Abstract
Nephrotoxicity is the most common adverse effect of gentamicin (GNT). This study aimed to investigate the possible nephroprotective effect of umbelliferone (UMB), against GNT-induced nephrotoxicity. Rats were allocated into the control group; UMB group (50 mg/kg/day, P.O. for 15 days); GNT group (100 mg/kg/day, i.p., for 8 days); and GNT + UMB group. By the end of the experimental period, serum creatinine, urea, and uric acid as well as urine KIM-1 and urine albumin/creatinine ratio were evaluated to estimate kidney function. Moreover, tissue samples were collected for assessment of ERK1/2, p-ERK1/2, TLR-4, p38 MAPK, NF-κB-p65, NLRP-3, IkBα, TNF-α, IL-1β, JAK1, STAT-3, p-STAT, and cleaved caspase-3. In support, the histopathological examination of renal tissues was performed. UMB improves kidney function through regulation of renal serum biomarkers, with alleviations of histological abrasions induced by GNT. Besides, UMB downregulates renal protein expressions of ERK1/ERK2, TLR-4, and p38MAPK, with subsequent suppression of NF-κB-p65/NLRP-3 inflammasome and JAK1/STAT-3 pathways as well as cleaved caspase-3. In parallel, UMB induced IkBα upregulation. Collectively, UMB markedly amended all GNT-induced renal changes. These nephroprotective outcomes could be attributed to its ability to impede TLR-4/NF-κB-p65/NLRP-3 inflammasome and JAK1/STAT-3 pathways activation, as well as to its anti-inflammatory property.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gentamicin (GNT) is an aminoglycoside bactericidal antibiotic that is effectively used to manage severe Gram-negative infections (Coutinho et al. 2018). However, its use is markedly restricted because of its ototoxicity and nephrotoxicity complications (Blunston et al. 2015). The GNT-induced nephrotoxicity occurs by selective accumulation of the drug in renal proximal convoluted tubules that lead to loss of its brush border integrity (Balakumar et al. 2010). Moreover, GNT is thought to bind to lysosomal membrane phospholipids and alters their functions resulting in lysosomal phospholipidosis and the distribution of GNT to different cellular organelles, producing their dysfunction (Abdeen et al. 2014; Lopez-Novoa et al. 2011). Interestingly, GNT nephrotoxicity is related to the production of renal free radical, reduction in antioxidant defense mechanisms along with acute tubular necrosis and glomerular congestion that resulting in diminished glomerular filtration rate, severe proximal renal tubular necrosis followed by deterioration of renal functions (Sahu et al. 2014).
Nephrotoxicity has been contributed to several oxidative and inflammatory cytokines’ pathological mechanisms. Despite the cell survival role of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), which are members of the mitogen-activated protein kinase superfamily, recent evidence illustrated the contribution of ERK1/2 activation in neuronal and renal epithelial cell death upon exposure to oxidative stress and toxicants (Huang et al. 2017).
Toll-like receptors (TLRs) are an important primary innate immunity barrier as a part of the defense mechanism invading microorganisms (Kawasaki and Kawai 2014). However, activation of TLRs targeting transcription of many inflammatory pathways including nuclear factor-kappa (NF-κ) B/nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) inflammasome and Janus kinase/signal transducers and activators of transcription (JAK /STAT) (González-Guerrero et al. 2017). NF-κB is a transcriptional factor that is expressed in inflammatory responses and involved in renal dysfunction (Woods et al. 2002). Reactive oxygen species (ROS)–mediated NF-κB activation may be significantly implicated in GNT nephrotoxicity. Also, ROS production is followed by NLRP-3 inflammasome activation. Upon activation, NLRP-3 oligomerizes and elicits the release of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) and mediate cell death (Mahmoud et al. 2019). Additionally, the JAK/STAT signaling is recently found to be important for the kidney response to injury and hence implicated in certain renal diseases (Si et al. 2013) and has a pivotal role in the transcription of many inflammatory responses involved in renal injury (Chuang and He 2010).
