Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by inflammation and joint destruction. In this study, we explored the effect of berberine on rats with bovine type II collagen-induced arthritis (CIA), an animal model for RA. Following treatment, berberine attenuates arthritic scores and suppresses collagen–specific immune responses in CIA rats. Compared with the un-treated CIA group, berberine reversed pathological changes, which showed a significant improvement in synovial hyperplasia and inflammatory infiltration. The expression levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-17 and vascular endothelial growth factor (VEGF) were obviously reduced in the sera of berberine-treated rats (all P < 0.05). Moreover, berberine showed marked inhibition of the expression of VEGF and CD34 (all P < 0.05). Interestingly, berberine significantly suppresses p-ERK, p-p38 and p-JNK activation (all P < 0.05), which may partially explain the anti-RA activity of berberine. These results suggest that berberine ameliorates CIA in rats associated with anti-inflammatory and anti-angiogenic effects, which might be of great therapeutic value for RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that may lead to joint damage, synovial membrane destruction, and cartilage and bone damage [1]. The pain, swelling, stiffness, and tissue destruction that accompany inflammatory disease result from a cascade of events that is initiated and propagated by the production of inflammation cytokines [2]. Pro-inflammatory cytokines located in the synovium, including interleukin (IL)-6, IL-1β, IL-17 and tumor necrosis factor (TNF)-α, have been known to play a major role in the progression of joint destruction and proliferation of synoviocytes [3]. A number of these cytokines were not randomly present but formed a network or hierarchy which controlled their expression. The importance of pro-inflammatory cytokines has been underscored by the success of biologics in treating disease by blocking the effects of cytokines such as TNF-α, IL-1β or IL-6, which are considered potential disease targets involved in the pathogenesis of RA [4].
Improved understanding of the molecular mechanisms supporting the pathogenesis of RA has placed new emphasis on the role of angiogenesis in the chronic inflammatory disease. Angiogenesis, the process of new blood vessel formation, is highly active in RA, particularly during the earliest stages of the disease [5]. Newly formed vessels can maintain the chronic inflammatory state by transporting inflammatory cells to sites of synovitis, and supply nutrients and oxygen to the pannus [6, 7]. Angiogenesis is strictly regulated by many inducers and inhibitors, and a number of pro-angiogenic factors have been suggested to be involved in neovascularization in RA joints. These include acidic and basic fibroblast growth factors, transforming growth factor (TGF)-β, angiopoietin, and placental growth factor (PlGF) in addition to vascular endothelial growth factor (VEGF) [8].
Berberine is an isoquinoline derivative alkaloid isolated from many medicinal herbs, such as Hydrastis canadensis (goldenseal), Cortex phellodendri (Huangbai), and Rhizoma coptidis (Huanglian) [9]. It has been used for the treatment of various conditions such as diarrhea and other gastrointestinal disorders for centuries in China, although its mechanism of action remains undefined [10]. Besides its anti-inflammatory effect, many studies have shown that berberine has anti-tumor activity, in which mechanisms include inhibited cell cycle progression, induced apoptosis, suppressed activation of NF-κB and inhibited angiogenesis [11, 12].
In the present study, we investigated the impact of berberine in CIA rat model. While special emphasis was placed on ascertaining the effect of berberine on inflammatory disease attenuation, novel information was also obtained concerning the relationship between berberine-induced anti-angiogenic and anti-arthritic activities. The results show that berberine has disease-modifying activity that is at least partly attributable to the inhibition of inflammation and neovasculature development. More interestingly, berberine impacts additional mechanisms involved in suppressing the activated expression of mitogen-activated protein kinases (MAPKs) for p-ERK, p-p38, and p-JNK.
MATERIALS AND METHODS
Materials
Berberine (purity >99.98 %) was obtained from Sigma-Aldrich (St. Louis, MO, USA), and was dissolved in 0.05 % DMSO (Sigma, St. Louis, MO, USA) to produce a stock solution.
Animals
Eighty female SD rats (age 4–5 weeks, body weight 120 ± 10 g) were purchased from the Laboratory Animal Services Center of the Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). The animals were given a period of 1 week to adjust to the new environment (room temperature, 20 ± 1 °C; relative humidity, 55 ± 15 %; 12 h light/12 h dark illumination cycle). Food and water were provided ad libitum throughout the experiments. All animals were treated in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals.
