Abstract
A comparative analysis of intermale aggression in the resident–intruder test was conducted with gray rats from a wild unselected population bred at the laboratory for three generations and gray rats selected for elimination (tame) and enhancement (aggressive) of aggressiveness towards human for 71–72 generations. Males from the laboratory line Wistar were used as neutral opponents. Rats from the tame line were characterized by reduced aggression manifest as longer attack latency, decreased number of attacks, upright postures, chases, kicks, and shorter total time of aggressive behavior compared to unselected males. There was no significant difference in the attack latency and the total time of aggression between rats of the aggressive line and unselected rats. A trend to decrease in the number of attacks, chases and upright postures and to increase in contribution of lateral threat postures to the total time of aggression was observed for males of the aggressive line. Plasma corticosterone in unselected males not presented with intruders and after their presentation was higher than in males of both selected lines. Comparative behavioral analysis of agonistic behaviors in rats from the aggressive and tame lines to opponents of different lines (Wistar, tame, aggressive) showed that the presence of an intruder from the aggressive line can enhance aggressive responses in residents from the tame line. Thus, selection for domestication of gray rats caused a significant attenuation of aggressive behavior without affecting the basic agonistic repertoire.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Domestication of animals was associated with numerous changes in physiology, morphology, and behavior. Behavioral adaptations relating to the ability of wild animals to coexist with humans in a captive environment was crucial for the success of domestication, particularly during its early steps. In the 1970s, experiments to domesticate wild gray rats were started (Belyaev and Borodin 1982). Lines of tame and aggressive rats were produced through genetic selection for attenuation and enhancement of aggressiveness towards human on the basis of performance in the glove test (Naumenko et al. 1989; Plyusnina, and Oskina 1997). This selection regime also affected physiological characteristics—notably those known to be closely associated with specific behavioral responses and the stress hormones (Naumenko et al. 1989; Plyusnina 2004; Plyusnina and Oskina 1997; Oskina et al. 2000, 2008). Taken together, these studies demonstrated that tame rats differed from aggressive rats in increased exploration, decreased anxiety in unknown situation, improved ability for spatial learning, also in decreased level of plasma adrenocorticotropic hormone (ACTH) and corticosterone in response to stress. Cross-fostering experiments showed that the maternal postnatal environment had no substantial effect on the behavioral responses of both tame and aggressive rats (Albert et al. 2008; Plyusnina et al. 2009). It has been established that long-term maintenance of wild rats in captivity is accompanied by weakening of their defensive responses and overall levels of aggression towards human (King 1939). According to Moyer (1968) the aggression towards human is fear-evoked and of a defensive nature. Wild gray rats that had been bred in a captive environment for 21–25 generations became much less fearful in the presence of humans; nevertheless, they were overtly and highly aggressive to intruders introduced into their colony, they also demonstrated hostility towards weak or young sexually mature males (King 1939). A later study demonstrated that a population of wild-type gray rats bred in the laboratory for 29 generations showed wide levels of variations in male offensiveness and retained individuals with extreme levels of intermale aggression in the resident–intruder (R–I) test (De Boer et al. 2003). In contrast, a population of laboratory Wistar rats had no males strongly expressing offensive aggression, an observation attributed to selection and inbreeding during domestication (De Boer et al. 2003). Tame rats that had been subjected to domestication for 13–19 generations did not differ from aggressive rats for the amount of the intermale aggression in an unfamiliar cage (Naumenko et al. 1989). However, in the test for irritable aggression, the number of attacks was significantly smaller in tame than in aggressive rats, with the difference increasing with each generation of selection. There are now over 70 generations of rats that have been subjected to selection for domestication and for the reverse, aggressiveness towards human. Rats of selected generation 71 showed territorial aggression in the R–I test to a neutral opponent (Wistar males) and spontaneous aggression in an unfamiliar cage scores that were considerably reduced in the tame compared with the aggressive rats (Plyusnina and Solov’eva 2010). However, the question of whether selection for elimination or enhancement of aggressiveness towards humans affected intraspecific aggression in gray rats remained open. Appropriate controls, rats of an unselected line, were necessary to answer this question. Despite numerous data indicating lower hypothalamic–pituitary adrenal (HPA) axis activity in tame rats relative to their aggressive counterparts, hormonal response to social stimuli in rats of selected lines remained unclear. Intermale confrontations were compared in the R–I test of tame and aggressive males using Wistar rats as neutral opponents mostly exhibiting freezing (Plyusnina and Solov’eva 2010). It is well known that the resident’s behavior during confrontation depends on the behavior of the intruder (Lucion and de Almeida 1991; Takahashi and Blanchard 1982). With this in mind, the goal of this study was twofold to (i) compare intermale aggression in the resident–intruder test for intruder Wistar males in tame, aggressive, and unselected rats, while comparing their corticosterone response to this social conflict situation (ii) estimate intermale interactions by using intruder males of the same line and males of the line selected in reverse.