Coumarins are natural phenolic compounds present in many kinds of medicinal plants and found in a rich source of human diet (Kostova et al. 2011). Umbelliferone (7-hydroxycoumarin; UMB) is a coumarin derivative that possessing anti-inflammatory and antioxidant activity with free radical scavenging properties (Vijayalakshmi and Sindhu 2017). Previous studies established the beneficial effects of UMB in different experimental models such as anti-hyperlipidemic and anti-diabetic effect against streptozotocin-induced diabetes mellitus (Kumar et al. 2013), anti-inflammatory activity against rheumatoid arthritis (Kumar et al. 2015b), antioxidant and hepatoprotective activity against liver injury (Germoush et al. 2018; Mahmoud et al. 2017), and in cerebral ischemia/reperfusion-induced damage (Wang et al. 2015). Previously, Wang et al. (2019) reported the renoprotective effect of UMB against diabetic nephropathy in rats. Besides, our previous study reported the protective effect of UMB against methotrexate-induced nephrotoxicity (Hassanein et al. 2018). However, our present study was conducted, for the first time, to explore the renoprotective effects of UMB against highly effective and widely used antibiotics; GNT with exploring the underlined mechanism differs from that reported in the previous studies.
Material and methods
Drugs and chemicals
Umbelliferone was purchased from Sigma-Aldrich (USA). GNT was used as a commercially used ampule (Gramycin® 80/2 ml ampule; Schering/Memphis, Egypt). Assay kits for urea (CAT# 318001), creatinine (CAT# 235002), and uric acid (CAT# 323002) were purchased from Spectrum Company (Egypt). The albumin kit (CAT# 210001) was purchased from the Egyptian Company for Biotechnology, Cairo, Egypt. Rat KIM-1 (CAT# E-EL-R3019, detection range 31.25-2000 pg/ml), TNF-α (CAT# E-EL-R0019, detection range 78.13–5000 pg/ml), and IL-1β (CAT# E-EL-R0012, detection range 31.25–2000 pg/ml) ELISA kits were purchased from Elabscience Company (USA). Rat p-ERK1/2 (CAT# RAB0349) ELISA kit was purchased from Sigma-Aldrich (USA). The primary mouse polyclonal antibodies for western blot analysis were purchased from Santa Cruz (USA) while the β-actin antibody was purchased from Thermo Fisher (USA). Polyvinylidene difluoride (PVDF) membrane was purchased from Sigma-Aldrich (USA). Trizol reagent (Invitrogen, USA) was used for total RNA extraction while the cDNA synthesis kit was obtained from Thermo Fisher (USA). Other chemicals and reagents were obtained from a certified local source with higher analytical grades.
Experimental animals
Adult male Wistar rats weighing 170–200 g were purchased from the central animal house, Faculty of Medicine, Assiut University (Assiut, Egypt). The animals were maintained at normal atmospheric temperature (24 ± 2 °C) and relative humidity of 50–60% on a 12-h light/dark cycle. The rats were supplied with a standard laboratory diet of known composition and water ad libitum. All experimental procedures were approved by the Ethics Committee of the Faculty of Medicine, Assiut University (No. IRB17300463), and followed by guidelines for the care and use of laboratory animals declared by the National Institutes of Health Guide and Laboratory Animals Use (NIH Publications No. 8023, revised 1978).
Experimental design
Thirty-two rats were allocated into 4 groups, each comprising 8 rats, as follows:
Control group: Rats received vehicle only (0.5% carboxymethyl cellulose (CMC) by oral gavage for 15 days). UMB group: Rats received 50 mg/kg UMB once daily suspended in 0.5% CMC by oral gavage for 15 days. GNT group: rats received 0.5% CMC for 7 days and then injected with GNT (100 mg/kg i.p), once daily for 8 days (Abd-Elhamid et al. 2018; Ali et al. 2020). UMB + GNT group: Rats received 50 mg/kg UMB for 7 days before GNT administration and continued for 8 days with GNT treatment. UMB dose was selected according to previously published studies (Germoush et al. 2018; Mahmoud et al. 2017) and was further confirmed on the basis of our preliminary pilot trials.