Induction of Bovine Type II Collagen-Induced Arthritis (CIA)
CIA was induced as previously reported [13, 14]. Briefly, rats were subcutaneously injected at the base of the tail with 200 μg bovine type II collagen (CII; Chondrex, Redmond, WA, USA) emulsified in complete Freund's adjuvant (Difco Labs., Detroit, MI, USA). The day of induction was designated as day 0. On day 7 after primary immunization, all the rats were given an intradermal booster injection of 100 μg CII in incomplete Freund's adjuvant on the back. The onset of arthritis in ankle joints usually became visually apparent between days 12 and 14.
Evaluation of CIA
Rats were clinically observed for characteristic signs and symptoms. Arthritis severity was evaluated by arthritis scores which were performed by two independent, blinded observers. The rats were scored by grading each paw, from 0 to 4 based on the erythema, swelling, and rigidity of the joint (0 = no erythema or swelling; 1 = erythema or swelling of one toe; 2 = erythema or swelling of two or more of the toes; 3 = erythema and swelling of the entire paw; 4 = complete erythema and swelling of the entire paw and incapacity to bend the ankle). All four legs were scored and the maximum possible score reached 16 (4 points for each paw). The threshold score of rats with established CIA was 2.
Berberine Treatment and Groups
In the therapeutic treatment protocol for established CIA, rats were treated with berberine from the day after onset of arthritis (day 14) until day 42 (the end of the experiment). The route of berberine delivery was oral administration intragastrically (200 mg/kg body weight) using syringe feeding.
Experimental SD rats were divided into four groups with equal number (n = 10): normal control group (Normal), CIA model group (CIA), CIA rats treated with PBS (Vehicle, orally administered), and CIA rats treated with berberine (Berberine).
Body Weight and Paw Swelling Dimension Observation
Rats were observed and their weights were recorded every 2 days. The volume of swelling was also measured in both hind paws with a foot volume meter (TK-105; Muromachi Kikai Co., Ltd., Tokyo, Japan) and the average of the volume in each rat was calculated.
Collection of Sera
Blood samples were collected when animals were killed (day 42) throughout the study and sera were prepared by centrifugation for 20 min at 500 × g in 4 °C. Aliquots were stored frozen at −18 °C until analyzed.
Assessment of Type II Collagen-Specific Immune Response
Antibody titers to type II collagen were assayed by enzyme-linked immunosorbent assay (ELISA). Nunc Maxisorb plates (Nunc, Roskilde, Denmark) were coated with 100 μl of bovine nasal collagen II (5 μg/ml in PBS) overnight at 4 °C, and then blocked (PBS/0.05 % bovine serum albumin; this solution was used for all further dilutions) for 2 h at 37 °C. Serum samples were diluted 1:1,000, and 100 μl was added to the coated 96-well plate and incubated at 37 °C for 2 h, followed by a 2-h incubation with a horseradish peroxidase-linked goat anti-rat IgG antibody (KPL, Gaithersburg, MD, USA) and mouse anti-rat IgG1, IgG2a antibody (Southern Biotech, Birmingham, AL, USA). At every step, plates were washed three times with 0.01 mol/l PBS containing 0.05 % Tween 20. Absorbance was read at 490 nm and values were expressed as mean ± standard error of the mean (SEM). Optical density was measured using Microplate computer software (Bio-Rad Laboratories).
Histopathological Assessment
Rats were sacrificed by cervical dislocation following the termination of the experiment, and the right hind knee was removed and fixed in 4 % neutral buffered formalin for 24 h. Following decalcification with 12.5 % EDTA (pH 7.0) for up to 1 month at 4 °C, the right hind knee was paraffin-embedded. Tissue sections (4 μm) were mounted on common slides for staining with hematoxylin and eosin (H&E). All sections were randomized and evaluated by two trained observers who were blinded to the treatment groups and the arthritis severity of each rat.
Immunohistochemical (IHC) Analysis of the Synovial Tissue
Sections (4 μm) from the paraffin-embedded, formalin-fixed synovial membrane tissues were fixed on the charged slides for IHC analysis. Primary mouse monoclonal antibodies to VEGF (dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit polyclonal antibodies to CD34 (dilution 1:100; Abcam, Cambridge, MA) were used in the study. All slides were deparaffinized with xylene and rehydrated through graded ethanol ending with distilled water. Then, endogenous peroxidase was blocked by 3 % hydrogen peroxide for 15 min. Sections for VEGF and CD34 for IHC analysis were subjected to microwave antigen retrieval with 0.1 M citrate buffer (pH 6.0) at 98 °C for 10 min, and were incubated overnight at 4 °C in a humidified chamber, followed by EnVision detection incubated for 30 min at room temperature. Staining was visualized by incubating with 3,3′-diaminobenzidine for 5 min at room temperature, then counterstained with hematoxylin. Negative (omission of primary antibody) and positive controls (paraffin sections) were carried out in parallel.