Materials and method
Animals
Experiments were performed with adult male gray rats (Rattus norvegicus) that have been selected for 71–72 generations for elimination (tame) and enhancement (aggressive) of aggressiveness towards human. Point scales for defensive responses of males and females at each generation from 2.5 to 3 months of age were based on the glove test (Naumenko et al. 1989; Plyusnina and Oskina 1997; Plyusnina et al. 2009). The glove test estimates behavior towards human by variations in point scores ranging from −4 to +4. These scores are based on the expression of negative or positive emotional responses to the gloved hand, depending on the distance between the gloved hand and the tested rat. For detailed description see Plyusnina et al. (2009). In addition, wild male adults of the third generation of an unselected population were used. Three females and five males were trapped in the vicinity of Akademgorodok. Two females and three males of the trapped animals gave offspring and served as founders of the unselected population. The neutral opponents in the resident–intruder test were naive Wistar adults from the IC&G animal facility.
The different lines were kept in separate rooms. The animals were housed in metal cages (50 × 33 × 20 cm) in groups of four from weaning 30 days after birth until the start (at the age of 90 days) of the experiments. At the age of 2.5–3 months, rats were tested for defensive responses in the glove test. Animals were kept under standard laboratory conditions in a natural 10:14 light–dark cycle with free access to food and water. Experiments were carried out in the light phase of day, from 14.00 to 18.00, local time. All experiments were performed according to the Guide for the Care and Use of Laboratory Animals approved by the Ministry of Public Health of Russia (Supplement to order N 267 of June 19, 2003).
Experimental design
To reveal the effects of experimental domestication, male rats of tame (n = 10) and aggressive (n = 10) lines and unselected rats (n = 8) were used as residents and Wistar males (n = 28) as intruders. These three groups of rats were used to study corticosterone response to social confrontation.
To analyze the effect of intruder genotype on territorial aggression, males of the tame (n = 24) and aggressive (n = 24) lines were used as residents, while males of Wistar (n = 20), tame (n = 14) and aggressive (n = 14) lines were used as intruders.
Resident–intruder (R–I) test
Rats were assessed for the display of offensive behaviors by the standard resident–intruder test, widely used for investigation of intraspecific intermale aggression (Koolhaas et al. 1980; De Boer et al. 1999). Adult tame, aggressive and unselected males were kept alone in observation cages with clear plastic tops for 7 days to facilitate territorial behavior. Thirty minutes prior to the start of the test, the cage with the male was removed to a special experimental room. After habituation, an unfamiliar, lighter male was introduced into the resident’s home cage for 5 min. The behavior of residents was videotaped and analyzed using a computer program, developed at the IC&G (Plyusnina et al. 2003) which allows for the assessment of the latency, the number and the total duration of behavioral patterns. Analysis was made by a trained observer blind to rat line. The status of a defined event was recorded by pressing keys on the computer keyboard. The following behavioral elements (Poshivalov 1978; De Boer et al. 1999) were scored:
-
(1)
Aggressive behavior. Attack: the resident initiates a series of actions that include pushing and biting the intruder. Chase: the resident pursues the fleeing intruder, this usually ends up with attack. Kick: the resident pushes the intruder with hind legs. Offensive upright: the resident stands on hind legs in reaction to approach or upright of the intruder. The resident and intruder may hold on to each others forelegs. Pinning: the resident turns the intruder into a supine posture, so that contact with the ground is released with all four intruder’s limbs. Aggressive grooming: the resident pushes down the intruder, nibbles the fur and the skin. Lateral threat: the resident pushes away, approaches or moves around the intruder with arched back and piloerection.
-
(2)
Social exploration. Moving toward: the resident moves and reduces the distance between him and the intruder. Sniffing occurs, while running speed is low or high. Sniffing: the resident sniffs the intruders’s flank, nasal or anogenital areas.
-
(3)
Non-social behavior. Locomotion associated or not with sniffing directed towards the environment. Rearing: the resident stands on hind legs. Auto-grooming: the resident washes their face with the forepaws; licks, nibbles and wipes the fur, tail and paws with tongue and teeth. Resting behavior: the resident lies down or sits, eyes open or closed.
The attack latency, the number of all agonistic patterns, the time of lateral threats, social exploration, auto-grooming, locomotion, rearing and resting behavior, and the total aggression time were measured. The aggression latency, expressing the latency time to the first aggressive act, was additionally measured. The percentage of freezing intruders was scored. Each resident–intruder pair was studied once.
