anti-mouse (1:1000, Alexa Fluor 488, A11001), goat anti-guinea pig (1:1000, Alexa Fluor 680, SA5-10098). All were obtained from Invitrogen, MA, USA.
VP axon projections collaterlization and co-localization
Images were analyzed using ImageJ to assess EGFP fluorescence intensity in major downstream targets and co-localization of axon terminal and Vglut2 or Vgat in LHb.
In situ hybridization
RNAscope multiplex fluorescent reagent kit v2 assay (Advanced Cell Diagnostics, Minneapolis, MN, USA) was used for mRNA expression detection. Briefly, brain slices (20 μm) were treated with tissue antigen retrieval reagent, then incubated with RNAScope protease III for 30 min at 40 °C, followed by mixed mRNA probes for 2 h at 40 °C, and then signals amplification was conducted to obtain effective detection performance.
Statistical analysis
All data were expressed as the mean ± SEM. Statistical significance was determined by two-tailed paired or unpaired Student’s t test, One-way ANOVA or Two-way ANOVA followed by Sidak’s post hoc test using GraphPad prism 9. A value of P < 0.05 was supposed to be statistically significant.
Results
Drug taking and seeking behavior in different phases of heroin addiction
The experimental procedure was shown in Fig. 1a. Briefly, rats were trained to self-administer heroin in context A for 12 d under FR1 schedule. The responses in active nose poke were gradually established under the dose of 100 μg/kg per infusion. When the dose of heroin decreased to 50 μg/kg per infusion, the number of active nose pokes was increased (Fig. 1b), demonstrating the reliable drug taking behavior. Then rats underwent extinction training in the context B for 12 d. The responses in active poke were gradually reduced to a low level and were relatively stable during the last three days, indicating the reduction of drug seeking motivation (Fig. 1c). Twenty-four hours after the last day of extinction, rats were randomly divided into two groups. One group was tested in context B (ABB), the other was back to context A (ABA). Drug seeking motivation was induced by heroin paired context, as the number of active pokes in the ABA group was magnificently increased compared with the ABB group, with slightly but not significantly increased inactive poke (Fig. 1d). We analyzed the number of active pokes in different time points during the 90 min relapse test and found that the drug seeking motivation was high in ABA group in the initial 30 min compared with ABB group, but gradually decreased to a low level, which was due to the absence of heroin. The number of active pokes in the first and second 30 min were significantly higher than that in the last 30 min in ABA group, and there was no significant difference between ABA group and ABB group in the last 30 min (Fig. 1e).
a Schematics of behavior training schedule. b Heroin self-administration training under FR1 schedule in context A. c Extinction training in context B. d Context-induced relapse test in context A (ABA group) and context B (ABB group). The number of active pokes was significantly increased in ABA group compared with ABB group. F(1,20) = 26.66, P < 0.0001, n = 6, Two-way ANOVA, ***P = 0.0002 compared with the number of active pokes in ABB group, Sidak’s post hoc test. e Number of active nose pokes in different time points. The drug seeking motivation was high in the initial 30 min and gradually reduced to a low level in the last 30 min. F(2,30) = 4.172, P = 0.0252, n = 6, Two-way ANOVA, ***P = 0.0009, *P = 0.0214 compared with ABB group, ##P = 0.0047 compared with the last 30 min in ABA group, Sidak’s post hoc test.
Dynamic calcium activity of VP glutamatergic neurons during different phases of heroin addiction
In order to investigate the response pattern of VP glutamatergic neurons (VPGlu) during different stages of heroin addiction, we employed fiber photometry to record the calcium signal of VPGlu in vivo in freely behaving rats. First, we validated the infection specificity of the adeno-associated virus (AAV) carrying Vglut2 promoter by fluorescence in situ hybridization (FISH). AAV-Vglut2-mCherry was injected to VP and FISH for Vglut2 and mCherry was conducted (Fig. 2a). The result showed that the vast majority of mCherry+ cells in VP were Vglut2+ cells (98.6% for mCherry+ cells) (Fig. 2b, c). Then the AAV-Vglut2-GCaMP6s vector was injected and an optic fiber was implanted into VP to record the fluorescence changes of GCaMP6s (Fig. 2d). We first examined the activity of VPGlu during heroin self-administration (Fig. 2e, f). We found that the frequency of the calcium signals was significantly reduced during self-administration in context A (Fig. 2g), and the averaged amplitude was also decreased (Fig. 2h). We then analyzed the peri-event change of the calcium signals around the active nose pokes, which showed that there was no significant alteration of averaged ΔF/F around the active poke behavior (Fig. 2i–k).
a Viral strategy of VP micro-injection. b Representative image of FISH for mCherry and Vglut2. Scale bar, 50 μm. White triangle: mCherry+ Vglut2+ cells. c Percentage of mCherry+ Vglut2+ cells of total mCherry+ cells. d Viral strategy and optic fiber implantation allowed recordings of GCaMP6s-expressing VPGlu. Scale bar, 200 μm. e Schematics of behavior training and fiber photometry recording schedule. f Representative traces of calcium signal of baseline and heroin self-administration recorded from GCaMP6s-expressing VPGlu. Tick marks above traces indicate threshold-detected events. g Event frequency of calcium signal during heroin self-administration was significantly decreased. **P = 0.0013 compared with baseline, t = 5.179, df = 7, n = 8, paired t test. h Averaged amplitude of calcium signal during heroin self-administration was significantly decreased *P = 0.0156 compared with baseline, t = 3.177, df = 7, n = 8, paired t test. i, j Representative Heatmap and histogram of the peri-event change of VPGlu calcium signal during heroin self-administration. k Area under curve of the averaged ΔF/F did not change between pre and post active poke. P = 0.2509 compared with pre active poke, t = 1.298, df = 5, n = 6, paired t test.
