Abstract
Rationale
Nicotine (NIC) potently increases operant responding for non-NIC reinforcers, and this effect may depend on drug-mediated increases in incentive motivation. According to this hypothesis, NIC should also potently increase approach to Pavlovian-conditioned stimuli associated with rewards.
Objective
The present studies explored the effects of NIC on Pavlovian-conditioned approach responses.
Method
To do so, liquid dippers were used to deliver an unconditioned stimulus (US; 0.1 ml sucrose) after presentation of a conditioned stimulus (CS; 30 s illumination of a stimulus light)—both the CS and US were presented in receptacles equipped to monitor head entries.
Results
In experiment 1, the CS and US were presented in the same receptacle, but NIC pretreatment (0.4 mg/kg base) did not increase conditioned approach responses. Delivery of the sucrose US was then shifted to receptacle in a different location. All rats learned to approach the new US location (goal-tracking) at similar rates. Approach to the CS receptacle (sign-tracking) declined for saline-pretreated rats, but NIC pretreatment increased sign-tracking. In experiment 2, NIC pretreatment increased sign-tracking when the CS and US were spatially separated during acquisition. In experiment 3, NIC pretreatments were replaced with saline, but the effect of NIC persisted for an additional 24 test sessions.
Conclusion
The findings suggest that NIC increases incentive motivation and that this effect is long-lasting, persisting beyond the pharmacological effects of NIC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotine is a weak primary reinforcer—in pre-clinical models, intravenous nicotine infusions support very low levels of operant behavior that are easily reversed (Le Foll and Goldberg 2006; Palmatier et al. 2007a). However, nicotine has potent reinforcement-enhancing effects (Caggiula et al. 2009)—nicotine administration robustly increases responding for other drug and non-drug reinforcers (Donny et al. 2003). Recent studies investigating the nature of the interaction between nicotine and non-nicotine reinforcers have converged on a new working hypothesis (Palmatier et al. 2012): that nicotine increases responding for non-drug rewards by amplifying the incentive properties of reward-associated stimuli. The term “incentive” refers to an anticipatory motivational state evoked by reward-associated stimuli (Robinson and Berridge 1993; Uslaner et al. 2008). According to this hypothesis, nicotine should increase responding for primary reinforcers (Chaudhri et al. 2006a; Donny et al. 2003) and conditioned reinforcers (Chaudhri et al. 2006a; Olausson et al. 2004a; Palmatier et al. 2007b). Also, nicotine should not increase operant responding in contexts with stimuli that have never been associated with a reward (Palmatier et al. 2007c, 2012).
An important feature of incentive models (Berridge and Robinson 1998; Robinson and Berridge 1993) is that an incentive stimulus “acquires” motivational properties. Unfortunately, operant behavior is guided and energized by stimuli with both inherent (primary reinforcer) and conditional motivational properties, making these paradigms less than ideal for investigating incentive motivation (Wyvell and Berridge 2000). However, if nicotine truly amplifies the effects of incentive stimuli, then the effects observed in operant conditioning paradigms should translate to other models of incentive motivation (Olausson et al. 2003; Thiel et al. 2009). Accordingly, nicotine enhances approach to contexts associated with social rewards (Thiel et al. 2009), and prior nicotine exposure increases approach evoked by an auditory/visual conditioned stimulus (CS) associated with a water reward (Olausson et al. 2003). Although the effects of nicotine were reliable in both studies, in the former study they were observed in adolescents but not adults (Thiel et al. 2009), and in the latter, they were only observed during the first 3 days of testing (Olausson et al. 2003).
The unexceptional effect of nicotine on Pavlovian-conditioned approach is somewhat problematic because conditioned approach directly measures behavior evoked by incentives (Robinson and Flagel 2009). Therefore, the goal of the present studies was to further explore the incentive amplifying effects of nicotine in a Pavlovian-conditioned approach paradigm. To do so, we attempted to optimize the procedures of Olausson et al. (2003) in several ways. First, drug treatment preceded testing by 15 min so that the pharmacological effects of nicotine overlapped with delivery of incentives. Second, the CS (illumination of a light) was presented in the same receptacle as the unconditioned stimulus (US; sucrose) to avoid conflict between “sign-tracking” and “goal-tracking” (Farwell and Ayres 1979; Hearst and Jenkins 1974). We also used different concentrations of sucrose as the US (0, 5, or 20 %) to investigate the effect of nicotine under different rates of acquisition, and we used a longer CS (30 s) to increase the period of time in which approach behavior was measured. An important feature of our apparatus was that two “goal” areas could be used and the CS and US could be presented in spatially contiguous or discontiguous locations. Based on the incentive amplification hypothesis, we predicted that nicotine would robustly increase approach to the CS during acquisition under these optimized procedures.
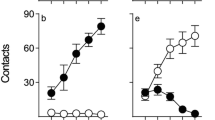
Average elevation scores (±1 SEM) during acquisition (A) and extinction (B) test sessions. Open symbols represent rats pretreated with placebo (SAL), and filled symbols represent rats pretreated with NIC. Circle (0 %), square (5 %), and triangle (20 %) symbols represent each level of the sucrose US. The dashed line on the ordinate represents a theoretical elevation score of 0, in which approach to the dipper during the CS and pre-CS periods is similar. #p < 0.05 indicates that rats with access to 20 % sucrose had significantly higher elevation scores than rats with access to 0 % sucrose. *p < 0.05 indicates that rats with access to 5 % sucrose had significantly higher elevation scores than rats with access to 0 % sucrose
Method
General method
Subjects
Eighty male Sprague Dawley rats (Charles River Labs, Portage, MI, USA) weighing 174–225 g on arrival were used for these experiments. The rats were housed in a temperature and humidity controlled colony room on a reverse 12:12-h light/dark cycle. Rats were allowed to habituate to the colony for 1 week in which feed and water was freely available. After habituation, feed was restricted so that all rats were maintained at 100 % of their free-feeding weight (minimum 250 g); food intake was controlled so that increases in bodyweight (5 g every 30 days) were slowed relative to free feeding. Rats were allowed to habituate to feed restriction for at least 3 days before testing began; water was freely available in the home cage throughout this habituation period and the remainder of each study. All experimental procedures were conducted in the dark portion of the cycle and were approved by the Kansas State University Institutional Animal Care and Use Committee (Animal Welfare Assurance #: A3609-01).
