Abstract
Berberine (BBR), a benzylisoquinoline alkaloid obtained from natural medicines such as coptidis rhizoma, has a wide range of pharmacological activities such as protecting the nervous system, protecting the cardiovascular system, anti-inflammatory, antidiabetic, antihyperlipidemic, antitumor, antibacterial, and antidiarrheal. However, factors such as poor solubility, low permeability, P-glycoprotein (P-gp) efflux, and hepatic-intestinal metabolism result in BBR having a low bioavailability (< 1%), which restricts its application in clinical settings. Therefore, improving its bioavailability is a prerequisite for its clinical applications. This review summarizes the various pharmacological effects of BBR and analyzes the main reasons for its poor bioavailability. It introduces methods to improve the bioavailability of BBR through the use of absorption enhancers and P-gp inhibitors, structural modification of BBR, and preparation of BBR salts and cocrystals as well as the development of new formulations and focuses on the bioavailability study of the new formulations of BBR. The research of BBR was also prospected in order to provide reference for the further research of BBR.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural products have been in the spotlight as an important source of inspiration for drug research and development over the past few decades, with remarkable success in the development of new drugs, especially in the search for new chemical structures. Natural products are characterized by their chemical and biological diversity, efficiency, safety, accessibility, and low cost, so they have significant advantages in modern drug research and development. With further research, many natural products have been shown to have a wide range of pharmacological activities and good therapeutic effects.
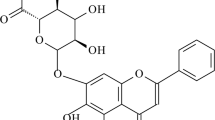
Berberine (BBR) is a natural benzylisoquinoline quaternary ammonium-type alkaloid, a yellow or orange crystalline powder with a weak special odor and characteristic bitterness (Singh et al. 2019), which is widely found in plants of Ranunculaceae, Berberidaceae, Papaveraceae, Rutaceae, Menispermaceae, and Rhamnaceae (Tillhon et al. 2012), and the chemical structure is shown in Figure 1. The content of BBR is high in phellodendri chinensis cortex and coptidis rhizoma, so coptidis rhizoma is often used as the main raw material for extracting BBR. BBR was first mainly used as an antimicrobial drug for the clinical treatment of gastrointestinal diseases, such as gastroenteritis and bacillary dysentery (Rabbani et al. 1987), and the modern pharmacological studies have shown that BBR has a wider range of pharmacological activities, such as anti-Alzheimer’s disease (AD), antidepressant, anti-Parkinson’s disease, antiarrhythmic, antihypertensive, treatment of heart failure, anti-inflammatory, antidiabetic, antihyperlipidemic, antitumor, and antidiarrheal. It is a drug with broad clinical application potential. However, BBR has the characteristics of low solubility and poor permeability, and it is also the substrate of the efflux transporter P-gp (Zhang et al. 2011). These factors make BBR difficult to be absorbed after oral administration, resulting in low bioavailability, which limits its clinical application. Therefore, improving its bioavailability is a prerequisite for its clinical role. This article provides a review from three aspects: the pharmacological activity and mechanism of BBR, the main obstacles to bioavailability, and methods to improve bioavailability. It focuses on the bioavailability research of various new formulations of BBR, in order to provide reference for further research on BBR.
Pharmacological activities
Protective effects on the nervous system
Anti-AD activity
AD is a common degenerative disease of the central nervous system in the middle-aged and elderly population, and its main clinical manifestation is memory and cognitive decline. It has been shown that BBR can significantly inhibit the pathogenesis of AD. β-Amyloid (Aβ) deposition and neuronal apoptosis are typical pathologic features in the pathogenesis of AD. It has found that BBR was able to improve the cognitive and learning abilities of AD model rats by affecting the signaling pathways of β-secretase 1 (BACE1) and eukaryotic translation initiation factor 2α (eIF2α), inhibiting the production of Aβ and promoting the clearance of Aβ (Zhu et al. 2011; Wu et al. 2021; Ghareeb et al. 2015). And BBR can also reduce Aβ accumulation and inhibit neuronal apoptosis by enhancing the expression of platelet-endothelial cell adhesion molecules, vascular endothelial growth factor (VEGF), and human angiopoietin-1 in the brain, promoting cerebral microvessel formation and restoration of cerebral blood flow, and ameliorating the cognitive dysfunction in the transgenic mouse model of AD (Ye, et al. 2021). Researchers also found that BBR could attenuate Aβ-induced neuronal damage in AD models by modulating the microRNA-188/nitric oxide (NO) synthase 1 pathway (Chen et al. 2020). In addition, the pathogenetic process of AD has been also related to the amyloid precursor protein (APP); Durairajan et al. found that BBR was able to reduce the high phosphorylation of APP, the C-terminal fragment of APP, and tau proteins through the activation of the protein kinase B (Akt)/glycogen synthase kinase 3 (GSK3) signaling pathway and consequently inhibit the production of Aβ (Durairajan et al. 2012). Neurofibrillary tangles (NFTs) are another typical pathological change in the pathogenesis of AD. Researchers have found that BBR is able to improve the status of NFTs by reducing the phosphorylation level of tau proteins, which in turn exerts its effect in preventing and treating AD (Liu et al. 2014). Wang et al. found that BBR was also able to restore D-ribose-induced mitochondrial dysfunction, mitochondrial autophagy through the PTEN-induced putative kinase 1 (PINK1)-Parkin pathway, thus alleviating D-ribose-induced cognitive deficits and exerting an anti-AD effect (Wang, et al. 2023). It has also been shown that BBR is able to exert anti-AD effects indirectly by affecting glucose metabolism, lipid metabolism, oxidative stress, inflammation, and neurotransmitters (Ji and Shen 2011; Jia et al. 2012; Lathe et al. 2014).
Antidepressant activity
Depression is a chronic mood disorder characterized by low mood, anxiety, insomnia, and lack of energy. It has been reported that BBR can exert its antidepressant effects by affecting the transporters, receptors, and related enzymes of noradrenaline (NE), serotonin (5-HT), and dopamine (DA) to increase the levels of NE, 5-HT, and DA in the whole brain of mice (Kulkarni and Dhir 2008; Hu et al. 2012; Peng et al. 2007). Researchers found that BBR was able to limit NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activity by promoting the binding of tripartite motif-containing 65 to NLRP3 and the ubiquitination of NLRP3, thereby alleviating depressive symptoms in chronic unpredictable mild stress (CUMS) mice (Yang et al. 2023). It was also reported that BBR was able to promote brain-derived unpredictable mild stress (BDNF) expression, which in turn reversed the effects of miR-34b-5p and miR-470-5p on depressive behavior and hippocampal neuronal growth in CUMS mice (Zhan et al. 2021). In addition, BBR can exert antidepressant effects by inhibiting oxidative stress. However, there are fewer studies validating the antidepressant effects of BBR from the perspective of oxidative stress, and the relationship between the two needs to be further explored in the future (Liu et al. 2017a).
Anti-Parkinson’s disease
Parkinson’s disease is a neurodegenerative disorder characterized by muscle stiffness, tremor, and changes in speech and gait. Studies have shown that inflammatory processes in Parkinson’s disease are closely related to NLPR3 inflammasome activation, and BBR can reduce the level of NLPR3 inflammasome activity by increasing autophagy activity and can also inhibit inflammation and apoptosis by regulating the long intergenic non-protein-coding RNA 00943 (LINC00943)/miR-142-5p/karyopherin alpha 4 (KPNA4)/nuclear factor-kappa B (NF-κB) pathway to inhibit inflammation and cell apoptosis, reduce neuronal damage, and ameliorate behavioral deficits in a mouse model of Parkinson’s disease (Li et al. 2021; Huang et al. 2020). Experts have also found that BBR can rapidly penetrate the blood-brain barrier and accumulate in the brain of the Parkinson’s disease zebrafish model, which in turn inhibits the accumulation of PINK1 protein and the overexpression of LC3 protein, thereby attenuating the loss of dopaminergic neurons and Parkinson’s symptoms (Wang et al. 2021a). In addition, BBR was able to significantly improve brain dopamine levels in a mouse model of Parkinson’s by upregulating levodopa synthesis in the intestinal flora, which in turn improved Parkinson’s symptoms (Wang et al. 2021b).
Treatment of other neurodegenerative diseases
Huntington’s disease is a neurodegenerative disease caused by misfolded proteins, and its main symptoms are chorea, dystonia, and cognitive decline. It has been found that BBR can promote the degradation of mutant huntingtin by enhancing autophagic function, thereby reducing the accumulation of mutant huntingtin, and BBR can also promote the clearance of neurotoxic misfolded proteins to play a therapeutic role in the treatment of neurodegenerative diseases (Jiang et al. 2015a; Rusmini, et al. 2020). In addition, BBR was able to improve dopamine levels by modulating the BDNF-tropomyosin receptor kinase B (TrkB)-PI3K/Akt signaling pathway and inhibiting NF-κB p65, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), which in turn ameliorated 3-nitropropionic acid-induced Huntington’s disease (Gendy et al. 2023).
Multiple sclerosis is a neurodegenerative disease characterized by multiple demyelinating lesions (Manu et al. 2021). BBR has been reported as a potential drug for the treatment of multiple sclerosis syndromes, and BBR is able to inhibit demyelination and loss of neurophysiological function by inhibiting the sphingosine kinase 1 (SPHK1)/sphingosine-1-phosphate (S1P) signaling pathway (Luo et al. 2017). In the mouse model of multiple sclerosis, BBR can also effectively alleviate the symptoms of the model by reducing the permeability of the blood-brain barrier and decreasing the expression and activity of matrix metalloproteinase-9 (MMP-9) (Ma et al. 2010).
Protective effects on the cardiovascular system
Antiarrhythmic activity
BBR is capable of broad-spectrum and effective treatment of cardiac arrhythmias. Researchers found that BBR had a protective effect on myocardial ischemia/reperfusion (I/R) injury. BBR was administered to rats at a dose of 100 mg/kg for 14 consecutive days. The results showed that myocardial I/R injury was reduced and the incidence of ventricular arrhythmias was decreased (Qin-Wei and Yong-Guang 2016). Wang et al. found that in the rat model of type 2 diabetic myocardial infarction, BBR can prevent and reduce arrhythmia by increasing the expression level of Kir2.1, restoring IK1 potassium current, and keeping the stability of resting membrane potential (Wang et al. 2011). In addition, BBR can also reduce ischemic arrhythmias by blocking adenosine triphosphate (ATP)-sensitive K+ (KATP) channels and shortening the action potential duration (APD) and effective refractory period (ERP) during ischemia (Wang et al. 1996).
Antihypertensive activity
BBR can improve endothelium-dependent vasodilation and enhance vascular endothelial synthesis by increasing the release of NO in endothelial cells, inhibiting the activity of angiotensin-converting enzyme (ACE), reducing aortic pulse wave velocity, and increasing the content of arterial media elastin fiber in the aortic media to achieve the purpose of dilating blood vessels and lowering blood pressure (Ko et al. 2000; Kang et al. 2002; Zhang et al. 2020a). Ma et al. found that BBR can improve vasodilation in diabetic rats by activating large-conductance Ca2+-activated K+ channel (BKca) in smooth muscle cells, which in turn leads to a reduction in blood pressure (Ma et al. 2017). Other studies have shown that BBR can also play a role in lowering blood pressure by inhibiting endothelium-dependent contraction, renin-angiotensin system (RAS), and inflammatory factors through activation of adenosine monophosphate-activated protein kinase (AMPK) (Guo et al. 2015; Liu et al. 2015). In addition, researchers found that BBR was able to improve symptoms in a mouse model of hypertension by modulating intestinal flora and inhibiting the production of oxidized trimethylamine (Wang et al. 2022a).
Treatment of heart failure
BBR is able to protect cardiac function by enhancing mitochondrial autophagy through upregulation of mitochondrial autophagy regulators PINK1 and Parkin (Abudureyimu et al. 2020) and also inhibits apoptosis of post-infarction heart failure cardiomyocytes through upregulation of Bc1-2/Bax and downregulation of caspase-3 expression (Liao et al. 2018), thus achieving the goal of treating heart failure. Other clinical studies have shown that BBR can reduce the frequency of ventricular premature beats, increase left ventricular ejection fraction, and improve heart failure in patients (Zeng et al. 2003).
Anti-inflammatory activity
The anti-inflammatory mechanism of BBR plays an active therapeutic role in a variety of diseases. In a mouse model of atherosclerosis, BBR can exert its anti-inflammatory effects by inhibiting NF-κB inflammatory signaling pathway and impeding the phosphorylation of IkB-α protein and the nuclear translocation of p65 protein (Feng et al. 2017). BBR also blocks injury-induced vascular smooth muscle cell (SMC) regeneration in vitro by inactivating the extracellular signal-regulated kinase (ERK)/early growth response gene (Egr-1) signaling pathway, thereby preventing injury-induced early signaling and attenuating inflammation to mitigate atherogenesis and restenosis (Liang et al. 2006).