Plasma corticosterone
Each rat of the control group was singly housed for 7 days before blood collection. Animals after the RI test and controls not subjected to it were sacrificed by rapid decapitation and blood was collected in microcentrifuge tubes containing EDTA for measurement of corticosterone level. Plasma corticosterone levels were determined by the competitive protein binding method (Murphy 1967; Tinnikov and Bazhan 1984) using rat corticosterone binding globulin, nonlabeled corticosterone (Sigma, St. Louis, MO) and [1,2,6,7-3H] corticosterone (specific activity 67 Ci/mmol) from Amersham Pharmacia Biotech UK. Intra- and interassay coefficients of variation were less than 5 and 10%, respectively.
Statistical analysis
Behavioral data were analyzed by nonparametric ANOVA Kruskal–Wallis with rat line as a factor. Line differences were analyzed by the Mann–Whitney U test. Corticosterone data were analyzed by one-way ANOVA with line as a factor, post-hoc comparisons were performed by the Neuman-Keuls test.
Results
The effect of domestication on territorial aggression
Resident–intruder test for neutral Wistar opponent
A significant line effect was shown for attack latency [H(2, N = 28) = 20.04, p = 0.0001], number of attacks [H(2, N = 28) = 19.54, p < 0.001], chases [H(2, N = 28) = 15.30, p < 0.001], kicks [H(2, N = 28) = 14.76, p < 0.001], upright postures [H(2, N = 28) = 9.33, p < 0.01], percentage time of aggressive behavior [H(2, N = 28) = 7.51, p < 0.05], lateral threats as percentage time of total aggression time [H(2, N = 25) = 7.88, p < 0.05].
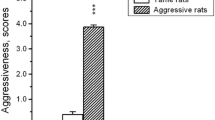
Statistical analysis demonstrated that males of the aggressive line did not noticeably differ from those of the unselected group in the attack latency and the total time of aggression. However, there was an insignificant trend to decrease in the number of attacks, chases and upright postures and to increase in the contribution of lateral threat postures to the total time of aggression for males of the aggressive line. The residents of the tame line differed considerably from the aggressive and unselected males in their behavior (Figs. 1a, b). The attack latency in the residents of the tame line was longer (Fig. 1a). The tame rats also showed a significantly shorter time of total aggressive behavior than the unselected and the aggressive males (Fig. 1a). The number of attacks (Fig. 1a), chases, kicks, and upright postures was significantly smaller in the tame compared with the aggressive and unselected males (Fig. 1b). The number of chases was significantly greater in the unselected compared with the residents of the tame line (Fig. 1b). There were no differences in pinning number between the groups. Analysis of the ratio of lateral threats to the other aggressive behaviors established prevalence of threatening postures for the tame rats, while patterns different from threats contributed mainly to aggressive behavior in the unselected rats. Thus, the lateral threat time expressed as percentage of total aggression time in the tame rats significantly surpassed that of their unselected counterparts (Fig. 1b). The aggressive males were intermediate in this regard, not significantly different from the tame and unselected rats in the lateral threats. No differences were found in the time of social exploration, rearing, grooming and resting behavior between residents of different lines (Table 1). Locomotion time was significantly shorter for unselected males compared to tame males (Table 1).
a Behavior of unselected, aggressive and tame rat males during the 5-min resident–intruder test to neutral Wistar opponent. Attack latency, total time of aggression and number of attacks are shown. * p < 0.05, *** p < 0.001 versus unselected rats, v p < 0.05, vvv p < 0.001 versus aggressive rats Mann–Whitney U test. b Behavior of unselected, aggressive and tame rat males during the 5-min resident–intruder test to neutral Wistar opponent. Number of chases, kicks, upright postures are shown. Lateral threat time is calculated as percentage of total time of aggressive behavior. * p < 0.05, ** p < 0.01, *** p < 0.001 versus wild rats, v p < 0.05, vv p < 0.01, vvv p < 0.001 versus aggressive rats Mann–Whitney U test
Changes in plasma corticosterone level in response to social confrontation
Figure 2 compares hormonal responses to a 5-min social stress in the RI test in the tame, aggressive, and unselected rats. Variance analysis demonstrated a significant effect of line (F(2,47) = 12.49, p < 0.001) and social confrontation (F(2,47) = 129.98, p < 0.01) on plasma corticosterone. Thus, its level after social stress significantly surpassed the control in the three lines. Plasma corticosterone levels under both the control and stress conditions were higher in the unselected line compared to both the aggressive and tame males (Fig. 2). The tame and aggressive males did not differ in corticosterone levels both in controls and stressed males.
