Abstract
The clinical use of gentamicin (GM) is restricted by its nephrotoxic effects. This study aimed for the first time to elucidate the ameliorative effects of the monoterpene linalool (Lin) against GM-mediated acute kidney injury in rats. A total of thirty-two rats were subdivided into four equal groups: control (saline), Lin (100 mg/kg/day), GM (100 mg/kg/day), and GM + Lin (100 and 100 mg/kg/day). Lin and GM were intraperitoneally administered for 12 days. Our results illustrated that Lin ameliorated GM-mediated renal histopathological abnormalities and reduced serum urea and creatinine levels in rats exposed to GM. Lin treatment mitigated oxidative stress in nephrotoxic animals as manifested by reducing serum and renal levels of malondialdehyde and increasing the activities of serum and renal glutathione peroxidase and renal catalase. Moreover, Lin markedly inhibited GM-triggered inflammation by downregulating NF-κB, iNOS, TNF-α, and IL-1β and reducing renal myeloperoxidase activity and nitric oxide levels. Interestingly, Lin repressed GM-induced apoptosis, as reflected by a marked downregulation of Bax and caspase-3 expression, concurrent with the upregulation of Bcl2 expression. Finally, Lin administration led to a significant downregulation of TGF-β expression in nephrotoxic animals. In summary, Lin ameliorated GM-mediated nephrotoxicity in rats, at least through its antioxidant, anti-inflammatory, and anti-apoptotic activities and by modulating TGF-β.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gentamicin (GM) is an effective aminoglycoside antibiotic that has been well-recognized for the treatment of infections caused by gram-negative bacteria for over 50 years (Tomşa et al. 2023). Despite its nephrotoxicity, GM is still widely used. Approximately 30% of patients who received GM for more than seven days had renal failure manifested by enhanced serum urea and creatinine levels (Medić et al. 2019; Huang et al. 2020; Tomşa et al. 2023). Additionally, administration of a single dose of GM and even the lowest therapeutically effective doses have the potential to induce acute kidney injury (AKI) (Morales et al. 2010; Medić et al. 2019).
GM is predominantly excreted through glomerular filtration in an unmetabolized state, is mainly concentrated in proximal tubule cells, and leads to acute tubular necrosis (ATN) (Khan et al. 2009; Srisung et al. 2017); however, the exact mechanisms underlying GM nephrotoxicity have not been fully elucidated, and oxidative stress has been recognized as a major player in the pathophysiology of GM-mediated AKI (Albalawi et al. 2023). High intracellular concentrations of GM dramatically decrease mitochondrial complex I activity, leading to the production of reactive oxygen species (ROS) (Yue et al. 2022). Numerous studies have demonstrated that elevated ROS levels after GM administration result in lipid peroxidation, inflammation, and apoptosis in the kidneys (Abd-Elhamid et al. 2018). Mechanistically, the generated ROS trigger the dissociation of NF-κB-p65 from IκB, and the unbound NF-κB-p65 relocates to the nucleus and induces inflammation-related genes, such as TNF-α, IL-1 (Öztopuz et al. 2019), and inducible nitric oxide synthase (iNOS) (Ali et al. 2020).
Apoptosis is a critical pathway in the development of GM-mediated nephrotoxicity (Adil et al. 2016; Abd-Elhamid et al. 2018). Elevated ROS levels after GM intoxication induce mitochondrial dysfunction, disrupting the balance between Bax and Bcl2 and resulting in the release of cytochrome C (Cyt C) into the cytoplasm. The released Cyt C triggers caspase-9 (Casp-9) and caspase-3 (Casp-3), which stimulates DNA degradation and apoptotic cell death (Adil et al. 2016).
Various reports have highlighted the role of GM-induced ROS overgeneration in activating transforming growth factor-β (TGF-β) (Bae et al. 2014; Beshay et al. 2020). Stimulated TGF-β promotes renal fibrosis in GM-mediated nephrotoxicity through different mechanisms (DiRenzo et al. 2016; Beshay et al. 2020). For example, TGF-β activation triggers the TGF-β/Smads signaling cascade, which induces fibrogenic genes in the nucleus (Beshay et al. 2020). Additionally, high levels of TGF-β enhance extracellular matrix (ECM) accumulation by increasing the epithelial-to-mesenchymal transition (EMT) (Song et al. 2017; Beshay et al. 2020).
Linalool (Lin; 3,7-dimethylocta-1,6-dien-3-ol) is a linear monoterpene of natural origin that is the main ingredient of essential oils obtained from various aromatic plant species, such as Coriandrum sativum L. and Lavandula angustifolia (Mohamed et al. 2021; Azırak and Özgöçmen 2023; Hosseini et al. 2023). In traditional medicine, Lin-rich botanical species have been used to treat acute and chronic diseases (Altlnok-Yipel et al. 2020; Hosseini et al. 2023). The safety of Lin for use in flavoring and as an adjuvant in human consumption has been acknowledged by the Food and Drug Administration (Bickers et al. 2003; Mohamed et al. 2021). Nevertheless, Lin has the potential to be highly toxic if consumed in excessive amounts, exceeding 500 mg/kg/day in the case of rodents (Politano et al. 2008). Lin has been reported to exhibit several pharmacological effects, such as antioxidant, anti-inflammatory, anti-atherogenic, anticancer, antinociceptive, and antimicrobial activities (Mączka et al. 2022). Lin’s potent pharmacological activities protect cells from oxidative injuries and inhibit mitochondrial damage by scavenging ROS (Salimi et al. 2022). Furthermore, Lin inhibits smoking-induced inflammatory responses in the lungs by suppressing NF-κB (Ma et al. 2015). Lin has also cardioprotective effects in rats with isoproterenol-induced infarction through its anti-inflammatory and anti-apoptotic activities (Mohamed et al. 2021). However, the possible ameliorative effects of Lin on kidney injury following GM administration remain to be elucidated. This study aimed to evaluate, for the first time, the possible role of Lin in alleviating histopathological abnormalities, oxidative stress, inflammation, apoptosis, and TGF-β dysregulation in rats with GM-mediated nephrotoxicity.