The positive cells of immunostaining for VEGF were reviewed and recorded according to the location of cytoplasm with or without positive nucleus and results are presented blindly by two independent observers. One hundred cells were randomly selected and counted from five representative fields of each section, and the expression of VEGF was assessed according to the percentage of immunoreactive cells.
Evaluation of Microvessel Density (MVD)
For quantitative evaluation of angiogenesis, synovial tissue sections were immunostained with rabbit anti-rat polyclonal antibody CD34. At low power field (×40), tissue sections were screened, and five areas with the most intense neovascularization were selected. Microvessel counts of these areas were performed at per high power field (HPF) (×200) by two investigators simultaneously. An automated microvessels count/field was computed in each spot, and the mean microvessel count of the five most vascular areas was taken as the MVD, which was expressed as the absolute number of microvessels/HPF. The MVD was measured based on Weidner’s method [15].
Enzyme-Linked Immunosorbant Assay
Sera were obtained from the rats on day 42. The expression levels of TNF-α, IL-1β, IL-6, IL-17 and VEGF in sera were detected by ELISA assay (R&D, USA) according to the manufacturer’s protocol. All experiments were done in triplicate.
Western Blot Analysis
Synovial tissue was extracted from the hind paws of the rats and were homogenized with lysis buffer (25 mM Tris/HCl (pH 7.4), 150 mM KCl, 5 mM EDTA, 1 % Nonidet P40, 0.5 % sodium deoxycholate and 0.1 % SDS) containing 1 mM PMSF and a cocktail of protease and phosphatase inhibitors (Cell Signaling Technology Inc., USA) on ice for 30 min and centrifuged at 4 °C at 12,000 × g for ~15 min. The supernatants were denatured with protein loading buffer following the determination of the protein concentration. Protein (45 μg) was resolved on 12 % sodium dodecyl sulfate-polyacrylamide gel, blotted onto a polyvinylidene fluoride (PVDF) membrane and blocked for 2 h with 5 % skimmed milk in Tris-buffered saline with Tween 20. Membranes were incubated with the desired primary antibody against p-ERK antibody (rabbit polyclonal antibody, dilution 1:100), p-p38 antibody (rabbit polyclonal antibody, dilution 1:200), p-JNK antibody (rabbit polyclonal antibody, dilution 1:100) (Cell Signaling Technology Inc., USA) or GAPDH antibody (rabbit polyclonal antibody, dilution 1:2000) (Santa Cruz Biotechnology Inc., USA) overnight at 4 °C and then with the appropriate HRP-conjugated secondary antibody for 50 min. Following washing, the membranes were visualized by enhanced chemiluminescence detection. Optical density for each band was assessed using ImageJ analysis software (National Institutes of Health, Bethesda, MD, USA). Sample loading was normalized by quantities of GAPDH detected parallel.
Statistical Analysis
Data are expressed as means ± SEM, and analyzed by SPSS 13.0 software. Differences between clinical and histological scores were analyzed with Mann–Whitney tests. Other data were analyzed with one-way ANOVA followed by Bonferroni's post-hoc tests or Student’s t-test. P values less than 0.05 were considered statistically significant.
RESULTS
Berberine Attenuates the Severity of Arthritis and Suppresses Type II Collagen-Specific Immune Responses in CIA Rats
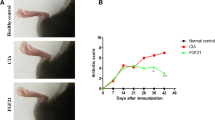
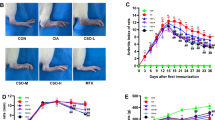
To investigate the effect of berberine on arthritis, the CIA model in SD rats was used. Oral administration of berberine (200 mg/kg body weight) once a day started from day 14 to the end of the experiment. Clinical scores of CIA rats were recorded every 2 days after first immunization. As Fig. 1a shows, the highest clinical arthritis scores were presented in CIA rats on day 24, and berberine treatment significantly decreased the mean clinic arthritis scores (P < 0.05). Consistent with the arthritis scoring, the assessment of paw swelling and body weight also showed berberine to be highly effective compared with untreated rats (P < 0.05) (Fig. 1b and c). In addition, berberine administration reduced serum anti-CII total IgG (P < 0.05), IgG1 (P < 0.05) and IgG2a (P < 0.01) levels with statistical significance relative to CIA group (Fig. 1d).