Plasma corticosterone in control group and after confrontation in unselected, aggressive, tame rats in the resident–intruder test to neutral opponent. Rats of control group were not subjected to RI test. ** p < 0.01 versus unselected rats, xxx p < 0.001 versus control group, post-hoc Neuman–Keuls test
Comparison of territorial aggression in tame and aggressive males to intruders of different lines
Comparison of agonistic interactions of residents from the tame line with intruders of different lines
Nonparametrical ANOVA Kruskal–Wallis statistics revealed a significant effect of intruder line on aggression latency [H(2, N = 24) = 5.49, p = 0.06] and a marginally significant effect on the aggression time [H(2, N = 24) = 4.76, p = 0.09] in residents of the tame line. Significant effects of intruder line on the number of offensive upright postures [H(2, N = 24) = 7.53, p < 0.05], kicks [H(2, N = 24) = 5.85, p = 0.05] were also demonstrated.
There was a decrease in aggression latency of residents from the tame line for a set of their interactions with intruders of Wistar, tame and aggressive lines (Fig. 3). The aggression latency of encounters of tame males with Wistar males was greater than that of tame males with the intruders of the same line, this being just a non-significant trend. However, the differences became significant when resident of the tame line encountered an intruder of the aggressive line. The total time of aggression toward the intruder of the aggressive line introduced to tame males was increased at a marginally significant level (p = 0.09) compared with Wistar intruders (Fig. 3). Furthermore, there was a distinct decrease in certain elements of aggressive behavior when Wistar males were introduced: the number of kicks was significantly reduced compared to the intruders of the tame line and marginally significant (p = 0.06) when the intruders were from the aggressive line (Fig. 3).When aggressive males were introduced the number of upright postures significantly exceeded those compared to the cases of introduction of tame or Wistar males (Fig. 3). When males of different lines were introduced, residents of the tame line did not differ significantly in the number of attacks, chases, aggressive grooming and attack latencies.
Behavior of aggressive (left columns) and tame (right columns) rats during the 5-min resident–intruder test to intruders of different lines. Attack latency, aggression latency, total time of aggressive behavior, number of attacks, kicks, upright postures are shown. * p < 0.05 versus Wistar opponent, x p < 0.05 versus resident of the aggressive line, v p < 0.05, vv p < 0.01 versus confrontation of resident and intruder from aggressive line
The total time of social exploration in residents of the tame line was shorter when intruders of the aggressive line were introduced as compared with Wistar males. This difference was marginally significant mainly due to a decrease in sniffing time of the intruder’s lower back. There were no differences for the time of non-social behaviors in residents of the tame line during interactions with intruders of different lines (Table 2).
Comparison of agonistic interactions of residents from the aggressive line with intruders of different lines
Nonparametric Anova Kruskal–Wallis statistics revealed a significant effect of intruder lines on the number of aggressive male attacks [H(2, N = 24) = 6.03, p < 0.05]. In interaction of residents from the aggressive line with intruders from the aggressive line, attack number was significantly smaller than in the case when Wistar males were introduced (Fig. 3). In interaction with tame males, this behavioral element was intermediate, not different from that for intruders of the aggressive and Wistar lines. The number of pinnings in residents of the aggressive line was smaller, being just a trend, in interaction with aggressive males compared with tame [z = 1.66, p = 0.1] and Wistar males [z = 1.66, p = 0.1]. We did not detect effects of intruder genotype on other elements of aggressive behavior and also on the time of non-social behaviors and social exploration in residents of the aggressive line (Table 2).
Comparison of agonistic behavior in residents of the aggressive and tame lines in interaction with intruders of different lines
The salient observations concerning interaction with a tame opponent are: a significant effect of resident line on the aggression latency [H(1, N = 14) = 5.59, p = 0.01] and upright posture numbers [H(1, N = 14) = 4.09, p < 0.05]. The aggression latency was significantly smaller, while the number of upright postures was significantly greater when tame males were introduced to residents of the aggressive and tame lines (Fig. 3). No other differences in elements of agonistic behaviors were found.
It is important to note that the tame and aggressive males did not differ in their interaction with the intruders of the aggressive line (Fig. 3).
When male residents and intruders were of the same line, there was a significant line effect on the aggression latency [H(1, N = 14) = 6.89, p < 0.01] and the number of offensive uprights [H(1, N = 14) = 7.64, p = 0.005]. The aggression latency was significantly longer and the number of upright postures was significantly smaller in interaction of tame male pairs in the R–I test as compared to the aggressive pairs (Fig. 3).
Aggressive grooming was expressed weakly in agonistic interaction and no significant interline differences in aggressive grooming were found. No significant differences were also found in the total time of social exploration and non-social behaviors between residents of the tame and aggressive lines (Table 2).
