Abstract
Background
The link between adolescent social stress and substance abuse is modeled in social defeat of adolescent male rats, at an age when social experiences are essential for neurobehavioral maturation.
Objective
We investigated the role of social experience and social defeat stress during adolescence on social behavior and cocaine self-administration (CocSelfAd) in early adulthood.
Methods
We manipulated social experience by housing male rats in pairs (PH) or singly (SH) on postnatal day (P) 21. In addition, rats were subjected to social defeat from P35–44. Social behavior was measured during the first and last social defeat in PH and SH adolescents and PH adults. After assessing the behavioral response to novelty and cocaine (P57–61), intrajugular catheters were implanted and CocSelfAd was analyzed.
Results
Residents were less aggressive toward PH adolescent intruders compared to PH adult intruders. Adults were submissive and defensive when attacked, whereas PH adolescents froze. In the course of repeated defeats, adolescent PH rats increased freezing, while SH rats decreased freezing. Longer attack-induced freezing after repeated defeats predicted escalated CocSelfAd in adulthood. PH controls acquired CocSelfAd more slowly than PH defeated and SH rats. Defeated PH rats increased CocSelfAd during progressive ratio schedules of reinforcement and during a 24-h continuous access binge compared to PH controls and SH defeated rats.
Conclusions
Social defeat in adolescence of PH rats caused persistent increases in adult CocSelfAd. Adolescent PH rats coped with attacks adaptively by increasing freezing behavior after repeated social defeats, a measure that predicted CocSelfAd in adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thirty percent of youths experience peer victimization, and about 10 % are victimized on a regular basis (Nansel et al. 2001; Newman et al. 2005). Experiencing the negative consequences of victimization, such as low self-esteem, increases severity of psychopathologies and substance abuse. Social defeat in laboratory rodents, in which the subjects (termed intruders) are confronted with aggressive conspecifics (termed residents) may model victimization in humans (Bjorkqvist 2001) and may help to identify neural mechanisms that cause stress-induced human pathologies, such as substance use disorder (Buwalda et al. 2005). Social defeat is an ethologically and etiologically relevant stressor for the rat (Miczek et al. 1991; Miczek et al. 2008) that elicits an intense glucocorticoid stress response (Koolhaas et al. 1997) that does not habituate over repeated confrontations (Covington and Miczek 2005; Watt et al. 2009). Episodes of social defeat in adulthood reliably increase cocaine self-administration in adult male rats (Covington and Miczek 2005; Miczek et al. 2011). As adolescence is a particularly sensitive period for social development, we measured cocaine self-administration following social defeat during adolescence.
Neural and behavioral development is frequently investigated in rodents during adolescence between postnatal days (P) 21 and 59 (reviewed in Burke and Miczek 2014). Adolescence is considered a period of hypersensitivity to stressful events as illustrated by slower return to baseline of stress-stimulated plasma corticosterone compared to adults (Goldman et al. 1973; Romeo 2010). Adolescent-typical social behaviors, such as play fighting, are essential for the development of normal adult social behaviors (Hall and Perona 2012; Lukkes et al. 2009c). The long-term effects of adolescent social deprivation and adolescent social defeat overlap because both sensitize the mesocorticolimbic dopamine system (Burke et al. 2010; Whitaker et al. 2013) and increase measures of psychostimulant reward (Burke et al. 2011; Howes et al. 2000). The interaction between social deprivation and defeat during adolescence was investigated in the present experiment.
Analysis of aggressive resident behavior during social defeat of socially housed male mice and female rats suggest that residents are less aggressive toward adolescents compared to adult intruders (Garcia-Pardo et al. 2014; Ver Hoeve et al. 2013). Further, social and nonsocial behaviors of socially housed adolescent intruders when confronted with an adult resident are different from adult intruder behaviors (Garcia-Pardo et al. 2014; Ver Hoeve et al. 2013). We now provide a detailed analysis of both resident and intruder behavior during adolescent and adult social defeat of pair-housed (PH) male rats.
As social deprivation during adolescence has short- and long-term effects on social behavior (Lukkes et al. 2009b; Panksepp et al. 1984), and single housing (SH) intensifies the negative effects of adult social defeat (de Jong et al. 2005; Nakayasu and Ishii 2008; Ruis et al. 1999; Von Frijtag et al. 2000), we investigated the role of housing conditions during adolescent social defeat on adult cocaine self-administration. Adolescent PH rats adopt submissive postures more quickly over repeated social defeat encounters exhibiting conditioned defeat behavior (Watt et al. 2009). We conducted a detailed analysis of adolescent rat behavior during the first and fourth social defeats to discover changes in social behaviors and investigated their relationship with adult behavioral phenotypes because individual differences in conditioned behavior during adolescence might predict adult behaviors.