Apparatus
All experiments were conducted in eight standard conditioning chambers measuring 30.5 × 25.4 × 30.5 (w × d × h). On one wall of each chamber was an intelligence panel with a house light and two liquid dipper receptacles mounted to it. The house light was located 2.5 cm below the ceiling on the center of the wall and provided ambient lighting during testing sessions where noted. The dipper receptacles were located on the left and right sides of the wall, approximately 2 cm from the floor and 2.5 cm from the rear (left) and front (right) side walls (approximately 11 cm separated the inside edges of the two receptacles). An infrared emitter detector unit was mounted approximately 1 cm above the bottom of each receptacle to monitor head entries. Stimulus lights were mounted in the top of each receptacle, and illumination of these stimulus lights served as the conditioned stimuli in these experiments. The stimulus and house lights were 1.12 W incandescent bulbs connected to a 28-V power supply. Liquid dippers with 0.1 ml cups were attached to the outside of the chamber on each side; activation of a motor raised the cup to the bottom of the receptacle. Sucrose delivered in the liquid dipper could be accessed through an aperture in the bottom of the receptacle (approximately 0.5 cm) and served as the US in these experiments.
Drugs and solutions
Sucrose was dissolved in de-ionized water at 0, 5, 10, or 20 % concentrations (w/v). (–) Nicotine hydrogen tartrate salt was dissolved in sterile saline (0.4 mg/ml, base). The pH of the solution was adjusted to 7.0 ± 0.2 with dilute NaOH and was then passed through a sterile filter and stored in sterilized serum vials capped with sterile septa. Sterile saline (0.9 %, w/v) served as the placebo and was filtered and stored in the same way. Subcutaneous injections (0.1 ml/kg) of the assigned drug solution were administered 15 min prior to testing sessions. After rats were habituated to the feed restriction schedule, they were randomly assigned to their drug exposure groups (NIC or SAL). Rats were injected with their assigned drug solution 1 day before shaping began to habituate them to the motor suppressant/aversive effects of nicotine. Rats were subsequently injected with their assigned drug solution prior to every subsequent test session (dipper training, acquisition, etc.), unless otherwise noted. No test occurred without a nicotine or saline pretreatment injection.
Procedures: experiment 1
Dipper training
Rats (N = 48) were randomly assigned to one of three sucrose concentrations (0, 5, or 20 %, n = 8 per concentration/drug condition) and initially trained to access this concentration of sucrose from the liquid dipper in the right receptacle within 5 s. Dippers were delivered on a random-time 1-min schedule, with 50 dipper deliveries during each 1 h session. All rats received two dipper-training sessions. For rats in the 5 and 20 % sucrose groups, head entries were recorded in at least one third of the total time that dippers were presented (83/250 s) during at least one of these sessions.
Acquisition and extinction
For the next 12 test sessions, a 30-s CS (illumination of the right dipper light) preceded delivery of the dipper (US). During each 60-min conditioning session, there were 20 trials (CS + US parings) which were separated by inter-trial intervals (US offset to next CS onset) of at least 2.5 min. At least 3 min of “empty” intervals (no CS or US) occurred at the beginning and end of each session. All acquisition sessions were conducted on consecutive days. During acquisition, we discovered that the bulb illuminating the CS was burnt out in one chamber, so two rats from the 5 % sucrose group (one NIC and one SAL rat) were dropped from the analyses. Extinction was conducted over 9 days following acquisition. During extinction, all test parameters were identical to acquisition except that illumination of the CS was not followed by activation of the dipper. The dipper was not activated at all during the extinction tests.
Spontaneous recovery, reinstatement, and reacquisition
Following extinction, rats were allowed to “rest” in the home cage for seven consecutive days (no injections were given during this rest period). A single test was then conducted in which three CS presentations occurred semi-randomly during a 60-min test period. All CS presentations were separated by at least 10 min. Following the spontaneous recovery test, rats were tested for reinstatement. During the first 20 min of the session, three semi-random US deliveries were presented; there were no programmed stimuli delivered during the second 20-min period; the final 20-min period included three semi-random presentations of the CS. Subsequent reacquisition tests were identical to acquisition, except that the house light was illuminated. The house light was illuminated to reduce visibility of the CS from other parts of the chamber in order to encourage more approach to the dipper. During reacquisition, the rats were also shifted to a 5-day/week testing protocol (M–F) which is more consistent with our previous operant work. As previously mentioned, pretreatment with assigned drug solutions (nicotine or saline) occurred 15 min prior to each test session during all test phases.
Goal shift
After approach behavior stabilized during reacquisition, the goal location was shifted to the left dipper receptacle, but the CS location (illumination of the stimulus light) remained in the right dipper. All other procedures were held constant.
Procedures: experiment 2
Dipper training
Dipper training was similar to experiment 1, except that 32 rats were shaped to access 10 % sucrose (w/v) from both right and left receptacles within 5 s. The 10 % solution was used to further investigate the possibility that nicotine pretreatment during acquisition would increase conditioned approach in rats with access to the CS and US in the same receptacle (SAME group). Dippers were delivered on a random-time 1-min schedule, with 52 dipper deliveries (26 on each side) during each 1-h session. All rats received two to four dipper-training sessions.
Acquisition
Following dipper training, rats in each drug pretreatment condition (NIC or SAL) were randomly assigned to one of two groups (SAME or DIFF, n = 8 per drug/group). Acquisition was similar to experiment 1, except that 24 test sessions were conducted under the 5-day/week testing protocol. For rats in the SAME groups, the CS and US both were delivered in the same receptacle. For the DIFF groups, the CS was presented in one dipper receptacle, and the US was presented in the alternate receptacle. The location of CS and US dipper receptacles (left vs. right) was semi-randomly assigned with the constraint that side preferences observed during shaping were balanced as much as possible. The house light was illuminated throughout these tests.