Materials and methods
Chemicals
Tris-HCl and Tris-EDTA were provided by Merck Company, Germany. Other chemicals, including hydrogen peroxide, NaN3, glutathione, K2HPO4, 2,4-Dinitro thiocyanate benzene (DNTB), o-dianisidine dihydrochloride, gentamicin sulfate (GM, CAS number: 1405-41-0, product number: G3632), and linalool (Lin, CAS number: 78-70-6, product number: L2602) were acquired from Sigma-Aldrich (St. Louis, USA).
Animals
Thirty-two Wistar rats, all adult males, weighing 190 ± 15 g were obtained from the Pasteur Institute, Tehran, Iran. The animals were transported to the animal laboratory at the Nutritional Health Research Center (Lorestan, Iran). During the study, the rats were maintained under controlled conditions (23 ± 2 °C; 12-hour light/12-hour dark cycle; 50% humidity) with unrestricted access to a pellet-based diet and tap water. The rats were maintained under the above conditions seven days prior to the initiation of the study to ensure compliance with these conditions.
Experimental design
Laboratory rats were randomly allocated to four groups (n = 8) as follows:
-
1.
Control group: Animals were intraperitoneally administered with 0.9% saline;
-
2.
Lin group: Lin (100 mg/kg/day) was intraperitoneally administered to the rats;
-
3.
GM group: Rats received intraperitoneal GM (100 mg/kg/day) (Tavafi and Ahmadvand 2011; Tavafi et al. 2012; Kang et al. 2013);
-
4.
GM + Lin group: Animals were intraperitoneally given GM (100 mg/kg/day) and Lin (100 mg/kg/day) (Mohamed et al. 2020, 2021; Altinoz et al. 2022).
All treatments were conducted for 12 successive days (Tavafi and Ahmadvand 2011; Kang et al. 2013). Lin was administrated one hour after the intraperitoneal GM treatment (Lee et al. 2012). The dose chosen for Lin (100 mg/kg/day) was based on previous studies (Mohamed et al. 2020, 2021; Altinoz et al. 2022) and our preliminary investigations. GM and Lin were dissolved in 0.9% saline immediately before injection. All experiments were performed in accordance with guidelines approved by the National Institutes of Health (NIH Publications No. 80 − 23, 1987). The study protocols were assessed and approved by the Ethics Committee of the Lorestan University of Medical Sciences (IR.LUMS.REC.1397.063) and Faculty of Veterinary Medicine, Lorestan University (lu.acri.2023.64).
Blood and tissue collection
After completion of the treatment protocol, all animals were exposed to deep anesthesia with intraperitoneal ketamine (10 mg/kg) and xylazine (100 mg/kg) (Abou-Abbass et al. 2016). The rats were decapitated and heart blood samples were gathered for serum biochemical analysis. Serum samples were isolated by centrifugation (3000 rpm, 15 min) and stored at − 20 °C. Before renal biochemical evaluation, kidney tissue was homogenized using cold PBS (pH 7.4) and centrifuged (18,000 × g, 4 °C, 15 min) to collect the supernatant. The left kidney was carefully detached and stored at − 80 °C for biochemical evaluation. The other kidney was stored in neutral buffered formalin (10%) for histopathology and immunohistochemistry studies.
Biochemical analysis
Evaluation of renal function parameters
Creatinine and urea in the serum samples were assessed using commercially available kits (Pars Azmoon Company, Tehran, Iran) and a biochemical autoanalyzer (Olympus AU-600, Tokyo, Japan).
Assessment of oxidative stress and inflammatory biomarkers
To evaluate lipid peroxidation, the concentration of MDA was determined using the thiobarbituric acid (TBA) method by measuring absorbance at 532 nm (Ohkawa et al. 1979). Spectrophotometric analysis of glutathione (GSH) concentrations in both the serum and kidney of rats was conducted using Ellman’s method at a wavelength of 412 nm (Ellman 1959). To measure nitric oxide (NO) levels, the end-product nitrite was estimated in the serum using the method established by Giustarini et al. (2008). The assessment of catalase (CAT) activity in the collected serum and supernatant was conducted using the colorimetric assay proposed by Sinha (1972). The spectrophotometric method of Rotruck et al. (1973) was used to evaluate glutathione peroxidase (GPX) activity in all samples. GPX activity was quantified as U/mg protein by measuring the absorbance of the sample using an ELISA reader (420 nm). Renal myeloperoxidase (MPO) activity was evaluated by spectrophotometry at 450 nm using hydrogen peroxide-induced oxidation of o-dianisidine dihydrochloride (Mullane 1989).
Histopathological study
Renal tissue samples were fixed in buffered formalin solution and embedded in paraffin. After a while, 4 μm sections were stained with hematoxylin and eosin (H&E). To assess ATN, the full thickness of the cortex was considered and divided into three different areas, including the subcapsular region (supracortex), cortex, and corticomedullary junction (subcortex), which were scored according to the degree of epithelial damage using a zero through five grading system: 0, no lesion; 1, ≤ 10% ATN; 2, 11–25% ATN; 3, 26–50% ATN; 4, 51–75% ATN; and 5, 76–100% ATN. Moreover, hyaline cast formation was analyzed as described in our previous study (Babaeenezhad et al. 2023).