We then recorded the calcium signal of VPGlu on the last day of extinction in context B, when the drug seeking behavior almost disappeared (Fig. 3a). The frequency of calcium signals was significantly increased compared with baseline (Fig. 3b, c), and the averaged amplitude was also up-regulated (Fig. 3d). We did not record the peri-event calcium activity due to the low level of active poke during the late period of extinction. Then we recorded during the first 30 min of context-induced relapse when the drug seeking motivation was high (Fig. 3e) and found that the frequency of the calcium activity of VPGlu was again down-regulated (Fig. 3f, g), with the averaged amplitude slightly but not significantly decreased (Fig. 3h). Surprisingly, we also observed a significantly inhibitory signal when rats responded in the active poke (Fig. 3i–k), indicating that there was a different peri-event pattern of VPGlu calcium activity between self-administration and context-induced relapse. These results suggest that VP glutamatergic neurons exhibited dynamic activity in different phases of opioid addiction and their activation and inhibition might relate to the reduction and augmentation of heroin taking and seeking motivation, respectively.
a Schematics of behavior training and fiber photometry recording schedule. b Representative traces of calcium signal of baseline and extinction recorded from GCaMP6s-expressing VPGlu. Tick marks above traces indicate threshold-detected events. c Event frequency of calcium signal during extinction was significantly increased. *P = 0.0343 compared with baseline, t = 2.888, df = 5, n = 6, paired t test. d Averaged amplitude of calcium signal during extinction was significantly increased. *P = 0.0240 compared with baseline, t = 3.198, df = 5, n = 6, paired t test. e Schematics of behavior training and fiber photometry recording schedule. f Representative traces of calcium signal of baseline and relapse recorded from GCaMP6s-expressing VPGlu. Tick marks above traces indicate threshold-detected events. g Event frequency of calcium signal during relapse was significantly decreased. *P = 0.0330 compared with baseline, t = 2.920, df = 5, n = 6, paired t test. h Averaged amplitude of calcium signal during relapse was slightly but not significantly decreased. P = 0.0606 compared with baseline, t = 2.414, df = 5, n = 6, paired t test. i, j Representative heatmap and histogram of the peri-event change of VPGlu calcium signal during context-induced relapse. k Area under curve of the averaged ΔF/F significantly decreased after active poke. **P = 0.0071 compared with pre active poke, t = 4.386, df = 5, n = 6, paired t test.
Chemogenetic manipulation of VP glutamatergic neurons modulated heroin taking and seeking behavior
To explore whether the activity of VPGlu regulates heroin taking and seeking behavior in different stages of addiction, we chemogenetically manipulated VPGlu by micro-injecting AAV-Vglut2-hM4Di-EGFP or the combination of AAV-Vglut2-Cre and EF1α-DIO-hM3Dq-mCherry vector into VP in order to inhibit or activate the glutamatergic neurons in vivo during behavioral test. The control group was micro-injected with AAV-Vglut2-EGFP or the combination of AAV-Vglut2-Cre and EF1α-DIO-mCherry vector.
As the result of fiber photometry showed that the calcium activity of VPGlu was increased after extinction, we assumed that the activation of VPGlu might attenuate the heroin addiction behavior. To test this, we first activated VPGlu during heroin self-administration (Fig. 4a–c). The effect of chemogenetic activation was verified by the increased co-localization of c-fos and mCherry in hM3Dq group (Fig. 4d, e). We found that the number of active pokes and heroin infusions were significantly decreased, with the inactive poke unchanged after VPGlu activation (Fig. 4f). The injection of clozapine in the control group had no effect (Fig. 4g). Reinstatement of drug seeking in heroin paired context was also blocked by VP glutamatergic activation (Fig. 4h).
a, b Viral strategy and representative micrograph showing the expression of AAV vector in VPGlu. Substance P was used to delineate the VP. Scale bar, 200 μm. c Schematics of behavior training and test schedule. d Representative micrographs showing the effect of chemogenetic activation. Scale bar, 20 μm. e Number of c-fos-mCherry co-localization cells was significantly increased by chemogenetic activation of VPGlu. ***P = 0.0001 compared with mCherry group, t = 15.51, df = 4, n = 3, unpaired t test. f Number of active pokes and infusions of heroin were significantly decreased by activation of VPGlu. F(1,36) = 19.64, P < 0.0001, n = 7, Two-way ANOVA, ***P = 0.0003, *P = 0.0261 compared with vehicle group, Sidak’s post hoc test, with inactive poke unchanged. P = 0.9459 compared with vehicle group, Sidak’s post hoc test. g Injection of clozapine had no effect on drug taking behavior in control (mCherry) group. F(1,12) = 0.2157, P = 0.6506, n = 5, Two-way ANOVA. h Number of active pokes was decreased by activation of VPGlu. F(1,16) = 39.18, P < 0.0001, n = 4–6, Two-way ANOVA, ****P < 0.0001 compared with mCherry gourp, Sidak’s post hoc test. Number of inactive pokes was unchanged. P = 0.2105 compared with mCherry gourp, Sidak’s post hoc test.