Li et al. induced mice with 2.2% dextrose sodium sulfate (DSS) to create a model of chronic recurrent colitis, and after 30 days of administration, BBR was able to dose-dependently reverse the upregulation of the helper T-cell 17 (Th17)-related cytokine IL-17 and was able to inhibit Th17 differentiation through downregulation of signal transducer and activator of transcription 3 (STAT3), which in turn reduced the severity of chronic recurrent colitis (Li et al. 2016). Other studies have shown that BBR can also reduce the levels of pro-inflammatory factors such as IL-6, IL-1β, IL-2, IL-8, IL-22, and TNF-α in serum and colonic tissue through the Keap1/Nrf2/NF-κB pathway, increase the levels of anti-inflammatory factors such as IL-10, IL-13, transforming growth factor-β (TGF-β), and IL-4, and restore the balance of Th1/Th17/regulatory T cells (Treg), thereby alleviating the symptoms of colitis (Li, et al. 2015; Li, et al. 2023; Jiang et al. 2021). In addition, Yu et al. found that BBR was able to inhibit the PLA2-cyclooxygenase-2 (COX-2)-PGE2-EP2 pathway by regulating the intestinal flora, reducing the levels of inflammatory factors, and thus reducing colonic inflammation (Yu et al. 2024). Therefore, the anti-inflammatory effect of BBR is of certain significance for the treatment of colitis.
Researchers found that BBR was able to attenuate the destruction of joint tissues by inflammatory cells by downregulating p-ERK, p-p38, and p-JNK activation, thus exhibiting antirheumatoid arthritis activity (Wang et al. 2014a). Another study showed that BBR hydrochloride (BH) was able to exert anti-inflammatory effects by inhibiting the infiltration of inflammatory cells, inhibiting the production of IL-6, IL-1β, and TNF-α, and activating the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway, in an LPS-induced mastitis mouse model (Wang et al. 2018). Furthermore, in a dry eye mouse model, BBR was able to inhibit inflammatory factors and ameliorate inflammation and cell apoptosis in dry eye mice by regulating the PI3K/Akt/NF-κB and mitogen-activated protein kinase (MAPK) pathways (Han et al. 2023). In summary, BBR can inhibit the secretion of inflammatory factors such as IL-17, IL-6, IL-1β, and TNF-α and promote the production of anti-inflammatory factors such as IL-10, IL-13, TGF-β, and IL-4 by regulating multiple signaling pathways such as NF-κB, MAPK, and PPARγ and exert its anti-inflammatory effects to achieve the treatment of various diseases.
Antidiabetic activity
Type 2 diabetes is a metabolic disease with endocrine system disorders characterized by hyperglycemia. BBR can play a hypoglycemic role by activating the AMPK signaling pathway, improving insulin sensitivity, promoting insulin and glucagon-like peptide-1 (GLP-1) secretion, and inhibiting hepatic gluconeogenesis, which is an effective drug for the treatment of type 2 diabetes.
BBR is able to improve insulin signaling by promoting protein phosphorylation through activation of AMPK, which in turn activates mitochondrial function to promote aerobic oxidation of intracellular glucose and lipids (Turner et al. 2008; Lee et al. 2006). BBR is also able to enhance insulin-induced glucose uptake and glucose transporter protein 4 (GLUT4) translocation by promoting tyrosine phosphorylation of insulin receptor substrate 1 (IRS1), activating the Akt and PI3K/Akt pathways, and inhibiting the MAPK pathway, thereby ameliorating insulin resistance (Liu et al. 2010a; Kong et al. 2009; Zhang et al. 2020b). In addition, BBR was able to increase GLUT2 expression through activation of the PPARγ-fibroblast growth factor 21 (FGF21)/GLUT2 signaling pathway and reverse insulin resistance, which in turn ameliorated the symptoms of type 2 diabetic mice (Chen et al. 2023a).
GLP-1 is an incretin, which can increase insulin release and reduce glucagon secretion in a glucose-dependent manner to improve glucose balance in the body. BBR can promote the biosynthesis of GLP-1 by upregulating the expression of glucagon and hormone-converting enzyme 3 genes (Yu et al. 2010). It was found that BBR was able to activate the β-catenin/TCF4 signaling pathway by decreasing miR-106b levels, thereby promoting the production of GLP-1 by intestinal L-cells and increasing the expression of GLP-1 in mouse colon tissues, and that BBR metabolites were also able to stimulate the secretion of GLP-1 by alleviating oxidative stress and mitochondrial dysfunction (Wang et al. 2021c; Yang et al. 2024). In addition, BBR can promote GLP-1 secretion through PKC-dependent signaling pathways and also stimulate GLP-1 secretion by activating intestinally expressed bitter taste receptors in a phospholipase C-dependent manner (Yu et al. 2015). Thus, BBR is able to promote insulin secretion by promoting the synthesis and secretion of GLP-1, which in turn reduces blood glucose levels (Lu et al. 2009).
It has been reported that BBR can inhibit hepatic gluconeogenesis and peripheral tissue gluconeogenesis by regulating the liver kinase B1 (LKB1)/AMPK/transcriptional coactivator 2 (TOROC2) signaling pathway, thereby improving blood glucose levels in diabetic rats (Jiang et al. 2015b; Xu et al. 2020). In a diabetic rat model, BBR was able to block the translocation of TORC2 from the cytoplasm to the nucleus by inducing the activation of AMPK, which in turn inhibited hepatic gluconeogenesis (Zhang et al. 2012). It has also been reported (Xia et al. 2011) that BBR was able to reduce ATP levels by inhibiting hepatocyte mitochondrial function and directly inhibit the expression of forkhead transcription factor O1 (FoxO1), sterol regulatory element-binding protein 1c (SREBP1) and carbohydrate responsive element-binding protein (ChREBP), and hepatic gluconeogenesis-related enzymes in the liver tissue of diabetic rats without relying on insulin, thereby inhibiting hepatic gluconeogenesis.
In addition, BBR can exert antidiabetic effects by reducing intestinal glucose absorption through modulation of oxidative stress, inflammatory responses, and enzyme activities (Pan and Kong 2018; Liu et al. 2010b).
Antihyperlipidemic activity
BBR is able to exert its hypolipidemic pharmacological effects by regulating cholesterol metabolism, inhibiting cholesterol absorption, and promoting cholesterol excretion in various ways. Studies have shown that BBR has a similar effect to statins in hyperlipidemic animals, which can significantly reduce low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglyceride (TG) levels and increase high-density lipoprotein cholesterol (HDL-C) levels. The results of clinical trials showed that BBR could significantly reduce cholesterol levels in patients with hypercholesterolemia without adverse effects (Pirillo and Catapano 2015; Chen et al. 2022a; Wei et al. 2024). In addition, BBR reduces blood glucose and plasma cholesterol levels in diabetics, as well as body mass index and waist circumference in patients with metabolic syndrome.
After 8 weeks of treatment with BBR in atherosclerotic rats, dietary cholesterol absorption was reduced by 40–50%, and plasma TC and non-high-density lipoprotein cholesterol (nonHDL-C) levels were reduced by 29–33% and 31–41%, respectively. As a result, plasma TC and nonHDL-C levels are significantly correlated with the rate of cholesterol absorption, whereas BBR is able to reduce plasma TC and nonHDL-C levels by inhibiting intestinal cholesterol absorption (Wang et al. 2014b; Wang et al. 2010). In Jian’s study, 3T3-L1 cells were treated with BBR, and the level of TG was significantly reduced. The results indicated that BBR was able to accelerate lipid metabolism by increasing the expression of adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) through the AMPK pathway (Jiang et al. 2016). It has also been reported that BBR can inhibit the activity of bile salt hydrolase (BSH) in the intestinal microflora, increase the excretion of bile acids, activate the intestinal farnesoid X receptor (FXR) signaling pathway, inhibit the expression of liver fatty acid translocase CD36 and the absorption of TG in the intestine, reduce the uptake of long-chain fatty acids by the liver, and exert its lipid-lowering effect (Sun et al. 2017). In addition, researchers also found that in hyperlipidemic hamsters, BBR can reduce serum TC, TG, and LDL-C levels by promoting cholesterol excretion from the liver into the bile, thereby exerting its hypolipidemic effect (Li et al. 2015). Researchers found that BBR was also able to upregulate hepatic low-density lipoprotein receptor (LDLR) levels by regulating intestinal flora, thereby promoting LDL uptake by hepatocytes and inhibiting lipid accumulation and lowering cholesterol levels, which then exerted a hypolipidemic effect (Yang et al. 2022; Wu et al. 2022; Wang et al. 2022b).
Antitumor activity
Malignant tumors are one of the common problems faced by mankind in today’s world. It has been shown that BBR has a wide range of antitumor effects and can be used to treat many types of cancers such as liver and intestinal cancers. It inhibits tumor cell proliferation, promotes tumor cell apoptosis, blocks the cell cycle, inhibits tumor cell invasion and migration, inhibits tumor angiogenesis, and regulates tumor cell autophagy, and BBR is able to target multiple signaling pathways such as AP-1, AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF, PI3K/Akt, and cytochrome-C/caspase to exert antitumor effects (Fu et al. 2013).
It has been reported that BBR can effectively inhibit the proliferation of tumor cells such as colon cancer, prostate cancer, lung cancer, and gastric cancer by activating autophagy protein expression, promoting tumor cell apoptosis, and blocking cell cycle (Katona et al. 2014; Huang et al. 2015; Chen et al. 2022b; Zhang et al. 2020c). BBR can interact with nucleic acids, especially DNA, to regulate the transcription and expression of relevant genes during tumor pathology and thus effectively induce apoptosis in tumor cells such as hepatocellular carcinoma and colon cancer (Yang and Huang 2013; Shukla et al. 2016), and BBR can also inhibit renal carcinogenesis by modulating the production of reactive oxygen species and inducing DNA breaks (Zhao et al. 2023a). In addition, BBR can also exert antitumor effects by interfering with microRNA expression, and BBR is able to interfere with JAK1/STAT3 signaling by upregulating miR-17-5p in bladder cancer cells, thereby exerting antitumor effects (Xia et al. 2021). During endometrial cancer treatment, BBR can inhibit endometrial cancer tumor growth via the circ_ZNF608/miR-377-3p/COX-2 axis (Liang et al. 2022).
Urokinase-type plasminogen activator (u-PA) and MMPs play important roles in tumor cell metastasis. BBR can inhibit the migration of tumor cells such as breast cancer, endometrial cancer, and human tongue squamous cell carcinoma by regulating the expression of u-PA and MMP-1, MMP-2, and MMP-9 (Agnarelli et al. 2018; Wu et al. 2012; Liu et al. 2017b; Ho et al. 2009; Kim et al. 2012; Kuo et al. 2012). The therapeutic effect of BBR is closely related to the mediation of intestinal flora (Habtemariam 2020). Researchers found that in AOM/dss-induced colorectal cancer model mice fed a high-fat diet, BBR was able to directly inhibit high-fat diet-associated colorectal cancer by modulating gut flora-mediated lysophosphatidylcholine levels (Chen et al. 2023b). In addition, BBR can also exert antitumor effects by inhibiting the NF-κB pathway and reducing the mRNA expression of IL-8, COX-2, inducible NO synthase (iNOS), and VEGF (Hamsa and Kuttan 2012).
Antimicrobial activity
As a broad-spectrum antimicrobial, BBR has a wide range of antimicrobial effects, and its efficacy and safety have been generally recognized. BBR has different degrees of inhibitory effects on Escherichia coli, Staphylococcus, Vibrio cholerae, Shigella, and Salmonella. However, its inhibitory mechanism is still unclear, and it may exert its inhibitory effect by inhibiting bacterial enzyme activity, inhibiting bacterial division, inhibiting bacterial nucleic acid function, and inhibiting bacterial adhesion.
It has been found that BBR can inhibit the adhesion of Gram-positive and Gram-negative bacteria to the body by reducing the number of pili on the bacterial surface, and it can also inhibit bacterial growth by affecting bacterial DNA synthesis through the inhibition of topoisomerase I and topoisomerase II activities (Li et al. 2000; Krishnan and Bastow 2000). In addition, BBR can also reduce the number of type I fimbriae of S. typhimurium, inhibit the adhesion of S. typhimurium, and prevent the formation of biofilm, thereby inhibiting its growth and reproduction (Xu et al. 2021). BBR can also exert its antistaphylococcal effects by increasing the membrane permeability of staphylococcal cell and disrupting their proton motive force (Zhao et al. 2023b; Zheng et al. 2023). Wojtyczka et al. evaluated the bacteriostatic activity of BH based on the inhibitory effects of commonly used antibiotics against 14 reference strains of staphylococci and assessed the degree of interaction of BBR with these antibiotics (Wojtyczka et al. 2014). The results showed that BBR and antibiotics had synergistic inhibitory effects on the coagulase-negative Staphylococcus reference strain, with the most significant synergistic effect of BBR in combination with linezolid, cefoxitin, and erythromycin. The synergistic effect between BBR and antibiotics suggests the potential application of compound combinations as effective novel therapeutic tools for antibiotic-resistant bacterial infections. Another study found that BBR is able to exert antimicrobial effects by disrupting carbohydrate metabolism and producing ROS to disrupt DNA, protein, and lipid biosynthesis (Du et al. 2020).