Immunohistochemistry (IHC) analysis
Replicated renal tissues of 3 μm thickness were deparaffinized and rehydrated. The samples were then immersed in a target retrieval solution (pH 9.0) and boiled in a water bath at 98 °C for 20 min to deliver the unmasked antigens. To block endogenous peroxidase activity, the sections were treated with 3% H2O2 in PBS for 15 min. Subsequently, the sections were incubated with 5% normal rabbit serum in PBS for 20 min to block non-specific background staining. Tissue sections were incubated with polyclonal rabbit anti-Casp-3 (orb 10,237, Biorbyt, UK) at 1:50 dilution; TGF-β (MBS462142, Mybiosource, USA) at 1:100 dilution; Bcl2 (orb 10,173, Biorbyt, UK); IL-1β (ab226918, Abcam, UK) at 1:100 dilution; TNF-α (ab6671, Abcam, UK) at 1:100 dilution; Bax (sc 7480, Santa Cruz, USA) at 1:50 dilution; NF-κB-p65 (orb312399, Biorbyt, UK) at 1:100 dilution; and monoclonal mouse anti-iNOS (C-11, sc 7271, Santa Cruz, USA) at 1:20 dilution. Thereafter, the sections were incubated with biotinylated goat anti-rabbit IgG (prediluted, Biocare, USA) for all markers, and subsequently underwent incubation with streptavidin horseradish peroxidase (sHRP) (prediluted, Biocare, USA) for 20 min. DAB solution was used to visualize the antibody-binding sites. Finally, the sections were subjected to counterstaining with Mayer’s hematoxylin (BioOptica, Italy).
Statistical analysis
All results are presented as the mean ± standard deviation (SD). Statistical analysis was conducted using SPSS software (version 27; SPSS Inc., Chicago, IL, USA). Data distribution was evaluated using the one-sample Kolmogorov–Smirnov test. Data exhibiting a normal distribution were analyzed using one-way ANOVA, followed by Tukey’s post hoc test. The non-parametric Kruskal–Wallis test was employed when the experimental groups showed non-normally distributed data. In this respect, data related to hyaline cast levels were analyzed using the Kruskal-Wallis test, whereas the data related to the other parameters were analyzed using one-way ANOVA. The level of statistical significance was set at p < 0.05.
Results
Effects of Lin on GM-mediated changes in renal function markers
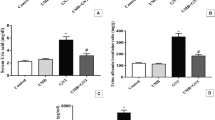
We measured the levels of urea and creatinine in the various experimental groups to evaluate the effects of Lin on GM-induced renal dysfunction. As shown in Fig. 1, GM injection in rats led to a remarkable increase (3.95-fold and 3.82-fold, respectively) in serum urea and creatinine levels compared with the control group; however, Lin administration after GM injection significantly decreased the serum urea and creatinine levels (36.17% and 41.54%, respectively) compared with the GM group (Fig. 1).
Effect of Lin on GM-induced oxidative stress
We examined the state of oxidative stress by measuring MDA, GSH, and antioxidant enzyme activities (GPX and CAT). Nephrotoxicity mediated by GM injection for 12 consecutive days led to a considerable elevation in serum and kidney MDA levels (1.80-fold and 9.04-fold, respectively), accompanied by a marked decrease in serum and renal GSH levels compared with those in the control group (1.62-fold and 1.57-fold, respectively) (Fig. 2). Additionally, the serum and renal activities of GPX (2.47-fold and 2.26-fold, respectively) and CAT (1.39-fold and 2.32-fold, respectively) in the untreated nephrotoxic animals were lower than those in the control group (Fig. 2). However, GM-intoxicated animals treated with Lin displayed significantly lower serum and renal MDA levels (35% and 31.56%, respectively) than the untreated nephrotoxic animals (Fig. 2). Conversely, Lin treatment in nephrotoxic animals remarkably promoted serum and renal GPX activities (93.37% and 83.01%, respectively), accompanied by a slight but significantly enhanced renal CAT activity (27.75%) compared to the untreated nephrotoxic rats (Fig. 2). Lin administration led to a modest but not significantly increased serum and renal GSH levels (28.64% and 13.19%, respectively) and serum CAT activity (0.38%) in the GM-intoxicated rats (p > 0.05, Fig. 2).
Effects of Lin on oxidative stress markers in rats with GM-induced nephrotoxicity. Lin; linalool (100 mg/kg), GM; gentamicin (100 mg/kg), Lin + GM; linalool (100 mg/kg) + gentamicin (100 mg/kg). Results are presented as mean ± standard deviation (SD). * p < 0.05 and **** p < 0.0001. NS: not significant
Effect of Lin on GM-induced histopathological alterations in the kidney
Microscopic evaluation of H&E-stained kidney sections from the control and Lin groups indicated a normal kidney architecture (Fig. 3a–d); however, animals that received daily GM for 12 days exhibited renal histopathological changes in favor of AKI. Several histopathological alterations were noted in the GM group, including ATN, hydropic degeneration, loss of the brush border, exfoliated epithelial cells, and leukocyte infiltration (Fig. 3e,f). These changes were considerably reduced after Lin treatment (Fig. 3g,h). As GM mainly affects the renal tubules, we analyzed ATN in the subcapsular, cortical, and subcortical areas. Receiving GM enhanced ATN in these regions (11.31-fold, 23.44-fold, and 17.66-fold, respectively) in nephrotoxic animals compared to the control group (Fig. 3j). Lin significantly mitigated ATN levels (63.55%, 63.92%, and 66.15%, respectively) in the nephrotoxic rats (Fig. 3j). Furthermore, the GM group showed profoundly higher (112.89-fold) hyaline cast levels than the control group (Fig. 3i). Hyaline cast levels were reduced by 42.4% but not significantly in the nephrotoxic rats treated with Lin (p > 0.05, Fig. 3i).