Next, we sought to see whether further inhibition of VPGlu could promote the drug taking and seeking behavior (Fig. 5a, b). The inhibitory effect of hM4Di was confirmed by the decreased co-localization of c-fos and EGFP in hM4Di group (Fig. 5c, d). However, chemogenetic inhibition of VP glutamatergic neurons during heroin self-administration did not increase the number of active pokes and infusions of heroin, but significantly increased the number of inactive pokes (Fig. 5e, f). The injection of clozapine in the control group had no effect (Fig. 5g). Then we inhibited VPGlu during relapse and found that the total number of both the active and inactive pokes tended to increase but the difference is not statistically significant (Fig. 5h, i). When we compared the number of active pokes in different time points during the 90 min relapse test, we found that the reduction of drug seeking behavior was delayed by VPGlu inhibition and the number of active pokes was significantly increased in the last 30 min (Fig. 5j, k) when the motivation was very low in the control group due to the absence of heroin, suggesting that the glutamatergic inhibition might extend the time of seeking drug. Therefore, we inhibited VPGlu on the last day of extinction training to see whether the drug seeking behavior could reappear in context B (Fig. 5l). The result showed that the number of active pokes was slightly but significantly increased by VPGlu inhibition (Fig. 5m), and the number of total responses was also raised (Fig. 5n), indicating that the VPGlu activation is necessary to the constraint of heroin seeking behavior. Taken together, these findings show that the activity of VP glutamatergic neurons bidirectionally modulates heroin taking and seeking behavior in different phases of heroin addiction.
a, b Viral strategy and representative micrograph showing the expression of AAV vector in VPGlu. Substance P was used to delineate the VP. Scale bar, 200 μm. c Representative micrographs showing the effect of chemogenetic inhibition. Scale bar, 20 μm. d Number of c-fos-EGFP co-localization cells was significantly decreased by chemogenetic inhibition of VPGlu. *P = 0.0491 compared with EGFP group, t = 2.794, df = 4, n = 3, unpaired t test. e Schematics of behavioral test during self-administration training. f Number of active pokes and infusions of heroin were not changed by inhibition of VPGlu, but the number of inactive pokes was slightly but significantly increased. F(1,24) = 0.08187, P = 0.7772, n = 9, Two-way ANOVA, *P = 0.0168 compared with vehicle group, Sidak’s post hoc test. g Injection of clozapine had no effect on self-administration behavior in control (EGFP) group. F(1,12) = 0.6807, P = 0.4255, n = 5, Two-way ANOVA. h Schematics of behavioral test during context-induced relapse. i Number of active pokes and inactive pokes were slightly but not significantly increased by inhibition of VPGlu during context-induced relapse. F(1,18) = 2.127, P = 0.1620, n = 4–7, Two-way ANOVA. j, k The time of drug seeking was extended by inhibition of VPGlu (j) and the number of active pokes was increased in the last 30 min of relapse (k). *P = 0.0224 compared with EGFP control, t = 2.753, df = 9, n = 4–7, unpaired t test. l Schematics of behavioral test during extinction training. m, n Number of active pokes (m) and total responses (n) was significantly increased by inhibition of VPGlu in the last day of extinction. m F(1, 27) = 6.255, P = 0.0188, n = 7–9, Two-way ANOVA, *P = 0.0133 compared with EGFP group, Sidak’s post hoc test; n *P = 0.0382 compared with EGFP group, t = 2.288, df = 14, n = 7–9, unpaired t test.
Activity of neurons in LHb was involved in the regulation of heroin addiction
The neural circuit by which VPGlu regulates the drug taking and seeking behavior is unknown. Thus, to find the possible projection target of VP glutamatergic neurons that is involved in heroin addiction, we first conducted the anterograde tracing by injecting the AAV-Vglut2-EGFP vector to VP (Fig. 6a, b). Among the several downstream targets, we found that the fluorescence intensity of VP glutamate axon terminals in LHb was significantly higher than those in other regions, including mediodorsal thalamus (MD), nucleus accumbens (NAc) and ventral tegmental area (VTA) (Fig. 6c, d). As the activity of VPGlu was increased during extinction, we sought to see whether the downstream targets were also activated by extinction training. We examined the c-fos expression after the last day of extinction, and found that the number of c-fos positive nuclei in LHb was significantly increased compared with the yoked saline group, but was unchanged in MD, NAc and VTA (Fig. 6e, f). LHb has been identified as a key brain region in epithalamus, mediating motivational and emotional behavior and the aversive effect of cocaine addiction [23,24,25,26], but its role in opioid taking and seeking behavior is poorly understood. Previous studies reported that the main type of neurons in LHb was glutamatergic [24, 27]. We confirmed this by fluorescence in situ hybridization and found that the glutamatergic neurons in LHb were mainly Vglut2 positive, with no Vgat and Vglut1 mRNA detected (Supplementary Fig. S1). Next, we examined the neural activity in LHb during different phases of heroin addiction by use of fiber photometry. AAV-hSyn-GCaMP6m vector was injected and an optic fiber was implanted to record the calcium signal of LHb neurons (Fig. 6g, h). The peri-event analysis around active nose poke revealed that during heroin self-administration, the calcium activity of LHb neurons was decreased after active poke (Fig. 6i–k). When we compared the area under curve (AUC) of z-score between three groups: extinction, heroin self-administration and yoked saline, we found that the calcium activity was significantly increased in the extinction group, while was attenuated during heroin self-administration (Fig. 6l, m). These results indicated that the activity of neurons in LHb was inhibited during self-administration, and was augmented after extinction. In order to examine whether activity of LHb neurons modulates heroin addiction, we micro-injected AAV-hSyn-hM3Dq-EGFP vector into LHb (Fig. 6n, o). Chemogenetic activation of LHb neurons abolished the heroin taking and seeking behavior during self-administration (Fig. 6p) and context-induced relapse (Fig. 6q), respectively. Thus, consistent with VPGlu, the activity of LHb neurons is involved in the heroin addiction, and its activation contributes to the reduction of heroin seeking motivation.