Berberine relieves the severity of arthritis and suppresses type II collagen (CII)-specific immune responses in CIA rats. a Berberine significantly decreased the mean arthritis scores compared with CIA rats. b Berberine treatment suppressed paw swelling in rats with CIA. c Berberine treatment increased body weight compared with untreated rats. d Anti-CII (total IgG, IgG1 and IgG2a) levels in serum from berberine-treated rats were markedly decreased, and the experiment was done in triplicate. Data are expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, comparison with the CIA group.
Berberine Treatment Prevents Arthritis Progression and Inhibits the Synovial Inflammation in CIA
Macroscopic evidence of arthritis such as erythema or swelling was shown in Fig. 2a and c, and berberine obviously attenuated arthritis severity in CIA rats on day 24 and day 42. Representative histopathological lesions in the synovial tissue of different groups were shown in Fig. 2b and c. Synovial hyperplasia, disorganized arrangement, infiltration of inflammatory cell, a number of small blood vessels and pannus formation were observed in CIA group. Histopathological changes were a significant improvement in the berberine-treated group to a different extent on day 24 and day 42, in which the infiltration of inflammatory cells and the hyperplasia of synovial cells were significantly decreased (Fig. 2b and c).
Berberine prevents arthritis progression and inhibits synovium inflammation in CIA. a Macroscopic evidence of arthritis in different groups such as erythema or swelling was observed on day 24 (×200). b Histological assessment of synovium in CIA rats treated with berberine on day 24 (×200). c Macroscopic evidence of arthritis in different group such as erythema or swelling was observed on day 42 (×200). d Histological assessment of synovium in CIA rats treated with berberine on day 42 (×200).
Berberine Treatment Inhibited Pro-inflammatory Cytokines in CIA
CIA is characterized by the marked expression of pro-inflammatory cytokines. To ascertain whether berberine inhibited this characteristic, CIA rats of different group were bled on day 42. Pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-17 and VEGF from sera were measured by ELISA. Berberine-treated rats showed significantly lower levels of TNF-α (P < 0.01), IL-1β (P < 0.01), IL-6 (P < 0.01), IL-17 (P < 0.05), and VEGF (P < 0.05) from sera than those in CIA group (Fig. 3). These results suggest that berberine may have a therapeutic effect in terms of CIA severity by inhibiting the production of pro-inflammatory cytokines.
Berberine decreases the expression of pro-inflammatory cytokines in sera of CIA rats. Sera levels of TNF-α, IL-1β, IL-6, IL-17, and VEGF in different group were measured by ELISA. All experiments were done in triplicate. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, comparison with the CIA group.
Berberine Ameliorates Angiogenesis in the Synovial Tissue in CIA Rats
Angiogenesis is known to play a prominent role in pannus formation in RA. Expression of VEGF and CD34 in the synovial tissue of CIA rats on day 24 and day 42 was observed by IHC staining (Fig. 4a and b), which are closely related to new blood vessel formation. As Fig. 5a shows, the rate of positive VEGF expression on day 42 was 72.56 ± 8.12 % in CIA rats and 40.42 ± 5.88 % in berberine-treated CIA rats, with a significant difference between them (n = 10, P < 0.01). MVD measurement has been shown to be a quantitative method of assessing angiogenesis. CD34 was used to mark vascular endothelial cell or endothelial cell clustering for MVD. The mean value of MVD was 60.28 ± 8.72/HPF in CIA rats on day 42, and MVD (38.85 ± 6.1/HPF) in berberine-treated group was obviously lower than that in CIA group (n = 10, P < 0.01) (Fig. 5b). These results suggest that berberine has a potent anti-angiogenesis activity in vivo.
Berberine reduced VEGF and CD34 expression in the synovium of CIA rat model. a Expression of VEGF and CD34 in synovial tissue of different group were observed by immunohistochemical staining on day 24 (×200). b Expression of VEGF and CD34 in synovial tissue of different group were observed by immunohistochemical staining on day 42 (×200).
Berberine decreased the vessel density in the synovial membrane of inflammation joint in CIA rats. a Berberine significantly reduced the VEGF positive cells in synovium of CIA rats. b Berberine significantly decreased the vessel density in synovium of CIA rats. Data are expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, comparison with the CIA group.