Histopathological assessment of renal tissues (a–h). Control group (a, b); normal tubular and glomerular structures. Linalool (Lin)-treated rats (c, d); similar to the control group, all structures are intact. Gentamicin (GM) group (e, f); extensive and uniform tubular coagulative necrosis (e, black arrows) and degenerated tubular cells (e, green arrows). Large numbers of tubular structures either are coagulated (black arrows) or to lesser extent are vacoulative degenerated, which are considered hydropic changes (green arrows). Additionally, a tubule with exfoliated epithelial cells stands out (f, green arrowhead). GM + Lin group (g, h); mild to moderate tubular damage from a few tubular destructions (g, black arrows), and vacuolated epithelial cells (g, green arrows) to hyaline cast formation (g, arrowheads). Although a few hyaline casts are visible at the center of tubules (h, arrows), the total structure is nearly well-preserved. H&E; Images a, c, e, g are at 100 μm and b, d, f, h (as higher magnification) are at 40 μm magnification. Hyaline cast and acute tubular necrosis (ATN) (i, j); a significant reduction in hyaline cast formation and ATN were observed after Lin administration in GM-intoxicated rats. Bars show mean ± standard deviation (SD). Bars with similar letters indicate no statistically significant difference
Effect of Lin on GM-mediated inflammation
Inflammation triggered by GM is an important factor in AKI development; therefore, we explored the effects of Lin on MPO activity, NO levels, and NF-κB-p65, TNF-α, IL-1β, and iNOS expression in GM-treated rats. Renal MPO activity, a biomarker of leukocyte infiltration, was significantly elevated (2.54-fold) in rats that received GM (Fig. 4). Conversely, daily Lin administration significantly decreased MPO activity compared with that in the GM group (Fig. 4). Lin treatment restored MPO activity similar to that of the control animals.
Effects of Lin on inflammatory biomarkers in rats with GM-induced nephrotoxicity. Lin; linalool (100 mg/kg), GM; gentamicin (100 mg/kg), Lin + GM; linalool (100 mg/kg) + gentamicin (100 mg/kg). Bars indicate mean ± standard deviation (SD). Bars with similar letters indicate no statistically significant difference
NF-κB-p65 immunohistochemically stained kidney sections from the normal and Lin groups indicated mild immunoreaction of a few tubular cells (Figs. 4 and 5). In the GM-treated rats, the expression level of NF-κB-p65 was significantly increased (18.28-fold) compared to that in the healthy rats, as a high population of both epithelial and inflammatory cells was positive (Figs. 4 and 5). In contrast, the NF-κB-p65 immunoexpression level was significantly reduced (73.95%) in the GM + Lin group (Fig. 4) and only a small number of renal tubules were partially stained (Fig. 5).
TGF-β immunostaining group (a, e, i, m); Control (a): a small number of tubules are reacted. Linalool (Lin) (e): many tubular cells in a single tubule are positive (arrow). Gentamicin (GM) (i): diffuse and relative membranous to cytoplasmic staining is demonstrated. GM + Lin (m): the staining is limited to few tubules. IL-1β immunolabeling in rats (b, f, j, n); Control (b): small number of cells are stained. Lin (f): one or two cells are stained around the glomerulus. GM (j): reaction is increased at the center of the population of infiltrating inflammatory cells. GM + Lin (n): negligible number of cells indicate cytoplasmic immunostaining. NF-κB immunoreaction group (c, g, k, o); Control (c): mild reaction in a few tubular cells. Lin (g): similar to the control group, a few stained cells are observed. GM (k): high population of both epithelial and inflammatory cells are positive staining. GM + Lin (o): partial staining of a single tubule is obvious. iNOS-immunopositive rats (d, h, l, p); Control (d): inconsiderable amount of epithelial cells are staining (arrow). Lin (h): small amount of cells have cytoplasmic reaction (arrow). GM (l): higher population of cells is positive among the inflammatory cells. GM + Lin (p): the positive reaction is prominently reduced (arrows) (a–p 40 μm)
IL-1β and TNF-α immunolabeling of kidney sections from the control and Lin-treated rats showed that a limited number of tubular cells were stained, albeit scattered (Figs. 4, 5 and 6); however, the immunoreactivity of IL-1β and TNF-α in the GM-treated rats was significantly more than in the control rats (3.04-fold and 66.20-fold, respectively), as reflected by the strong immunoreactivity of tubular cells for TNF-α and elevated immunostaining for IL-1β in the center of the densely populated region of infiltrating inflammatory cells (Figs. 4, 5 and 6). Interestingly, the treatment of nephrotoxic rats with Lin significantly reduced the immunoexpression levels of IL-1β and TNF-α compared with those in the GM group (44.93% and 66.11%, respectively), as manifested by the lower number of tubules with immunoreactivity for IL-1β and TNF-α (Figs. 4, 5 and 6).
Bax immunostaining group (a, e, i, m); Control (a): staining is limited in some glomeruli. Linalool (Lin) (e): similar to the control group, only glomeruli are partially stained. Gentamicin (GM) (i): diffuse and strong staining of tubular cells. GM + Lin (m): only a few of tubular cells are weakly stained .Bcl2 immunolabeling rats (b, f, j, n); Control (b): intensive and diffuse staining of epithelial cells. Lin (f): extensive immunopositive in renal structures. GM (j): a few tubular structures are stained faintly. GM + Linalool (n): focal but strong positive reaction of tubules. Caspase-3 (Casp-3) immunoreaction group (c, g, k, o); Control (c): only the glomeruli are staining. Lin (g): resembles the control group, the labeling is restricted to glomeruli. GM (k): intensive positive tubular cells. GM + Lin (o): the positivity of tubular cells is reduced prominently and pale in contrast to the GM rats. TNF-α immunopositive rats (d, h, l, p); Control (d): very few and scattered tubular cells are positive thoroughly (arrow). Lin (h): scant number of cells are immunostaining (not shown). GM (l): massive and strong reaction of tubular cells. GM + Lin (p): immunolabeling is confined to many of tubules (a–p 100 μm)
iNOS immunoreactivity was only observed in a minimal number of tubular cells in the control and Lin groups (Figs. 4 and 5). Daily administration of GM significantly promoted iNOS immunoexpression (9.73-fold) compared with the control group (Figs. 4 and 5). A greater proportion of cells were iNOS-positive among the inflammatory cells in the rats exposed to GM (Fig. 5). Lin prominently rescued iNOS immunoexpression (71.38%) in the nephrotoxic rats compared to that in the untreated nephrotoxic animals (Figs. 4 and 5).