a, b Viral strategy of VPGlu anterograde tracing and representative micrograph showing the expression of AAV vector in VPGlu. Scale bar, 200 μm. c Representative micrographs showing the fluorescence of VPGlu axon terminal in downstream targets. Scale bar, 100 μm. d The intensity of the fluorescence in LHb was higher than other regions, including MD, NAc and VTA. F(3,8) = 6.385, P = 0.0162, n = 3, One-way ANOVA, *P = 0.0297 compared with MD, *P = 0.0257 compared with NAc, *P = 0.0148 compared with VTA, Sidak’s post hoc test. e Representative micrographs of c-fos expression in downstream targets of VPGlu. Scale bar, 20 μm. f Number of c-fos positive nuclei in LHb, but not MD, NAc and VTA was increased after extinction. F(1,16) = 5.363, P = 0.0342, n = 3, Two-way ANOVA, **P = 0.0038 compared with yoked saline group, Sidak’s post hoc test. g, h Viral strategy and optic fiber implantation (g) allowed recordings of GCaMP6m-expressing LHb neuorns (h). Scale bar, 100 μm. i, j Representative heatmap and histogram of calcium signal in LHb during heroin self-administration. k Area under curve of the averaged ΔF/F significantly decreased after active poke during heroin self-administration. *P = 0.0424 compared with pre active poke, t = 2.940, df = 4, n = 5, paired t test. l Representative traces of LHb calcium signal in yoked saline, heroin self-administration and extinction groups. m The calcium activity of LHb in extinction group was increased, while was decreased in heroin self-administration group. F(2,7) = 31.15, P = 0.0003, n = 3–4, One-way ANOVA, **P = 0.0026 compared with yoked saline group, ***P = 0.0002 compared with heroin SA group, Sidak’s post hoc test. n, o Viral strategy and representative micrograph showing the expression of AAV vector in LHb. Scale bar, 100 μm. p Chemogenetic activation of LHb neurons blocks the heroin self-administration behavior. F(1,21) = 16.20, P = 0.0006, n = 8, Two-way ANOVA, **P = 0.0063 compared with vehicle group, Sidak’s post hoc test. q Chemogenetic activation of LHb neurons blocks the drug seeking during context-induced relapse. F(1,22) = 18.47, P = 0.0003, n = 6–7, Two-way ANOVA, **P = 0.0020 compared with saline group, Sidak’s post hoc test.
VPGlu regulated heroin addiction via direct innervation to LHb
We have demonstrated that the VPGlu and its projection target LHb exhibited the same change of activity during different phases of heroin addiction. Thus, to explore whether VPGlu modulates heroin addiction via direct projection to LHb, we first micro-injected the AAV-Vglut2-GCaMP6s vector into VP and implanted optic fiber to LHb, in order to record the VPGlu axon terminal in LHb during the process of heroin addiction (Fig. 7a, b). We found that both the frequency and averaged amplitude of calcium signals of VPGlu axon terminal in LHb was significantly attenuated during heroin self-administration (Fig. 7c–e). However, the frequency was increased after extinction of drug seeking motivation, with the averaged amplitude unchanged (Fig. 7f–h). We also analyzed the peri-event change of ΔF/F around active nose pokes during heroin self-administration and context-induced relapse (Fig. 7i–l), which showed that the active poke behavior during both processes was closely associated with the inhibition of VPGlu axon terminal in LHb (Fig. 7m, n). These results suggested that there was dynamic activity of VPGlu-LHb circuit during different phases of heroin addiction. Next, in order to see whether VPGlu could regulate the activity of LHb neurons, we micro-injected the combination of AAV-Vglut2-Cre and EF1α-DIO-hM3Dq-mCherry vector into VP (Fig. 7o). Three weeks later, we chemogenetically activated VP glutamatergic neurons by injection of clozapine (i.p.), and conducted immuofluorescene to examine the c-fos expression in LHb. We found that activation of VPGlu increased the number of c-fos positive nuclei in LHb (Fig. 7p, q), indicating that there was a functional connection between VPGlu and LHb neurons. Then we sought to determine the functional role of VPGlu-LHb circuit in modulating drug taking and seeking behavior. We micro-injected AAV-retro-DIO-flp into LHb and the combination of AAV-Vglut2-Cre and AAV-fDIO-hM3Dq-EGFP or AAV-fDIO-EGFP into VP, by which we could chemogenetically activate VPGlu-LHb circuit during self-administration and relapse (Fig. 7r). We found that activation of this glutamate circuit only partly decreased the drug taking behavior during self-administration (Fig. 7s), but significantly reduced drug seeking motivation during context-induced relapse (Fig. 7t), suggesting that inhibition of VPGlu to LHb projection was necessary to drug seeking behavior, and its activation resulted in the reduction of motivation. Taken together, these results illustrate that VPGlu-LHb circuit plays a pivotal role in drug-seeking motivation.