Disease Attenuation Following Berberine Treatment Is Partly Attributable to Inhibition of p-ERK, p-p38 and p-JNK Activation
MAPK signaling pathway is a well-known key regulator in inflammation and angiogenesis.
To investigate the potential mechanism through which berberine exerts its anti-RA effect, we further detected the protein expression levels of p-ERK, p-p38, and p-JNK by western blot analysis on day 24 and day 42 (Fig. 6a). Compared with those in CIA rats, berberine treatment significantly suppressed p-ERK, p-p38, and p-JNK (all P < 0.05) activation on day 24 and day 42 (Fig. 6b), which may partially explain the anti-RA activity of berberine.
Berberine inhibits p-ERK, p-p38, and p-JNK activation in CIA. a Berberine suppressed p-ERK, p-p38 and p-JNK activation in synovium of CIA rats by western blot. b The expression levels of p-ERK, p-p38 and p-JNK in different group were assessed with semiquantitative analysis. All experiments were done in triplicate. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, comparison with the CIA group.
DISCUSSION
Berberine, a clinically important natural isoquinoline alkaloid derived from the Berberis species, has been reported to exhibit multiple pharmacological activities including anti-inflammatory, anti-tumor, anti-hypertensive, and anti-diabetic effects [16]. In the current study, we observed the potential therapeutic effect of berberine on CIA in rat. The results showed that berberine treatment could effectively attenuate clinical symptoms, plasma TNF-α, IL-1β, IL-6, IL-17, and VEGF levels, synovial tissue VEGF production, and CD34 expression of CIA rats. In addition, the inhibition of p-ERK, p-p38, and p-JNK activation might be involved in anti-RA activity of berberine. Our findings suggest that berberine exerts a potent curative effect in CIA.
Pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-17, and VEGF play critical roles in RA, and these cytokines initiate, amplify, and perpetuate the inflammatory response in RA [17]. TNF-α, mainly produced by monocyte/macrophages, can elicit the inflammatory cascade in RA [18]. IL-6 also plays important roles in joint inflammation and cartilage destruction in various forms of arthritis, and elevated TNF-α and IL-6 were observed in serum and synovial fluid from patients with RA [19]. IL-1β promotes inflammation and destruction in the synovial tissue, bone, cartilage and joints in patients with RA [20]. IL-1β is a crucial mediator in the generation of synovial inflammation and pannus. In addition, increased level of IL-1β in the synovial tissue was correlated with histological features of arthritis [21]. IL-17 promotes inflammation by enhancing the production of cytokines such as IL-1β, TNF-α and IL-6 [22]. Plenty of evidence shows that IL-17 contributes to the inflammation in the pathogenesis of RA. In CIA, reminiscent in several aspects to RA, IL-17 level is elevated in inflamed synovium and could act on osteoblasts [23]. IL-17 deficiency prevents the induction of CIA in mice [24]. In line with the previous findings, this study showed that berberine treatment caused a marked decrease in the expression levels of TNF-α, IL-1β, IL-6, and IL-17 in sera of CIA rats. Furthermore, histopathological analysis indicated that berberine treatment reduced the synovial inflammation and bone destruction in CIA rats.
Angiogenesis, as a critical component of disease progression in RA, involves the formation and maintenance of the infiltration of synovial membrane [25]. In recent years, many studies have demonstrated that angiogenesis is an essential event in perpetuating inflammatory and immune responses, as well as supporting pannus growth and the development of RA [26, 27]. VEGF is a dimeric glycoprotein that induces the proliferation and migration of endothelial cells to form new blood vessels, and which increases vascular permeability. Several recent reports have demonstrated that VEGF is also implicated in the pathogenesis of RA [28, 29]. VEGF in the synovial fluids is significantly more increased in RA than in osteoarthritis, and serum levels of VEGF correlate well with RA disease activity, particularly with swollen joint counts [30]. VEGF protein and mRNA are expressed by synovial macrophages and synovial fibroblasts in the synovial tissues of RA patients, and cultured synovial cells are able to secrete VEGF under hypoxic conditions or when stimulated with IL-1, IL-6, IL-17, IL-18, prostaglandin, or TGF-β, or by CD40 ligation [31, 32]. Furthermore, VEGF knockout mice showed reduced pathology and synovial angiogenesis in antigen-induced models of arthritis [33]. These findings strongly suggest that the inhibition of the angiogenic action of VEGF is likely to suppress rheumatoid inflammation. In the present study, berberine reduced VEGF and CD34 expression in synovium of CIA rats by IHC staining assessment, which was closely related to new blood vessel formation. Besides the anti-inflammatory effect, our results showed that berberine could inhibit angiogenesis in the synovium tissue of CIA rats.