Serum NO levels were spectrophotometrically determined in various groups and were dramatically enhanced (3.65-fold) in the GM-treated rats (Fig. 4). Daily treatment with Lin in the nephrotoxic animals considerably decreased (69.20%) serum NO levels compared to the untreated nephrotoxic rats (Fig. 4).
Effect of Lin on GM-mediated apoptosis
To investigate the effect of Lin on GM-mediated apoptosis in nephrotoxic rats, we immunohistochemically studied Bax, Bcl2, and Casp-3 expression in the kidney sections of the experimental groups. Kidney sections with Bax and Casp-3 immunolabeling in the control and Lin groups showed partial staining of a few glomeruli (Fig. 6). Conversely, GM-treated rats exhibited diffuse and strong immunoreactivity in tubular cells. The immunoexpression of Bax and Casp-3 was significantly higher (13.25-fold and 33.49-fold, respectively) in the nephrotoxic rats than in the healthy rats (Figs. 6 and 7). In contrast, renal sections from the GM + Lin group showed a few tubular cells with weak immunostaining for Bax and Casp-3 (Fig. 6). Receiving Lin in the nephrotoxic rats caused a significant reduction in Bax and Casp-3 expression (65.02% and 77.16%, respectively) compared to the GM group (Figs. 6 and 7).
Effects of Lin on apoptotic biomarkers and TGF-β in rats with GM-induced nephrotoxicity. Lin; linalool (100 mg/kg), GM; gentamicin (100 mg/kg), Lin + GM; linalool (100 mg/kg) + gentamicin (100 mg/kg). Results are presented as mean ± standard deviation (SD). Bars with similar letters indicate no statistically significant difference
Next, we examined the expression of Bcl2, an anti-apoptotic protein, in the kidney samples from the studied groups. Tubular epithelial cells in the control and GM groups showed high Bcl2 expression (Fig. 6). Significantly lower Bcl2 expression (5.89-fold) was detected in kidney sections from GM-treated rats—in which a few tubular structures were weakly stained—than that in the control group (Figs. 6 and 7). Nephrotoxic rats treated with Lin for 12 days showed remarkably higher Bcl2 expression (3.17-fold) than the rats in the GM group (Figs. 6 and 7). Kidney sections from the Lin + GM group indicated a focal but strong positive reaction in the tubules (Fig. 6).
Effect of Lin on GM-induced TGF-β dysregulation
TGF-β expression levels varied among the experimental groups. The control and Lin groups exhibited low TGF-β expression and partial immunoreactivity in a few tubules (Figs. 5 and 7). TGF-β immunoexpression in GM-treated animals was notably higher (3.76-fold) than in the control group (Figs. 5 and 7). Tubular epithelial cells showed diffuse and relative membranous to cytoplasmic immunostaining in the GM group (Fig. 5). Administration of Lin markedly reduced TGF-β expression (54.85%) in nephrotoxic animals, as immunostaining was limited to a few tubules (Figs. 5 and 7).
Discussion
This experimental study is most likely the first to examine the renoprotective potential and beneficial effects of Lin on GM-mediated nephrotoxicity in rats. The findings of this study revealed that GM-induced AKI was noticeably alleviated after 12 d of Lin administration. Lin treatment considerably improved the majority of the altered parameters in nephrotoxic rats.
The findings illustrated that exposure to GM for 12 days successfully induced AKI in rats, as manifested by a marked increase in serum urea and creatinine levels and kidney histopathological abnormalities. Numerous studies have shown that GM increases tubular blockade and reduces the glomerular filtration rate (GFR), leading to an increase in serum urea and creatinine levels (Lopez-Novoa et al. 2011; Abd-Elhamid et al. 2018; Tomşa et al. 2023). Lin treatment considerably mitigated GM-induced histopathological alterations and renal dysfunction. These findings clearly show the renoprotective effects of Lin and are in line with the study of Altinoz et al., illustrating the beneficial effects of Lin on renal failure (Altinoz et al. 2022). The reduction in serum urea and creatinine levels via Lin treatment was likely achieved by regenerating kidney histopathological changes and GFR in GM-exposed rats, as reported for other natural antioxidants with nephroprotective effects (Khan et al. 2009; Adil et al. 2016; Tomşa et al. 2023).
GM triggering oxidative stress has been identified as a key node in AKI pathogenesis, as demonstrated in this study and other in vivo reports (Morales et al. 2010; Yue et al. 2022; Tomşa et al. 2023). The results showed that daily treatment with Lin remarkably ameliorated lipid peroxidation and promoted the activity of the antioxidant enzymes GPX and CAT in GM-exposed rats. These findings are consistent with those of previous studies, highlighting the potent antioxidant activity of the monoterpene Lin (Sabogal-guáqueta et al. 2019; Altinoz et al. 2022; Azırak and Özgöçmen 2023). The effectiveness of Lin in ameliorating oxidative stress may be attributed to its potential to directly eliminate ROS (Gunaseelan et al. 2017). Furthermore, Mohamed et al. (2021) showed that Lin increases nuclear Nrf2, a redox-sensitive transcription factor that translocates from the cytoplasm to the nucleus to induce the expression of antioxidant enzyme genes during oxidative stress (Mohamed et al. 2021). The reduction in oxidative stress by Lin in GM-exposed rats may be associated with its ability to remove ROS and/or upregulate nuclear Nrf2. This needs to be further explored in an animal model of GM-induced nephrotoxicity.