A frequently documented consequence of peer victimization is increased illicit drug use (Hoffmann et al. 2000; Tharp-Taylor et al. 2009). Victimization of adolescents by adults also increases substance abuse (Nelson et al. 1995). Animal studies demonstrate that stress history in adulthood increases measures of drug reinforcement, preference, and locomotion, but capturing this link in adolescent rodents is investigated less (Burke and Miczek 2014). A history of brief intermittent social defeat episodes in adult rats promotes greater voluntary self-administration of cocaine according to fixed or progressive ratio schedules of reinforcement (PR), and particularly during a 24-h continuous access binge (Covington and Miczek 2005; Miczek et al. 2011). Adolescent social defeat stress increases amphetamine-stimulated locomotion and conditioned place preference in rats (Burke et al. 2013; Burke et al. 2011). In the present study, we investigated cocaine self-administration under several schedule conditions after adolescent social defeat. Single housing increases measures of drug self-administration, but only when the rat is isolated from weaning onward (Lopez et al. 2011; Robbins et al. 1996; Schenk et al. 1990). We investigated how housing conditions interacted with social defeat during adolescence and their effects on cocaine self-administration in adulthood using limited and extended access conditions.
The current study aimed to identify the long-term effects of social deprivation and social defeat during adolescence on novelty and cocaine-stimulated locomotion, acquisition of cocaine self-administration, motivation for cocaine, and cocaine self-administration during a 24-h binge in early adulthood. Furthermore, we sought to compare and contrast adolescent social defeat behaviors as a function of age and housing conditions. The overarching hypotheses were that adolescent social deprivation and social defeat would increase cocaine self-administration and pair housing would serve as a social buffer against the negative effects of social defeat stress.
Experimental methods
Animals and environment
Long-Evans rats from Charles River Laboratories (Wilmington, MA) were housed in a vivarium that was maintained at 21 ± 1 °C with 35–40 % humidity on a reverse light/dark cycle (lights on 2000 to 0800 hours). Non-littermate male rats (N = 96, eight separate one-day shipments over 18 months) arrived on P20 or P21 and were housed singly or in pairs in standard polycarbonate cages (45 × 24 × 20 cm) with wood chip and Crink-l’Nest (The Andersons, Maumee, OH) bedding. Adult non-littermate rats arrived on P58 (N = 12) and were pair housed (PH). Food (Purina laboratory rodent chow) and tap water were available ad libitum. Reliably aggressive adult male (500–700 g) rats (N s= 20) were housed with females in large stainless steel cages (71 × 46 × 46 cm) filled with the same bedding as intruders in a separate room, but within the same vivarium. All adolescent and adult intruder rats were handled and weighed Monday–Friday from P32 onward. Facilities and procedures were approved by the Tufts Institutional Animal Care and Use Committee in adherence with the guidelines established by the National Institutes of Health (National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals et al. 2011).
Social stress
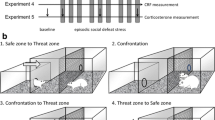
Residents were confronted once per day for 12 days with nonexperimental rats prior to experimental social defeat to identify the most reliably aggressive residents. The six most consistently aggressive residents were selected based on latency to attack, aggressive postures, and frequency of attacks/bites. Adolescents were defeated four times over 10 days, following the intermittent episodic social defeat design of previous experiments in adults (Boyson et al. 2014; Covington and Miczek 2005), occurring specifically on P35, P38, P41, and P44 (Fig. 1). Controls remained undisturbed during each defeat procedure. Cage-mates of defeated and control rats had the same treatment. The females and any pups were removed a few minutes before the defeat procedure. The experimenter recorded the latency and frequency of bites and supine postures, the duration of each supine posture, and the total duration of the interaction (termed latency to submission). The interaction was terminated 5 min after the first attack bite or earlier if the intruder displayed a submissive supine posture for greater than 4 s or if more than 12 attack bites occurred. The intruder was then removed and placed in a protective mesh cage (20 × 30 × 20 cm), which was then placed inside the resident’s home cage for 10 min. Intruders were exposed to a different resident during each episode of defeat. If no attack bite was observed within 5 min, the intruder was placed into a different resident’s cage. A group of PH adult rats underwent the same procedure and were exposed to the same residents used for adolescent defeats but at different ages (P65, P68, P71, and P74).
Rat behavior was measured using The Observer XT v9.0.436 (Noldus Information Technology, The Netherlands) that allowed for recording of simultaneous and overlapping events. A trained observer (intra-observer reliability r > 0.93) measured previously described intruder and resident behaviors (Buwalda et al. 2013; Koolhaas et al. 1980; Plyusnina et al. 2011) during separate observations. Nonsocial exploration (walking, rearing, and sniffing) and nonambulatory motor behavior were quantified for all rats. The following resident behaviors were quantified: attack bite (usually directed at rump and flank and included weaker nips), aggressive posture (pin), other aggression (foreleg attack, hind leg attack, dragging), frontal threat, lateral sideways threat, pursuit, allogrooming, and anogenital investigation. Intruder behaviors quantified were supine posture, escape from supine, upright defensive posture, freezing posture, walking slowly, tail rattle, follow and crawl under resident, approach resident, and social investigation (sniffing resident). Since the duration of the fights (latency to submission) varied, all Observer-coded data were expressed as a percentage of total time or number per minute. A time lag of −3 to +5 s from the attack bite was used to determine the probability of intruder behaviors occurring in response to an attack bite.