Reversal
After acquisition, there were 12 reversal tests in which the goal locations were shifted for all rats, but the CS location remained constant. For the SAME groups, this procedure replicates the shift from experiment 1, the sucrose US was delivered in the previously inactive receptacle, and the CS was presented in the same receptacle as acquisition. The goal of the reversal was to replicate the findings from experiment 1 in the SAME groups after acquisition—without the additional potential confounding influences of the extinction, spontaneous recovery, reinstatement, and reacquisition tests. For the DIFF groups, the goal shift meant that the CS and sucrose US were presented in the same receptacle; the receptacle in which the CS was presented during acquisition. The goal of the reversal phase for the DIFF groups was to demonstrate that any retardation observed during acquisition (SAL rats) was attributed to a difference in performance (e.g., sitting between the two dippers during the CS), rather than an associative learning deficit.
Procedures: experiment 3
Return to baseline for DIFF groups
Rats from the second replication of experiment 2 (n = 16) were used in this experiment (four from each group/drug condition). Following the reversal phase, all 16 rats were habituated to testing procedures in which the CS and US locations differed for eight testing sessions. Sign-tracking and goal-tracking stabilized and approach did not differ statistically for rats in different groups (see “Results”) at the end of the habituation phase (average from days 6–8). Rats were then collapsed so that drug pretreatment (NIC vs. SAL, see Fig. 6b) was the only between subjects variable. Testing began the day after experiment 2 ended, and all other experimental procedures were identical to those previously described.
Placebo replacement tests
Beginning on day 9 of experiment 3, rats in the NIC group were shifted to saline pretreatment injections. Rats in the SAL group were maintained on placebo injections for comparison. All other testing procedures were identical to those previously described.
Data analyses
For both experiments, head entries were recorded in 30 s intervals (except during the 5-s US). Intervals immediately preceding the CS (Pre-CS) were used to measure basal head-entry behavior. The principal dependent measure was the average elevation score from each session (Palmatier et al. 2004). Elevation scores were calculated by subtracting head-entries during the Pre-CS from head entries during the CS on each trial. When the US location differed from the location of the CS, elevation scores were calculated for both receptacles, but only reflect head entries that occurred during presentation of the CS. Elevation scores for each side represent approach behavior during the time that the CS was illuminated. Thus, an elevation score for the “sign” receptacle reflects the number of head entries that occurred during the illumination of the light in that receptacle. An elevation score for the “goal” receptacle reflects the number of head entries that occurred during the illumination of the light, even if the light was illuminated in a different receptacle. Because the location of the CS and US varied, the elevation scores are described as “goal-tracking” or “sign-tracking,” depending on which event(s) occurred in each location.
Mixed analyses of variance (ANOVAs) were used to determine statistical reliability of the effects of manipulations and repeated tests on elevation scores. Because of the complex design and the added manipulation of separating the goal location from the CS, ANOVAs were constrained to three factors (one within and two between) to avoid interpretation of four-way interactions. Thus, separate ANOVAs for sign-tracking and goal-tracking were conducted when session was included as a repeated measure. To directly investigate the role of the sign vs. goal location, elevation scores for each subject were averaged across the last three sessions of a particular phase of the experiment (e.g., acquisition or reversal). Three-way ANOVAs including location (sign vs. goal) were then used to investigate whether the topography of elevation scores interacted with drug pretreatment and US (experiment 1) or group (experiment 2). Follow-up analyses included simple effects analyses, Tukey’s honestly significant differences (HSD) tests, and t tests with Bonferroni’s correction where appropriate. An alpha criterion of p ≤ 0.05 was used for significance tests.
Results
Experiment 1
Acquisition
Elevation scores were the highest for rats that received 20 % sucrose, moderate for rats with access to 5 % sucrose, and did not differ from a theoretical mean of 0 for rats that received 0 % sucrose (Fig. 1a). There was also a tendency for rats in the NIC pretreatment conditions to respond slightly more than their respective SAL control groups; however, this initial difference was not observed at the end of acquisition testing (Fig. 1a). These conclusions were confirmed with repeated measures ANOVA which revealed statistically significant main effects of US, drug, and session (F’s ≥ 5.92, p’s ≤ 0.02). The significant session × US [F(22, 440) = 26.5, p < 0.001] interaction confirmed that rats with higher US concentrations displayed higher elevation scores across testing sessions. A post hoc Tukey’s HSD test confirmed that elevation scores for rats with access to each sucrose concentration significantly differed from the elevation scores in the other two concentrations (20 > 5 > 0 %; p’s < 0.001); analyses across sessions (t tests with Bonferroni’s correction) confirmed that rats with access to 20 % sucrose displayed greater elevation scores than 0 % controls throughout testing (sessions 1–12, p’s < 0.05), whereas rats with access to 5 % sucrose acquired a bit more slowly with significantly higher elevation scores on sessions 3 and 5–12 (p’s ≤ 0.04).
Average elevation scores (±1 SEM) during the spontaneous recovery (a), reinstatement (b), and reacquisition (c) test sessions. In a, filled bars represent elevation scores from the spontaneous recovery test session, and open bars represent elevation scores from the last day of extinction and are included for comparison purposes. The break on the abscissa separates NIC and SAL pretreated rats on this test. In b, filled and open bars represent rats pretreated with NIC or SAL, respectively. In c, circles (0 %), squares (5 %), and triangles (20 %) represent each level of the sucrose US, and filled/open symbols represent NIC vs. SAL pretreatment, respectively. The asterisk denotes significantly higher elevation scores in NIC pretreated rats relative to SAL controls
The lack of significant interactions involving drug was surprising based on previous research (Olausson et al. 2003). Visual inspection of the data (Fig. 1a) suggests that we replicated the findings of Olausson et al. (2003), in which NIC increased acquisition of goal-tracking during the first 3 days of testing. However, this was masked in omnibus analyses including all levels of sucrose concentration and all acquisition sessions. Therefore, an additional two-way ANOVA contrasting drug across session was performed for each of the US conditions independently and including only the first 3 days of acquisition. There were significant main effects of session, but only for rats with access to 5 or 20 % sucrose (p’s ≤ 0.01); however, there were no significant main effects of drug or significant interactions with this factor. Within-session analyses of the first test day (trial as repeated measure) confirmed these findings; there were no significant main effects or interactions of drug (p’s ≥ 0.24).