a, b Viral strategy and representative micrograph allowed recording of the VPGlu axon terminal in LHb. Scale bar, 100 μm. c Representative traces of calcium signal of baseline and heroin self-administration recorded from GCaMP6s-expressing VPGlu axon terminal in LHb. Tick marks above traces indicate threshold-detected events. d Event frequency of calcium signal during heroin self-administration was significantly decreased. *P = 0.0392 compared with baseline, t = 2.773, df = 5, n = 6, paired t test. e Averaged amplitude of calcium signal during heroin self-administration was significantly decreased. *P = 0.0170 compared with baseline, t = 3.513, df = 5, n = 6, paired t test. f Representative traces of calcium signal of baseline and extinction recorded from GCaMP6s-expressing VPGlu axon terminal in LHb. Tick marks above traces indicate threshold-detected events. g Event frequency of calcium signal during extinction was significantly increased. *P = 0.0366 compared with baseline, t = 3.606, df=3, n = 4, paired t test. h Averaged amplitude of calcium signal during extinction was unchanged P = 0.3073 compared with baseline, t = 1.227, df=3, n = 4, paired t test. i, j Representative heatmap and histogram of calcium signal of VPGlu axon terminal in LHb during heroin self-administration. k, l Representative heatmap and histogram of calcium signal of VPGlu axon terminal in LHb during context-induced relapse. m, n Area under curve of the averaged ΔF/F significantly decreased after active poke during both heroin self-administration (m) and context-induced relapse (n). m *P = 0.0430 compared with pre active poke, t = 2.926, df = 4, n = 5, paired t test; n **P = 0.0064 compared with pre active poke, t = 6.854, df = 3, n = 4. o Viral strategy allowed the chemogenetic manipulation of VPGlu. p Representative micrographs showing the LHb c-fos expression in mCherry and hM3Dq group. Scale bar, 20 μm. q Activation of VPGlu increased the number of c-fos positive nuclei in LHb. *P = 0.0173 compared with mCherry group, t = 3.917, df = 4, n = 3, unpaired t test. r Viral strategy allowed the chemogenetic manipulation of VPGlu-LHb circuit. s Activation of VPGlu-LHb circuit partly block the drug taking behavior during heroin self-administration. F(1,18) = 2.545, P = 0.1280, n = 7, Two-way ANOVA, Sidak’s post hoc test. t Activation of VPGlu-LHb circuit significantly attenuated the drug seeking behavior during context-induced relapse (t). F(1,24) = 7.209, P = 0.0129, n = 7–8, Two-way ANOVA, *P = 0.0102 compared with EGFP group, Sidak’s post hoc test.
Extinction of heroin seeking motivation changed the balance of VP glutamate/GABA transmission to LHb
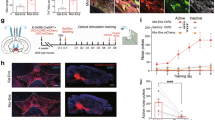
Based on our findings that reduction of heroin seeking behavior was mediated by the activation of VPGlu-LHb circuit, we sought to know whether the glutamate transmission from VP to LHb had adaptive change which resulted from the extinction training. The presynaptic accumulation and release of neurotransmitter are mainly regulated by the expression of vesicle transporter [28]. Thus, we first micro-injected AAV-Vglut2-mCherry vector into VP to label the glutamatergic neurons and their axon terminals in downstream target (Fig. 8a–c). Then, after the last day of extinction, we conducted immunofluorescence to examine the expression of Vglut2 protein in the axon terminal of VP glutamatergic neurons that projected to LHb. We found that the co-localization of VPGlu axon terminal and Vglut2 resulted in a significant increase in LHb after extinction (Fig. 8d, e), indicating that extinction led to the enhancive glutamate transmission from VP to LHb.
a Viral strategy allowed labeling of VPGlu and their axon terminals in LHb. b, c Representative micrographs showing the expression of AAV vector in VPGlu (Scale bar, 200 μm) and the axon terminals in LHb (Scale bar, 100 μm). d Representative micrographs showing the co-localization of Vglut2 and VPGlu axon terminals in LHb. Scale bar, 10 μm. e The co-localization of Vglut2 and VPGlu axon terminals in LHb was increased after extinction of heroin seeking motivation. *P = 0.0304 compared with yoked saline group, t = 2.520, df = 10, n = 4–8, unpaired t test. f Viral strategy allowed labeling of VPGABA and their axon terminals in LHb. g, h Representative micrographs showing the expression of AAV vector in VPGABA (Scale bar, 200 μm) and the axon terminals in LHb (Scale bar, 100 μm). i Representative micrographs showing the co-localization of Vgat and VPGABA axon terminals in LHb. Scale bar, 10 μm. j The co-localization of Vgat and VPGABA axon terminals in LHb was decreased after extinction of heroin seeking motivation. **P = 0.0072 compared with yoked saline group, t = 3.453, df = 9, n = 5–6, unpaired t test.