Adult locomotion testing
All locomotor testing occurred in the vivarium, but in a separate room. Previously defeated rats were undisturbed except for handling and weighing until the open field test on P57. The open field test was conducted under red lighting between 0900 and 1600 hours in plastic cages that measured 54.0 × 35.5 × 48.5 cm or 48.3 × 29.3 × 43.2 cm. Two different-sized open fields were used because identical replacements were unavailable mid-experiment. A computer equipped with EthoVision XT v.8.5.614 (Noldus) measured motor behavior. A center rectangle (37.5 × 21.6 cm larger cage; 31.8 × 17.1 smaller cage) was outlined with EthoVision. The dependent variables “distance moved” and “time in center” were closely similar in the two different open fields (p > 0.05). Immediately after the open field test, rats were placed into custom-built acrylic chambers (30 × 30.5 × 24.5 cm; one rat per chamber), which served as the home cage until cocaine self-administration was initiated.
From P57 to P59, all rats received daily saline injections (1 ml/kg, i.p.). On P60, each rat was injected with saline and returned to the home cage illuminated by red lighting, and then a tracking software (HomeCageScan v3.0, CleverSys Inc., Reston, VA, USA) was started a few seconds later. After 10–12 min, the rat was injected with cocaine (10 mg/kg, i.p.) and placed back in the home cage. Cocaine hydrochloride (Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD, USA) was dissolved in sterile 0.9 % saline for all experiments. The behaviors defined by CleverSys “walk to the left” and “walk to the right” from 6 to 10 min after i.p. injection were combined into one measurement and used as the dependent variable, “walking duration (s)”. Walking for 6 to 10 min after i.p. saline was compared with the corresponding 5 min time bin after i.p. cocaine consistent with previous studies (Covington and Miczek 2005; Miczek et al. 2011).
Cocaine self-administration
Surgery
Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (6 mg/kg). An indwelling catheter (Silastic silicone tubing, Dow Corning, ID 0.63 mm, OD 1.17 mm) was implanted into the right jugular vein as previously described (Covington and Miczek 2001). Following 5 days of recovery, rats were placed in a different custom-built chamber (30 × 30.5 × 24.5 cm) within a sound- and light-attenuating enclosure.
Procedures
All self-administration procedures and equipment were identical to previous experiments using adult rats (Boyson et al. 2014; Covington and Miczek 2001; Miczek et al. 2011). MED-PC IV (Med Associates Inc., St. Albans, VT) controlled experimental events and recorded operant responding.
Acquisition protocol
Immediately after the rats were placed into the self-administration chamber, the experimental session was started, and rats were allowed to self-administer cocaine (0.75 mg/kg per infusion) according to a fixed ratio (FR) 1 schedule with only the active lever present. The session was terminated after 5 h or 15 infusions, whichever came first. Rats were considered to have acquired the task when they obtained 15 infusions in two consecutive sessions, after which the FR was increased. If rats did not achieve this requirement within 3 days/15 h of access to cocaine, the experimenter shaped the rat’s behavior by placing female urine on the active lever. Palatable food was used as a secondary technique. No behavioral shaping was used during measurement of dependent variables. Ninety-six percent of PH controls, 73 % of the PH defeat group, 55 % of SH controls, and 43 % of the SH defeat group required shaping. After acquisition, the FR was slowly increased to 5, and after three stable days of FR5 responding, the motivation to self-administer cocaine was studied according to a PR. Responses per minute were calculated by dividing the total responses while cocaine was available by the duration of the session for all procedures.
Progressive ratio schedule of cocaine reinforcement
Rats were given access to cocaine on a PR that required an increasing number of responses to be reinforced by an infusion of cocaine (0.3 mg/kg/infusion). The schedule permitted all rats to reach their breakpoint within 5 h, using the following progression 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 118, 145, 178, …, 402, 492, 603….etc. (Richardson and Roberts 1996). A lower dose of cocaine was used for this phase (0.3 mg/kg/infusion) based on previous studies suggesting that reliable augmented self-administration following adult social defeat is more consistently observed at this lower dose (Covington and Miczek 2001; 2005). The session terminated when no cocaine infusion was delivered for 60 min. Three PR sessions were conducted on different days separated by FR5 maintenance sessions.
Twenty-four-hour binge protocol
After stable FR5 responding was observed following the third PR session, rats were given access to cocaine (0.3 mg/kg/infusion) for 24 h on an FR5 schedule.