Extinction
Elevation scores decreased rapidly across initial testing session and did not vary as a function of US or drug during extinction (Fig. 1b). This was confirmed by repeated measures ANOVA with a significant main effect of session [F(8, 320) = 24.1, p < 0.001], a significant main effect of US [F(2, 40) = 6.64, p < 0.01], and a significant session × US interaction [F(16, 320) = 7.96, p < 0.001]; no other main effects or interactions reached statistical significance. Post hoc analyses confirmed that the 0 % groups had lower elevation scores than the 5 or 20 % groups; however, this was constrained to the first 2 days of extinction testing (p’s < 0.05).
Spontaneous recovery
Elevation scores increased on the spontaneous recovery test, and this increase was selective to the 20 and 5 % groups; however, NIC pretreatment did not alter spontaneous recovery (Fig. 2a). This was confirmed by repeated measures ANOVA with a significant main effect of test [last day of extinction vs. spontaneous recovery test, F(1, 40) = 31.9, p < 0.001] and a significant test × US interaction [F(2, 40) = 5.96, p < 0.01]. No other main effects or interactions were significant. Follow-up contrasts confirmed that only rats with access to 5 or 20 % sucrose increased their elevation scores on the spontaneous recovery test relative to the last day of extinction (p’s ≤ 0.01).
Average elevation scores (±1 SEM) during the goal switch test sessions. a Elevation scores calculated from the goal dipper receptacle during the CS and Pre-CS across the ten test sessions. b Elevation scores from the sign receptacle during the same test period. In a and b, circles (0 %), squares (5 %), and triangles (20 %) represent each level of the sucrose US, and filled/open symbols represent NIC vs. SAL pretreatment, respectively (see Fig. 1). In c, filled and open bars represent goal- and sign-tracking, respectively. The break in the abscissa separates SAL pretreatment groups from NIC pretreatment groups. ^p < 0.05 denotes significantly higher elevation scores for rats receiving the 20 % sucrose US, relative to 0 % controls. *p < 0.05 denotes significantly higher elevation scores for rats receiving the 5 or 20 % sucrose US, relative to 0 % controls. #p < 0.05 denotes significantly greater sign-tracking, relative to goal-tracking
Reinstatement
NIC pretreatment potentiated elevation scores on the reinstatement test, but only in rats with access to 20 % sucrose (Fig. 2b). Rats with access to 0 % sucrose did not demonstrate increased elevation scores on the reinstatement test, and no effect of NIC pretreatment was observed in these subjects. These findings were confirmed by univariate ANOVA with significant main effects of drug and US (F’s ≤ 14.4, p’s < 0.001), as well as a significant drug × US interaction [F(2, 46) = 3.33, p = 0.04]. Follow-up contrasts confirmed that NIC only increased elevation scores for rats with access to 20 % sucrose (p = 0.03).
Reacquisition
Reacquisition was comparable to the initial acquisition phase; elevation scores increased selectively in rats with access to 5 and 20 % sucrose, but NIC pretreatment did not alter this increase in responding (Fig. 2c). This finding was confirmed by repeated measures ANOVA with significant main effects of test and US and a significant test × US interaction (F’s ≥ 3.03, p’s ≤ 0.01). No other main effects or interactions were significant. Tukey’s HSD test confirmed that elevation scores from each level of US (0, 5, or 20 %) differed significantly from the elevation scores of rats at both other levels (p’s < 0.001). Once again, the trend during reacquisition (Fig. 2c) appeared to follow that of Olausson et al. (2003); however, additional analyses of early sessions and individual trials did not confirm any statistically significant effect of drug nor drug × session/trial interactions (p’s ≥ 0.12).
Goal switch: goal-tracking
Both SAL and NIC rats learned to approach the new US dipper during the CS presentation (goal-tracking). This new learning did not differ as a function of NIC pretreatment and was selective for rats with the 20 % sucrose US (Fig. 3a). This was confirmed by repeated measures ANOVA on elevation scores calculated in the goal receptacle with significant main effects of test, US, and a significant test × US interaction (F’s ≥ 8.11, p’s < 0.001). No other main effects or interactions were significant. Tukey’s HSD post hoc test confirmed that rats with the 20 % US had significantly higher elevation scores than rats with the 5 or 0 % US (p’s < 0.01); however, rats with 5 and 0 % sucrose USs did not differ from each other (p = 0.36). Follow-up contrasts confirmed that rats with 20 % sucrose US had higher elevation scores in the goal receptacle than 0 % controls from sessions 35–41 (tests 4–10, p’s ≤ 0.02).
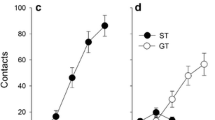
Average elevation scores (±1 SEM) during experiment 2. a Elevation scores calculated from the active (SAME groups) and goal (DIFF groups) dipper receptacle during the CS and Pre-CS across the 24 acquisition sessions. b Elevation scores from the sign receptacle during the same test period (DIFF groups). In a and b, symbol shapes represent group (SAME vs. DIFF); filled symbols represent rats pretreated with nicotine (NIC); open symbols represent rats pretreated with saline (SAL). In c, filled and open bars represent goal- and sign-tracking, respectively. The asterisk denotes significantly higher elevation scores for NIC pretreated rats, relative to SAL controls
Goal switch: sign-tracking
Approach to the CS that predicted delivery of 5 or 20 % sucrose was selectively sustained by NIC pretreatment but tended to extinguish across testing sessions in rats that were pretreated with SAL (Fig. 3b). This conclusion was confirmed by repeated measures ANOVA with a significant test × drug × US interaction [F(18, 360) = 2.13, p < 0.01]. All three main effects (test, drug, and US) and two-way interactions were significant (F’s ≥ 3.4, p’s < 0.001), except the drug × US interaction, which approached significance [F(2, 40) = 2.8, p = 0.07]. To further investigate the three-way interaction, separate contrasts for each level of US investigated the effect of drug pretreatment on each test session. Bonferroni’s correction was used to control alpha inflation. For rats with the 5 or 20 % US, NIC pretreatment significantly increased sign-tracking on sessions 36–41 (tests 5–10, p’s ≤ 0.03). For rats with the 0 % US, NIC pretreatment did not alter sign-tracking on any test session (p’s ≥ 0.2).