Antidiarrheal
BBR has been used over the counter as a treatment for diarrhea and is widely used in clinical practice. Clinical studies have found that BBR-based nutritional supplements significantly reduce diarrhea events by 50–70% even after 30 days of administration of the treatment, and after 90 days, this reduction increases to 70–80%, with a reduction in the number of bowel movements per week of more than 60% and more than 50% of treated subjects behaving normally (Pierro et al. 2020). Another clinical study showed that after 8 weeks of treatment with BBR and placebo, respectively, the number of diarrhea, abdominal pain, and urgently needed bowel movements were significantly reduced in BBR-treated patients, resulting in a better therapeutic outcome compared to placebo. In addition, BBR was well tolerated (Chen et al. 2015). The antidiarrheal effects of BBR may be related to the following mechanisms: reversal of water and electrolyte secretion stimulated by cholera toxin and related toxins of Escherichia coli (Swabb et al. 1981), regulation of intestinal motility by decreasing intestinal smooth muscle contraction and delaying intestinal transit time, and increasing expression levels of Na+/H+ exchanger 3 and water channel protein 4, which ameliorates damage to intestinal epithelial tight junctions and restores intestinal barrier function (Li et al. 2010; Dubreuil 2013). However, researchers have found that while BBR is widely used clinically as an antidiarrheal drug, it can itself cause mild diarrhea by causing dysbiosis in the gut flora (Yue et al. 2019). The pharmacological activities of BBR are shown in Figure 2.
Barriers of bioavailability
Solubility and permeability
BBR has the characteristics of low solubility and poor permeability, which limits its absorption in the gastrointestinal tract, thereby reducing its bioavailability. Under physiological conditions, BBR exists mainly in an ionized form and is prone to self-aggregation in the acidic environment of the gastrointestinal tract, which in turn reduces its solubility. In a pH gradient test, the solubility of BBR in aqueous solution at 37 °C changed with the change in pH. The maximum solubility of BBR in phosphate buffer at pH 7.0 was 9.69 ± 0.37 mM and decreased with the change in pH. The solubility at pH 1.2 was only 5% of that at pH 7.0, and approximately 56% of BBR was not absorbed by the gastrointestinal tract due to aggregation (Spinozzi et al. 2014; Battu et al. 2010). Due to the lipophobic nature of BBR, passage through the enterocytes is hindered and its effective permeability coefficient in the rat intestinal mucosa is only 0.178 × 10−4 cm/s, thus confirming the low permeability of BBR (Chen et al. 2011; Liu et al. 2010c).
P-gp-mediated efflux of macromolecules
P-gp, also known as ABCB1 protein, is a transmembrane protein with a molecular weight of approximately 170 kDa encoded by the multidrug resistance gene 1 and belongs to the family of energy-dependent ABC-transporting hyperproteins. It is expressed at high levels in tissues such as the apical surface of mature intestinal epithelial cells, hepatocyte tubular membranes, and renal and cerebral vascular endothelial cells. P-gp as a transporter protein that rapidly and non-specifically pumps a large number of drug molecules out of the cell, thereby protecting the cell from harmful external molecules. Also, P-gp actively translocates certain compounds to blood-to-mucosa direction and restricts their translocation in the direction of absorption, thus limiting the absorption of these drugs and reducing their bioavailability. BBR has been proved to be the substrate of P-gp (Zhang et al. 2011); P-gp efflux has a great influence on the transport of BBR and is considered to be an important reason for the low bioavailability of BBR. Pan et al. found that the P-gp inhibitor cyclosporine A can significantly inhibit the efflux of BBR in the Ussing lumen in vitro (Pan et al. 2002). In the in situ recirculation perfusion model, the amount of BBR passing through the ileum was increased by 14.8% and 17.2% in the presence of the P-gp inhibitor cyclosporine A and verapamil, respectively, compared to the BBR group. Researchers studied the intestinal absorption of BBR and found that in Caco-2 cells, the presence of verapamil resulted in a 1.45-fold increase in BBR absorption and a 16.5% and 42.7% decrease in efflux and efflux ratios, respectively (Xu et al. 2019). In the presence of P-gp inhibitors, the efflux of BBR was significantly reduced and the bioavailability was significantly improved. It can be concluded that P-gp efflux is an important factor affecting the bioavailability of BBR.
Other factors
Re-excretion of BBR may also reduce its oral absorption due to hepatic and intestinal circulatory processes. It was found that only 7.8 × 10−5% of BBR was excreted through the bile within 24 h, and the amount of BBR entering the portal vein was only 0.5%. However, hepatobiliary system excretion, especially re-excretion, is still considered an important factor affecting the bioavailability of BBR (Liu et al. 2010c; Ma et al. 2013; Tsai and Tsai 2004). Li et al. found through an in vivo pharmacokinetic study in mice that, after injection of radioiodinated BBR, the gallbladder concentration-time curves appeared to have two peaks at 15 min and 2 h, respectively, and their corresponding Cm were 0.10% and 0.71%, which were in perfect agreement with the Cm of BBR in the small intestine (Liu et al. 2016). Re-excretion by the hepatobiliary system was thus found to be an important cause of low bioavailability of BBR.
To study first-pass elimination in the stomach, intestine, and liver, researchers administered BBR through four different routes: intragastric, intraduodenal, intraportal, and intravenous. It was found that after intragastric administration, approximately 50% of BBR was completely passed through the gastrointestinal tract, and the other half was disposed of by the small intestine, resulting in extremely low oral bioavailability of BBR (Liu et al. 2010c). Researchers have also found that rectal administration can significantly increase the bioavailability of BBR compared to oral administration (Mori et al. 2023). Therefore, the intestinal first-pass effect of BBR is considered to be one of the major barriers to its oral bioavailability.
Methods to improve BBR bioavailability
Absorption enhancer
Low permeability is a key factor affecting the oral bioavailability of BBR, and the use of intestinal absorption enhancers is an important approach to improve the oral bioavailability of low-permeability drugs. A large number of well-known substances have been shown to alter intestinal permeability including spices, peptide-based promoters, surfactants, and polymers.
Sodium caprate is an anionic surfactant and has been shown to be a safe and effective absorption enhancer (Anderberg et al. 1993). Sodium caprate can improve the permeability of BBR in the intestine by increasing its lipid solubility and expanding the tight junctions of the intestinal epithelium, thereby improving its bioavailability. In a study by Lv, it was suggested that an oral formulation of BBR supplemented with sodium caprate at a dose of 100 mg/kg increased the AUC of BBR by 28% and decreased the area under the glycemic curve by 22.5% compared to an oral formulation of BBR without sodium caprate, suggesting that sodium caprate can significantly increase the bioavailability of BBR and contribute to improve its antidiabetic effect (Lv et al. 2010).
Chitosan is a cationic polysaccharide, and its enhanced absorption effect is dose dependent. Chitosan facilitates BBR uptake by two pathways. First, it interacts with the anionic component of epithelial cell surface glycoproteins, altering the relative concentration of ions within the tightly juxtaposed hydrated internal channels, leading to relaxation and opening of the tight junctions and improving the BBR paracellular pathway in the intestinal tract. Second, chitosan is a high molecular polymer with adhesive properties that increase the retention of the drug on the mucosal surface, thus facilitating the absorption and improving the bioavailability of BBR. The AUC values of orally administered BBR in rats were reported to increase by 1.9, 2.2, and 2.5-fold when 0.5%, 1.5%, and 3.0% chitosan was added to the formulation, and the best enhancement was observed when the concentration of chitosan was 3%, with an AUC of 91.13 ± 51.29 ng∙h/mL (Chen et al. 2012). Researchers have also prepared BBR spray-dried preparations containing chitosan using a dual-channel spray gun technology. In vitro Caco-2 cell permeability experiments showed that the permeability of BBR was significantly enhanced (Godugu et al. 2014).
Absorption enhancers such as glycerol and menthone also played an important role in improving the permeability of BBR, with a 6-fold increase in AUC values and a 2.46-fold increase in Cmax compared to free BBR (Godugu et al. 2014). Gao et al. used an in situ closed-loop method to study the effect of DSPE-PEG polymers on the absorption of BBR in the rat small intestine, and the results showed that DSPE-PEG polymers were able to loosen the tight junctions of the intestinal epithelial cells, which increased the paracellular absorption of BBR in rats. Therefore, DSPE-PEG polymer is a very promising absorption enhancer, especially in improving the oral bioavailability of BBR (Gao et al. 2023). The above studies have shown that absorption enhancers are able to improve drug permeability and thus oral bioavailability of drugs by modulating tight junctions, increasing drug retention time and improving drug solubility in various ways.
P-gp inhibitors
As mentioned previously, P-gp expressed in the stomach and intestine can efflux its substrates, thereby limiting the absorption of these substrates and reducing their bioavailability. BBR has been shown to be a substrate for P-gp, and P-gp-mediated molecular efflux is a major factor in the low bioavailability of BBR (Maeng et al. 2002); therefore, the addition of P-gp inhibitors is a common strategy to improve the oral bioavailability of BBR. Common P-gp inhibitors include tocopheryl polyethylene glycol succinate (TPGS), polyoxyethylenated castor oil, poloxamer, cyclodextrin, and polyethylene glycol. In addition, natural compounds such as silymarin, tetrandrine, and curcumin also have P-gp inhibitory function (Dewanjee, et al. 2017).
Zhang et al. combined TPGS with BBR phospholipid complex and the results showed that the relative oral bioavailability of BBR in this formulation was 322.66% of that of BBR alone in rats. After oral administration of BBR containing 2.5% TPGS, the Cmax and AUC of BBR in rats increased by 1.9-fold and 2.9-fold, respectively, without damaging the epithelium and intact villus structure (Zhang et al. 2014). In a study by Shan, tetrandrine, a P-gp inhibitor, was used to improve the bioavailability of BBR. Tetrandrine significantly inhibited the efflux of Caco-2 intestinal cells (from 74.6 to 46.5%) and increased the uptake of BBR by Caco-2 intestinal cells. The Cmax and AUC values of BBR increased by 0.62-fold and 0.61-fold, respectively. Compared with BBR alone, the hypoglycemic effect of tetrandrine was more significant (Shan et al. 2013a). In addition, silymarin and gelatin are also potential P-gp inhibitors, both of which were able to inhibit P-gp-mediated efflux and promote BBR absorption when used in combination with BBR, and gelatin showed better hypoglycemic effects when used in combination with BBR (Pierro et al. 2015; Sun et al. 2018).
Structural modification
BBR has a unique structure of methylenedioxy ring and aromatic quaternary ammonium isoquinoline alkaloid structure with multiple structural modification sites, and modification of its structure can change its physicochemical properties and improve its pharmacological activity. Thus, many modified BBR derivatives or analogs have better pharmacological activity and bioavailability compared to BBR.
Shan et al. synthesized the BBR analog pseudoberberine (IMB-Y53) (Figure 3A) and proved that it has low affinity for P-gp. In Caco-2 cells, the retention time of IMB-Y53 was significantly longer than that of BBR and was not affected by the P-gp inhibitor tetrandrine. Pharmacokinetic studies showed that the Cmax and AUC of IMB-Y53 in Wistar rats were 1.61-fold and 2.27-fold higher than those of BBR, respectively (Shan et al. 2013b). Ge et al. synthesized novel tetrahydroprotoberberine derivatives (Figure 3B), which showed a bioavailability of 65.1% in SD rats, which is a great improvement compared to BBR, and it also showed a significant therapeutic effect for the treatment of high-fat diet in an obese mouse model (Ge et al. 2021). The bioavailability of 8,8-dimethyldihydroberberine (Figure 3C), a dihydroberberine derivative synthesized by Cheng et al. was 10.03% in obese and diabetic mouse models, which was significantly higher than that of dihydroberberine (2.65%). At the same dose, its effects of improving glucose tolerance, reducing insulin resistance, and reducing plasma glycerol were also better than those of dihydroberberine (Cheng et al. 2010). The researchers also designed and synthesized new BBR-type NO donor derivatives, which were found to have improved gastrointestinal absorption, blood-brain barrier permeability, and bioavailability and exhibited some glycerol inhibitory activity compared to BH (Wang et al. 2024).