Statistical analysis
Intruder behaviors in adult PH rats were compared to intruder behaviors of adolescent PH rats using a one-way ANOVA because there were no significant effects of time for adults. If one group had no variance because the behavior was not observed, Kruskal-Wallis ANOVA on ranks was performed. Intruder behaviors by adolescents were analyzed with a repeated measures (RM) two-way ANOVA (housing [pair vs. single] × time [P35 first vs. P44 fourth defeat]). The average weight from P53–56 (early adult post stress) minus the average weight from P32–35 (pre defeat) for each individual calculated body weight gain, which was analyzed with a two-way ANOVA (housing × treatment [social defeat vs. control]). Open field behavior was analyzed with a two-way ANOVA (housing × treatment). Walking in response to i.p. injections was analyzed with a three-way ANOVA (housing × treatment × drug [saline vs. cocaine]) followed by two-way ANOVAs for each housing condition and post hoc pairwise comparisons using the Holm-Sidak method. Acquisition of the operant response for cocaine was analyzed by comparing the proportion of rats to have reached our criterion for acquisition. Kaplan-Meier 1-survival analysis of the first 15 h of access followed by log-rank (Mantel-Cox) tests which determined differences between treatment groups. Rats that performed zero responses during the first 15 h of access were excluded from this analysis (N = 19, 13 in PH and 6 in SH) because cocaine availability was unknown. The total number of cocaine infusions obtained under the PR was averaged over the three PR sessions. For the 24-h binge, the total number of cocaine infusions obtained was the dependent variable. Two-way ANOVAs (housing × treatment) were applied to PR and binge data. Significant main effects and interactions were followed up by pairwise comparisons (Holm-Sidak). Separate linear regression analyses were used to assess potential relationships between defeat behavior and cocaine self-administration. Change in attack-induced freezing was calculated by subtracting the probability of freezing during an attack bite on P35 from the same measure on P44. Separate Grubbs’ tests were applied to data sets where appropriate (resident behavior, intruder behavior, and locomotion) to identify outliers (Grubbs 1969). Statistical outliers were removed only if the value was >2.25 standard deviations above or below the mean for the PR (n = 1) and binge (n = 1). Survival analysis and log ranks were performed using IBM SPSS Statistics v21 (IBM Corp.). Sigma Plot v11 (Systat Software Inc.) was used for the remainder of statistical tests. The alpha level was always 0.05 for statistical significance.
Results
Social defeat behaviors
Adult vs. adolescent defeat (statistics in Table 1)
One-way ANOVA indicated that residents attacked adult intruders faster than adolescent intruders (Table 1). The rate of attack bites per minute was significantly higher when confronted with adults versus adolescent intruders. The adult residents spent more time in the lateral sideways threat posture when confronting an adult intruder compared to an adolescent. In contrast, the adult resident engaged in significantly more frontal threat postures when confronted with an adolescent intruder. The latency for the first attack bite, supine posture, and full submission was significantly longer when the resident was confronted with an adolescent intruder. Adult intruders spent a significantly greater percentage of their time in the supine posture, while adolescents spent a significantly greater percent of time following/crawling under the resident and approaching the resident. In response to an attack bite, adult intruders were significantly more likely to adopt the supine and upright defensive posture, whereas the adolescent intruders were more likely to freeze or remain immobile.
Resident behaviors with PH vs. SH adolescent rats (statistics in Table 2)
Residents spent significantly more time engaged in aggression (attack bite and other aggressive behaviors) and sniffing the SH rats (Table 2). However, residents spent significantly more time engaged in frontal threat postures, allogrooming, and anogenitally investigating the PH compared to SH rats. The latency to attack bite declined significantly from the first to the last defeat. The percent of time engaged in anogenital investigation of PH intruders on the P35 defeat was significantly greater than the last defeat and also greater than in SH intruder on P35. The percent of time engaged in nonsocial exploration reduced over the four defeats when confronted with PH rats, such that nonsocial exploration was lower than when confronted with SH rats by P44.
PH vs. SH adolescent intruder behavior (statistics in Table 2)
During the 10 days of repeated confrontations with aggressors, the number of escapes from supine, upright defensive postures, and immobility increased, while latency to submission, social investigation of the resident, and nonsocial exploration decreased (statistics in Table 2). The latency to freeze significantly decreased from P35 to P44 in PH rats only, and on P44, PH rats were quicker to freeze than SH intruders. The percent of time engaged in freezing behavior significantly increased from P35 to P44 in the PH intruders only, and on P44, PH rats spent more time freezing than SH intruders (Table 2 and Fig. 2). Single-housed intruders spent more time following/crawling under the resident and approaching the resident. In response to an attack bite, PH rats were less likely to freeze during the first defeat, but significantly more likely to freeze during the fourth defeat, both compared to SH intruders (Table 2 and Fig. 2). Single-housed intruders actually reduced freezing in response to an attack bite on P35 compared to P44. Figure 2 highlights selected data from Table 2. In general, from the first to the last social defeat, PH rats showed an increased probability of freezing in response to an attack bite, increased overall freezing, and decreased latencies to freeze and supine posture, none of which were observed in SH rats.
Summary of selected adolescent intruder behaviors from Table 1. The relative probability of freezing after an attack bite is represented by the thickness of the arrow between “Bite” and “Freeze”. The size of the gray circle represents the relative percent of time adopting the freezing posture. The length of the dotted black lines (latency to freezing posture) and gray lines (latency to supine posture) represent the relative amount of time from introduction to the resident until the behavior was first observed
Body weight
From P32 to P61, the PH control group gained 190 ± 4.4 g, PH stress group gained 198 ± 5.6 g, SH control 188 ± 3.7 g, and SH stress 182 ± 2.2 g (N = 22–26/group). Two-way ANOVA revealed significantly less weight gain in SH compared to PH rats (F(1,91) = 4.364, p = 0.039), an effect that appears driven differences in weight gain among stressed rats.