Goal switch: sign-tracking vs. goal-tracking
Rats that were pretreated with NIC tended to approach the sign (CS receptacle) more than the goal (US receptacle) during presentation of the CS at the end of the goal switch. In contrast, rats that were pretreated with SAL tended to display the opposite pattern, with greater approach to the goal relative to the sign (Fig. 3c). This pattern was confirmed by ANOVA contrasting elevation scores on the CS side with elevation scores on the US side. To do so, elevation scores from each side were averaged over the final three test sessions (39–41), and these average scores were analyzed using mixed ANOVA with location (sign vs. goal, df = 1, 40) as a within subjects factor, drug (NIC vs. SAL, df = 1, 40), and US (0 vs. 5 vs. 20 % sucrose, df = 2, 40) were between subjects factors. There were significant main effects of drug and US and a significant drug × US interaction (F’s ≥ 4.5, p’s ≤ 0.02). The main effect of location was also statistically significant (p = 0.05), and there was a significant location × drug interaction (F = 17.4, p < 0.001). No other interactions were significant. The pattern of two-way interactions suggested that the effect of NIC was specific to the 5 and 20 % sucrose USs and differed by location. Therefore, follow-up analyses contrasted topography of responding (goal vs. sign-tracking) at each level of US and drug. Because neither topography developed in the 0 % US groups, they were excluded from follow-up analyses to avoid unnecessary alpha inflation. For rats pretreated with NIC, significantly greater sign-tracking was observed, relative to goal-tracking in both 5 and 20 % sucrose US groups (p’s < 0.05). For rats pretreated with SAL, there was a numerical bias toward goal-tracking (Fig. 3c); however, this did not reach statistical significance (p’s > 0.05).
Experiment 2
Acquisition: goal-tracking
Rats in the SAME groups readily learned to goal track as the CS and US were delivered in the same location. Rats in the DIFF groups acquired the goal-tracking response more slowly, relative to SAME groups, and DIFF rats pretreated with SAL acquired the goal-tracking response more slowly than rats pretreated with NIC (Fig. 4a). This was confirmed by repeated measures ANOVA with significant main effects of group (Same vs. DIFF), drug (NIC vs. SAL), and session (session, F’s ≥ 4.1, p’s ≤ 0.05). There were also significant session × group and session × drug interactions [F’s(23, 644) ≥ 2.1, p’s ≤ 0.002]. No other interactions were significant. Because both between-subjects factors (group and drug) interacted with the repeated measure (session), simple effects analyses compared drug (NIC vs. SAL) in each group. The simple effects confirmed that NIC and SAL pretreated rats acquired the goal-tracking response at similar rates in the SAME group (F < 1). However, for the DIFF group, NIC pretreated rats acquired the goal-tracking response more rapidly relative to the SAL pretreated rats [F(1, 14) = 6.6, p = 0.02]. As illustrated in Fig. 4a, goal-tracking elevation scores in the SAL pretreated DIFF rats only marginally increased above 0. To determine whether these rats “acquired” a goal-tracking response, a one-sample t test was used to compare average elevation score on at the end of acquisition (average of tests 22–24) to a theoretical mean of 0 (equivalent approach to the goal receptacle during CS and pre-CS). Elevation scores at the end of acquisition were significantly greater than 0 [t(7) = 3.24, p = 0.01], confirming that rats in the SAL DIFF group acquired a weak goal-tracking response.
Average elevation scores (±1 SEM) during the reversal phase of experiment 2. a Elevation scores calculated from the goal dipper receptacle during the CS and Pre-CS across the 12 test sessions. b Elevation scores from the sign receptacle during the same test period. In a and b, square symbols represent DIFF groups and triangles represent SAME groups; filled/open symbols represent NIC vs. SAL pretreatment, respectively. In c, filled and open bars represent elevation scores from the NIC and SAL pretreatment groups, respectively. The break in the abscissa separates SAME and DIFF groups. *p < 0.05 denotes significantly higher elevation scores for rats DIFF rats pretreated with NIC, relative to SAL controls. ^p < 0.05 denotes significantly higher elevation scores for SAME rats pretreated with NIC, relative to SAL pretreated controls. #p < 0.05 denotes significantly higher elevation scores for NIC pretreated rats in both groups, relative to SAL controls
Acquisition: sign-tracking
For the DIFF groups, rats that were pretreated with NIC acquired a sign-tracking response more rapidly than rats pretreated with SAL, who never acquired a sign-tracking response during the 24 acquisition tests (Fig. 4b). This was confirmed by repeated measures ANOVA with significant main effects of drug and session, as well as a significant drug × session interaction (F’s ≥ 3.7, p’s ≤ 0.001). Follow-up analyses confirmed that DIFF rats pretreated with NIC had higher elevation scores in the CS receptacle on sessions 12, 14, and 16–24 relative to SAL pretreated DIFF rats (p’s < 0.05). Elevation scores at the end of acquisition confirmed that DIFF rats pretreated with SAL did not acquire a sign-tracking response [t(7) = 1.82, p = 0.1].
Acquisition: sign- vs. goal-tracking
NIC pretreatment facilitated goal- and sign-tracking equally, there were no differences in approach responses based on location (Fig. 4c). This was confirmed by mixed factors ANOVA on elevation scores for the DIFF group averaged over the last 3 days of acquisition; location (goal vs. sign receptacle) and drug (NIC vs. SAL) were the independent factors. There was a significant main effect of drug [F(1, 14) = 14.19, p < 0.01], but the main effects of location and the drug × location interaction were not significant.
Reversal: goal-tracking
During the reversal, goal-tracking increased across sessions for all groups, except for the DIFF rats pretreated with NIC, who were already approaching the goal dipper at a high rate because the CS was presented in this receptacle during acquisition (Fig. 5a). This was confirmed by repeated measures ANOVA with significant main effects of session, drug, and group (F’s ≥ 8, p’s ≤ 0.008) as well as significant session × group, session × drug, and session × group × drug interactions [F’s(11, 308) ≥ 1.9, p’s ≤ 0.04]. To explore the three-way interaction, elevation scores for rats pretreated with NIC or SAL were contrasted within each group. For DIFF groups, SAL rats rapidly acquired the goal-tracking response as elevation scores were only significantly lower than NIC pretreated rats on sessions 25, 26, and 28 (reversal tests 1, 2, and 4, p’s < 0.05). For SAME groups, the rate of acquisition did not appear to differ; however, SAL pretreated rats had significantly lower elevation scores in the goal receptacle on session 29, relative to NIC pretreated rats (p < 0.05).