Modulation of angiogenesis may alter arthritis, which to some extent is linked with the imbalance of angiogenesis inducers and inhibitors in inflammation states. Angiogenesis and inflammation are interdependent processes, and inflammatory mediators have significant effects on angiogenesis [34]. For example, the chronic transgenic delivery of PlGF to murine epidermis resulted in a significant increase in inflammatory response. In addition, VEGF165 has a direct pro-inflammatory role in the pathogenesis of RA [35]. The synovial fluid mononuclear cells of RA patients showed a greater response to VEGF165 stimulation than the peripheral blood mononuclear cells of healthy controls. Moreover, anti-VEGF reduces the onset and severity of arthritis as well as joint angiogenesis in mouse CIA [36]. These findings suggest that VEGF may act as a pro-inflammatory mediator and as an angiogenic stimulator in RA joints, and thus, they indicate that VEGF is an important link between angiogenesis and the inflammatory process. Most angiogenic mediators described above, including growth factors, pro-inflammatory cytokines and chemokines are abundantly produced in the RA synovium. Furthermore, there are autocrine loops in the RA synovium leading to the perpetuation of angiogenesis associated with inflammation [37]. Disrupting the formation of new blood vessel can prevent the delivery of nutrients into the inflammatory site, and can also contribute to vessel regression and disease reversal. Therefore, the inhibition of angiogenesis has been proposed as a novel therapeutic strategy for RA.
RA is a chronic autoimmune disease in which imbalances in pro- and anti-inflammatory cytokines promote the induction of autoimmunity, inflammation and joint destruction [38]. A great number of pro-inflammatory cytokines including VEGF, TNF-α, IL-1β, IL-6, IL-17 and IL-8 govern angiogenesis in RA. These factors play important roles in the development of neovasculature by interacting with each other. The VEGF-dependent signaling system is necessary for neoangiogenesis [39]. In this study, our data showed that berberine could suppress the levels of TNF-α, IL-1β, IL-6, IL-17, and VEGF in sera of CIA rats, suggesting the inhibitory effect of berberine on the VEGF-mediated signal pathway. MAPKs have been implicated as playing key regulatory roles in the production of pro-inflammatory cytokines and downstream signaling events leading to joint inflammation and destruction [40]. Recent studies had demonstrated that berberine suppressed pro-inflammation response through the inhibition of the phosphorylation of MAPK activation in macrophages via AMP-activated protein kinase stimulation [41, 42]. Consistent with these findings, we also observed that berberine could inhibit the phosphorylation of MAPKs, such as ERK, p38, and JNK, which may partially explain the anti-RA effect of berberine.
In conclusion, our findings suggest that berberine ameliorates CIA in rats contributed to anti-inflammatory and anti-angiogenic effects, which are associated with the reduction of arthritis severity, joint destruction, serum anti-type II collagen antibodies levels, serum pro-inflammatory cytokines levels, and expression of VEGF and CD34 in rats with CIA. Notably, these effects were at least partly due to the inhibition of p-ERK, p-p38, and p-JNK activation. Further studies are required to determine the pharmacological effects and precise molecular mechanisms of berberine.
Abbreviations
- RA:
-
Rheumatoid arthritis
- CIA:
-
Collagen-induced arthritis
- CII:
-
Type II collagen
- VEGF:
-
Vascular endothelial growth factor
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-α
- ELISA:
-
Enzyme-linked immunosorbent assay
References
Parolini, O., L. Souza-Moreira, F. O'Valle, M. Magatti, P. Hernandez-Cortes, E. Gonzalez-Rey, et al. 2014. Therapeutic effect of human amniotic membrane-derived cells on experimental arthritis and other inflammatory disorders. Arthritis and Rheumatism 66: 327–339.
Bombardier, C., M. Barbieri, A. Parthan, D.J. Zack, V. Walker, D. Macarios, et al. 2012. The relationship between joint damage and functional disability in rheumatoid arthritis: a systematic review. Annals of the Rheumatic Diseases 71: 836–844.
Moreland, L.W., and J.R. Curtis. 2009. Systemic nonarticular manifestations of rheumatoid arthritis: focus on inflammatory mechanisms. Seminars in Arthritis and Rheumatism 39: 132–143.