Discussion
Study of domestications effect on intraspecific aggression in rats has been mainly based on comparisons of laboratory with wild-type rats (Boice 1973; De Boer et al. 2003; Price 1978, 1984; Takahashi and Blanchard 1982). A comprehensive analysis of territorial aggression has been conducted with Wistar and wild-type rats bred in captivity for 29 generations (De Boer et al. 2003). A trimodal distribution of phenotypes for intensity of territorial aggression was characteristic of the wild-type rats; in contrast, the number of medium aggressive laboratory counterparts was decreased and no highly aggressive group was distinguished. Artificial selection, the absence of natural selection pressure, domestication, and inbreeding were all implicated as causes of elimination of high aggression in laboratory rats (De Boer et al. 2003; Koolhaas et al. 2010). It should be noted that the domestication of laboratory rats and of other rodents was different (Robinson 1965). Tameness of rats was achieved by their laboratorization, being reared in laboratory cages. True, they were no longer completely free animals, but they did not establish themselves as household pets in the sense that they have not been subjected to direct selection for their response towards human. Our model of the domesticated rat was derived from selective breeding for elimination of aggressiveness towards human. This culminated in rat generations that consistently exhibited no aggression towards human, moreover, they are highly tolerant to handling (Naumenko et al. 1989; Plyusnina 2007). Thus, the results of the glove test in tame rats for the last 10 generations showed that the rat tameness was scored as +3.1 ± 0.03, whereas the behavior towards human in Wistar rats (n = 70) from the IC&G facility was scored as +1.4 ± 0.06. Aggressiveness towards human in rats of the aggressive line was scored as high as −3.3 ± 0.02. Comparison of intermale aggression has demonstrated not only its attenuation in tame males, but also their increasing trend towards demonstrative threats (Plyusnina and Solov’eva 2010). This raised the question of whether aggression attenuation was possibly brought about by domestication. To answer it, intermale aggression in tame and wild rats not subjected to selection for behavior had to be additionally compared. To do so, we used unselected males that had been bred in captivity for 3 generations.
Aggressive intermale interactions involve various behavioral responses culminating in demonstrative threats, chasing, biting, and sometimes injury (Poshivalov 1978). The aggressive intentions included threatening postures without attacks and other elements of aggressive behavior. According to our data elements such as attacks, upright postures, kick, chase, pinning, and aggressive grooming were closely related so that one element can be substituted by another. All the three lines displayed patterns of intermale aggression; however, the tame males had longer attack latency, shorter aggressive behavior, less number of attacks among other elements in comparison with the unselected and aggressive rats. Lateral threat postures have been related to demonstration of aggressive intentions (Poshivalov 1978), being harmless, they are only the primary elements of adaptive offensive behavior. Piloerection and lateral threats as manifestations of offensive aggression give the opponent chances to bypass or to switch to submissiveness, thereby to avoid physical confrontation (Blanchard and Blanchard 1977; Blanchard et al. 2003). Judging by our current results, lateral threat postures contributed more to the total aggression in tame than in unselected rats. Thus, the evidence to date indicates an attenuation of intraspecific aggression in male rats selectively bred for domestication. As for laboratory rats (Neumann et al. 2010), selectively bred for high (HAB) and low (LAB) anxiety-related behavior, they also showed a high level of lateral threats in the RI test, like the tame rats. Interestingly, aggressive rats that had been bred for generations for enhancement of aggressiveness towards human did not differ from the unselected rats in such main parameters of aggressive behavior as attack latency and total time of aggression in the RI test. The aggressive males tended to show a decrease in the number of attacks, chases and upright postures when compared to the unselected rats. These observations warrant detailed analysis.
As known, glucocorticoids may facilitate the manifestation of aggressive behavior. Thus, administration of corticosterone to rats promoted their agonistic confrontations (Haller et al. 1997; Mikics et al. 2004), whereas the inhibitor metyrapone in contrast decreased them (Mikics et al. 2004). Differences in the HPA activity between tame and aggressive rats have been repeatedly demonstrated using different non-social stimuli. It has been noted that plasma glucocorticoid levels under basal and stress conditions were lower in tame than in aggressive rats. Here, we demonstrated for the first time a higher plasma corticosterone level after a 5-min social confrontation in unselected, aggressive, and tame rats. This elevation in response to social stress in the three rat groups is fully consistent with numerous reported observations (Sgoifo et al. 1996; Kruk et al. 2004; Veenema et al. 2007; Veenema and Neumann 2007). While unselected rats showed the highest level of corticosterone response to confrontation, aggressive and tame rats did not differ in this response. Plasma corticosterone was measured promptly after exposure to the R–I test in current experiments. It may be suggested that unselected males, in this particular situation, would show a higher HPA axis responsiveness than males of the selected lines. De Boer et al. (2003) have demonstrated that corticosterone response pattern to a 15-min social defeat differed, depending on aggressiveness level, in wild-type male rats. Tame and aggressive rats differed significantly in the time course of changes in corticosterone level in response to acute non-social stress (Oskina et al. 2000) and possibly their corticosterone level in response to social stress might have followed a different course, too. It cannot be ruled out that corticosteroid response patterns to social and non-social stimuli would show variation within a line as well. Thus, the more aggressive LAB rats responded to non-social stimuli by a lower corticosterone when compared to the less aggressive HAB rats (Veenema et al. 2007). Nevertheless, in response to social stress LAB rats responded by a highly stress-provoked ACTH level in comparison with HAB rats. Higher ACTH and corticotropin-releasing hormone (CRH) levels and enhanced aggression were related in the social defeat test, as confirmed by experiments with CRH antisense treatment (Skutella et al. 1994). As for gray rats, their selection for elimination of aggressive defensive responses towards human is associated with a lower level of ACTH (Oskina and Plyusnina 2000; Oskina et al. 2008), also that of mRNA CRH in the hypothalamus (Amel’kina, unpublished data) compared to rats selected for enhancement of aggressiveness towards human. Diminished activities of the peripheral and central links of the HPA could potentially have attenuated intraspecific intermale aggression in tame rats.