Sample collection and preparation
At the end of the experiment, rats were sacrificed under ketamine (100 mg/kg, i.p) anesthesia and samples were collected via cardiac puncture. Blood samples were left to coagulate for serum preparation to assay urea, creatinine, and uric acid. Immediately after sacrifice, the kidneys were rapidly excised, rinsed in ice-cold saline, and weighed. Each kidney was cut into three parts. One part was fixed in 10% neutral buffered formalin for histopathological studies. The other part was rinsed and stored in RNA later solution (Ambient, UK) for real-time quantitative reverse transcription-polymerase chain reaction (Q-RT-PCR) for ERK1, ERK2, JAK1, STAT-3, TLR-4, NF-κB-p65, NLRP-3, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes. The last part from the kidney was kept frozen in liquid nitrogen and stored at - 80 °C for western blot analysis for NF-κB-p65, p38 mitogen–activated protein kinase (p38MAPK), STAT-3, p-STAT-3, nuclear factor of kappa light polypeptide gene enhancer in B cell inhibitor, alpha (IkBα), cleaved caspase-3, and β-actin.
Determination of renal somatic index (RSI)
The renal somatic index (RSI) was determined according to the previously described method (Ali et al. 2018a). In brief, kidneys were isolated and washed with phosphate buffer saline, dried with ashless filter paper, and weighed. Then, the RSI was calculated as follows:
RSI = kidney weight (g)/final body weight of rat (g) × 100
Determination of kidney function biomarkers
Serum urea, creatinine, and uric acid were assayed using commercial kits according to the manufacturer’s instructions. Serum creatinine level was measured depending on a method of Bartels et al. (1972), while the urea level was measured in serum by using a method of Fawcett and Scott (1960). Serum uric acid was assayed using the method was described by Fossati et al. (1980). Urine creatinine was measured as previously mentioned (Delanghe and Speeckaert 2011). Urine albumin was measured according to Doumas et al. (1971). The urine albumin/creatinine ratio defines the urine albumin excretion.
Determination of KIM-1 and pro-inflammatory cytokines, TNF-α, and IL-1β
The determination of urine KIM-1, as well as renal TNF-α, IL-1β, and p-ERK1/2, was performed in the kidney homogenate using commercial ELISA kits according to the manufacturer’s instructions following the principle of the method described by Van Weemen and Schuurs (1971).
Gene expression analyses by qRT-PCR
To evaluate the effect of UMB on GNT-induced nephrotoxicity, gene expressions of ERK1, ERK2, JAK1, STAT-3, TLR-4, NF-κB-p65, NLRP-3, and GAPDH were assayed using qRT-PCR as we previously reported (Hassanein et al. 2019). Total RNA was isolated from the kidney tissues using Trizol reagent (Invitrogen, USA). The quantity and quality of RNA samples were estimated using a SPECTROstarNano spectrometer (BMG Labtech, France) with A260/280 ratio of 1.9 or more and were selected for cDNA synthesis. Negative control was performed to test RNA contamination. cDNA was done using a reverse transcription kit (Thermo Fisher, USA) and then amplified using SYBR green master mix (Thermo Fisher, USA) and the primer set is listed in Table 1. Briefly, single-stranded cDNA was synthesized from 2 μg total RNA. The produced cDNA was used for quantifying target mRNA expression using real-time thermocycler PCR amplification. A total reaction of 20 μl was performed in the RT-PCR plate. The amplification reaction mixture contains 2.5 μl of cDNA, 2 μl of each primer (10 μM), 10 μl SYBR green master mix, and 3.5 μl of DNase-free water. The samples were incubated in the RT-PCR instrument as per one cycle and for 35 cycles of the following reactions: initial denaturation (95 °C, 3 min), denaturation (95 °C, 30 s), annealing (varies depending on the gene, 30 s), extension (72 °C, 40 s), and final extension (72 °C, 5 min). Each sample was measured in three parallel wells. Non-template blank wells were used for the identification of DNA contamination. The 2−ΔΔCt method (Livak and Schmittgen 2001) was used to analyze the qRT-PCR data and results were normalized to GAPDH.