As the calcium activity of VPGlu-LHb circuit was inhibited during context induced relapse, we asked whether the enhancive Vglut2 expression in VPGlu-LHb axon terminal could be reversed by relapse behavior. Therefore, we examined the co-localization of VPGlu axon terminal and Vglut2 after the last day of extinction training (ABB) or 30 min context induced relapse test (ABA) during which the heroin seeking motivation was high. However, we did not see any significant change between ABB and ABA group, indicating that the increased expression of Vglut2 in axon terminal of VPGlu could not be reversed by acute exposure to the drug paired context and drug seeking behavior (Supplementary Fig. S2).
The major type of neurons in VP is GABAergic (VPGABA). Evidence suggested that VPGABA mediated the increased motivation for reward and cocaine [18, 29]. Thus, we supposed that VP GABAergic transmission would not change when the motivation for heroin was down-regulated. We examine the Vgat expression in VP GABAergic terminal in LHb by the same way after extinction (Fig. 8f–h). Surprisingly, the co-localization of Vgat and GABAergic terminal in LHb was significantly decreased after extinction (Fig. 8i, j), indicating the down-regulated GABA transmission from VP to LHb. These data implied that the reduction of drug-seeking motivation by extinction changed the balance of GABA/glutamate transmission from VP to LHb, with glutamte enhanced and GABA decreased, and this out-of-balance may lead to the activation of LHb neurons and contributes to the attenuation of drug seeking behavior.
Discussion
Activity of VP glutamatergic neurons have been previously suggested mediating the emotion and motivation [18]. Recently, study by Levi et al. indicated that VPGlu might be involved in the aversive emotion symptoms caused by abstinence from drug addiction, but the function and neurocircuit mechanism of VPGlu mediating the pathological motivation for drug during the process of opioid addiction remains to be elucidated. As opioid addiction, to some extent, share the same neural circuit with natural reward [2], VP glutamatergic neurons may also play an important role in the motivation of opioid taking and seeking. But their precise function underlying opioid addiction is unknown. In the present study, using ABA renewal paradigm combined with fiber photometry, we revealed that the activity of VPGlu was decreased in drug paired context A during heroin self-administration and context-induced relapse, but was increased after extinction training of heroin seeking behavior in context B, which demonstrated that VPGlu was inhibited by heroin taking and seeking but activated after context-dependent reduction of opioid seeking motivation. Chemogenetic activation of glutamatergic neurons abolished the drug taking and seeking behavior. However, inhibition of VPGlu did not increase the drug taking behavior. We ascribe this to the marked reduction of the VPGlu activity during heroin self-administration, which might occlude the function of the further chemogenetic inhibition of these neurons. Although inhibition of VPGlu did not significantly increase the number of active pokes during relapse either, especially during the first 30 min when the drug seeking motivation was relatively high, it extended the time of drug seeking in the last 30 min when the seeking behavior was significantly decreased in control group due to the absence of heroin, indicating that the high activity of VPGlu might be necessary to the restraint of drug seeking behavior. We further confirmed this by inhibiting VPGlu in the last day of extinction, which resulted in the increased drug seeking behavior in context B. These results suggest that the dynamic activity of VPGlu mediates the drug taking and seeking behavior in different phases of opioid addiction and its augmented activity is critically involved in the reduction of opioid seeking motivation.
Different from the findings of Heinsbroek et al. towards cocaine [29], our results showed that the activity of VPGlu was inhibited during context-induced heroin relapse. One possible explanation of this discrepancy may be the different paradigm used in two studies. Heinsbroek et al. used cue-induced relapse, by which mice were trained in the same context during self-administration and extinction, and the drug seeking motivation was induced by drug paired cues, such as light or sound. In our study, we employed ABA renewal paradigm, with rats trained self-administration in context A and extinction in a new context B, and the relapse of heroin seeking was induced by re-exposure to drug paired context A. Therefore, the different change of VPGlu activity might be due to the factor of context. However, so far, there is no evidence suggesting the distinct mechanism underlying cue and context-induced relapse. Another most likely reason is the different pharmacological effect between cocaine and opioids. Cocaine produces the reward effect through blocking the re-uptake of monoamine [30], while heroin increases the release of dopamine by binding with μ-opioid receptor on VTA GABAergic interneurons and disinhibition of adjacent dopamine neurons [31]. It was reported that cocaine also produced aversive effect. Animals that self-administered cocaine in runway model developed approach-avoidance conflict behavior [32] and injection of cocaine has anxiogenic effect evaluated by plus-maze test [33]. The molecular mechanism underlying the aversive effect of cocaine is unclear. But it’s worth noting that LHb neurons are activated after a single injection of cocaine when the function of cocaine is predominantly aversive [34]. Electrophysiological study in vitro reveals that cocaine could depolarize LHb neurons, increase their firing rate, and enhance the glutamatergic excitation to LHb [35]. Moreover, the excitatory input from rEPN to LHb drives cocaine avoidance behavior [36]. These results indicate that the activation of LHb and its excitatory projection are involved in the administration of cocaine. And it is well known that the increased activity of LHb leads to the negative emotions [37]. Therefore, we infer that, during cocaine addiction, the drug paired context and drug seeking behavior may also be associated to the aversive aspect of cocaine besides the reward effect, which leads to the activation of LHb and its excitatory upstream nucleus, such as VPGlu. On the contrary, our data showed that VPGlu and LHb were both inhibited during heroin self-administration. The inhibition of LHb is often thought to be related to the cue that predicting the reward outcome [38]. Thus, the drug paired context might only associate to the reward effect of heroin, which decreased the activity of excitatory circuit of LHb during context-induced relapse. More work is needed to further explore the discrepant function of cocaine and opioid in regulating neural activity in this circuit.