Average elevation scores (±1 SEM) from experiment 3. a Average elevation scores calculated from the CS and US dippers in each group (SAME vs. DIFF); the break in the abscissa separates NIC and SAL pretreatment conditions. Each bar represents an average score from the last 3 days of testing (sessions 6–8). b Elevation scores from the CS and US receptacles across the 24 days of placebo challenge tests (all rats pretreated with saline injections). In b, symbol shapes represent elevation scores from the CS and US receptacles, and filled/open symbols represent prior NIC vs. SAL pretreatment
Reversal: sign-tracking
Approach to the sign tended to decline in the SAME group, but only for rats pretreated with SAL. Approach to the sign was potentiated by NIC pretreatment in the SAME group, replicating the findings from experiment 1 (Fig. 5b). This was confirmed by repeated measures ANOVA with significant main effects of drug and session, and a significant drug × session interaction (F’s ≥ 2.1, p’s ≤ 0.03). Follow-up contrasts confirmed that rats in the NIC pretreatment group sign-tracked more than the SAL group on sessions 32–36, p’s ≤ 0.05.
The reversal also allowed us to explore the potential role of location cues as “sign” stimuli, for rats in the DIFF groups; the CS and US were presented in the same location during the reversal phase, but the US was delivered in the alternate dipper receptacle during acquisition. Approach to this “inactive” receptacle was maintained by NIC administration, suggesting that the features of the goal receptacle acquired some incentive salience during acquisition. This was confirmed by repeated measures ANOVA with a significant main effect of drug [F(1, 14) = 9.22, p < 0.01] and a drug × session interaction [F(11, 154) = 2.4, p = 0.01]. Follow-up contrasts confirmed that rats in the NIC pretreatment group sign tracked more than rats in the SAL group on sessions 33–36, p’s < 0.05.
Reversal: sign- vs. goal-tracking
NIC increased sign-tracking when the US was shifted away from the CS; however, sign-tracking and goal-tracking did not differ for NIC pretreated groups (SAME groups; Fig. 5c). This was confirmed with mixed ANOVA for the SAME group with a significant main effect of drug [F(1, 14) = 12, p < 0.01] and a significant drug × location interaction [F(1, 14) = 6.44, p = 0.02]. Follow-up contrasts confirmed that approach to the CS receptacle (sign-tracking) was increased by NIC pretreatment (p < 0.01); approach to the US receptacle was statistically similar across drug treatments.
Reversal: sign/goal-tracking vs. approach to a US-associated location
NIC increased approach to the location cues that previously predicted delivery of the US (DIFF groups; Fig. 5c). A mixed ANOVA for the DIFF group revealed a significant main effect of location [F(1, 14) = 35.3, p < 0.001] and a significant location × drug interaction [F(1, 14) = 5.3, p = 0.04]. Follow-up contrasts confirmed that approach to the receptacle previously associated with the US (goal) was increased by NIC pretreatment (p < 0.01); approach to the active receptacle was statistically similar across drug pretreatments.
Experiment 3
Return to baseline for DIFF groups
After eight sessions with the goal location shifted back to the original receptacle for the DIFF group, there were no differences in approach to the CS or US receptacles between DIFF and SAME groups (Fig. 6a). This was confirmed by three-way ANOVA performed on average elevation scores in the goal and sign receptacles from the last 3 days (6–8) of the return to baseline tests. There were no significant main effects or interactions involving group (F’s ≤ 2.4, p’s ≥ 0.15). The main effects of drug and location were significant (F’s ≥ 7, p’s ≤ 0.02), and there was also a significant drug × location interaction [F(1, 12) = 4.9, p = 0.05]. Elevation scores were collapsed across group (SAME and DIFF) for subsequent analyses.
Placebo replacement tests
Prior exposure to nicotine did not alter goal-tracking but increased sign-tracking in a manner that did not dissipate over testing (Fig. 6b). This was confirmed by three-way ANOVA with a significant main effect of location [F(1, 322) = 6.9, p = 0.02] and a significant drug × location interaction [F(1, 322) = 11.66, p < 0.01]. No other main effects or interactions were significant (F’s ≤ 2.9, p’s ≥ 0.11). Simple effects confirmed that rats previously exposed to nicotine had significantly higher elevation scores in the sign receptacle [F(1, 14) = 26, p < 0.001], but not the goal receptacle [F(1, 14) = 1.4, p = 0.25], relative to rats who had only been exposed to saline.
Discussion
Prior research suggests that the stimulus effects of nicotine can guide both sign- and goal-tracking response forms (Besheer et al. 2004; Dion et al. 2011; Palmatier et al. 2004); however, the present studies are the first to demonstrate that nicotine pretreatment directly shifts the form of the response from goal- to sign-tracking. In the present studies, nicotine pretreatment facilitated approach to incentives associated with a sucrose reward, but only when those incentives were spatially or temporally dissociated from the US (reinstatement). Experiment 2 confirmed that this was not an artifact of test procedures and that nicotine could facilitate approach to a location associated with sucrose in the past. Experiment 3 demonstrated that this effect of nicotine persisted for at least 24 test sessions after nicotine pretreatments were discontinued. These findings confirm that nicotine potently increases anticipatory approach behavior evoked by incentive stimuli and establish a paradigm in which the incentive amplifying effects of nicotine are robust, long-lasting, and do not depend on a contingency between operant behavior and reward delivery. They also prompt several further questions about the nature of the synergism between nicotine and incentives. What is the role of incentive stimuli in the response elevating effects of nicotine observed in operant conditioning paradigms? What features of the incentives make them more or less likely to be “enhanced” by nicotine and what features of nicotine exposure produce these enduring changes in behavior?