Truly, agonistic interactions in the RI test were weakened in tame relative to aggressive and unselected rats. Nevertheless, tame rats retained their aggressive repertoire. Our current experiments using males of the aggressive line as intruders brought this into prominence. As known, the behavior of the intruder has considerably affects on resident behavior (Lucion and de Almeida 1991; Takahashi and Blanchard 1982). To illustrate, a resident male stopped attacking when an intruder started to show little or no movements. Here, we compared the territorial aggression of tame and aggressive rats in interaction with intruders of the tame, aggressive, and Wistar lines. The effect of the intruder genotype on the behavior of resident from the aggressive line was significant for attack number only. The attack number was greater in interactions with Wistar intruders than with the intruders of the same line. The largest, although insignificant, increase in upright postures was for interactions of resident from the aggressive line with the intruder of the same line. Intruders of the aggressive line appear to be more active in defense than Wistar intruders. Effect of intruder genotype on behavior was more prominent in the residents of the tame than of the aggressive line. Thus, the aggression latency in interaction with the intruder of the aggressive line was significantly shorter than in interaction with Wistar intruder, this was associated with an appreciable increase, albeit insignificant, in the total time of aggression, the number of kicks, and significant increase in the number of upright postures. The attack latency and the number of attacks did not change in interaction of resident from the tame line with intruders from different lines presumably because attack is not the main strategy of tame rats during agonistic interactions. It is of interest that, as in the case of resident from the aggressive line, the number of upright postures significantly increased in interaction of the tame male with an intruder from the aggressive line, and that this pattern was absent during interaction with the intruder of the same line and Wistar. Brought together, the current data showed that aggressive interactions of tame males with the intruders of the aggressive line were more intense than with the Wistar intruders. There is no doubt that the defensive behavior of intruders contributes to aggressive behavior of residents. Further study of this relationship appears promising and would clarify certain aspects of intermale aggression in tame rats. Our current results are in agreement with those reported in the literature (Blanchard et al. 1975; Boice 1973, 1999). Aggressive behavior of domestic Norway rats is comparable to levels of savageness observed in free-living rats (Blanchard et al. 1975). In an early analysis of behavior of laboratory hooded rats housed with wild rats, Boice (1999) observed enrichment of social behavior with an escalation of threat postures, injurious fighting and resultant deaths. Our data on enhancement of aggression in tame males in interactions with intruders of the aggressive line, as well as absence of significant differences in territorial aggression between tame and aggressive rats in the case of an opponent from the aggressive line support and extend the early suggestions (Boice 1973; Hale 1969) that rat domestication was associated with weakening of intermale aggression which raised the threshold for intermale aggression. However, the presence of an opponent from aggressive line lowers the threshold for intense agonistic response.
The aggressive rats do not differ from tame and unselected rats in social activity and locomotion. Therefore, only levels of aggression have been altered under selection in this line. Taking this into account, the aggressive line of gray rats developed at the IC&G may be a good model for the consideration of the neurobiological mechanisms of aggression. It is relevant to note that aggressive behavioral responses either are weakly expressed in many inbred and outbred lines of rats and mice (De Boer et al. 2003, Robertoux et al. 1999; Parmigiani et al. 1999; Guillot and Chapouthier 1996) or are accompanied by general disturbances in social behavior (Veenema and Neumann 2007).
Consideration of the central mechanisms regulating aggression of different types has focused on the serotonergic system. It has been established that serotonin exerts an inhibitory influence on aggressive behaviors (for a review, see Miczek et al. 2007). Selection of gray rats for elimination of defensive aggression towards human was accompanied by high activation of the serotonergic system (Naumenko et al. 1989; Popova et al. 1991, 2005). There is a reason to believe that these changes were consequential in contributing to weakening intraspecific aggression in rats successfully bred for domestication. An aggressive line was developed through long-term reverse selection. Rats of this line displayed high levels of defensive aggression towards human and offensive aggression in R–I test.