To improve the bioavailability of BBR, researchers synthesized hydrophilic BBR derivatives such as 9-O-glucosyl berberine (Figure 3D), which showed a 10.1-fold increase in the AUC value of 9-O-glucosyl berberine compared to BBR, thus suggesting that hydrophilic modification can improve the bioavailability of BBR (Han et al. 2019). On this basis, carbohydrate-modified berberine derivatives synthesized by Han et al. showed higher bioavailability and better antidiabetic effect than BBR, among which the solubility of mannose-modified berberine derivative (Figure 3E) was 5.1-fold higher than that of BBR, and it showed better antidiabetic activity and lower cytotoxicity in HepG2 cells (Han et al. 2019). In addition, the researchers also used some disaccharide groups to modify the structure of BBR. Among them, the diglucose-modified berberine derivative (Figure 3F) also showed strong antidiabetic activity in the zebrafish model, and it can significantly improve the uptake of zebrafish even at very low concentrations (Wang et al. 2020).
In summary, the structural modification of BBR can significantly improve the bioavailability of BBR, which in turn makes the modified BBR derivatives have stronger pharmacological activity. Therefore, BBR derivatives are candidates for BBR-specific treatment strategies, but they still need to be compared with BBR in terms of bioavailability and distribution.
Salt formation modifications
Salt-forming modification can keep the parent structure of BBR unchanged and only change the anion, thus changing the physicochemical properties of BBR, which is an effective way to improve the bioavailability and pharmacological activity of BBR.
Xu et al. prepared four BBR fatty acid salts from BH, and the chemical structures are shown in Figure 4. The equilibrium solubility of the four BBR fatty acid salts was improved compared with that of BH, among which the equilibrium solubility of berberine caproate was increased 2-fold, and all of them showed high anticancer activity (Xu et al. 2022). In addition, the researchers also prepared berberine fumarate, berberine malate, berberine succinate, and berberine citrate, four kinds of berberine organic acid salts (BOAs), and their pharmacokinetics were studied, which showed that the bioavailability of BOAs was higher than that of BH, and the bioavailability of berberine fumarate and berberine succinate increased by 1.278-fold and 1.313-fold, respectively, and showed good pharmacological effects on type 2 diabetes (Cui, et al. 2018). BBR-silymarin salt has been reported to improve the permeability of BBR and silymarin and enhance the absorption of both components, resulting in a significant increase in the bioavailability of BBR and silymarin, which in turn demonstrated better hypolipidemic activity (Ma et al. 2024). The above studies demonstrated that salt-forming modifications could effectively improve the bioavailability of BBR and enhance its therapeutic effects in anticancer, antidiabetic, and hypolipidemic areas by increasing drug solubility and permeability and facilitating drug absorption.
Intestinal microbiota
The human intestinal microbiota consists of thousands of bacteria and archaea. It is considered an “invisible organ” associated with the pathogenesis of cardiovascular disease, obesity, and diabetes (Feng et al. 2015) and plays a key role in the absorption and metabolism of oral drugs. Intestinal microbiota can influence the metabolism, first-pass effect, and hepatic and intestinal recirculation of orally administered drugs by altering intestinal permeability, which in turn affects the bioavailability of orally administered drugs (Mujtaba et al. 2022). It has been reported that nitroreductase in the intestinal flora can convert BBR to an absorbable dihydroberberine. Compared to BBR, dihydroberberine has a lower efflux ratio. The intestinal absorption rate in animals is 5-fold that of BBR, and its bioavailability in humans is also significantly higher than that of BBR. However, dihydroberberine is unstable in solution and is oxidized to BBR in the intestinal tissue and enters the blood. It was found that intestinal flora promoted the intestinal absorption of BBR and improved its oral bioavailability (Feng et al. 2015; Moon et al. 2021).
Eutectic
Eutectic refers to a new crystalline form formed by the combination of the active ingredient of the drug and the eutectic formers in non-covalent bonds such as hydrogen bond, van der Waals force, and ionic bond. The formation of eutectic can change the physicochemical properties of the drug, such as solubility, dissolution rate, and stability, thus improving the bioavailability and efficacy of the drug. Lu et al. prepared BH-citric acid eutectic using citric acid as a eutectic former and showed that the eutectic improved the solubility, dissolution rate, and physical stability of BH while reducing its hygroscopicity (Lu et al. 2019). BBR-fumaric acid eutectic was prepared by combining BBR and fumaric acid in a stoichiometric ratio of 2:1, and the solubility and dissolution rate of BBR were also improved (Yang et al. 2020). The researchers also prepared BH-emodin eutectic and BH-2-emodin-ethanol eutectic, and it was found that after the formation of the eutectic, BH could achieve sustained release in water, and the AUC and Cmax of emodin in the eutectic were improved (Yanping et al. 2018). In addition, researchers have also synthesized a new BH cocrystal with significantly improved solubility compared to BH. The cocrystal exhibits good stability and overall excellent performance, making it a suitable solid form for the development of BBR oral tablets (Guo et al. 2023). In summary, BBR improves its solubility and dissolution rate by forming eutectics with eutectic formers, which in turn improves the bioavailability of BBR.
New formulations
Natural deep eutectic solvents (NADES)
NADES is an important environmentally friendly solvent consisting of primary metabolites of plants that are non-toxic and safe for humans and can be used in the food and pharmaceutical sectors. NADES has great potential as a solubilizer to improve the bioavailability of poor solubility drugs (Li and Lee 2016; Faggian et al. 2017).
Sut et al. prepared different NADES using food-grade ingredients as the main raw material, and three NADES/BBR solutions and an aqueous suspension were administered at a dose of 50 mg/kg, respectively, and it was found that there was a significant increase in the solubility of NADES/BBR as compared to BBR, and the blood concentration of BBR was determined by LC-MS/MS method. The results showed that the AUC of NADES/BBR increased by 2–20-fold (Sut, et al. 2017). Zhao et al. first demonstrated the presence of NADES in coptidis rhizoma extract and systematically investigated its effect on the pharmacokinetics of orally administered BH. The results showed that NADES mainly affected the absorption process of orally administered BH and that NADES was able to promote intestinal absorption in mice in vivo in a concentration-dependent manner, which was manifested by a significant increase in the AUC and Cmax of BH in the portal vein of mice (Zhao et al. 2021). This study demonstrated that the primary metabolites of coptidis rhizoma extract can form “endogenous” NADES, confirming for the first time that NADES can be extracted and prepared from herbal extracts and can improve the pharmacokinetic properties of coexisting active ingredient.
Microsphere system
Microspheres refer to the skeletal, tiny spherical entities formed by the drug dispersed or dissolved in carrier excipients. Microspheres, as the carriers for drug encapsulation, slow release, controlled release, and targeted release, are characterized by a high encapsulation rate, a high loading capacity, and an improved drug stability (Sokolik et al. 2018). As a drug carrier, it also has a good application in improving the bioavailability of drugs.
In order to increase the effective concentration of BH in the gastrointestinal tract, researchers prepared novel pH-responsive composite hydrogel microspheres. It was found that at pH 7.4, the novel composite hydrogel microspheres showed the highest dissolution rate and cumulative release, which significantly increased the effective concentration of BH in the gastrointestinal tract (Gao et al. 2020). The researchers also developed microspheres containing sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC) to improve the bioavailability of BH, and the results showed that SNAC significantly increased the intestinal permeability of BH, with a 9.87-fold increase in the AUC of the mixture of BH and SNAC and a 14.14-fold increase in the AUC of the microspheres loaded with BH-SNAC, compared with BH alone (Li and Zhu 2020). Another study found that lipid microspheres loaded with BBR showed a significant increase in half-life and an 8.3-fold increase in bioavailability relative to BBR injection in rats and exhibited good pharmacological activity with a tumor inhibition rate of 91.55% (Qi and Liu 2021). In addition, researchers found that microspheres can make BBR achieve sustained release, effectively improving its bioavailability, so that it can better play anti-inflammatory, antibacterial, and other pharmacological effects (Sang et al. 2023; Chen et al. 2023c). Studies have shown that microspheres as drug carriers can improve the intestinal permeability of BBR and further improve its bioavailability and pharmacological activity by prolonging its release time.
Microemulsion, nanoemulsion, and self-emulsifying systems
Microemulsion, nanoemulsion, and self-emulsifying systems are thermodynamically stable systems composed of surfactants, cosurfactants, and oil and water phases. Due to its low surface tension, it can increase the solubility of the drug and promote the absorption of the drug in the human intestinal lining, so it shows a good effect in improving the bioavailability of poorly soluble drugs.
Gui et al. developed an oral BBR microemulsion and performed a pharmacokinetic study on it and found that the bioavailability of oral BBR microemulsion was 6.47-fold higher than that of BBR suspension (Gui et al. 2008). Another study found that the microemulsions loaded with BBR and decorated with cannabidiol, prepared by a one-step emulsion method, were also able to significantly enhance the bioavailability and pharmacological effects of BBR (Fan et al. 2023). In order to improve the stability and oral bioavailability of BH, researchers developed a water-in-oil nanoemulsion system of BH, and monolayer assessment of Caco-2 cells showed a significant increase in intestinal permeability and a significant decrease in efflux of BH, and the relative bioavailability of this formulation was 440.40% compared to unencapsulated BH, and it had good stability (Li et al. 2017). Xu et al. developed a new nanoemulsion with a particle size of 30.56 ± 0.35 nm and good stability. Cell transport and intestinal perfusion studies showed that, compared with the BBR control group, the P-gp efflux of the preparation was reduced by 50%, the permeability was increased by 5.5-fold, and the bioavailability in rats was increased by 212.02% and that the preparation reduced the blood glucose level of diabetic mice by 1/3 (Xu et al. 2019).
Pund et al. developed BBR self-nanoemulsifying drug delivery systems in both solid and liquid forms and found that the systems had the effect of enhancing drug solubility, dissolution, and in vivo pharmacological activity (Pund et al. 2014). Li et al. developed a novel water-in-oil self-nanoemulsifying drug delivery system by low-energy emulsification methods, and the relative oral bioavailability of this formulation in rabbits was 3.41-fold higher than that of BBR; moreover, the Caco-2 cell monolayer transport study showed that the formulation could enhance the permeability of BBR and inhibit its efflux, and in the MV4-11 transplanted leukemia mouse model, the survival time of mice treated with the formulation was significantly longer than that of the BBR treatment group (Li et al. 2018). In a study by Zhu, rats were orally administered BBR self-microemulsion formulation and BBR commercial tablets at a dose of 25 mg∙kg−1, respectively; the Cmax and AUC in rats orally administered BBR self-microemulsion formulation were 163.4% and 154.2% of those of BBR tablets administered orally, respectively, with a relative bioavailability of 242% (Zhu et al. 2013).
Therefore, microemulsion, nanoemulsion, and self-emulsifying drug delivery systems are able to improve the bioavailability and pharmacological effects of BBR by reducing P-gp efflux and increasing the solubility and permeability of BBR and are an effective delivery mode for BBR.
Nanoparticles
Nanoparticles are solid colloidal particles with a particle size on the order of nanometers (0.1–100 nm) made of natural or synthetic polymeric materials. With the development of nanotechnology, nanoparticles have shown significant application in the field of drug delivery. The reduction of BBR particles to the nanometer range is conducive to the improvement of their bioavailability and better performance of their pharmacological effects.
To improve the solubility of BBR, Kohli et al. prepared BBR-loaded chitosan-alginate nanoparticles by ion gelation methods, and pharmacokinetic studies showed a 4.1-fold increase in the bioavailability of BBR in this formulation compared to BBR (Zhang et al. 2021). In addition, BBR nanoparticles prepared by antisolvent precipitation method also significantly improved the solubility of BBR, and the Cmax and AUC of BBR nanoparticles were 3.97-fold and 3.88-fold higher than those of BBR, respectively, and showed better hepatoprotective effects (Sahibzada et al. 2021). Khan et al. co-delivered BBR and doxorubicin by poly(lactic-co-glycolic acid) nanoparticles using a conjugation/encapsulation strategy. The excretion of BBR in the urine of patients was significantly reduced, and its retention time in the body was also significantly prolonged. Compared with BBR, the AUC of BBR in plasma increased 2-fold after nanoparticle administration, and its toxicity to breast cancer cells increased 14-fold (Khan et al. 2019). Protein nanoparticles are naturally present in the extract of coptidis rhizoma, which can act as concentration-dependent carriers to promote the absorption of BBR and enhance the plasma exposure of BBR. Therefore, the aqueous solubility, in vitro absorption, and plasma exposure of BBR in coptidis rhizoma extract were significantly better than those of pure BBR (Ma et al. 2016). The researchers also prepared biodegradable nanoparticles loaded with BBR, which showed better pharmacological activity with a 4.4-fold increase in bioavailability and a 2-fold increase in antioxidant capacity compared to BBR (Mir et al. 2023).