Protein determination by western blot
Kidney samples were homogenized in RIPA buffer supplemented with proteinase inhibitors for studying the effect of UMB on the NF-κB-p65, IkBα, p38MAPK, STAT-3, p-STAT-3, and cleaved caspase-3 expression. Tissue homogenates were centrifuged at 12000 rpm for 10 min and the protein was determined according to the previously described method (Ali et al. 2018b). In total, 50 μg protein of each sample was subjected to SDS-PAGE followed by transfer onto PVDF membranes. The membranes were blocked in 5% skimmed milk in tris buffer saline/tween 20 (TBST) for 1 h at room temperature and then probed with primary antibodies for NF-κB-p65, IkBα, p38MAPK, STAT-3, p-STAT-3, cleaved caspase-3, and β-actin (Santa Cruz, USA) diluted 1:1000 in 0.1% TBST milk. After overnight incubation at 4 °C, the membranes were washed three times with TBST and incubated with alkaline phosphatase-conjugated secondary antibody (Santa Cruz, USA, dilution 1:5000). Bands were visualized by BCIP/NBT substrate detection Kit (Genemed Biotechnologies, San Francisco, USA). The produced bands were analyzed using the ImageJ® software (National Institutes of Health, Bethesda, USA). The results were normalized to β-actin and presented as a percent of control.
Tissue histopathology
For histopathological examination, kidney samples were fixed in buffered formalin solution (0.4% sodium phosphate monobasic, 0.64% sodium phosphate dibasic, and 10% formaldehyde in double-distilled water). Paraffin-embedded sections of each tissue (4 μm) were prepared and stained with hematoxylin and eosin (H&E) before light microscope viewing (Olympus CX21®, Japan). Each renal section was investigated blindly by a professional histopathologist to determine the changes in the tissue sections. Scoring of histopathological changes of kidney tissues stained with H&E was performed (Derelanko 2017). The scoring system was conducted as the following: normal appearance (−), mild changes (+), moderate changes (++), and severe changes (+++) and presented is in Table 2.
Statistical analysis for histological scoring was done for different fields, where each field was assigned a score concerning (degenerative and necrotic changes, blood vessel congestion, inflammatory cell infiltration, and intraluminal casts). Scoring followed the principle described earlier by Gibson-Corley et al. (2013), where scores 0, 1, 2, 3, and 4 denoted percentages of tissue area or cell number affected as being 0%, 1–25%, 26–50%, 51–75%, and 76–100%, respectively. Data of the histopathological scoring were presented as counts of ordinal values.
Statistical analysis
All statistical comparisons were made using the one-way ANOVA test followed by Tukey’s test post hoc analysis using GraphPad Prism (GraphPad Software, CA, USA). The results were presented as mean ± standard error of the mean (SEM). Statistical analysis of the assigned histopathological scores was performed using the chi-squared test. P value < 0.05 was considered significant.
Results
Effect of UMB on kidney function biomarkers in GNT-induced nephrotoxicity
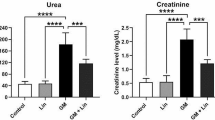
To evaluate the impact of GNT administration and treatment effect of UMB on kidney function of rats, we assessed serum creatinine, urea, and uric acid levels as well as urine KIM-1 and albumin/creatinine ratio. As clarified in Fig. 1, GNT administration to rats at a dose of 100 mg/kg significantly increased the serum levels of each creatinine, urea, and uric acid as well as urine KIM-1 level and albumin/creatinine ratio by 298.8%, 416.8%, 154.4%, 331.1%, and 192.5% respectively, compared to normal control rats, indicated kidney dysfunctions. On the other hand, GNT rats that received treatment with UMB exhibited a significant decrease in serum creatinine and urea levels as well as urine KIM-1 and albumin/creatinine ratio by 46.9%, 52.1%, 53.9%, and 47.3% respectively, compared to GNT induction rats. A remarkable decrease in uric acid nearly back to the normal level was also noticed in GNT rats that were treated with UMB.