Electrophysiological study in vitro indicated that one of the major projection targets of VPGlu is LHb [20]. Indeed, according to our result of anterograde tracing, LHb received more intense projection from VP glutamatergic neurons than other VP downstream targets like NAc, VTA and MD. Compelling evidence has suggested the key role of LHb in cocaine seeking. Cocaine increased the activity of LHb neurons and inactivation of either LHb or its glutamatergic input decreased cocaine seeking behavior [39, 40], indicating that the activation of LHb and its excitatory input are important to cocaine seeking motivation. However, in the present study, fiber photometry recording revealed that LHb neurons were inhibited during heroin addiction but were activated after extinction in context B, which was consistent with the activity of VPGlu. Chemogenetic activation of LHb abolished the drug taking and seeking behavior during self-administration and context-induced relapse. These findings demonstrate that the inhibition of LHb is necessary to the motivation for opioids, and activation of LHb leads to the reduction of opioid seeking motivation. These results provide new perspective to the different neural mechanism between opioids and cocaine.
Studies have found that VPGlu mediated the avoidance behavior and negative reinforcement via innervation to LHb [41]. But the function of this circuit in drug addiction is unknown. We showed that the activity of VPGlu-LHb circuit had negative correlation with the motivation for heroin. Activation of this circuit reduced the heroin seeking motivation during context-induced relapse. Nevertheless, we only observed partial reduction of the drug taking behavior during heroin self-administration, suggesting that the restraining effect on heroin taking of VPGlu activation during self-administration might also involve other neural circuits.
Meye et al. found that cocaine withdrawal decreased the GABAergic transmission from EPN to LHb and this shifted GABA/glutamate balance contributes to the withdrawal symptom and stress induced cocaine reinstatement [25]. VP mainly contains two subtypes of neurons. A small amount is glutamatergic and the most is GABAergic. Whether there is a shift of the two transmitters in the downstream target that underlying the change of drug-seeking motivation is unknown. Hence, we combined the AAV tracing and immunofluorescence to investigate the expression of Vglut2 and Vgat in the VP-LHb projection. We found that after the extinction of heroin seeking motivation, the expression of Vglut2 in VP-LHb glutamatergic axon terminal was increased, while the expression of Vgat was decreased in the GABAergic terminal. However, the acute exposure to drug paired context and the heroin seeking behavior did not reverse the increased Vglut2 expression. These results demonstrate that the prolonged extinction of heroin seeking behavior changes the inhibitory/excitatory transmission of VP-LHb and indicate that the reduction of heroin seeking motivation results from the out-of-balance of VP-LHb circuit
In conclusion, our study revealed that the activity of VP glutamatergic neurons modulated drug taking and seeking motivation in different phases of heroin addiction. The activation of VP glutamatergic neurons mediated the reduction of heroin taking and seeking behavior, while the inhibition of VP glutamatergic neurons partly contributed to the increase of heroin seeking motivation. Moreover, VP glutamatergic neurons restrained heroin seeking behavior via activating LHb neurons and the heroin seeking motivation was related to the balance of GABA/glutamate transmission from ventral pallidum to LHb. Thus, the present study identified the VP glutamatergic neurons and their projection to LHb as a potential target for intervention of heroin addiction.
References
Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–43.
Cooper S, Robison AJ, Mazei-Robison MS. Reward circuitry in addiction. Neurotherapeutics. 2017;14:687–97.
Kupchik YM, Prasad AA. Ventral pallidum cellular and pathway specificity in drug seeking. Neurosci Biobehav Rev. 2021;131:373–86.
Richard JM, Ambroggi F, Janak PH, Fields HL. Ventral pallidum neurons encode incentive value and promote cue-elicited instrumental actions. Neuron. 2016;90:1165–73.
Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–49.
Ottenheimer D, Richard JM, Janak PH. Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens. Nat Commun. 2018;9:4350.
Prasad AA, McNally GP. Ventral pallidum output pathways in context-induced reinstatement of alcohol seeking. J Neurosci. 2016;36:11716–26.
Prasad AA, Xie C, Chaichim C, Nguyen JH, McClusky HE, Killcross S, et al. Complementary roles for ventral pallidum cell types and their projections in relapse. J Neurosci. 2020;40:880–93.