Locomotion
Pair-housed rats moved less in the open field (F(1,92) = 22.56, p < 0.001) and spent more time in the center of the open field (F(1,92) = 16.09, p < 0.001; Fig. 3a) in early adulthood (P57) compared to SH rats. Cocaine increased walking in PH controls (p = 0.004), PH defeated rats (p = 0.026), and SH controls (p = 0.003), but not in SH defeated rats as indicated by post hoc tests based on the a priori hypothesis that i.p. cocaine increases walking compared to i.p. saline. There was a significant main effect of housing (F(1181) = 10.488, p = 0.001) and drug (F(1181) = 20.28, p < 0.001; 3 B).
a Total distance moved (mean ± SEM) and time spent in the center of the 60-min open field test on P57 for social defeat (stress) and control treatment groups that were pair-housed or single-housed since P21 (N = 22–26/group). *Significant effect of housing (p < 0.001). b Total duration of walking (mean ± SEM) 5–10 min after i.p. injection of saline and cocaine (10 mg/kg; N = 23–26/group). #Significantly different from saline of same treatment group (p < 0.01)
Cocaine self-administration
Pair-housed defeated rats, SH controls, and SH defeated rats acquired the iv. cocaine self-administration task significantly faster in adulthood compared to PH controls (respectively, χ 2 = 4.5, p = 0.034; χ 2 = 10.1, p = 0.001; χ 2 = 16.5, p < 0.001; Fig. 4). Furthermore, a greater proportion of SH defeated rats acquired the task than PH defeated rats (χ 2 = 5.5, p = 0.019). There was no difference between rats that acquired with or without behavioral shaping in subsequent measures of cocaine taking (p > 0.05). There was no effect of housing or treatment on maintenance as measured by the average responses per minute during the three FR5 sessions prior to the first PR session (p > 0.05).
One minus survival plot of the cumulative proportion of pair control (N = 18), pair defeat (N = 18), single control (N = 17), and single defeated rats (N = 19) reaching the acquisition criterion during the first 15 hours (over 3 days) of access to cocaine (0.75 mg/kg/infusion). *Significant difference from pair-housed control group (p < 0.05). #Significant difference between pair- and single-housed defeated rats (p < 0.05)
For the infusions obtained during PR, there was a significant main effect of housing (F(1,61) = 5.01, p = 0.029) and a significant interaction between housing and treatment (F(1,61) = 6.445, p = 0.014; Fig. 5). Post hoc tests showed that defeat increased cocaine infusions in PH rats (p = 0.043). Among the defeated rats, PH rats took more infusions than SH rats (p = 0.002).
Left, number of cocaine infusions (mean ± SEM) obtained by pair-housed control (N = 16), pair-housed defeat (N = 13), single-housed control (N = 18), and single-housed defeated rats (N = 19) averaged across progressive ratio (PR) schedule of reinforcement sessions. Right, number of cocaine infusions (mean ± SEM) accumulated by pair control (N = 14), pair defeat (N = 11), single control (N = 13), and single defeated rats (N = 13) during the 24-h binge. *Significantly different from pair-housed control group. #Significantly different from single-housed defeat group
There was a significant interaction between housing and stress treatment for total binge infusions obtained (F(1,46) = 4.55, p = 0.038; Fig. 5). Post hoc tests showed that in pair-housed rats, social defeat in adolescence increased the number of binge infusions (p = 0.038). Furthermore, PH defeated rats obtained more cocaine infusions than SH defeated rats (p = 0.019).
The average proportion of freezing in response to an attack bite on P44 (fourth defeat) was positively correlated with the responses per minute during the binge (p = 0.007, r 2 = 0.30; Fig. 6, top) and during PR (p = 0.012, r 2 = 0.020; data not shown). The change in freezing from P35 to P44, calculated by subtracting the freezing during attack bite on P35 from the corresponding value on P44, was positively correlated with total infusions obtained during the 24-h binge (p = 0.032, r 2 = 0.20; Fig. 5, middle) and during PR (p = 0.005, r 2 = 0.25; data not shown). Cocaine self-administration data were split at zero into rats that increased freezing from P35 to P44 and those that decreased freezing, and rats that increased freezing over repeated social defeats obtained more cocaine infusions during the binge (F(1,21) = 4.789, p = 0.040) and PR (F(1,29) = 6.069, p = 0.020) compared to decreased freezing group (Fig. 5, bottom).
Top panel, the probability that an intruder freezes during an attack bite on P44 is positively correlated with responses per minute during the 24-h continuous access binge (p < 0.01). Middle panel, the change in the probability that an intruder freezes during attack bite from first (P35) to fourth (P44) defeat is positively correlated with total cocaine infusions during a 24-h binge (p < 0.05). Values above zero were termed increased freezing and below zero, decreased freezing. Bottom panel, individuals that increased freezing from P35 to P44 demonstrated increased cocaine self-administration during the binge and under a progressive ratio schedule of reinforcement (PR) (p < 0.05)
Discussion
This is the first study to demonstrate that episodes of social defeat during adolescence escalate cocaine intravenous self-administration in adulthood. Defeated PH rats consumed more cocaine compared to SH defeated rats, suggesting that social experience plays a pivotal role escalation of cocaine self-administration in socially defeated adolescents. PH controls acquired cocaine self-administration more slowly than PH defeated and SH rats. Cocaine self-administration in adulthood was predicted by greater attack-induced freezing after repeated social defeats in adolescence, suggesting a relationship between adolescent social behavior and adult cocaine taking. We confirmed and expanded upon recent studies by showing that residents engaged in a higher attack rate toward adult versus adolescent intruders (Garcia-Pardo et al. 2014; Ver Hoeve et al. 2013; Zou et al. 2014). Intruders coped with the aggressor differently depending on age and housing conditions. For example, adult intruders were more likely to adopt the supine posture after an attack bite, whereas adolescent intruders were more likely to freeze. Notably, PH rats modified their behavior from the first to the last defeat by increasing freezing, whereas SH rats actually decreased freezing in response to an attack bite.