Therefore, shrinking BBR particles to the nanoscale can improve the solubility and permeability of BBR, prolong its retention time in the body, promote its absorption, and then improve its bioavailability so that it can better perform its pharmacological effects.
Solid lipid nanoparticles (SLNs)
SLNs are a certain proportion of solid lipids as the structural core and solid structured carriers accompanied by surfactants, which is characterized by low toxicity, high biodegradability, and high biocompatibility and is suitable for a wide range of administration routes, with great potential for application in the pharmaceutical field (Santonocito and Puglia 2022; Ghasemiyeh and Mohammadi-Samani 2018).
BBR-SLNs prepared by Xue et al. showed significantly higher bioavailability in rats compared to BBR, and the AUC and Cmax of BBR-SLNs could be up to 2-fold and 4-fold of those of BBR, respectively, and BBR-SLNs showed better effects in inhibiting body weight gain and fasting blood glucose levels in db/db diabetic mice (Xue et al. 2013). In addition, BBR-SLNs also have some slow release effects, and BBR can enter the bloodstream rapidly after release from BBR-SLNs, thus preventing the reaggregation and precipitation of BBR in the gastrointestinal tract, so the slow release property of BBR-SLNs can improve the uptake of BBR in the circulatory system, thus improving the bioavailability of BBR (Van et al. 2023). In addition, BBR-SLNs were able to increase the bioavailability of BBR by improving its solubility and were better able to exert anti-inflammatory effects and attenuate Adriamycin-induced inflammation in H9c2 rat cardiomyocytes in vitro (Rawal et al. 2022). It can be seen that SLNs can be used as an effective carrier for slow release BBR formulations, which provides a reference for achieving long-term release of BBR in vivo, prolonging the retention time within the drug carrier, and improving the bioavailability and pharmacological activity of BBR.
Liposomes
Liposome is a kind of ultra-micro-spherical carrier preparation encapsulated by single or multilayer lipid bilayer in the form of concentric circles. It is a typical representative of nanodrug delivery system. Liposomal materials resemble the cell membranes of living organisms and are characterized by biocompatibility, degradability, targeting, enhanced drug solubility, and low toxicity (Wang et al. 2017; Uma Maheswari et al. 2023). Liposomes have been evaluated as potential delivery vehicles for drugs with poor oral bioavailability, and nanostructured lipid carriers (NLCs) are also included (Zhuang et al. 2010).
Kutbi et al. prepared BBR hyaluronate-based liposomes by film hydration methods, which provided better sustained release and higher stability compared to BBR. Pharmacokinetic studies revealed that the Cmax and AUC of orally administered BBR were 21.5 ± 4.71 ng∙mL−1 and 194.8 min∙ng∙mL−1, respectively, whereas those of BBR hyaluronate-based liposomes were 67.79 ± 1.12 ng∙mL−1 and 854 min∙ng∙mL−1, respectively, which were significantly higher compared to BBR. The relative bioavailability of BBR hyaluronate-based liposomes was increased approximately 4.38-fold compared to BBR (Kutbi, et al. 2021). The researchers also prepared liposomes loaded with BBR by an air-suspension coating (layering) method, and the oral bioavailability of this formulation in rats was increased by 628% compared to BBR, and it had the effect of prolonging the release time of BBR, and the oral administration of this BBR liposome at a dose of 100 mg/kg reduced the TC, TG, and LDL-C of hyperlipidemic mice by 15.8%, 38.2%, and 57.0% respectively, indicating its potential for the treatment of hyperlipidemia (Duong et al. 2022).
Yin et al. prepared selenium coatings on the surface of NLCs by in situ reduction technique and then prepared BBR-loaded selenium NLCs (BBR-SeNLCs) with a particle size of about 160 nm by hot-melt dispersion/homogenization methods. BBR solution, BBR-NLCs, and BBR-SeNLCs were administered to rats by gavage, and pharmacokinetic studies were performed. The results showed that the BBR-SeNLCs exhibited better sustained release compared with the normal NLCs, and the bioavailability of BBR was increased by 6.63-fold. The sustained release and good intestinal absorption of the drug, coupled with the synergistic effect of selenium, resulted in a significantly better hypoglycemic effect of BBR-SeNLCs than that of BBR-NLCs and BBR solution (Yin et al. 2017). The researchers also prepared BBR-NLCs (BBR-CTS-NLCs) overlaid with chitosan. Pharmacokinetic and brain accumulation experiments showed that the drug level of BBR-CTS-NLCs in animal brain was significantly improved. Compared with BBR, the AUC and Cmax of BBR-CTS-NLCs were significantly improved, and BBR-CTS-NLCs had stronger targeting ability (Abo El-Enin, et al. 2022).
The above studies have shown that liposomes, as a typical nanodrug delivery system, are able to improve the absorption of BBR and enhance its bioavailability by prolonging the drug release time, which in turn enables it to better perform its pharmacological effects such as hypolipidemic and antidiabetic effects.
Phospholipid complexes
Phospholipid complexes are a new technology that can improve drug bioavailability and pharmacological activity by increasing the solubility and permeability of drugs (Kuche et al. 2019). The BBR complex bound to phospholipids has shown great improvement in drug dissolution and absorption, so phospholipid complexes are an ideal choice to improve the oral bioavailability of drugs.
For the first time, researchers have prepared a BBR-phospholipid complex (P-BBR) using a rapid solvent evaporation method and self-assembly technique. In vivo pharmacokinetic studies showed that the oral bioavailability of P-BBR was increased approximately 3-fold compared to oral BBR. In a db/db diabetic mouse model, after 4 weeks of treatment with P-BBR, the fasting blood glucose of the mice was significantly reduced and the antidiabetic effect was significantly enhanced (Yu et al. 2016). On this basis, P-BBR-loaded polymer-lipid hybrid nanoparticles (PEG-lipid-PLGA NPs) were developed to improve the oral bioavailability of BBR. Pharmacokinetic studies showed that the relative oral bioavailability and Cmax of PEG-lipid-PLGA NPs/BBR-soybean phosphatidylcholine complex (BBR-SPC) were 3.4-fold and 3.2-fold higher, respectively, than those of BBR in rats (Yu et al. 2017). Zhang et al. prepared a solid dispersion (BPTS-SD) consisting of P-BBR, TPGS1000, and silicon dioxide (SiO2) by solvent evaporation technique, and intestinal perfusion studies showed that the absorption of P-BBR was increased 1.4–2.0-fold compared to BBR, while the absorption of BPTS-SD was further increased compared to P-BBR. Pharmacokinetic studies showed a significant increase in Cmax and AUC for both P-BBR and BPTS-SD and a 322.66% increase in relative bioavailability of BPTS-SD compared to BBR (Zhang et al. 2014). In addition, researchers have prepared BPC nanoaggregate-embedded thiolated chitosan hydrogels, which are formulations with long drug release time and appropriate residence time, which can further improve the bioavailability of BBR (Hashtrodylar et al. 2023). The above studies have shown that P-BBR can enhance the intestinal absorption of BBR by improving its lipid solubility, and at the same time, it can be administered with the help of a variety of new drug delivery systems to further enhance the bioavailability of BBR by taking advantage of the dual strengths of P-BBR and drug delivery systems to better utilize the pharmacological effects of BBR.
Solid dispersions
Solid dispersions, as an intermediate for pharmaceutical formulations that can improve the dissolution and solubility of insoluble drugs by increasing the effective contact area with the dissolution medium (Han et al. 2011), are an effective way to improve drug bioavailability, delay drug release, and increase drug stability.
Meng et al. prepared an amorphous solid dispersion of BBR with sodium citrate as an absorption enhancer by solvent evaporation method, which was used to improve the dissolution and oral bioavailability of BBR. Pharmacokinetic studies showed that compared with BBR, the permeability of the solid dispersion was increased 3-fold, the in situ intestinal perfusion was increased 4-fold, the in vivo bioavailability was increased 5-fold, and it also had a good effect on glucose and lipid metabolism in type 2 diabetic rats (Zhaojie et al. 2014). In addition, the amorphous solid dispersion of BBR prepared by hydrogenated phosphatidylcholine also has the effect of improving permeability and intestinal absorption, and the AUC and Cmax of this formulation in rats were 3.62-fold and 4.32-fold higher than those of BBR, respectively, and the bioavailability was significantly improved (Shi et al. 2015a). Mishra et al. prepared solid dispersions of BH using different ratios of hydrophilic carriers glycerol ester 44/14 and glycerol ester 50/13, the solubility of BH in this formulation was significantly improved compared to free BH, the 2 h drug release was increased from 24.39 to 98.59%, and the oral bioavailability was increased 2.32-fold (Mishra and Dhole 2019). In summary, solid dispersions can improve the bioavailability of BBR by increasing its solubility and permeability and then make it better play the pharmacological effects.
Micelles
Micelles are amphiphilic colloidal structures whose core consists of amphiphilic hydrophobic fragments while their shell consists of hydrophilic fragments (Parikh et al. 2018). Because micelles have a unique structure capable of solubilizing hydrophobic drugs, they are able to improve the bioavailability and efficacy of drugs without disrupting the drug formulation (Jin et al. 2017; Patra et al. 2018). Therefore, micelles are well suited to improve oral drug delivery (Tian and Mao 2012).
Kwon et al. prepared a BBR-loaded mixed micelle formulation with ratios of BBR:pluronic P85:tween-80 of 1:5:0.5 (w/w/w). This formulation increased the solubility, permeability, and AUC of BBR by 8-fold, 3.64-fold, and 14.58-fold, respectively, and significantly inhibited P-gp-mediated BBR efflux, resulting in a decrease in the efflux ratio from 7.54 to 1.05, and the formulation had no apparent cytotoxicity in the range of 100 μm (Kwon, et al. 2020). Shen et al. prepared polymeric micelles containing TPGS, which improved the delivery of BBR to tumors, resulting in a 3-fold increase in the solubility of BBR and a 37-fold increase in its AUC in a healthy canine model compared to free BBR (Shen et al. 2016).
Kang et al. prepared BBR-loaded hybrid micelles by a solvent diffusion method and found that the mixed micelles had good intestinal permeability and cellular uptake, which significantly improved the pharmacokinetic profile of BBR, and its bioavailability was increased by 317.17% and efficacy was increased by 3.44-fold compared with BBR. The results showed that the micellar formulations loaded with BBR could significantly improve the solubility and permeability of BBR and also had a certain inhibitory effect on P-gp efflux (Kang et al. 2020). In addition, researchers also developed a new food-grade BBR delivery system, namely, BBR-LipoMicel. The study found that compared with BBR, the aqueous solubility of the preparation increased by 1.4-fold and AUC and Cmax increased by 5.84-fold and 9.46-fold, respectively, which can effectively promote the absorption of BBR and improve its bioavailability (Solnier, et al. 2023). Therefore, micellar formulations are an effective way to improve the bioavailability and pharmacological activity of BBR.
Dendrimers
Dendrimers, also known as highly branched polymers, are macromolecules with a high density of surface functional groups that are highly branched and monodisperse. It consists of three main parts: molecular center core, branched unit inner layer, and functional group outer layer (Choudhary et al. 2017). It has become a widely concerned structure because of its solubilization performance.
Cupta et al. covalently coupled BBR to a terminal amine G4 PAMAM dendrimer and compared it with free BBR and found that BBR-conjugated dimer had smaller particle size and better solubility, and in vivo pharmacokinetic studies using adult female albino rats as a model showed that the AUC of BBR-conjugated dimer was increased by 73% compared to free BBR, and in addition, an in vitro anticancer study showed that BBR-conjugated dimer had better anticancer activity and potential (Gupta et al. 2017). The researchers also prepared PEGylated PAMAM dendrimers loaded with BBR, and the results of the study showed significant cellular uptake and the ability to control the release of the drug, with a significant inhibitory effect on breast cancer cell line (Yadav et al. 2023). The above studies have shown that dendrimers are able to improve the bioavailability of BBR by increasing the solubility of the drug as well as controlling the release of the drug, which in turn leads to a better performance of its pharmacological effects.
Nanocrystals
Drug nanocrystals, also known as nanosuspensions, are nanoscale carrier-free colloidal systems with a theoretical drug loading capacity of 100%. Drug nanocrystals are easy to prepare and have strong adhesion, which can effectively solve the problems of short retention time, slow release, and low bioavailability of water-insoluble drugs (Shi et al. 2015b).