Taken together, our current results demonstrate that domestication of gray rats caused by selection for elimination of defensive aggression towards human is associated with the attenuation of intraspecific intermale aggression. As in the case of laboratorization, the repertoire of agonistic behaviors is unaffected. However, the presence of an opponent from the aggressive line might have enhanced agonistic responses.
References
Albert FW, Shchepina O, Winter C, Rompler H, Teupser D, Palme R, Ceglarek U, Kratzsch J, Sohr R, Trut LN, Thiery J, Morgenstern R, Plyusnina IZ, Schoneberg T, Paabo S (2008) Phenotypic differences in rats selected for tameness and aggression are associated with differences in stress response. Horm Behav 53:413–421
Belyaev DK, Borodin PM (1982) The influence of stress on variation and its role in evolution. Biol Zent 100:705–714
Blanchard RJ, Blanchard DC (1977) Aggressive behavior in the rat. Behav Biol 21:197–224
Blanchard RJ, Fukunaga K, Blanchard DC, Kelley MJ (1975) Conspecific aggression in the laboratory rat. J Comp Physiol Psychol 89:1204–1209
Blanchard RJ, Wall PM, Blanchard DC (2003) Problems in the study of rodent aggression. Horm Behav 44:161–170
Boice R (1973) Some behavioral tests of domestication in Norway rats. Behaviour 3(4):198–231
Boice R (1999) Domestication. Psychol Bull 80(3):215–230
De Boer SF, Lesourd M, Mocaer E, Koolhaas JM (1999) Selective antiaggressive effects of alnespirone in resident–intruder test are mediated via 5-hydroxytryptamine 1A receptors: a comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635. J Pharmacol Exp Ther 288(3):1125–1133
De Boer SF, Van der Vegt BJ, Koolhaas JM (2003) Individual variation in aggression of feral rodent strains: a standard for the genetics of aggression and violence? Behav Genet 33(5):485–501
Guillot PV, Chapouthier G (1996) Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res 77:211–213
Hale EB (1969) Domestication and the evolution of behavior. In: Hafez ESE (ed) The behavior of domestic animals. Williams and Wilkins, Baltimore, pp 22–45
Haller J, Albert I, Macara GB (1997) Acute behavioral effects of corticosterone rhythm and the propensity to behave aggressively in male rats. J Neuroendocrinol 12:937–941
King HD (1939) Life processes in gray Norway rats during fourteen years in captivity. Am Anat Mem No 17. Cited from Robinson R (1965) Genetics of the Norway rat. Pergamon Press Ltd, Oxford, pp 1–804
Koolhaas JM, Schurman T, Wiepkema PR (1980) The organization of intraspecific agonistic behaviour in the rat. Prog Neurobiol 15(3):247–268
Koolhaas JM, de Boer SF, Coppens CM, Buwalda W (2010) Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31(3):307–321
Kruk MR, Halasz J, Meelis W, Haller J (2004) Fast positice feedback between the adrenal stress response and a brain mechanism in aggressive behavior. Behav Neurosci 118:1062–1070
Lucion AB, de Almeida RMM (1991) Role of the intruder in the aggressive behaviour of colonies of wild rats (Rattus norvegicus). In: Animal models in psychopharmacology, advances in pharmacological sciences. Birkhauser Verlag, Basel, pp 347–356
Miczek KA, de Almeida RMM, Kravitz EA, Rissman EF, de Boer SF, Raine A (2007) Neurobiology of escalated aggression and violence. J Neurosci 27(44):11803–11806
Mikics E, Kruk MR, Haller J (2004) Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology 29(5):618–635
Moyer KE (1968) Kinds of aggression and their physiological basis. Commun Behav Biol 2:65–87
Murphy BE (1967) Some studies of the protein binding of steroids and their application to the routine micro and ultramicro measurements of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab 27:973–990
Naumenko EV, Popova NK, Nikulina EM, Dygalo NN, Shishkina GT, Borodin PM, Markel AL (1989) Behavior, adrenocortical activity, and brain monoamines in Norway rats selected for reduced aggressiveness towards man. Pharmacol Biochem Behav 33:85–91
Neumann ID, Veenema AH, Beiderbeck DI (2010) Aggression and anxiety: social context and neurobiological links. Behav Neurosci 4(12):1–16
Oskina IN, Plyusnina IZ (2000) The hypothalamus–pituitary–adrenal system during selection of animals for tame behavior. In: Shumnii VK, Markel AL (eds) Modern concepts of evolutionary genetics, vol 22–45. IC & G, Novosibirsk, pp 327–333 (Russian)