UMB restored renal function biomarkers in the GNT-induced nephrotoxicity model. Serum levels of urea (a), creatinine (b), and uric acid (c) as well as urine albumin/creatinine ratio (d) were assessed colorimetric while urine KIM-1 (e) was assessed by ELISA. Data were presented as mean ± S.E.M (n = 8). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multi-comparison test. *Significantly different from the control group at P < 0.05. #Significantly different between the GNT and UMB + GNT group at P < 0.05. UMB, umbelliferone; GNT, gentamicin; KIM-1, kidney injury molecule-1
Effect of UMB on RSI in GNT-induced nephrotoxicity
Concerning initial body weights, there are no significant differences between control, UMB, GNT, and UMB + GNT groups. The RSI value in the GNT group was significantly higher than that of the normal control group by 46% indicating renal edema and inflammation. On the contrary, RSI was revealed back to the normal value in the GNT + UMB group in comparison to the GNT induction group (Table 3).
Effect of UMB on tissue expression of ERK1/ERK2 and cleaved caspase-3 in GNT-induced nephrotoxicity
To illustrate the role of ERK1/ERK2 expression in GNT-induced nephrotoxicity, gene expressions of ERK1 and ERK2 were determined by qRT-PCR as well as p-ERK1/2 by ELISA. As illustrated in Fig. 2, mRNA expressions of ERK1 and ERK2 as well as protein expression of p-ERK1/2 were significantly increased by 9.09-, 9.52-, and 4.26-fold, respectively, in rats that received GNT alone when compared to normal control rats. On the other hand, treatment with UMB significantly reduced tissue expressions of ERK1 by - 0.40-fold, ERK2 by - 0.77-fold, and p-ERK1/2 by - 0.63-fold when compared with rats that received GNT alone. Additionally, our data revealed that UMB successfully decreased the cleaved caspase-3 expression by - 0.88-fold as compared to the GNT control group, and hence, the mechanism by which UMB protects against apoptosis injury may be via the ERK1/2/caspase-3 pathway in GNT-induced nephrotoxicity in rats (Fig. 4).
UMB downregulated ERK1/2 expressions in the GNT-induced nephrotoxicity model. The mRNA expressions of ERK1 (a) and ERK2 (b) were determined by qRT-PCR while p-ERK1/2 (c) was measured using ELISA. Data were presented as mean ± S.E.M (n = 6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multi-comparison test. *Significantly different from the control group at P < 0.05. #Significantly different between the GNT and UMB + GNT group at P < 0.05. UMB, umbelliferone; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; GNT, gentamicin
Effect of UMB on tissue expression of TLR-4/p38MAPK in GNT-induced nephrotoxicity
TLR-4/p38MAPK was assessed to evaluate its impact on nephrotoxicity pathogenesis. The mRNA expression of TLR-4 (Fig. 3b) and the protein expression of p38MAPK (Fig. 4) were markedly increased by 10.03- and 2.79-fold, respectively, in the kidney of rats that received GNT as compared to the control rats. Rats received 50 mg/kg of UMB with GNT showed a significant downregulation of the expression of TLR-4 by - 0.70-fold and p38MAPK by - 0.71-fold as compared to GNT control rats.