Sahibzada et al. prepared BBR semicrystalline nanoparticles by using antisolvent precipitation with a syringe pump and evaporative precipitation of nanosuspension and performed histopathological studies and blood biochemical analyses in rabbits, which showed a 3.88-fold increase in AUC and 3.97-fold increase in Cmax of BBR semicrystalline nanoparticles compared to BBR. And the animals treated with BBR nanoparticles achieved significant improvement in liver function test enzyme levels and liver histopathology (Sahibzada et al. 2021; Sahibzada et al. 2018). Wang et al. prepared BBR nanocrystal suspensions with a mean particle size of 73.1 nm using high-pressure homogenization technique; the solubility and efficacy of this formulation were significantly improved compared to BBR, and the formulation showed significant antidiabetic activity in a diabetic mouse model with fewer side effects (Wang et al. 2015). In order to improve the intestinal absorption of BBR, the researchers also developed a Brij-S20-modified nanocrystalline formulation, which further improved the absorption of BBR by increasing the solubility of BBR and inhibiting P-gp-mediated efflux, and a pharmacokinetic study demonstrated a 404.1% increase in the relative bioavailability of BBR in the formulation compared to BBR alone (Xiong et al. 2018). It was reported that Li et al. prepared baicalin-BBR (BA-BBR) complex nanocrystals by high-pressure homogenization, and the BA-BBR complex nanocrystals improved the bioavailability of BA and BBR by increasing their dissolution rate and degree of dissolution. In vitro dissolution evaluation showed that the dissolution of BA and BBR in BA-BBR complex nanocrystals was 3.30-fold and 2.35-fold higher than that in BA-BBR complex, respectively. The pharmacokinetic results showed that the AUC of BA and BBR in BA-BBR complex nanocrystals was 7.49-fold and 2.64-fold that in BA-BBR complex, respectively (Li et al. 2022).
The above studies have shown that drug nanocrystals are able to enhance the bioavailability and pharmacological activity of BBR by improving its solubility and dissolution as well as inhibiting the efflux of P-gp, which makes them a good choice for improving the bioavailability of BBR.
Nanogels
Nanogels are three-dimensional hydrogel materials in the nanoscale size range formed by crosslinked swellable polymer networks with high water-holding capacity, high biocompatibility, high mechanical stability, and ease of modification, which have broad application prospects in biomedicine and other fields (Soni et al. 2016).
Fread et al. developed BBR oleate liquid crystal nanoparticle (BBR-OL-LCNP) hydrogel, which showed a 3-fold increase in drug accumulation and approximately a 10-fold increase in drug penetration in rats compared to BBR, and in vivo studies revealed that topical application of BBR-OL-LCNP hydrogel significantly alleviated psoriasis symptoms and reduced the levels of psoriatic inflammatory cytokines (Freag et al. 2019). In addition, the researchers also found that the manganese-doped albumin-gelatin composite nanogel loaded with BBR has good dispersibility and sustained release effect, and its sustained release effect can significantly improve the bioavailability of BBR and enhance its role in relieving oxidative stress and inhibiting inflammation (Sun et al. 2023). It has been reported that BBR-loaded chitosan-alginate nanocomposite gel can effectively control the release of BBR, prolong the release time, enhance its cellular uptake, improve the bioavailability of BBR, and effectively improve the antitumor and anti-inflammatory activities of BBR (Singh et al. 2024; Akhter et al. 2023). Therefore, nanogel encapsulated BBR can improve its bioavailability by improving its permeability and prolonging its release time, thereby improving its pharmacological activities, which is an effective drug delivery method.
Summary and prospect
There are many unknown active ingredients in natural medicines, and in-depth excavation of monomeric components in natural medicines is an effective way for drug development. BBR, as a natural extract, has a wide range of pharmacological activities (Table 1), such as protecting the nervous system, protecting the cardiovascular system, anti-inflammatory, antidiabetic, antihypolipidemic, antitumor, antibacterial, and antidiarrheal. The main reasons for the low bioavailability of BBR include poor solubility, low permeability, P-gp-mediated macromolecular efflux, and hepatic-intestinal metabolism. In order to solve this problem, researchers have proposed a variety of methods, including the use of absorption enhancers and P-gp inhibitors, structural modification of BBR, preparation of BBR salts and cocrystals, and development of new formulations to improve its bioavailability (Table 2). The results showed that the various methods were useful in improving the pharmacological activities and bioavailability of BBR, thus it is highly promising to convert BBR and its derivatives, which have a wide range of pharmacological activities and therapeutic potentials, into a product that can be widely used in clinical treatments.
BBR can exert a wide range of pharmacological effects by affecting specific enzymes, receptors, and signaling pathways and can produce synergistic effects when used in combination with other drugs with different pharmacological effects, and BBR can also be used for the treatment of metabolic diseases by regulating disorders of the intestinal microbiota, which shows that BBR has a complex mechanism of action. However, its biomolecular targets and molecular mechanisms in the human body are still unclear, which seriously limits its development in the pharmaceutical field. Therefore, further research is needed to support the clinical application of BBR.
The long-term safety of BBR is based on low bioavailability, and toxicological studies have shown that BBR is toxic to gastrointestinal function, immune function, and the heart. Therefore, its safety must be emphasized when it is widely absorbed and used over a long period of time. To date, various methods to increase the bioavailability of BBR have only been validated at the animal and cellular level without clinical trials. Therefore, large-scale clinical trials are needed to assess the safety and clinical value of BBR and to validate the clinical feasibility of methods to enhance its bioavailability.
Data availability
No datasets were generated or analyzed during the current study.
References
Abo El-Enin HA et al (2022) Lipid nanocarriers overlaid with chitosan for brain delivery of berberine via the nasal route. Pharmaceuticals (Basel) 15(3):281
Abudureyimu M et al (2020) Berberine promotes cardiac function by upregulating PINK1/Parkin-mediated mitophagy in heart failure. Front Physiol 11:565751
Agnarelli A et al (2018) Cell-specific pattern of berberine pleiotropic effects on different human cell lines. Sci Rep 8(1):10599
Akhter MH et al (2023) Enhanced drug delivery and wound healing potential of berberine-loaded chitosan-alginate nanocomposite gel: characterization and in vivo assessment. Front Public Health 11:1238961
Anderberg EK, Lindmark T, Artursson P (1993) Sodium caprate elicits dilatations in human intestinal tight junctions and enhances drug absorption by the paracellular route. Pharm Res 10(6):857–864
Battu SK et al (2010) Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech 11(3):1466–1475
Chen W et al (2011) Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 12(2):705–711
Chen W et al (2012) Enhancing effects of chitosan and chitosan hydrochloride on intestinal absorption of berberine in rats. Drug Dev Ind Pharm 38(1):104–110
Chen C et al (2015) A randomized clinical trial of berberine hydrochloride in patients with diarrhea-predominant irritable bowel syndrome. Phytother Res 29(11):1822–1827
Chen M et al (2020) Berberine attenuates Aβ-induced neuronal damage through regulating miR-188/NOS1 in Alzheimer’s disease. Mol Cell Biochem 474(1–2):285–294
Chen Y et al (2022a) Integrated lipidomics and network pharmacology analysis to reveal the mechanisms of berberine in the treatment of hyperlipidemia. J Transl Med 20(1):412
Chen Q et al (2022b) Berberine induces non-small cell lung cancer apoptosis via the activation of the ROS/ASK1/JNK pathway. Ann Transl Med 10(8):485
Chen Y et al (2023a) Berberine protects mice against type 2 diabetes by promoting PPARγ-FGF21-GLUT2-regulated insulin sensitivity and glucose/lipid homeostasis. Biochem Pharmacol 218:115928
Chen H et al (2023b) Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. Int J Biol Sci 19(7):2097–2113
Chen L et al (2023c) Berberine-encapsulated poly(lactic-co-glycolic acid)-hydroxyapatite (PLGA/HA) microspheres synergistically promote bone regeneration with DOPA-IGF-1 via the IGF-1R/PI3K/AKT/mTOR pathway. Int J Mol Sci 24(20):15403
Cheng Z et al (2010) 8,8-Dimethyldihydroberberine with improved bioavailability and oral efficacy on obese and diabetic mouse models. Bioorg Med Chem 18(16):5915–5924
Choudhary S et al (2017) Impact of dendrimers on solubility of hydrophobic drug molecules. Front Pharmacol 8:261
Cui HX et al (2018) Preparation and evaluation of antidiabetic agents of berberine organic acid salts for enhancing the bioavailability. Molecules 24(1):103
Dewanjee S et al (2017) Natural products as alternative choices for P-glycoprotein (P-gp) inhibition. Molecules 22(6):871
Di Pierro F et al (2015) Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins. Diabetes Metab Syndr Obes 8:89–96
Di Pierro F et al (2020) Role of a berberine-based nutritional supplement in reducing diarrhea in subjects with functional gastrointestinal disorders. Minerva Gastroenterol Dietol 66(1):29–34
Du GF et al (2020) Proteomic investigation into the action mechanism of berberine against Streptococcus pyogenes. J Proteomics 215:103666
Dubreuil JD (2013) Antibacterial and antidiarrheal activities of plant products against enterotoxinogenic Escherichia coli. Toxins (basel) 5(11):2009–2041
Duong TT et al (2022) Berberine-loaded liposomes for oral delivery: preparation, physicochemical characterization and in-vivo evaluation in an endogenous hyperlipidemic animal model. Int J Pharm 616:121525
Durairajan SS et al (2012) Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol Aging 33(12):2903–2919
Faggian M et al (2017) Natural deep eutectic solvents (NADES) as a tool for bioavailability improvement: pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: possible application in nutraceuticals. Molecules 21(11):1531
Fan X et al (2023) Cannabidiol-decorated berberine-loaded microemulsions improve ibs-d therapy through ketogenic diet-induced cannabidiol receptors overexpression. Int J Nanomedicine 18:2839–2853
Feng R et al (2015) Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci Rep 5:12155
Feng M et al (2017) Comparative effect of berberine and its derivative 8-cetylberberine on attenuating atherosclerosis in ApoE(-/-) mice. Int Immunopharmacol 43:195–202
Freag MS et al (2019) Liquid crystalline nanoreservoir releasing a highly skin-penetrating berberine oleate complex for psoriasis management. Nanomedicine (lond) 14(8):931–954
Fu L et al (2013) Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS ONE 8(7):e69240
Gao J et al (2020) Preparation and application of pH-responsive composite hydrogel beads as potential delivery carrier candidates for controlled release of berberine hydrochloride. R Soc Open Sci 7(11):200676
Gao Y et al (2023) DSPE-PEG polymer enhanced berberine absorption specifically in the small intestine of rats through paracellular passway. J Pharm Pharmacol 75(7):931–939
Ge H et al (2021) Design, synthesis, and biological evaluation of novel tetrahydroprotoberberine derivatives to reduce SREBPs expression for the treatment of hyperlipidemia. Eur J Med Chem 221:113522
Gendy AM et al (2023) New insights into the role of berberine against 3-nitropropionic acid-induced striatal neurotoxicity: possible role of BDNF-TrkB-PI3K/Akt and NF-κB signaling. Food Chem Toxicol 175:113721
Ghareeb DA et al (2015) Berberine reduces neurotoxicity related to nonalcoholic steatohepatitis in rats. Evid Based Complement Alternat Med 2015:361847
Ghasemiyeh P, Mohammadi-Samani S (2018) Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci 13(4):288–303
Godugu C et al (2014) Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 9(3):e89919
Gui SY et al (2008) Preparation and evaluation of a microemulsion for oral delivery of berberine. Pharmazie 63(7):516–519
Guo Z et al (2015) Anti-hypertensive and renoprotective effects of berberine in spontaneously hypertensive rats. Clin Exp Hypertens 37(4):332–339
Guo H, Liu S, Sun CC (2023) Modulating pharmaceutical properties of berberine chloride through cocrystallization with benzendiol isomers. Pharm Res 40(12):2791–2800
Gupta L et al (2017) Dendrimer encapsulated and conjugated delivery of berberine: a novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int J Pharm 528(1–2):88–99
Habtemariam S (2020) Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res 155:104722
Hamsa TP, Kuttan G (2012) Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem Toxicol 35(1):57–70
Han HK, Lee BJ, Lee HK (2011) Enhanced dissolution and bioavailability of biochanin A via the preparation of solid dispersion: in vitro and in vivo evaluation. Int J Pharm 415(1–2):89–94