The greater probability of freezing in response to an attack bite of adolescent rats is specific to social stress because adolescents freeze to a similar extent to adults in nonsocial contexts (Broadwater and Spear 2013). Single-housed rats exhibited more freezing when confronted with the resident for the first time on P35 in agreement with other tests conducted in adult rats after adolescent SH (Lukkes et al. 2009a; Lukkes et al. 2009b; van den Berg et al. 1999). Adolescent PH rats increased attack-induced freezing, total freezing behavior, and were quicker to freeze or adopt supine postures, which was in sharp contrast to SH rat behavior (summarized in Fig. 2). The PH adolescent’s behavior may be considered to be adaptive because for adult rats increased freezing in the presence of the resident is thought to reduce the probability of further attacks (Buwalda et al. 2012; Nocjar et al. 2012; Paul et al. 2011). Rats with a genetic predisposition for anxiety exhibit greater freezing when confronted with an aggressor, perhaps driven by a high-level anticipatory anxiety (Frank et al. 2006). The change in freezing during adolescence over repeated confrontations may be interpreted as an adaptive fear or anxiety response, and this adaptive coping behavior during adolescence predicts some cocaine self-administration behaviors approximately 40 days later in early adulthood.
The increase in social exploration of residents in adulthood following adolescent SH is thought to provoke further attacks from aggressors (van den Berg et al. 1999). The increased approaching, following, and crawling under the resident observed in SH intruders confirms previously reported SH effects on social behavior of adolescent rats (Buwalda et al. 2013; Varlinskaya and Spear 2008) and is one possible explanation for increased attacks and other aggression directed toward SH intruders. Thus, we interpret the reduced attack-induced freezing over repeated defeats observed in SH adolescents as a maladaptive behavior.
Defeat in adolescence did not cause hyperactivity in a novel locale, which conflicts with our previous studies in Sprague Dawley rats (Burke et al. 2010; Burke et al. 2011; Watt et al. 2009). A different social defeat schedule, strain of rat, novel apparatuses, and different treatment of controls may account for the conflicting results. The increased locomotion for SH rats compared to PH rats agrees with other studies during adolescence (Levine et al. 2007; Meng et al. 2010) and adulthood (Chappell et al. 2013; Lapiz et al. 2003; Powell et al. 2002; Wright et al. 1991). Less time in the center of the open field of SH rats suggests greater anxiety (Prut and Belzung 2003) and replicates a previous report (Meng et al. 2010). There was no significant correlation between novelty- or cocaine-stimulated locomotion and cocaine self-administration (p > 0.05; data not shown), in agreement with a previous report (Thomsen and Caine 2011). The current dose of cocaine (10 mg/kg, i.p.) causes cross-sensitization after adult social defeat (Covington et al. 2005; Covington and Miczek 2001; Miczek et al. 2011). Social defeat in adolescence did not enhance cocaine hyperactivity, similar to some earlier results with amphetamine (Burke et al. 2013; Burke et al. 2010). Overall, brief adolescent social defeat did not impact behavior in the novel open field, but social deprivation throughout adolescence caused hyperactivity and anxiety-like behavior. There might be age differences in social defeat cross-sensitization to a cocaine challenge.
Single housing during adolescence increased the acquisition of cocaine self-administration in adulthood, confirming several previous studies (Baarendse et al. 2014; Bardo et al. 2001; Ding et al. 2005; Howes et al. 2000). More than 20 days after four episodes of social defeat applied to PH adolescent rats, we observed an increased rate of acquisition of cocaine self-administration. Adult social defeat only increases acquisition a few days after repeated defeats (Haney et al. 1995; Kabbaj et al. 2001; Tidey and Miczek 1997), but not a couple weeks later (Covington et al. 2005; Covington and Miczek 2001). These data support the hypothesis that social deprivation and social defeat during adolescence both increase acquisition of cocaine taking in early adulthood.
Adolescent social defeat of PH rats increased the motivation to lever press for cocaine during the PR and compulsive cocaine taking during a 24-h binge compared to PH controls and SH defeated rats. A similar intermittent social defeat protocol applied to adult rats also increases these same measures (Covington and Miczek 2005; Covington et al. 2008), suggesting social defeat increases cocaine taking under these parameters regardless of age. In these previous experiments, the adult rats were reared in groups by the supplier until early adulthood and then SH upon arrival and throughout the social defeat experiences. The effect of adult social defeat on cocaine self-administration of socially housed rats remains to be investigated. In the current study, PH could be interpreted as social buffer against drug taking only during the acquisition phase of cocaine self-administration.