UMB suppressed NLRP-3 inflammasome activation in the GNT-induced nephrotoxicity model. The mRNA expressions of NLRP-3 (a), TLR-4 (b), and NF-κB-p65 (c) were investigated by qRT-PCR. Data were presented as mean ± S.E.M (n = 6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multi-comparison test. *Significantly different from the control group at P < 0.05. #Significantly different between the GNT and UMB + GNT group at P < 0.05. UMB, umbelliferone; NLRP-3, nucleotide-binding domain (NOD)-like receptor protein-3; GNT, gentamicin; TLR-4, toll-like receptor-4; NF-κB-p65, nuclear factor-kappa B-p65
UMB inhibited the inflammatory burden in the GNT-induced nephrotoxicity model. The protein expressions of p38 MAPK, NF-κB-p65, IκBα, and cleaved caspase-3 were investigated by western blot analysis (a). Densitometric analyses of p38 MAPK (b), NF-κB-p65 (c), IκBα (d), and cleaved caspase-3 (e) were performed relative to β-actin. Data were presented as mean ± S.E.M (n = 6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multi-comparison test. *Significantly different from the control group at P < 0.05. #Significantly different between the GNT and UMB + GNT group at P < 0.05. UMB, umbelliferone; GNT, gentamicin; p38 MAPK, p38 mitogen-activated protein kinase; NF-κB-p65, nuclear factor-kappa B-p65; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha
Effect of UMB on activation of NF-κB/IκBα/NLRP-3 inflammasome pathway in GNT-induced nephrotoxicity
To investigate the possible involvement of NF-κB-p65/NLRP-3 inflammasome axis in GNT-induced nephrotoxicity and the therapeutic role of UMB, the protein expressions of NF-κB-p65 and IκBα, as well as the mRNA expressions of NF-κB-p65 and NLRP-3, were determined in the kidney. As represented in Figs. 3 and 4, the administration of GNT resulted in a significant upregulation of NF-κB- p65 expression by 2.73-fold and NLRP-3 expression by 4.39-fold in rat kidney when compared to the kidney of normal rats. Treatment with UMB (50 mg/kg) produced a notable suppression of kidney expressions of NF-κB-p65 by - 0.63-fold and NLRP-3 by - 0.64-fold as compared to rats that received GNT alone. Moreover, the mechanism of the inhibitory effect of UBM on the GNT-induced nephrotoxicity of IκBα was clarified, where rats received GNT alone exhibited a significant suppression in IκBα expression by - 0.64-fold when compared to normal control rats. Conversely, in comparing with the GNT control group, GNT rats that were co-treated with UMB significantly augmented the protein expression level of IκBα (Fig. 4).
Effect of UMB on kidney levels of TNF-α and IL-1β in GNT-induced nephrotoxicity
Renal inflammatory biomarkers represented as TNF-α and IL-1β were significantly induced by injection of GNT to rats (136.8% and 271.4%, respectively) when compared to normal control rats. Besides, the administration of UMB to rats that previously received GNT significantly reduced tissue levels of both TNF-α and IL-1β by 37.5% and 36.6% respectively, in comparison to rats that received GNT alone. Moreover, tissue levels of TNF-α and IL-1β were nearly restored to normal levels in the GNT + UMB group in comparison to those in the normal control group (Fig. 5a and b).
UMB inhibited inflammatory response in the GNT-induced nephrotoxicity model. The protein expressions of TNF-α (a) and IL-1β (b) were estimated by ELISA. Data were presented as mean ± S.E.M (n = 8). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multi-comparison test. *Significantly different from the control group at P < 0.05. #Significantly different between the GNT and UMB + GNT group at P < 0.05. UMB, umbelliferone; GNT, gentamicin; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1 beta
Effect of UMB on activation of JAK1/STAT-3 pathway in GNT-induced nephrotoxicity
To explore the possible pathogenic role of the JAK1/STAT-3 pathway and the potential therapeutic effect of UMB in GNT-induced nephrotoxicity, mRNA and protein expressions were assessed. As represented in Fig. 6, GNT administration produced a significant upregulation of mRNA expressions of JAK1 and STAT-3 by 19.18- and 13.55-fold, respectively, as well as the protein expressions of STAT-3 and p-STAT-3 by 4.45- and 4.84-fold, respectively, in rats in comparison to normal controls. UMB treatment significantly counteracts this upregulation and suppressed kidney levels of JAK1, STAT-3, and p-STAT-3 nearly back to normal levels when compared to the GNT induction group.