Han L et al (2019) Novel carbohydrate modified berberine derivatives: synthesis and in vitro anti-diabetic investigation. Medchemcomm 10(4):598–605
Han Y et al (2023) Berberine ameliorate inflammation and apoptosis via modulating PI3K/AKT/NFκB and MAPK pathway on dry eye. Phytomedicine 121:155081
Hashtrodylar Y et al (2023) Berberine-phospholipid nanoaggregate-embedded thiolated chitosan hydrogel for aphthous stomatitis treatment. Nanomedicine (lond) 18(19):1227–1246
Ho YT et al (2009) Berberine suppresses in vitro migration and invasion of human SCC-4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and -9. Cancer Lett 279(2):155–162
Hu Y et al (2012) Berberine and evodiamine influence serotonin transporter (5-HTT) expression via the 5-HTT-linked polymorphic region. Pharmacogenomics J 12(5):372–378
Huang ZH et al (2015) Berberine targets epidermal growth factor receptor signaling to suppress prostate cancer proliferation in vitro. Mol Med Rep 11(3):2125–2128
Huang S et al (2020) Berberine protects against NLRP3 inflammasome via ameliorating autophagic impairment in MPTP-induced Parkinson’s disease model. Front Pharmacol 11:618787
Ji HF, Shen L (2011) Berberine: a potential multipotent natural product to combat Alzheimer’s disease. Molecules 16(8):6732–6740
Jia L et al (2012) Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J Pharm Pharmacol 64(10):1510–1521
Jiang W et al (2015a) Therapeutic effect of berberine on Huntington’s disease transgenic mouse model. PLoS ONE 10(7):e0134142
Jiang SJ et al (2015b) Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J Gastroenterol 21(25):7777–7785
Jiang D et al (2016) Berberine increases adipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis 15(1):214
Jiang Y et al (2021) Exploring the mechanism of berberine intervention in ulcerative colitis from the perspective of inflammation and immunity based on systemic pharmacology. Evid Based Complement Alternat Med 2021:9970240
Jin Q et al (2017) Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood-brain barrier permeability. Theranostics 7(4):884–898
Kalaiarasi A et al (2016) Plant isoquinoline alkaloid berberine exhibits chromatin remodeling by modulation of histone deacetylase to induce growth arrest and apoptosis in the A549 cell line. J Agric Food Chem 64(50):9542–9550
Kang DG et al (2002) Effects of berberine on angiotensin-converting enzyme and NO/cGMP system in vessels. Vascul Pharmacol 39(6):281–286
Kang H, Yao Y, Zhang X (2020) Mixed micelles with galactose ligands for the oral delivery of berberine to enhance its bioavailability and hypoglycemic effects. J Biomed Nanotechnol 16(12):1755–1764
Katona BW et al (2014) EZH2 inhibition enhances the efficacy of an EGFR inhibitor in suppressing colon cancer cells. Cancer Biol Ther 15(12):1677–1687
Khan I et al (2019) Nano-co-delivery of berberine and anticancer drug using PLGA nanoparticles: exploration of better anticancer activity and in vivo kinetics. Pharm Res 36(10):149
Kim S et al (2012) Berberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-α in breast cancer cells. J Surg Res 176(1):e21–e29
Ko WH et al (2000) Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol 399(2–3):187–196
Kong WJ et al (2009) Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism 58(1):109–119
Krishnan P, Bastow KF (2000) The 9-position in berberine analogs is an important determinant of DNA topoisomerase II inhibition. Anticancer Drug Des 15(4):255–264
Kuche K et al (2019) Drug-phospholipid complex-a go through strategy for enhanced oral bioavailability. AAPS PharmSciTech 20(2):43
Kulkarni SK, Dhir A (2008) On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol 589(1–3):163–172
Kuo HP et al (2012) Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J Agric Food Chem 60(38):9649–9658
Kutbi HI et al (2021) Optimization of hyaluronate-based liposomes to augment the oral delivery and the bioavailability of berberine. Materials (Basel) 14(19):5759
Kwon M et al (2020) Enhanced intestinal absorption and pharmacokinetic modulation of berberine and its metabolites through the inhibition of P-glycoprotein and intestinal metabolism in rats using a berberine mixed micelle formulation. Pharmaceutics 12(9):882
Lathe R, Sapronova A, Kotelevtsev Y (2014) Atherosclerosis and Alzheimer–diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr 14:36
Lee YS et al (2006) Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55(8):2256–2264
Li Z, Lee PI (2016) Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives. Int J Pharm 505(1–2):283–288
Li TK et al (2000) Human topoisomerase I poisoning by protoberberines: potential roles for both drug-DNA and drug-enzyme interactions. Biochemistry 39(24):7107–7116
Li N et al (2010) Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. Eur J Pharm Sci 40(1):1–8
Li C et al (2015) Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Mol Immunol 67(2 Pt B):444–454
Li XY et al (2015) Effect of berberine on promoting the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters. J Transl Med 13:278
Li YH et al (2016) Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res 110:227–239
Li YJ et al (2017) Nanoemulsion-based delivery system for enhanced oral bioavailability and caco-2 cell monolayers permeability of berberine hydrochloride. Drug Deliv 24(1):1868–1873
Li J et al (2018) Self-nanoemulsifying system improves oral absorption and enhances anti-acute myeloid leukemia activity of berberine. J Nanobiotechnology 16(1):76
Li X et al (2021) Berberine Attenuates MPP(+)-Induced Neuronal Injury by Regulating LINC00943/miR-142-5p/KPNA4/NF-κB Pathway in SK-N-SH Cells. Neurochem Res 46(12):3286–3300
Li Z et al (2022) Baicalin-berberine complex nanocrystals orally promote the co-absorption of two components. Drug Deliv Transl Res 12(12):3017–3028
Li C et al (2023) Oxyberberine ameliorates TNBS-induced colitis in rats through suppressing inflammation and oxidative stress via Keap1/Nrf2/NF-κB signaling pathways. Phytomedicine 116:154899
Li Y, Zhu C (2020) Development and in vitro and in vivo evaluation of microspheres containing sodium n-[8-(2-hydroxybenzoyl)amino]caprylate for the oral delivery of berberine hydrochloride. Molecules 25(8):1957
Liang KW et al (2006) Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem Pharmacol 71(6):806–817
Liang H et al (2022) Berberine inhibits the development of endometrial cancer through circ_ZNF608/miR-377-3p/COX2 axis. Autoimmunity 55(7):485–495
Liao Y et al (2018) Berberine inhibits cardiac remodeling of heart failure after myocardial infarction by reducing myocardial cell apoptosis in rats. Exp Ther Med 16(3):2499–2505
Liu LZ et al (2010a) Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol Cell Endocrinol 317(1–2):148–153
Liu L et al (2010b) Berberine suppresses intestinal disaccharidases with beneficial metabolic effects in diabetic states, evidences from in vivo and in vitro study. Naunyn Schmiedebergs Arch Pharmacol 381(4):371–381
Liu YT et al (2010c) Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Dispos 38(10):1779–1784
Liu X et al (2014) Berberine attenuates axonal transport impairment and axonopathy induced by Calyculin A in N2a cells. PLoS ONE 9(4):e93974
Liu L et al (2015) Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem Biophys Res Commun 458(4):796–801
Liu CS et al (2016) Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 109:274–282
Liu YM et al (2017a) Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res Bull 134:220–227
Liu L et al (2017b) Berberine inhibits the LPS-induced proliferation and inflammatory response of stromal cells of adenomyosis tissues mediated by the LPS/TLR4 signaling pathway. Exp Ther Med 14(6):6125–6130
Lu SS et al (2009) Berberine promotes glucagon-like peptide-1 (7–36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol 200(2):159–165
Lu Q et al (2019) Improving solid-state properties of berberine chloride through forming a salt cocrystal with citric acid. Int J Pharm 554:14–20
Luo J et al (2017) The effects of berberine on a murine model of multiple sclerosis and the SPHK1/S1P signaling pathway. Biochem Biophys Res Commun 490(3):927–932
Lv XY et al (2010) Enhancement of sodium caprate on intestine absorption and antidiabetic action of berberine. AAPS PharmSciTech 11(1):372–382
Ma X et al (2010) Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS ONE 5(10):e13489
Ma JY et al (2013) Excretion of berberine and its metabolites in oral administration in rats. J Pharm Sci 102(11):4181–4192
Ma BL et al (2016) Naturally occurring proteinaceous nanoparticles in coptidis rhizoma extract act as concentration-dependent carriers that facilitate berberine absorption. Sci Rep 6:20110
Ma YG et al (2017) Berberine reduced blood pressure and improved vasodilation in diabetic rats. J Mol Endocrinol 59(3):191–204
Ma X et al (2024) Berberine-silybin salt achieves improved anti-nonalcoholic fatty liver disease effect through regulating lipid metabolism. J Ethnopharmacol 319(Pt 2):117238
Maeng HJ et al (2002) P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci 91(12):2614–2621
Manu MS, Hohjoh H, Yamamura T (2021) Extracellular vesicles as pro- and anti-inflammatory mediators, biomarkers and potential therapeutic agents in multiple sclerosis. Aging Dis 12(6):1451–1461
Mir MA et al (2023) Design-of-experiment-assisted fabrication of biodegradable polymeric nanoparticles: in vitro characterization, biological activity, and in vivo assessment. ACS Omega 8(42):38806–38821
Mishra R, Dhole S (2019) Lipid-based floating multiparticulate delivery system for bioavailability enhancement of berberine hydrochloride. J Appl Pharmaceutical Sci 9(11):36–47
Moon JM et al (2021) Absorption kinetics of berberine and dihydroberberine and their impact on glycemia: a randomized, controlled, crossover pilot trial. Nutrients 14(1):124
Mori N et al (2023) Comparison of berberine bioavailability between oral and rectal administrations in rats. Biol Pharm Bull 46(11):1639–1642
Mujtaba MA et al (2022) An updated review on therapeutic potential and recent advances in drug delivery of berberine: current status and future prospect. Curr Pharm Biotechnol 23(1):60–71
Pan Y, Kong LD (2018) High fructose diet-induced metabolic syndrome: Pathophysiological mechanism and treatment by traditional Chinese medicine. Pharmacol Res 130:438–450
Pan GY et al (2002) The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol 91(4):193–197
Parikh A et al (2018) Curcumin-loaded self-nanomicellizing solid dispersion system: part I: development, optimization, characterization, and oral bioavailability. Drug Deliv Transl Res 8(5):1389–1405
Patra JK et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 16(1):71
Peng WH et al (2007) Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci 81(11):933–938
Pirillo A, Catapano AL (2015) Berberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studies. Atherosclerosis 243(2):449–461
Pund S, Borade G, Rasve G (2014) Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine 21(3):307–314
Qi Y, Liu G (2021) Berberine-10-hydroxy camptothecine-loaded lipid microsphere for the synergistic treatment of liver cancer by inhibiting topoisomerase and HIF-1α. Drug Deliv 28(1):171–182
Qin-Wei Z, Yong-Guang LI (2016) Berberine attenuates myocardial ischemia reperfusion injury by suppressing the activation of PI3K/AKT signaling. Exp Ther Med 11(3):978–984
Rabbani GH et al (1987) Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. J Infect Dis 155(5):979–984
Rawal S et al (2022) Solid lipid nanoformulation of berberine attenuates doxorubicin triggered in vitro inflammation in H9c2 rat cardiomyocytes. Comb Chem High Throughput Screen 25(10):1695–1706
Rusmini P et al (2020) Enhanced clearance of neurotoxic misfolded proteins by the natural compound berberine and its derivatives. Int J Mol Sci 21(10):3443
Sahibzada MUK et al (2018) Berberine nanoparticles with enhanced in vitro bioavailability: characterization and antimicrobial activity. Drug Des Devel Ther 12:303–312
Sahibzada MUK et al (2021) Bioavailability and hepatoprotection enhancement of berberine and its nanoparticles prepared by liquid antisolvent method. Saudi J Biol Sci 28(1):327–332
Sang S et al (2023) Sprayable berberine-silk fibroin microspheres with extracellular matrix anchoring function accelerate infected wound healing through antibacterial and anti-inflammatory effects. ACS Biomater Sci Eng 9(6):3643–3659
Santonocito D, Puglia C (2022) Applications of lipid-based nanocarriers for parenteral drug delivery. Curr Med Chem 29(24):4152–4169
Shan YQ et al (2013a) Tetrandrine potentiates the hypoglycemic efficacy of berberine by inhibiting P-glycoprotein function. Biol Pharm Bull 36(10):1562–1569
Shan YQ et al (2013b) Berberine analogue IMB-Y53 improves glucose-lowering efficacy by averting cellular efflux especially P-glycoprotein efflux. Metabolism 62(3):446–456
Shen R et al (2016) Development and evaluation of vitamin E d-α-tocopheryl polyethylene glycol 1000 succinate-mixed polymeric phospholipid micelles of berberine as an anticancer nanopharmaceutical. Int J Nanomedicine 11:1687–1700
Shi C et al (2015a) Preparation, characterization and in vivo studies of amorphous solid dispersion of berberine with hydrogenated phosphatidylcholine. Eur J Pharm Sci 74:11–17
Shi J et al (2015b) Progress in the study of drug nanocrystals. Pharmazie 70(12):757–764