The most parsimonious unifying explanation of the effects observed in the present studies, in related paradigms (Olausson et al. 2003; Thiel et al. 2009), and in operant conditioning paradigms (Palmatier et al. 2007c) is that nicotine amplifies the incentive properties of reward-associated stimuli. In the Pavlovian conditioning paradigms, nicotine increased approach to contexts (Thiel et al. 2009) and discrete stimuli associated with rewards (Olausson et al. 2003). Facilitation of conditioned approach was most easily measured in the present studies when “sign-tracking” was dissociated from “goal-tracking.” Approach to reward-associated incentives is probably also the target of nicotine effects in operant conditioning paradigms. Sign-tracking is an element of positively reinforced operant behavior; performing the response requires orienting toward and approaching reward-associated stimuli (e.g., Brown and Jenkins 1968; Pithers 1982; Reid 2009). In operant conditioning paradigms, the reinforcement-enhancing effect of nicotine appears to depend on nicotine-facilitated sign-tracking (Palmatier et al. 2012). Additional studies are needed to confirm this hypothesis; however, the alternative that nicotine increases operant behavior and sign-tracking via independent processes does not seem parsimonious.
If these effects of nicotine can be applied more generally to reward-associated incentive stimuli (Hogarth et al. 2010; Olausson et al. 2003; Palmatier et al. 2012; Paterson et al. 2008; Raiff and Dallery 2008; Thiel et al. 2009), then the descriptor we have used most often “reinforcement-enhancing effect” (Chaudhri et al. 2006b; Donny et al. 2003; Palmatier et al. 2006) may no longer be useful. The term “reinforcement” is overly restrictive with regard to behavioral test paradigm; a reinforcement-enhancing effect of nicotine can only be observed in paradigms that include contingencies between a well-defined behavior and delivery of a reinforcer. This term cannot be applied to paradigms such as place conditioning (Thiel et al. 2009) and Pavlovian-conditioned approach (e.g., Olausson et al. 2003; present studies). Also, reinforcement includes a complex set of motivational, associative, and sensorimotor processes (e.g., Timberlake 1993), any or all of which could be inflated by nicotine exposure. The statement that nicotine functions as a “reinforcement enhancer” could follow from an alteration in motivation, associative learning, and/or sensorimotor function. Recent findings have converged on incentive stimuli as the principal target for these effects of nicotine (Hogarth and Duka 2006), and therefore, a more precise and less restrictive way to label these effects of nicotine might be “incentive amplifying” (Bevins and Palmatier 2004).
One important feature of the present studies was that when the sign and goal were separated, pretreatment with nicotine usually resulted in more approach to the sign than the goal. The notable exception was that during acquisition (DIFF groups, experiment 2), approach to both locations was comparable. There are two potential explanations for this finding. First, the saline pretreated rats acquired a very weak goal-tracking CR during this phase. The optimal strategy was most likely to wait between the dippers during the CS and then approach the goal after the dipper was engaged. Adoption of a similar strategy could have reduced sign-tracking in the nicotine-pretreated rats. Another explanation is that location cues may also be considered incentives in that the stimuli around the goal location are associated with sucrose delivery. One feature of sign- vs. goal-tracking paradigms is that both response forms represent approach to incentive stimuli—there is no benefit to approaching the goal unless the stimuli surrounding the goal have also been associated with the US. Unless the US is surgically implanted (e.g., intravenous administration; Uslaner et al. 2006, 2008), there will always be a consistent set of stimuli which identify the location that the US will be delivered. If nicotine increases approach to incentives, then it is not surprising that approach to incentives that provide information about the timing of the US (light illumination) and the location of the US (goal dipper) were comparable. The findings from this group during the reversal—in which NIC rats approached the old goal more than SAL rats during the CS—support this hypothesis. This was part of our rationale for shifting the location of the US, rather than the CS in experiments 1 and 2—changing the location of the illuminated light would not change the localized incentive stimuli (features of the receptacle) already associated with sucrose.
Experiment 3 also prompts questions about the contributions of prior drug exposure and the behavioral testing paradigm to the long-term changes in behavior engendered by nicotine. In paradigms that employ a visual stimulus as a reinforcer, response-elevating effects rapidly decline after nicotine treatment is withdrawn (Palmatier et al. 2006, 2008), and prior nicotine exposure does not increase responding during later testing (Weaver et al. 2012). However, brief nicotine exposure can have long-lasting effects on incentive motivation when tested in other paradigms (Cohen et al. 2005; Harrod et al. 2012; Lacy et al. 2012; Olausson et al. 2004b). For example, gestational exposure to intravenous nicotine results in increased motivation to obtain sucrose (Lacy et al. 2012) and increased sensitivity to the reinforcing effects of methamphetamine (Harrod et al. 2012) in offspring tested as adults. Similar effects (Cohen et al. 2005; Olausson et al. 2004b) do not depend on nicotine exposure during critical developmental periods. The emerging pattern suggests that the effect of prior nicotine exposure is moderated by the status of the incentives (i.e., associative strength) within the test context. In agreement with this, incentive-amplifying effects of nicotine are more robust when incentive stimuli are associated with more potent rewards (Palmatier et al. 2012). In rats responding for visual reinforcers (Palmatier et al. 2007c; Weaver et al. 2012), the incentives are weak in comparison with stimuli that precede sucrose (Lacy et al. 2012; present studies) and methamphetamine (Harrod et al. 2012). Finally, when the incentive status of the CS was purposefully manipulated in the present studies (extinction), the facilitative effect of nicotine was not observed. However, during the reinstatement test, when both the CS and US were presented in a temporally distinct manner, nicotine facilitated approach to the CS. Thus, with a strong enough incentive, prior nicotine exposure is probably sufficient to engender increased approach behavior. This may be further strengthened if a stimulus acquires incentive properties within the context of nicotine’s pharmacological effects. If nicotine alters what is learned about incentive-stimuli during acquisition, this could help to explain the assiduous nature of smoking “cues.”