The NF-κB pathway is critically involved in the development of GM-mediated AKI and amplifies the inflammatory responses (Albalawi et al. 2023). In this study, animals in the GM group showed a higher expression of NF-κB-p65, IL1β, and TNF-α than those in the other groups. Daily Lin administration one hour after GM led to a noticeable decrease in NF-κB-p65 expression and pro-inflammatory cytokines IL-1β and TNF-α. These findings are in accordance with those of previous studies identifying the anti-inflammatory effects of Lin through suppression of the NF-κB pathway in smoke-induced lung inflammation (Ma et al. 2015) and ultraviolet-B radiation-induced skin injuries (Gunaseelan et al. 2017). The activation of NF-κB has been found to be intricately related to the regulatory role played by the mitogen-activated protein kinase (MAPK) pathway as its upstream (Ozbek et al. 2009; Lee et al. 2013). Several reports have intriguingly demonstrated that Lin effectively represses MAPK signaling cascades (Huo et al. 2013; Gunaseelan et al. 2017). Nevertheless, the anti-inflammatory effects of Lin may be due to the inactivation of the MAPK/NF-κB pathway. The potential suppressive effects of Lin on MAPK signaling pathways require further investigation in rats with GM nephrotoxicity.
NO plays conflicting roles in the modulation of kidney function. At normal levels, NO acts as a vasodilator and modulates tubular functions (Radermacher et al. 1992). In contrast, the elevated levels of NO caused by iNOS are related to oxidative damage in the kidneys (Araujo and Welch 2006; Christo et al. 2011). Similar to previous studies (Ozbek et al. 2009; El-Kashef et al. 2015), high levels of NO were detected in rats exposed to GM, accompanied by marked kidney injury. A dramatic increase in NO levels in GM-intoxicated rats has been linked to iNOS overexpression (Ozbek et al. 2009; Lee et al. 2012), as detected by immunohistochemistry in this study. Additionally, NF-κB-p65 activation induces iNOS expression in inflammatory states, including GM nephrotoxicity (Abd-Elhamid et al. 2018). Lin effectively mitigated GM-induced iNOS overexpression and elevated NO levels in the nephrotoxic rats. These results are supported by previous studies demonstrating that Lin reduces iNOS expression and NO levels under inflammatory pathological conditions (Kim et al. 2019).
Kidney MPO activity and leukocyte infiltration were markedly increased in the nephrotoxic animals. Lin significantly inhibited the increase in renal MPO activity and leukocyte infiltration in the GM + Lin group. As found in this study and previous reports, Lin has the potential to downregulate NF-κB-p65 and pro-inflammatory cytokines (Huo et al. 2013; Ma et al. 2015; Gunaseelan et al. 2017), most probably leading to decreased MPO activity and leukocyte infiltration in nephrotoxic rats. Overall, these results showed that the anti-inflammatory effects of Lin may be mediated by suppression of the NF-κB/iNOS/TNF-α/IL-1β pathway in GM nephrotoxicity.
Activation of apoptosis is a fundamental mechanism underlying the development of GM-mediated AKI (Adil et al. 2016; Abd-Elhamid et al. 2018). In line with previous studies (Yue et al. 2022; Albalawi et al. 2023), GM activated the intrinsic mitochondrial apoptotic pathway in nephrotoxic animals; however, Lin antagonized the pro-apoptotic effects of GM, as manifested by the downregulation of the pro-apoptotic markers Bax and Casp-3, and the upregulation of the anti-apoptotic protein Bcl2. These findings clearly elaborate on the anti-apoptotic effects of Lin, which are in agreement with the results of previous studies (Mohamed et al. 2020, 2021). The intrinsic apoptotic pathway is activated by oxidative stress and NF-κB activation during GM toxicity (Abd-Elhamid et al. 2018; Albalawi et al. 2023). Surprisingly, Lin noticeably inhibited oxidative stress and reduced NF-κB-p65 expression in GM-exposed animals. Therefore, Lin prevented GM-induced apoptosis, possibly by suppressing oxidative stress and/or NF-κB activation.
TGF-β has been implicated in renal fibrosis during GM nephrotoxicity (Deepa and Anuradha 2013; Bae et al. 2014; Beshay et al. 2020). In a previous study, Beshay et al. (2020) illustrated that increasing TGF-β expression in the kidneys of rats with GM nephrotoxicity promoted renal fibrosis and EMT by activating the TGF-β/Smad pathway. Similarly, TGF-β expression was higher in the nephrotoxic rats than that in the healthy rats. However, Lin administration markedly reduced TGF-β expression in the nephrotoxic animals. It has been identified that ROS-activated NF-κB is a main inducer of TGF-β (Deepa and Anuradha 2013; Dursun et al. 2018; Beshay et al. 2020). Similar to these results, Deepa and Anuradha (2013) demonstrated that Lin reduced renal fibrosis by ameliorating oxidative stress and suppressing NF-κB-p65 and TGF-β. Altogether, Lin may exert antifibrotic effects in nephrotoxic rats by inhibiting TGF-β through its antioxidant and anti-inflammatory activities.
Conclusions
The findings of this study revealed that Lin ameliorated oxidative stress and improved kidney function parameters, accompanied by regeneration of renal histopathological alterations. Additionally, Lin decreased the GM-induced inflammatory response by inhibiting the NF-κB/iNOS/TNF-α/IL-1β pathway, MPO activity, and NO levels. Lin also mitigated GM-induced intrinsic apoptosis and TGF-β dysregulation. Overall, the beneficial effects of Lin on GM-mediated AKI appear to be mediated by its antioxidant, anti-inflammatory, and anti-apoptotic effects, and the modulation of TGF-β (Fig. 8). Further studies are crucial for comprehensively elucidating and refining the effectiveness of Lin as a promising therapeutic target for clinical use. This study may give a new perspective on the potential of Lin to ameliorate GM-mediated nephrotoxicity in rats.
Data availability
No datasets were generated or analysed during the current study.