Pribiag H, Shin S, Wang EH, Sun F, Datta P, Okamoto A, et al. Ventral pallidum DRD3 potentiates a pallido-habenular circuit driving accumbal dopamine release and cocaine seeking. Neuron. 2021;109:2165–82.e10.
Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700.
Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacol (Berl). 1982;78:204–9.
Galaj E, Han X, Shen H, Jordan CJ, He Y, Humburg B, et al. Dissecting the role of GABA neurons in the VTA versus SNr in opioid reward. J Neurosci. 2020;40:8853–69.
Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res. 1990;508:20–9.
Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–88.
Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected]. J Comp Neurol. 2005;483:351–73.
Faget L, Zell V, Souter E, McPherson A, Ressler R, Gutierrez-Reed N, et al. Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat Commun. 2018;9:849.
Liu B, Cao Y, Wang J, Dong J. Excitatory transmission from ventral pallidum to lateral habenula mediates depression. World J Biol Psychiatry. 2020;21:627–33.
Stephenson-Jones M, Bravo-Rivera C, Ahrens S, Furlan A, Xiao X, Fernandes-Henriques C, et al. Opposing contributions of GABAergic and Glutamatergic ventral pallidal neurons to motivational behaviors. Neuron. 2020;105:921–933.e5.
Wang F, Zhang J, Yuan Y, Chen M, Gao Z, Zhan S, et al. Salience processing by glutamatergic neurons in the ventral pallidum. Sci Bull (Beijing). 2020;65:389–401.
Levi LA, Inbar K, Nachshon N, Bernat N, Gatterer A, Inbar D, et al. Projection-specific potentiation of ventral pallidal glutamatergic outputs after abstinence from cocaine. J Neurosci. 2020;40:1276–85.
Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–30.
Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–73.
Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018;554:323–7.
Proulx CD, Aronson S, Milivojevic D, Molina C, Loi A, Monk B, et al. A neural pathway controlling motivation to exert effort. Proc Natl Acad Sci USA. 2018;115:5792–7.
Meye FJ, Soiza-Reilly M, Smit T, Diana MA, Schwarz MK, Mameli M. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat Neurosci. 2016;19:1019–24.
Valentinova K, Tchenio A, Trusel M, Clerke JA, Lalive AL, Tzanoulinou S, et al. Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nat Neurosci. 2019;22:1053–6.
Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–66.
Fattorini G, Verderio C, Melone M, Giovedì S, Benfenati F, Matteoli M, et al. VGLUT1 and VGAT are sorted to the same population of synaptic vesicles in subsets of cortical axon terminals. J Neurochem. 2009;110:1538–46.
Heinsbroek JA, Bobadilla AC, Dereschewitz E, Assali A, Chalhoub RM, Cowan CW, et al. Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Rep. 2020;30:2018–2027.e3.
Vitcheva V. Cocaine toxicity and hepatic oxidative stress. Curr Med Chem. 2012;19:5677–82.
Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8.
Freeman KB, Konaklieva MI, Riley AL. Assessment of the contributions of Na+ channel inhibition and general peripheral action in cocaine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2005;80:281–8.
Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–3.
Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–12.
Zuo W, Chen L, Wang L, Ye JH. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology. 2013;70:180–9.
Li H, Eid M, Pullmann D, Chao YS, Thomas AA, Jhou TC. Entopeduncular nucleus projections to the lateral habenula contribute to cocaine avoidance. J Neurosci. 2021;41:298–306.
Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–7.
Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5.
Mathis VP, Williams M, Fillinger C, Kenny PJ. Networks of habenula-projecting cortical neurons regulate cocaine seeking. Sci Adv. 2021;7:eabj2225.
Nair SG, Smirnov DS, Estabrook MM, Chisholm AD, Silva PR, Neumaier JF. Effect of chemogenetic inhibition of lateral habenula neuronal activity on cocaine- and food-seeking behaviors in the rat. Addict Biol. 2021;26:e12865.
Wulff AB, Tooley J, Marconi LJ, Creed MC. Ventral pallidal modulation of aversion processing. Brain Res. 2019;1713:62–9.
Acknowledgements
This research was supported by the Major Project of the Science and Technology Innovation 2030 of China (2021ZD0203500, STI2030-Major Projects 2021ZD0202900); National Natural Science Foundation of China (82030112, 81773710, 82371533, 82301677); Science and Technology Commission of Shanghai Municipality (20ZR1468200); Natural Science Foundation of Shanghai (22ZR1449300); Program of Shanghai Academic/Technology Research Leader (22XD1402400) and Shenzhen-Hong Kong Institute of Brain Science—Shenzhen Fundamental Research Institutions (NYKFKT2019015).
Author information
Authors and Affiliations
Contributions
ZQL, RSC and JL conceived this study and design the experiment; RSC and JL conducted the experiments with the assistance of YJW and KN; RSC and JL analyzed the data and wrote the manuscript; ZQL and JGL revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Rs., Liu, J., Wang, Yj. et al. Glutamatergic neurons in ventral pallidum modulate heroin addiction via epithalamic innervation in rats. Acta Pharmacol Sin 45, 945–958 (2024). https://doi.org/10.1038/s41401-024-01229-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-024-01229-4
- Springer Nature Singapore Pte Ltd.