Shukla S et al (2016) Concurrent acetylation of FoxO1/3a and p53 due to sirtuins inhibition elicit Bim/PUMA mediated mitochondrial dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol Appl Pharmacol 291:70–83
Singh AK et al (2019) Berberine: a plant-derived alkaloid with therapeutic potential to combat Alzheimer’s disease. Cent Nerv Syst Agents Med Chem 19(3):154–170
Singh N et al (2024) Chitosan/alginate nanogel potentiate berberine uptake and enhance oxidative stress mediated apoptotic cell death in HepG2 cells. Int J Biol Macromol 257(Pt 2):128717
Sokolik CG et al (2018) Proteinaceous microspheres as a delivery system for carvacrol and thymol in antibacterial applications. Ultrason Sonochem 41:288–296
Solnier J et al (2023.) Characterization and pharmacokinetic assessment of a new berberine formulation with enhanced absorption in vitro and in human volunteers. Pharmaceutics 15(11)
Soni KS, Desale SS, Bronich TK (2016) Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release 240:109–126
Spinozzi S et al (2014) Berberine and its metabolites: relationship between physicochemical properties and plasma levels after administration to human subjects. J Nat Prod 77(4):766–772
Sun R et al (2017) Orally administered berberine modulates hepatic lipid metabolism by altering microbial bile acid metabolism and the intestinal FXR signaling pathway. Mol Pharmacol 91(2):110–122
Sun J et al (2018) Improvement of intestinal transport, absorption and anti-diabetic efficacy of berberine by using Gelucire44/14: In vitro, in situ and in vivo studies. Int J Pharm 544(1):46–54
Sun J et al (2023) Manganese-doped albumin-gelatin composite nanogel loaded with berberine applied to the treatment of gouty arthritis in rats via a SPARC-dependent mechanism. Int J Biol Macromol 253(Pt 3):126999
Sut S et al (2017) Natural deep eutectic solvents (NADES) to enhance berberine absorption: an in vivo pharmacokinetic study. Molecules 22(11)
Swabb EA, Tai YH, Jordan L (1981) Reversal of cholera toxin-induced secretion in rat ileum by luminal berberine. Am J Physiol 241(3):G248–G252
Tian Y, Mao S (2012) Amphiphilic polymeric micelles as the nanocarrier for peroral delivery of poorly soluble anticancer drugs. Expert Opin Drug Deliv 9(6):687–700
Tillhon M et al (2012) Berberine: new perspectives for old remedies. Biochem Pharmacol 84(10):1260–1267
Tsai PL, Tsai TH (2004) Hepatobiliary excretion of berberine. Drug Metab Dispos 32(4):405–412
Turner N et al (2008) Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 57(5):1414–1418
Uma Maheswari RT et al (2023) CD44 tagged hyaluronic acid - chitosan liposome carrier for the delivery of berberine and doxorubicin into lung cancer cells. Int J Biol Macromol 253(Pt 2):126599
Van NH, Manh Khoa LN, Ngoc Mai NC (2023) Spray-dried solid lipid nanoparticles for enhancing berberine bioavailability via oral administration. Curr Pharm Des
Wang YX, Zheng YM, Zhou XB (1996) Inhibitory effects of berberine on ATP-sensitive K+ channels in cardiac myocytes. Eur J Pharmacol 316(2–3):307–315
Wang X et al (2009) Effect of berberine on Staphylococcus epidermidis biofilm formation. Int J Antimicrob Agents 34(1):60–66
Wang Y et al (2010) Berberine and plant stanols synergistically inhibit cholesterol absorption in hamsters. Atherosclerosis 209(1):111–117
Wang LH et al (2011) Berberine elicits anti-arrhythmic effects via IK1/Kir2.1 in the rat type 2 diabetic myocardial infarction model. Phytother Res 25(1):33-7
Wang Z et al (2014a) Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation 37(5):1789–1798
Wang Y et al (2014b) Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism 63(9):1167–1177
Wang Z et al (2015) Berberine nanosuspension enhances hypoglycemic efficacy on streptozotocin induced diabetic C57BL/6 mice. Evid Based Complement Alternat Med 2015:239749
Wang X et al (2017) Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes. J Pharm Pharmacol 69(6):625–632
Wang X et al (2018) Anti-Inflammatory Effects of berberine hydrochloride in an LPS-induced murine model of mastitis. Evid Based Complement Alternat Med 2018:5164314
Wang L et al (2020) Synthesis of disaccharide modified berberine derivatives and their anti-diabetic investigation in zebrafish using a fluorescence-based technology. Org Biomol Chem 18(18):3563–3574
Wang L et al (2021a) Treatment of Parkinson’s disease in zebrafish model with a berberine derivative capable of crossing blood brain barrier, targeting mitochondria, and convenient for bioimaging experiments. Comp Biochem Physiol C Toxicol Pharmacol 249:109151
Wang Y et al (2021b) Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct Target Ther 6(1):77
Wang J et al (2021c) Berberine activates the β-catenin/TCF4 signaling pathway by down-regulating miR-106b to promote GLP-1 production by intestinal L cells. Eur J Pharmacol 911:174482
Wang Z et al (2022a) Berberine improves vascular dysfunction by inhibiting trimethylamine-N-oxide via regulating the gut microbiota in angiotensin II-induced hypertensive mice. Front Microbiol 13:814855
Wang S et al (2022b) Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: a double blinded placebo controlled randomized study. Gut Microbes 14(1):2003176
Wang C et al (2023) Berberine rescues D-ribose-induced Alzheimer’s pathology via promoting mitophagy. Int J Mol Sci 24(6)
Wang K et al (2024) The study on synthesis and vitro hypolipidemic activity of novel berberine derivatives nitric oxide donors. Fitoterapia 176:105964
Wei G et al (2024) Tetrahydroberberine alleviates high-fat diet-induced hyperlipidemia in mice via augmenting lipoprotein assembly-induced clearance of low-density lipoprotein and intermediate-density lipoprotein. Eur J Pharmacol 968:176433
Wojtyczka RD et al (2014) Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Staphylococcus strains in vitro. Molecules 19(5):6583–6596
Wu YQ, Chen XS, Chai JB (2012) The involvement of the CD40-CD40L pathway in activated platelet-induced changes in HUVEC COX-2 and PPARα expression. Inflammation 35(3):1184–1190
Wu Y et al (2021) Berberine Reduces Aβ(42) Deposition and tau hyperphosphorylation via ameliorating endoplasmic reticulum stress. Front Pharmacol 12:640758
Wu C et al (2022) Gut microbiota specifically mediates the anti-hypercholesterolemic effect of berberine (BBR) and facilitates to predict BBR’s cholesterol-decreasing efficacy in patients. J Adv Res 37:197–208
Xia X et al (2011) Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 6(2):e16556
Xia Y et al (2021) Berberine suppresses bladder cancer cell proliferation by inhibiting JAK1-STAT3 signaling via upregulation of miR-17-5p. Biochem Pharmacol 188:114575
Xiong W et al (2018) Dual-functional Brij-S20-modified nanocrystal formulation enhances the intestinal transport and oral bioavailability of berberine. Int J Nanomedicine 13:3781–3793
Xu HY et al (2019) Nanoemulsion improves hypoglycemic efficacy of berberine by overcoming its gastrointestinal challenge. Colloids Surf B Biointerfaces 181:927–934
Xu XH et al (2020) Berberine inhibits gluconeogenesis in skeletal muscles and adipose tissues in streptozotocin-induced diabetic rats via LKB1-AMPK-TORC2 signaling pathway. Curr Med Sci 40(3):530–538
Xu C et al (2021) Targeting effect of berberine on type I fimbriae of Salmonella Typhimurium and its effective inhibition of biofilm. Appl Microbiol Biotechnol 105(4):1563–1573
Xu F et al (2022) Improvement of anticancer effect of berberine by salt formation modifications. Phytomedicine 104:154314
Xue M et al (2013) Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int J Nanomedicine 8:4677–4687
Yadav D, Semwal BC, Dewangan HK (2023) Grafting, characterization and enhancement of therapeutic activity of berberine loaded PEGylated PAMAM dendrimer for cancerous cell. J Biomater Sci Polym Ed 34(8):1053–1066
Yang X, Huang N (2013) Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol Med Rep 8(2):505–510
Yang D et al (2020) Solubility and stability advantages of a new cocrystal of berberine chloride with fumaric acid. ACS Omega 5(14):8283–8292
Yang YN et al (2022) The berberine-enriched gut commensal Blautia producta ameliorates high-fat diet (HFD)-induced hyperlipidemia and stimulates liver LDLR expression. Biomed Pharmacother 155:113749
Yang L et al (2023) Berberine attenuates depression-like behavior by modulating the hippocampal NLRP3 ubiquitination signaling pathway through Trim65. Int Immunopharmacol 123:110808
Yang WL et al (2024) Berberine metabolites stimulate GLP-1 secretion by alleviating oxidative stress and mitochondrial dysfunction. Am J Chin Med 52(1):253–274
Yanping D et al (2018) Preparation, crystal structures, and oral bioavailability of two cocrystals of emodin with berberine chloride. Cryst Growth Des 18(12):7481–7488
Ye C et al (2021) Berberine improves cognitive impairment by simultaneously impacting cerebral blood flow and β-amyloid accumulation in an APP/tau/PS1 mouse model of Alzheimer’s disease. Cells 10(5):1161
Yin J et al (2017) Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect. Int J Nanomedicine 12:8671–8680
Yu Y et al (2010) Modulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies. Biochem Pharmacol 79(7):1000–1006
Yu Y et al (2015) Berberine induces GLP-1 secretion through activation of bitter taste receptor pathways. Biochem Pharmacol 97(2):173–177
Yu F et al (2016) Monodisperse microparticles loaded with the self-assembled berberine-phospholipid complex-based phytosomes for improving oral bioavailability and enhancing hypoglycemic efficiency. Eur J Pharm Biopharm 103:136–148
Yu F et al (2017) PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv 24(1):825–833
Yu H et al (2024) Berberine alleviates inflammation and suppresses PLA2-COX-2-PGE2-EP2 pathway through targeting gut microbiota in DSS-induced ulcerative colitis. Biochem Biophys Res Commun 695:149411
Yue SJ et al (2019) Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed Pharmacother 116:109002
Zeng XH, Zeng XJ, Li YY (2003) Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 92(2):173–176
Zhan Y et al (2021) Berberine suppresses mice depression behaviors and promotes hippocampal neurons growth through regulating the miR-34b-5p/miR-470-5p/BDNF axis. Neuropsychiatr Dis Treat 17:613–626
Zhang X et al (2011) Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: involvement of P-glycoprotein. Xenobiotica 41(4):290–296
Zhang M et al (2012) Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis. Mol Cell Endocrinol 363(1–2):122–130
Zhang Z et al (2014) Solid dispersion of berberine-phospholipid complex/TPGS 1000/SiO2: preparation, characterization and in vivo studies. Int J Pharm 465(1–2):306–316
Zhang G et al (2020a) Berberine reduces endothelial injury and arterial stiffness in spontaneously hypertensive rats. Clin Exp Hypertens 42(3):257–265
Zhang N et al (2020b) Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: Dual regulation of the PI3K/AKT and MAPK pathways. Regul Toxicol Pharmacol 110:104544
Zhang Q et al (2020c) Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed Pharmacother 128:110245
Zhang X et al (2021) The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm Sin B 11(7):1789–1812
Zhao J et al (2021) Intra-herb interactions: primary metabolites in coptidis rhizoma extract improved the pharmacokinetics of oral berberine hydrochloride in mice. Front Pharmacol 12:675368
Zhao Y et al (2023a) Berberine inhibits the progression of renal cell carcinoma cells by regulating reactive oxygen species generation and inducing DNA damage. Mol Biol Rep 50(7):5697–5707
Zhao N et al (2023b) Berberine disrupts staphylococcal proton motive force to cause potent anti-staphylococcal effects in vitro. Biofilm 5:100117
Zhaojie M et al (2014) Amorphous solid dispersion of berberine with absorption enhancer demonstrates a remarkable hypoglycemic effect via improving its bioavailability. Int J Pharm 467(1–2):50–59
Zheng T et al (2023) Co-assembled nanocomplexes comprising epigallocatechin gallate and berberine for enhanced antibacterial activity against multidrug resistant Staphylococcus aureus. Biomed Pharmacother 163:114856
Zhu F et al (2011) Decrease in the production of β-amyloid by berberine inhibition of the expression of β-secretase in HEK293 cells. BMC Neurosci 12:125
Zhu JX et al (2013) Development of self-microemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Dev Ind Pharm 39(3):499–506
Zhuang CY et al (2010) Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm 394(1–2):179–185
Acknowledgements
All authors have conveyed their sincere gratitude to scientists and researchers who had worked in nanotraditional Chinese medicine and contributed their valuable results in the literature. All authors expressed their sincere thanks to all the expert reviewers for their valuable time and suggestions for shaping up this manuscript.
Author information
Authors and Affiliations
Contributions
Lingjun Li had the idea for the article; Yulong Cui performed the literature and wrote the first draft of the manuscript; Quanying Zhou, Min Jin, Siqi Jiang, Peizhao Shang, and Xiaofan Dong critically revised the work. All authors read and approved the final manuscript. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, Y., Zhou, Q., Jin, M. et al. Research progress on pharmacological effects and bioavailability of berberine. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03199-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03199-0