References
Abd-Elhamid TH, Elgamal DA, Ali SS et al (2018) Reno-protective effects of ursodeoxycholic acid against gentamicin-induced nephrotoxicity through modulation of NF-κB, eNOS and caspase-3 expressions. Cell Tissue Res 374:367–387. https://doi.org/10.1007/s00441-018-2886-y
Abou-Abbass H, Bahmad H, Abou-El-Hassan H et al (2016) Deciphering glycomics and neuroproteomic alterations in experimental traumatic brain injury: comparative analysis of aspirin and clopidogrel treatment. Electrophoresis 37:1562–1576. https://doi.org/10.1002/elps.201500583
Adil M, Kandhare AD, Dalvi G et al (2016) Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren Fail 38:996–1006. https://doi.org/10.3109/0886022X.2016.1165120
Albalawi RS, Binmahfouz LS, Hareeri RH et al (2023) Parthenolide phytosomes attenuated gentamicin-induced nephrotoxicity in rats via activation of Sirt-1, Nrf2, OH-1, and NQO1 axis. Molecules 28:2741. https://doi.org/10.3390/molecules28062741
Ali F, Hassanein E, Bakr A et al (2020) Ursodeoxycholic acid abrogates gentamicin-induced hepatotoxicity in rats: role of NF-κB-p65/TNF-α, Bax/Bcl-xl/Caspase-3, and eNOS/iNOS pathways. Life Sci 254:117760. https://doi.org/10.1016/j.lfs.2020.117760
Altinoz E, Oner Z, Elbe H et al (2022) Linalool exhibits therapeutic and protective effects in a rat model of doxorubicin-induced kidney injury by modulating oxidative stress. Drug Chem Toxicol 45:2024–2030. https://doi.org/10.1080/01480545.2021.1894751
Altlnok-Yipel F, Tekeli IO, Özsoy OY et al (2020) Hepatoprotective activity of linalool in rats against liver injury induced by carbon tetrachloride. Int J Vitam Nutr Res 90:302–308. https://doi.org/10.1024/0300-9831/A000581
Araujo M, Welch WJ (2006) Oxidative stress and nitric oxide in kidney function. Curr Opin Nephrol Hypertens 15:72–77. https://doi.org/10.1097/01.mnh.0000191912.65281.e9
Azırak S, Özgöçmen M (2023) Linalool prevents kidney damage by inhibiting rifampicin-induced oxidative stress and apoptosis. Tissue Cell 82:102097. https://doi.org/10.1016/j.tice.2023.102097
Babaeenezhad E, Dezfoulian O, Hadipour Moradi F et al (2023) Exogenous glutathione protects against gentamicin-induced acute kidney injury by inhibiting NF-κB pathway, oxidative stress, and apoptosis and regulating PCNA. Drug Chem Toxicol 46:441–450. https://doi.org/10.1080/01480545.2022.2049290
Bae EH, Kim IJ, Joo SY et al (2014) Renoprotective effects of the direct renin inhibitor aliskiren on gentamicin-induced nephrotoxicity in rats. J Renin-Angiotensin-Aldosterone Syst 15:348–361. https://doi.org/10.1177/1470320312474853
Beshay ON, Ewees MG, Abdel-Bakky MS et al (2020) Resveratrol reduces gentamicin-induced EMT in the kidney via inhibition of reactive oxygen species and involving TGF-β/Smad pathway. Life Sci 258:118178. https://doi.org/10.1016/j.lfs.2020.118178
Bickers D, Calow P, Greim H et al (2003) A toxicologic and dermatologic assessment of linalool and related esters when used as fragrance ingredients. Food Chem Toxicol 41:919–942. https://doi.org/10.1016/S0278-6915(03)00016-4
Christo JS, Rodrigues AM, Mouro MG et al (2011) Nitric oxide (NO) is associated with gentamicin (GENTA) nephrotoxicity and the renal function recovery after suspension of GENTA treatment in rats. Nitric Oxide 24:77–83. https://doi.org/10.1016/j.niox.2010.12.001
Deepa B, Anuradha CV (2013) Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol Mech Methods 23:223–234. https://doi.org/10.3109/15376516.2012.743638
DiRenzo DM, Chaudhary MA, Shi X et al (2016) A crosstalk between TGF-β/Smad3 and Wnt/β-catenin pathways promotes vascular smooth muscle cell proliferation. Cell Signal 28:498–505. https://doi.org/10.1016/j.cellsig.2016.02.011
Dursun M, Sahin S, Besiroglu H et al (2018) Protective effect of nebivolol on gentamicin-induced nephrotoxicity in rats. Bratisl Med J 119:718–725. https://doi.org/10.4149/BLL_2018_128
El-Kashef DH, El-Kenawi AE, Suddek GM, Salem HA (2015) Protective effect of allicin against gentamicin-induced nephrotoxicity in rats. Int Immunopharmacol 29:679–686. https://doi.org/10.1016/j.intimp.2015.09.010
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Giustarini D, Rossi R, Milzani A, Dalle-Donne I (2008) Nitrite and nitrate measurement by Griess reagent in human plasma: evaluation of interferences and standardization. Methods Enzymol 440:361–380
Gunaseelan S, Balupillai A, Govindasamy K et al (2017) Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS ONE 12:e0176699. https://doi.org/10.1371/journal.pone.0176699
Hosseini A, Pourheidar E, Rajabian A et al (2023) Linalool attenuated ischemic injury in PC12 cells through inhibition of caspase-3 and caspase-9 during apoptosis. Food Sci Nutr 11:249–260. https://doi.org/10.1002/fsn3.3057
Huang H, Jin WW, Huang M et al (2020) Gentamicin-induced acute kidney injury in an animal model involves programmed necrosis of the collecting duct. J Am Soc Nephrol 31:2097–2115. https://doi.org/10.1681/ASN.2019020204
Huo M, Cui X, Xue J et al (2013) Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J Surg Res 180:e47–e54. https://doi.org/10.1016/J.JSS.2012.10.050
Kang C, Lee H, Hah DY et al (2013) Protective effects of Houttuynia cordata Thunb. On gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicol Res 29:61–67. https://doi.org/10.5487/TR.2013.29.1.061
Khan SA, Priyamvada S, Farooq N et al (2009) Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacol Res 59:254–262. https://doi.org/10.1016/J.PHRS.2008.12.009