UMB attenuated the renal JAK1/STAT-3 pathway in the GNT-induced nephrotoxicity model. The mRNA expressions of JAK1 (a) and STAT-3 (b) were measured by qRT-PCR. The protein expressions of STAT-3 and p-STAT-3 were investigated by western blot analysis (c). Densitometric analysis of STAT-3 (d) and p-STAT-3 (e) were performed relative to β-actin. Data were presented as mean ± S.E.M (n = 6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multi-comparison test. *Significantly different from the control group at P < 0.05. #Significantly different between the GNT and UMB + GNT group at P < 0.05. UMB, umbelliferone; JAK1, janus kinase 1; STAT-3, signal transducers and activators of transcription-3; GNT, gentamicin
Histopathological findings
The ameliorative effect of UMB on GNT-induced nephrotoxicity was further confirmed by the histological findings. As revealed in Fig. 7 and Table 2, the examination of kidney’s sections of the negative control group and UMB group revealed normal morphological features of renal parenchyma with apparent intact nephron segments at different zones as well as renal corpuscle and normal vasculatures. In contrast, rats administered GNT alone showed severe diffuse degenerative and necrotic changes of lining tubular epithelium (arrow) accompanied by many dilated and congested intertubular blood vessels (star). Moderate interstitial inflammatory cell infiltration was also recorded (red arrow) along with occasional intraluminal eosinophilic casts (dashed arrow). On the other hand, kidney sections of the UMB + GNT group showed a significant protective efficacy with a decrease of degenerative changes records in some tubular epithelium, and minimal intraluminal cast formation was recorded.
UMB attenuated renal histological changes in the GNT-induced nephrotoxicity model. a A photomicrograph of renal tissue obtained from the control group demonstrated normal morphological features of renal parenchyma with apparent intact nephron segments at different zones as well as renal corpuscle and normal vasculatures. b A photomicrograph of renal tissue obtained from the UMB group exhibited the same histological structure as the control group. c1, c2, and c3 Photomicrographs of renal tissue obtained from the GNT control group showed severe diffuse degenerative and necrotic changes of lining tubular epithelium accompanied by many dilated and congested intertubular blood vessels, moderate interstitial inflammatory cell infiltration, and occasional intraluminal eosinophilic casts. d A photomicrograph of renal tissue obtained from the UMB + GNT group showed a significant protective efficacy with a decrease of degenerative change records in some tubular epithelium and minimal intraluminal cast formation was recorded. Scale bar = 20 μm. e Represents statistical analysis for histological scoring of different fields. Data were presented as mean ± S.E.M (n = 6). Statistical analysis of the assigned histopathological scores was performed using the chi-squared test. UMB, umbelliferone; GNT, gentamicin
Discussion
Nephrotoxicity is a common kidney feature in which kidney-specific detoxification and excretion do not work properly due to the damage or destruction of kidney function owing to exogenous or endogenous nephrotoxic agents (Perazella and Moeckel 2010). Drug-induced nephrotoxicity is a common complication of several medications which contributed to up to 20% of injured kidney hospital admissions (Kim and Moon 2012; McWilliam et al. 2017). One of the most nephrotoxic agents is aminoglycoside which was developed in the 1940s and is known as one of the oldest antibiotics used to treat serious infections caused by Gram-negative and some Gram-positive bacteria (Wargo and Edwards 2014).
Despite the nephrotoxic effect of aminoglycosides, the emergence of bacterial resistance to commonly used antibiotics has necessitated retention of the aminoglycosides as a viable treatment option (Katary and Salahuddin 2017; Mingeot-Leclercq and Tulkens 1999). Consequently, these encouraged more researches to force against possible ways to ameliorate the nephrotoxic effect of aminoglycosides.
In the present study, aminoglycosides represented by GNT were used as a proven model for nephrotoxicity induction in rats. Intraperitoneal injection of GNT caused severe renal injury and impaired kidney function evidenced by a significant increase in serum creatinine, urea, and uric acid levels. In the same manner, previous studies proved the alteration of kidney function and induction of nephrotoxicity by GNT (Stojiljkovic et al. 2012). UMB treatment significantly improves GNT-induced elevations in serum levels of creatinine, urea, and uric acid as well as effectively decreased urine KIM-1 level and albumin/creatinine ratio. In agreement, Garud and Kulkarni (2017) reported that UMB decreased the level of serum creatinine and urea in the diabetic rat model. Also, Hassanein et al. (2018) stated that UMB significantly reduced serum levels of creatinine and urea in nephrotoxicity induced by methotrexate in rats (Hassanein et al. 2018). In this regard, the present results demonstrated that UMB can restore kidney functions particularly due to attenuation of the inflammatory process and consequence stabilization of renal tubular functions indicating nephroprotective effect.