The current data confirm the observation that SH during adolescence increases acquisition of intravenous self-administration without effects on subsequent measures of drug taking (Lu et al. 2003). This was the first study that investigated the possible role of housing conditions on the escalation of drug taking caused by social defeat. We expected synergism between SH and adolescent defeat based on previous studies in adults suggesting reduced stress reactivity in socially defeated PH rats (de Jong et al. 2005; Nakayasu and Ishii 2008; Ruis et al. 1999; Von Frijtag et al. 2000). However, there was no compounding effect of SH and adolescent defeat on cocaine self-administration during PR or the 24-h binge. Some social behaviors, which peak during rat mid-adolescence, are rewarding (Trezza et al. 2010), and SH adolescents exhibit increased preference for social contact and engage in social behavior more than group-housed adolescents (Douglas et al. 2004; Varlinskaya et al. 1999; Yates et al. 2013). Thus, social interaction with the resident might be less aversive for SH intruders in the current study to explain the absence of escalated cocaine taking in adulthood. Some of our data support this hypothesis; SH rats exhibited more social behaviors during social defeat than PH rats, such as approach, follow, and crawl under the resident. Single-housed rats also failed to increase fear- and anxiety-related behaviors over the course of repeated social defeats (summarized in Fig. 2).
Social defeat of PH adolescents increased all measures of cocaine self-administration in early adulthood, while social deprivation during adolescence increased only the acquisition of cocaine self-administration. Behavior during repeated confrontations with an aggressor of PH rats was adaptive, while the behavior of SH rats was maladaptive based on the higher rate of aggression toward SH intruders. Social defeat stress procedures provide episodes of discrete salient events that activate stress-related neurocircuitry including the mesocorticolimbic dopamine system, while social deprivation does not (reviewed in Burke and Miczek 2014). We have identified an adolescent social experience (PH defeat) that increased cocaine taking in early adulthood and may serve useful for identifying stress-induced neural mechanisms and possible interventions for social stress escalated substance abuse. Our ongoing studies investigate the corticotropin releasing factor system in the adolescent ventral tegmental area, which is implicated in escalated cocaine taking after adult social defeat (Boyson et al. 2014; Boyson et al. 2011)
References
Baarendse PJ, Limpens JH, Vanderschuren LJ (2014) Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology (Berl) 231:1695–1704
Bardo MT, Klebaur JE, Valone JM, Deaton C (2001) Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology 155:278–284
Bjorkqvist K (2001) Social defeat as a stressor in humans. Physiol Behav 73:435–442
Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, Miczek KA (2014) Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration. J Neurosci 34:6659–6667
Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA (2011) Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology 218:257–269
Broadwater M, Spear LP (2013) Age differences in fear retention and extinction in male Sprague–Dawley rats: effects of ethanol challenge during conditioning. Behav Brain Res 252:377–387
Burke AR, Forster GL, Novick AM, Roberts CL, Watt MJ (2013) Effects of adolescent social defeat on adult amphetamine-induced locomotion and corticoaccumbal dopamine release in male rats. Neuropharmacology 67:359–369
Burke AR, Miczek KA (2014) Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 231:1557–1580
Burke AR, Renner KJ, Forster GL, Watt MJ (2010) Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Res 1352:147–156
Burke AR, Watt MJ, Forster GL (2011) Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience 197:269–279
Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM (2005) Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev 29:83–97
Buwalda B, Scholte J, de Boer SF, Coppens CM, Koolhaas JM (2012) The acute glucocorticoid stress response does not differentiate between rewarding and aversive social stimuli in rats. Horm Behav 61:218–226
Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM (2013) Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience 26:258–270
Chappell AM, Carter E, McCool BA, Weiner JL (2013) Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res 37(Suppl 1):E394–E403
Covington HE 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP Jr, Miczek KA (2005) Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology 30:310–321
Covington HE 3rd, Miczek KA (2001) Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology 158:388–398
Covington HE 3rd, Miczek KA (2005) Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology 183:331–340
Covington HE 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA (2008) NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology 197:203–216
de Jong JG, van der Vegt BJ, Buwalda B, Koolhaas JM (2005) Social environment determines the long-term effects of social defeat. Physiol Behav 84:87–95
Ding YJ, Kang L, Li BM, Ma L (2005) Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav 82:673–677
Douglas LA, Varlinskaya EI, Spear LP (2004) Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol 45:153–162
Frank E, Salchner P, Aldag JM, Salome N, Singewald N, Landgraf R, Wigger A (2006) Genetic predisposition to anxiety-related behavior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav Neurosci 120:60–71
Garcia-Pardo MP, Rodriguez-Arias M, Maldonado C, Manzanedo C, Minarro J, Aguilar MA (2014) Effects of acute social stress on the conditioned place preference induced by MDMA in adolescent and adult mice. Behav Pharmacol 25:532–546
Goldman L, Winget C, Hollingshead GW, Levine S (1973) Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology 12:199–211
Grubbs FE (1969) Procedures for detecting outlying observations in samples. Technometrics 11:1
Hall FS, Perona MT (2012) Have studies of the developmental regulation of behavioral phenotypes revealed the mechanisms of gene-environment interactions? Physiol Behav 107:623–640
Haney M, Maccari S, Le Moal M, Simon H, Piazza PV (1995) Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698:46–52
Hoffmann JP, Cerbone FG, Su SS (2000) A growth curve analysis of stress and adolescent drug use. Subst Use Misuse 35:687–716
Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ (2000) Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 151:55–63
Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H (2001) Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology 158:382–387
Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A (1997) Social stress in rats and mice. Acta Physiol Scand 640:69–72
Koolhaas JM, Schuurman T, Wiepkema PR (1980) The organization of intraspecific agonistic behaviour in the rat. Prog Neurobiol 15:247–268
Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA (2003) Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol 33:13–29
Levine JB, Youngs RM, MacDonald ML, Chu M, Leeder AD, Berthiaume F, Konradi C (2007) Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience 145:42–55
Lopez MF, Doremus-Fitzwater TL, Becker HC (2011) Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6 J mice. Alcohol 45:355–364
Lu L, Shepard JD, Hall FS, Shaham Y (2003) Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev 27:457–491
Lukkes J, Vuong S, Scholl J, Oliver H, Forster G (2009a) Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci 29:9955–9960
Lukkes JL, Mokin MV, Scholl JL, Forster GL (2009b) Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav 55:248–256
Lukkes JL, Watt MJ, Lowry CA, Forster GL (2009c) Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci 3:18
Meng Q, Li N, Han X, Shao F, Wang W (2010) Peri-adolescence isolation rearing alters social behavior and nociception in rats. Neurosci Lett 480:25–29
Miczek KA, Nikulina EM, Shimamoto A, Covington HE 3rd (2011) Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci 31:9848–9857
Miczek KA, Thompson ML, Tornatzky W (1991) Subordinate animals: behavioral and physiological adaptations and opioid tolerance. In: Brown MR, Koob GF, Rivier C (eds) Stress neurobiology and neuroendocrinology. Marcel Dekker Inc., pp 323–357
Miczek KA, Yap JJ, Covington HE 3rd (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120:102–128
Nakayasu T, Ishii K (2008) Effects of pair-housing after social defeat experience on elevated plus-maze behavior in rats. Behav Processes 78:477–480
Nansel TR, Overpeck M, Pilla RS, Ruan WJ, Simons-Morton B, Scheidt P (2001) Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA 285:2094–2100
National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington, D.C
Nelson DE, Higginson GK, Grant-Worley JA (1995) Physical abuse among high school students. Prevalence and correlation with other health behaviors. Arch Pediatr Adolesc Med 149:1254–1258
Newman ML, Holden GW, Delville Y (2005) Isolation and the stress of being bullied. J Adolesc 28:343–357
Nocjar C, Zhang J, Feng P, Panksepp J (2012) The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience 218:138–153
Panksepp J, Siviy S, Normansell L (1984) The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev 8:465–492
Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA (2011) Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol Behav 104:272–282
Plyusnina IZ, Solov'eva MY, Oskina IN (2011) Effect of domestication on aggression in gray Norway rats. Behav Genet 41:583–592
Powell SB, Swerdlow NR, Pitcher LK, Geyer MA (2002) Isolation rearing-induced deficits in prepulse inhibition and locomotor habituation are not potentiated by water deprivation. Physiol Behav 77:55–64
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33
Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Robbins TW, Jones GH, Wilkinson LS (1996) Behavioural and neurochemical effects of early social deprivation in the rat. J Psychopharmacol (Oxford, England) 10:39–47
Romeo RD (2010) Adolescence: a central event in shaping stress reactivity. Dev Psychobiol 52:244–253
Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM (1999) Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 24:285–300
Schenk S, Gorman K, Amit Z (1990) Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol 7:321–326
Tharp-Taylor S, Haviland A, D'Amico EJ (2009) Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav 34:561–567
Thomsen M, Caine SB (2011) Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol 19:321–341
Tidey JW, Miczek KA (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology 130:203–212
Trezza V, Baarendse PJ, Vanderschuren LJ (2010) The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci 31:463–469
van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM (1999) Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol 34:129–138
Varlinskaya EI, Spear LP (2008) Social interactions in adolescent and adult Sprague–Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res 188:398–405
Varlinskaya EI, Spear LP, Spear NE (1999) Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiol Behav 67:475–482
Ver Hoeve ES, Kelly G, Luz S, Ghanshani S, Bhatnagar S (2013) Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience 249:63–73
Von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM (2000) Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res 117:137–146
Watt MJ, Burke AR, Renner KJ, Forster GL (2009) Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci 123:564–576
Whitaker LR, Degoulet M, Morikawa H (2013) Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron 77:335–345
Wright IK, Upton N, Marsden CA (1991) Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav 50:1129–1132
Yates JR, Beckmann JS, Meyer AC, Bardo MT (2013) Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug Alcohol Depend 129:240–246
Zou S, Funk D, Shram MJ, Le AD (2014) Effects of stressors on the reinforcing efficacy of nicotine in adolescent and adult rats. Psychopharmacology (Berl) 231:1601–1614
Acknowledgments
The authors acknowledge support from NIH R01DA031734 (KAM) and F32DA032226 (ARB). We are grateful to Tufts University research assistants Ashleigh L. Matthews, Punit N. Matta, Enshu Chawla, Lauren M. Behlke, Jacob Seiden, Zachary Butzin-Dozier, Rachel E. Moss, and Johann M. Schmidt. We thank Christopher O. Boyson for assistance. The authors declare no conflicts of interest.
Conflict of interest
The authors declare no conflicts of interest. The authors acknowledge support from the National Institute of Health R01DA031734 (KAM) and F32DA032226 (ARB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burke, A.R., Miczek, K.A. Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology 232, 3067–3079 (2015). https://doi.org/10.1007/s00213-015-3947-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3947-5