Oskina IN, Plyusnina IZ, Sysoletina AIu (2000) Effect of selection for behavior on the hypophyseal-adrenal function in the gray rats Rattus norvegicus during postnatal ontogenesis. Zh Evol Biokhim Fiziol 36:120–126 (Russian)
Oskina IN, Herbeck YuE, Shikhevich SG, Plyusnina IZ, Gulevich RG (2008) Alterations in the hypothalamus–pituitary–adrenal and immune systems during selection of animals for tame behavior. The Herald Vavilov Soc Genet Breed Sci 12:39–49
Parmigiani S, Palanza P, Rodgers J, Ferrari PF (1999) Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev 23:957–970
Plyusnina IZ (2004) Locomotor activity–exploration or “panic”? Rossisky Fiziologichesky Zhurnal 90:84 (Russian)
Plyusnina IZ (2007) The behavior of wild grey rats at the modern step of domestication. Abstract 4 Russian conference on animal behaviour, Moscow, pp 596–597 (Russian)
Plyusnina IZ, Oskina IN (1997) Behavioral and adrenocortical responses to open-field test in rats selected for reduced aggressiveness toward humans. Physiol Behav 61(3):381–385
Plyusnina IZ, Solov’eva MYu (2010) Intraspecific intermale aggression in tame and aggressive grey rats. Zh Vyssh Nerv Deiat Im I P Pavlov 60(2):175–183 (Russian)
Plyusnina IZ, Trut LN, Karpushkeeva NI, Alekhina TA, Oskina IN (2003) Some behavioral and physiological characteristics of nonagouti mutation in gray rats during breeding for aggressiveness. Zh Vyssh Nerv Deiat Im I P Pavlov 53(5):613–621 (Russian)
Plyusnina IZ, Oskina IN, Tibeikina MA, Popova NK (2009) Cross-fostering effects on weight, exploratory activity, acoustic startle reflex and corticosterone stress response in Norway gray rats selected for elimination and for enhancement of aggressiveness towards human. Behav Genet 39:202–212
Popova NK, Kulikov AV, Nikulina EM, Kozlachkova EY, Maslova GB (1991) Serotonin metabolism and 5-HT receptors in Norway rats selected for low aggressiveness to man. Aggress Behav 17:207–213
Popova NK, Naumenko VS, Plyusnina IZ, Kulikov AV (2005) Reduction in 5-HT1A receptor density, 5-HT1A mRNA expression, and functional correlates for 5-HT1A receptors in genetically defined aggressive rats. J Neurosci Res 80(2):286–292
Poshivalov VP (1978) Ethological atlas for pharmacological study on rodents (mice, rats). VINITI, Moscow, pp 1–43
Price EO (1978) Genotype versus experience effects on aggression in wild and domestic Norway rats. Behaviour 64:340–353
Price EO (1984) Behavioral aspects of animal domestication. Q Rev Biol 59(1):1–32
Robertoux PL, Mortaud S, Perez-Diaz F, Tordjman S (1999) Measuring aggression in the mouse. In: Crusio WE, Gerlai RT (eds) Handbook of molecular-genetic techniques for brain and behavior research. Elsevier, Amsterdam, pp 696–709
Robinson R (1965) Genetics of the Norway rat. Pergamon Press Ltd, Oxford
Sgoifo A, De Boer SF, Haller J, Koolhaas JM (1996) Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: relationship with aggression. Physiol Behav 60(6):1403–1407
Skutella T, Montkowski A, Stohr T, Probst JC, Landgraf R, Holsboer F, Jirikowski GF (1994) Corticotropin-releasing hormone (CRH) antisense oligodeoxynucleotide treatment attenuates social defeat-induced anxiety in rats. Cell Mol Neurobiol 14(5):579–588
Takahashi LK, Blanchard RJ (1982) Attack and defense in laboratory and wild Norway and Black rats. Behav Process 7:49–62
Tinnikov AA, Bazhan NM (1984) Estimation of glucocorticoids in plasma and incubation medium by competitive binding of hormones by proteins without preliminary extraction. Lab Delo 12:709–713 Russian
Veenema AH, Neumann ID (2007) Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain Behav Evol 70:274–285
Veenema AH, Bredewold R, Neumann ID (2007) Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 32:437–450
Acknowledgments
The authors are grateful to A. Fadeeva for help during preparation of the manuscript. We also thank R. Kozhemyakina for her assistance with experimental procedure. This study was supported by grant 08-04-01412 from the Russian Fund of Basic Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Stephen Maxson.
Rights and permissions
About this article
Cite this article
Plyusnina, I.Z., Solov’eva, M.Y. & Oskina, I.N. Effect of Domestication on Aggression in Gray Norway Rats. Behav Genet 41, 583–592 (2011). https://doi.org/10.1007/s10519-010-9429-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-010-9429-y