Miller, A.M., and I.B. McInnes. 2011. Cytokines as therapeutic targets to reduce cardiovascular risk in chronic inflammation. Current Pharmaceutical Design 17: 1–8.
Schroeder, M., L. Viezens, I. Fuhrhop, W. Rüther, C. Schaefer, B. Schwarzloh, et al. 2013. Angiogenic growth factors in rheumatoid arthritis. Rheumatology International 33: 523–527.
Semerano, L., E. Duvallet, N. Belmellat, N. Schall, M. Monteil, A. Starzec, et al. 2014. A1.36 Active immunisation against peptides of VEGF improves joint inflammation and destruction in collagen-induced arthritis. Annals of the Rheumatic Diseases 73(Suppl 1): A15–A16.
Marrelli, A., P. Cipriani, V. Liakouli, F. Carubbi, C. Perricone, R. Perricone, et al. 2011. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmunity Reviews 10: 595–598.
Szekanecz, Z., and A.E. Koch. 2008. Targeting angiogenesis in rheumatoid arthritis. Current Rheumatology Reviews 4: 298–303.
Kong, W., J. Wei, P. Abidi, M. Lin, S. Inaba, C. Li, et al. 2004. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nature Medicine 10: 1344–1351.
Qin, X., B.T. Guo, B. Wan, L. Fang, L. Lu, L. Wu, et al. 2010. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. Journal of Immunology 185: 1855–1863.
Kuo, C.L., C.W. Chi, and T.Y. Liu. 2004. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Letters 203: 127–137.
Yang, X., and N. Huang. 2013. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Molecular Medicine Reports 8: 505–510.
Remmers, E.F., B. Joe, M.M. Griffiths, D.E. Dobbins, S.V. Dracheva, A. Hashiramoto, et al. 2002. Modulation of multiple experimental arthritis models by collagen-induced arthritis quantitative trait loci isolated in congenic rat lines: different effects of non-major histocompatibility complex quantitative trait loci in males and females. Arthritis and Rheumatism 46: 2225–2234.
Rosloniec, E.F., M. Cremer, A. Kang, and L.K. Myers. 2001. Collagen-induced arthritis. Curr Protoc Immunol Chapter 15: Unit 15.5.
Weidner, N. 1995. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Research and Treatment 36: 169–180.
Sun, Y., K. Xun, Y. Wang, and X. Chen. 2009. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 20: 757–769.
Brennan, F.M., and I.B. McInnes. 2008. Evidence that cytokines play a role in rheumatoid arthritis. Journal of Clinical Investigation 118: 3537–3545.
Bertolini, D.R., G.E. Nedwin, T.S. Bringman, D.D. Smith, and G.R. Mundy. 1986. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319: 516–518.
van Leeuwen, M.A., J. Westra, P.C. Limburg, P.L. van Riel, and M.H. van Rijswijk. 1995. Interleukin-6 in relation to other proinflammatory cytokines, chemotactic activity and neutrophil activation in rheumatoid synovial fluid. Annals of the Rheumatic Diseases 54: 33–38.
Tak, P.P., and B. Bresnihan. 2000. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis and Rheumatism 43: 2619–2633.
Ying, X., X. Chen, S. Cheng, Z. Zhao, X. Guo, H. Chen, et al. 2013. SeMet inhibits IL-1β-induced rheumatoid fibroblast-like synoviocytes proliferation and the production of inflammatory mediators. Biological Trace Element Research 153: 437–445.
Benedetti, G., and P. Miossec. 2014. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. European Journal of Immunology 44: 339–347.
Azizi, G., F. Jadidi-Niaragh, and A. Mirshafiey. 2013. Th17 Cells in immunopathogenesis and treatment of rheumatoid arthritis. International Journal of Rheumatic Diseases 16: 243–253.
Park, M.J., H.S. Park, H.J. Oh, J.Y. Lim, B.Y. Yoon, H.Y. Kim, et al. 2012. IL-17-deficient allogeneic bone marrow transplantation prevents the induction of collagen-induced arthritis in DBA/1J mice. Experimental and Molecular Medicine 44: 694–705.
Isozaki, T., J.H. Ruth, M.A. Amin, P.L. Campbell, P.S. Tsou, C.M. Ha, et al. 2014. Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation. Arthritis Research and Therapy 16: R28.
Thairu, N., S. Kiriakidis, P. Dawson, and E. Paleolog. 2011. Angiogenesis as a therapeutic target in arthritis in 2011: learning the lessons of the colorectal cancer experience. Angiogenesis 14: 223–234.