Kim MG, Kim SM, Min JH et al (2019) Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int Immunopharmacol 74:105706. https://doi.org/10.1016/j.intimp.2019.105706
Lee IC, Kim SH, Lee SM et al (2012) Melatonin attenuates gentamicin-induced nephrotoxicity and oxidative stress in rats. Arch Toxicol 86:1527–1536. https://doi.org/10.1007/s00204-012-0849-8
Lee KE, Kim EY, Kim CS et al (2013) Macrophage-stimulating protein attenuates gentamicin-induced inflammation and apoptosis in human renal proximal tubular epithelial cells. Biochem Biophys Res Commun 434:527–533. https://doi.org/10.1016/j.bbrc.2013.03.108
Lopez-Novoa JM, Quiros Y, Vicente L et al (2011) New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 79:33–45
Ma J, Xu H, Wu J et al (2015) Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int Immunopharmacol 29:708–713. https://doi.org/10.1016/J.INTIMP.2015.09.005
Mączka W, Duda-Madej A, Grabarczyk M, Wińska K (2022) Natural compounds in the battle against Microorganisms—Linalool. Molecules 27:6928. https://doi.org/10.3390/molecules27206928
Medić B, Stojanović M, Rovčanin B et al (2019) Pioglitazone attenuates kidney injury in an experimental model of gentamicin-induced nephrotoxicity in rats. Sci Rep 9:13689. https://doi.org/10.1038/s41598-019-49835-1
Mohamed ME, Abduldaium YS, Younis NS (2020) Ameliorative effect of linalool in cisplatin-induced nephrotoxicity: the role of HMGB1/TLR4/NF-κB and NRF2/HO1 pathways. Biomolecules 10:1488. https://doi.org/10.3390/biom10111488
Mohamed ME, Abduldaium MS, Younis NS (2021) Cardioprotective effect of linalool against isoproterenol-induced myocardial infarction. Life 11:120. https://doi.org/10.3390/life11020120
Morales AI, Detaille D, Prieto M et al (2010) Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int 77:861–869. https://doi.org/10.1038/KI.2010.11
Mullane K (1989) Neutrophil-platelet interactions and post-ischemic myocardial injury. Prog Clin Biol Res 301:39–51
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ozbek E, Cekmen M, Ilbey YO et al (2009) Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kappaB pathways. Ren Fail 31:382–392. https://doi.org/10.1080/08860220902835863
Öztopuz Ö, Türkön H, Sehitoglu MH et al (2019) Hyperbaric oxygen treatment ameliorates gentamicin-induced nephrotoxicity and expression of kidney injury molecule 1 in the rat model. Undersea Hyperb Med 46:125–133
Politano VT, Lewis EM, Hoberman AM et al (2008) Evaluation of the developmental toxicity of linalool in rats. Int J Toxicol 27:183–188. https://doi.org/10.1080/10915810801977948
Radermacher J, Klanke B, Schurek HJ et al (1992) Importance of NO/EDRF for glomerular and tubular function: studies in the isolated perfused rat kidney. Kidney Int 41:1549–1559. https://doi.org/10.1038/ki.1992.225
Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: biochemical role as a component of glatathione peroxidase. Science 179:588–590. https://doi.org/10.1126/science.179.4073.588
Sabogal-guáqueta AM, Hobbie F, Keerthi A et al (2019) Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed Pharmacother 118:109295. https://doi.org/10.1016/j.biopha.2019.109295
Salimi A, Khodaparast F, Bohlooli S et al (2022) Linalool reverses benzene-induced cytotoxicity, oxidative stress and lysosomal/mitochondrial damages in human lymphocytes. Drug Chem Toxicol 45:2454–2462. https://doi.org/10.1080/01480545.2021.1957563
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Song Y, Peng C, Lv S et al (2017) Adipose-derived stem cells ameliorate renal interstitial fibrosis through inhibition of EMT and inflammatory response via TGF-β1 signaling pathway. Int Immunopharmacol 44:115–122. https://doi.org/10.1016/j.intimp.2017.01.008
Srisung W, Teerakanok J, Tantrachoti P et al (2017) Surgical prophylaxis with gentamicin and acute kidney injury: a systematic review and meta-analysis. Ann Transl Med 5:100. https://doi.org/10.21037/atm.2017.03.06
Tavafi M, Ahmadvand H (2011) Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell 43:392–397. https://doi.org/10.1016/j.tice.2011.09.001
Tavafi M, Ahmadvand H, Toolabi P (2012) Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis 6:25–32
Tomşa AM, Răchişan AL, Pandrea SL et al (2023) Curcumin and vitamin C attenuate gentamicin-induced nephrotoxicity by modulating distinctive reactive species. Metabolites 13:49. https://doi.org/10.3390/metabo13010049
Yue L, Yang YR, Ma WX et al (2022) Epigallocatechin gallate attenuates gentamicin-induced nephrotoxicity by suppressing apoptosis and ferroptosis. Molecules 27:8564. https://doi.org/10.3390/molecules27238564
Acknowledgements
We would like to thank the Nutritional Health Research Center of Lorestan Medical University, Lorestan, Iran.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
EB, OD, MMS, and HA conceived and designed research. EB and OD conducted experiments. MMS and HA contributed new reagents or analytical tools. EB wrote the manuscript. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Lorestan University of Medical Sciences (IR.LUMS.REC.1397.063) and Faculty of Veterinary Medicine, Lorestan University (lu.acri.2023.64).
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babaeenezhad, E., Dezfoulian, O., Moradi Sarabi, M. et al. Monoterpene linalool restrains gentamicin-mediated acute kidney injury in rats by subsiding oxidative stress, apoptosis, and the NF-κB/iNOS/TNF-α/IL-1β pathway and regulating TGF-β. Naunyn-Schmiedeberg's Arch Pharmacol 397, 5701–5714 (2024). https://doi.org/10.1007/s00210-024-02978-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-024-02978-z