Sign-tracking has become a popular technique for investigating the incentive motivational effects of abused drugs (Anderson and Spear 2011; DiFeliceantonio and Berridge 2012; Doremus-Fitzwater and Spear 2011; Uslaner et al. 2006). Preferential approach to sign stimuli in some individuals has been proposed as part of an endophenotype of substance dependence (Flagel et al. 2008; Robinson and Flagel 2009), and comparable behavioral and neurobiological bases of sign-tracking, substance dependence, and incentive motivation (Tomie et al. 2008) have invigorated research in this area. The incentive amplifying effects of nicotine and their ability to increase approach to reward associated stimuli may be relevant to the development of tobacco dependence in humans. For example, flavor and odor additives with incentive properties (e.g., cinnamon, vanilla) are often included in tobacco products. Co-delivery of these incentives with nicotine could increase their ability to evoke approach responses—promoting smoking behavior. Our hypothesis that nicotine amplifies the effects of these incentives suggests that co-presentation of an incentive stimulus with nicotine infusions should invigorate volitional nicotine intake (Caggiula et al. 2009; Palmatier et al. 2007b). The effects of nicotine on sign-tracking also varied between individuals; although the present studies did not have the power required to analyze these individual differences, they may predict susceptibility to dependence or the amount of nicotine self-administered in pre-clinical models. Refinement of the techniques used to investigate incentive motivation is needed, but these preliminary findings suggest that the incentive amplifying effects of nicotine may be critical to promoting tobacco use and dependence.
References
Anderson RI, Spear LP (2011) Autoshaping in adolescence enhances sign-tracking behavior in adulthood: impact on ethanol consumption. Pharmacol Biochem Behav 98:250–260
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Besheer J, Palmatier MI, Metschke DM, Bevins RA (2004) Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology 172:108–117
Bevins RA, Palmatier MI (2004) Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev 3:143–158
Brown PL, Jenkins HM (1968) Auto-shaping of the pigeon's key-peck. J Exp Anal Behav 11:1–8
Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009) The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv 55:91–109
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF (2006a) Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology 189:27–36
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006b) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology 184:353–366
Cohen C, Perrault G, Griebel G, Soubrie P (2005) Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30:145–155
DiFeliceantonio AG, Berridge KC (2012) Which cue to ‘want’? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res 230:399–408
Dion AM, Reichel CM, Bevins RA (2011) Sign- vs. goal-tracking in a feature positive discrimination task with nicotine: importance of spatial location of the conditional stimulus. Behav Brain Res 218:341–345
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology 169:68–76
Doremus-Fitzwater TL, Spear LP (2011) Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behav Neurosci 125:661–667
Farwell B, Ayres JJ (1979) Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (goal tracking) in rats. Learn Motiv 10:295–312
Flagel SB, Watson SJ, Akil H, Robinson TE (2008) Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res 186:48–56
Harrod SB, Lacy RT, Morgan AJ (2012) Offspring of prenatal IV nicotine exposure exhibit increased sensitivity to the reinforcing effects of methamphetamine. Front Pharmacol 3:116
Hearst E, Jenkins HM (1974) Sign-tracking: the stimulus–reinforcer relation and directed action. Austin: Psychonomic Society
Hogarth L, Duka T (2006) Human nicotine conditioning requires explicit contingency knowledge: is addictive behaviour cognitively mediated? Psychopharmacology (Berl) 184:553–566
Hogarth L, Dickinson A, Duka T (2010) The associative basis of cue-elicited drug taking in humans. Psychopharmacology (Berl) 208:337–351
Lacy RT, Hord LL, Morgan AJ, Harrod SB (2012) Intravenous gestational nicotine exposure results in increased motivation for sucrose reward in adult rat offspring. Drug Alcohol Depend 124:299–306
Le Foll B, Goldberg SR (2006) Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology (Berl) 184:367–381
Olausson P, Jentsch JD, Taylor JR (2003) Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology 28:1264–1271
Olausson P, Jentsch JD, Taylor JR (2004a) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology 171:173–178
Olausson P, Jentsch JD, Taylor JR (2004b) Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology 173:98–104
Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA (2004) Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol 15:183–194
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF (2006) Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 184:391–400
Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF (2007a) The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology 32:1098–1108
Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF (2007b) Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology 195:235–243
Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF (2007c) The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend 89:52–59
Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF (2008) Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology 33:2139–2147
Palmatier MI, O'Brien LC, Hall MJ (2012) The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology (Berl) 219:1119–1131
Paterson NE, Balfour DJ, Markou A (2008) Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res 10:995–1008
Pithers RT (1982) The roles of S-R contiguity and reinforcement in autoshaping and omission responding. Aust J Psychol 34:1–16
Raiff BR, Dallery J (2008) The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology 201:305–314
Reid AK (2009) Resistance to change within heterogeneous response sequences. J Exp Psychol Anim Behav Process 35:293–311
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Robinson TE, Flagel SB (2009) Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry 65:869–873
Thiel KJ, Sanabria F, Neisewander JL (2009) Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 204:391–402
Timberlake W (1993) Behavior systems and reinforcement: an integrative approach. J Exp Anal Behav 60:105–128
Tomie A, Grimes KL, Pohorecky LA (2008) Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev 58:121–135
Uslaner JM, Acerbo MJ, Jones SA, Robinson TE (2006) The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res 169:320–324
Uslaner J, Dell'Orco J, Pevzner A, Robinson T (2008) The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology 33:2352–2361
Weaver MT, Geier CF, Levin ME, Caggiula AR, Sved AF, Donny EC (2012) Adolescent exposure to nicotine results in reinforcement enhancement but does not affect adult responding in rats. Drug Alcohol Depend 125:307–312
Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130
Acknowledgments
The research was conducted at Kansas State University and all of the protocols and procedures were approved by the Kansas State University Institutional Animal Care and Use Committee (Animal Welfare Assurance #: A3609-01). We thank Dr. Rick Bevins for his comments on a previous version of this manuscript. We thank Dr. Matt McBee for assistance with data analysis. We thank Jessica Jones, Ryan Floyd, Skyler Gross, and Taylor Montgomery for their assistance conducting these studies. The studies were partially supported by the Johnson Center for Basic Cancer Research at Kansas State University (SA Jones) and NIH grant DA-24801 (MI Palmatier).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palmatier, M.I., Marks, K.R., Jones, S.A. et al. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology 226, 247–259 (2013). https://doi.org/10.1007/s00213-012-2892-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2892-9