Kong, X., Y. Zhang, C. Liu, W. Guo, X. Li, X. Su, et al. 2013. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS One 8: e77513.
Vordenbäumen, S., P. Sewerin, T. Lögters, F. Miese, C. Schleich, E. Bleck, et al. 2014. Inflammation and vascularisation markers of arthroscopically guided finger joint synovial biopsies reflect global disease activity in rheumatoid arthritis. Clinical and Experimental Rheumatology 32: 117–120.
Yoo, S.A., S.K. Kwok, and W.U. Kim. 2008. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediators of Inflammation 2008: 129873.
Yoo, S.A., D.G. Bae, J.W. Ryoo, H.R. Kim, G.S. Park, C.S. Cho, et al. 2005. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. Journal of Immunology 174: 5846–5855.
Cho, C.S., M.L. Cho, S.Y. Min, W.U. Kim, D.J. Min, S.S. Lee, et al. 2000. CD40 engagement on synovial fibroblast up-regulates production of vascular endothelial growth factor. Journal of Immunology 164: 5055–5061.
Kasama, T., F. Shiozawa, K. Kobayashi, N. Yajima, M. Hanyuda, H.T. Takeuchi, et al. 2001. Vascular endothelial growth factor expression by activated synovial leukocytes in rheumatoid arthritis: critical involvement of the interaction with synovial fibroblasts. Arthritis and Rheumatism 44: 2512–2524.
Mould, A.W., I.D. Tonks, M.M. Cahill, A.R. Pettit, R. Thomas, N.K. Hayward, et al. 2003. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis and Rheumatism 48: 2660–2669.
Ferrara, N., H.P. Gerber, and J. LeCouter. 2003. The biology of VEGF and its receptors. Nature Medicine 9: 669–676.
Oura, H., J. Bertoncini, P. Velasco, L.F. Brown, P. Carmeliet, and M. Detmar. 2003. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood 101: 560–567.
Kong, J.S., S.A. Yoo, J.W. Kim, S.P. Yang, C.B. Chae, V. Tarallo, et al. 2010. Anti-neuropilin-1 peptide inhibition of synoviocyte survival, angiogenesis, and experimental arthritis. Arthritis and Rheumatism 62: 179–190.
Rabquer, B.J., and A.E. Koch. 2013. NK4 therapy: a new approach to target angiogenesis and inflammation in rheumatoid arthritis. Arthritis Research and Therapy 15: 119.
Chen, Z., S. Tu, Y. Hu, Y. Wang, Y. Xia, and Y. Jiang. 2012. Prediction of response of collagen-induced arthritis rats to methotrexate: an (1)H-NMR-based urine metabolomic analysis. Journal of Huazhong University of Science and Technology. Medical Sciences 32: 438–443.
Tang, X., Y. Yang, H. Yuan, J. You, M. Burkatovskaya, and S. Amar. 2013. Novel transcriptional regulation of VEGF in inflammatory processes. Journal of Cellular and Molecular Medicine 17: 386–397.
Kong, X., C. Liu, C. Zhang, J. Zhao, J. Wang, H. Wan, et al. 2013. The suppressive effects of Saposhnikovia divaricata (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-κB and mitogen activated protein kinases activation on collagen-induced arthritis model. Journal of Ethnopharmacology 148: 842–850.
Jia, L., J. Liu, Z. Song, X. Pan, L. Chen, X. Cui, et al. 2012. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. Journal of Pharmacy and Pharmacology 64: 1510–1521.
Jeong, H.W., K.C. Hsu, J.W. Lee, M. Ham, J.Y. Huh, H.J. Shin, et al. 2009. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. American Journal of Physiology - Endocrinology and Metabolism 296: E955–E964.
Conflict of Interest
The authors declared no competing interests exist.
Authors’ Contribution
Z.G.W., Z.C. and S.H.T. designed the research, Z.G.W. and Z.C. finished experiments and wrote the manuscript. Y.W. and Z.Y.H. performed partial research. S.S.Y. and J.F.G. analyzed the data. S.H.T. and Z.G.R. helped revise the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Z. Wang and Z. Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Z., Chen, Z., Yang, S. et al. Berberine Ameliorates Collagen-Induced Arthritis in Rats Associated with Anti-inflammatory and Anti-angiogenic Effects. Inflammation 37, 1789–1798 (2014). https://doi.org/10.1007/s10753-014-9909-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9909-y