Our present study revealed that GNT induced several histopathological changes including severe diffuse degenerative and necrotic changes of lining tubular epithelium with many dilated and congested intertubular blood vessels along with infiltration of inflammatory cells and occasional intraluminal eosinophilic casts. Additionally, these results were confirmed by increasing RSI of the GNT induction group which indicates remarkable kidney hypertrophy and inflammation. Interestingly, UMB treatment alleviated all histopathological changes induced by GNT in parallel with the reduction in RSI. Modification effects of UMB on renal function tests, histopathological findings, and RSI indicate the renoprotective properties of UMB against GNT-induced renal injury. These results are in accordance with other previous reports (Abd-Elhamid et al. 2018; Wang et al. 2019).
Much attention has been focused on the molecular mechanisms and mediators underlying the inflammatory injury of GNT-induced nephrotoxicity. Despite the role of ERK in cell proliferation and survival, accumulated evidence demonstrated a crucial role of ERK activation in cephaloridine-induced renal cell injury (Kohda et al. 2003; Kohda et al. 2005). Additionally, the upregulation of ERK has been confirmed to induce renal damage through activation of caspase-3 and apoptotic reactions (Huang et al. 2017). The current study also demonstrated that UMB effectively decreased the elevated level of cleaved caspase-3 and hence the mechanism by which UMB protects against apoptosis injury may be via the ERK1/2/caspase-3 pathway in GNT-induced nephrotoxicity in rats. In accordance with that, this study documented a significant increase in renal tissue expression of ERK1 and ERK2 following GNT injection in rats that illustrated the evident role of ERK1 and ERK2 in GNT-induced renal damage. However, treatment of GNT rats with UMB exhibited an obvious effect on suppressing ERK1 and ERK2 renal expression. Previous studies demonstrated the inhibitory effect of UMB on ERK expression in many inflammatory disorders like rheumatoid arthritis via modulation of the MAPK/NF-κB signaling pathway (Ouyang et al. 2019).
Toll-like receptors have an essential impact on the innate inflammatory process. TLR family contains 10 members (TLR1-TLR10), and among which TLR-4 is presented as the most famous one (Moresco et al. 2011). Stimulation of TLR-4/p38MAPK was conducted to the inflammatory cascade along with activation of the key downstream effector NF-κB and its mediated inflammatory responses (Dou et al. 2013). NF-κB exists in the cytoplasm as an inactive complex with the inhibitory protein IkBα. Activation of NF-κB involves IkBα phosphorylation, with NF-κB translocation to the nucleus (Lisi et al. 2012). Phosphorylation and nuclear translocation of the NF-κB p65 subunit are required for NF-κB transcriptional activity which in turn regulate a diverse array of cellular processes including immunological responses, inflammation, apoptosis, growth, and development reactions that are involved in renal injury (Kumar et al. 2015a). The current study stated that GNT administration to rats significantly increased renal expression of TLR-4/p38MAPK and NF-κB-p65. These results matched with the results of Lee et al. (2014) who illustrated that acute renal injury is associated with TLR-4/NF-κB pathway activation in different kidney models including the GNT induction model. Dependently, GNT causes a significant inhibition in IkBα phosphorylation which negatively regulates NF-κB translocation. Again, this data was confirmed by the results of Stacey et al. (1997) who reported that promotion of the activation of transcriptional factor NF-kB depends on interference with its plasmic inhibitor IkBα. On the contrary, our results revealed that UMB treatment resulted in the suppression of the TLR-4/NF-kB-p65 pathway with concomitant stimulation of the plasmic inhibitor IkBα. Similarly, Zambon and Vincent (2008) indicated that UMB exerted a significant protective effect on lipopolysaccharide-induced acute lung injury by inhibiting the activation of the TLR-4/Myeloid differentiation primary response 88/NF-κB pathway. From these data, we speculated that UMB can protect against GNT-induced renal injury through the inhibition of the TLR-4/p38MAPK and TLR-4/NF-kB-p65 pathways.
Side by side, the upregulation of NF-κB-p65 signaling plays a major role in many inflammatory and autoimmune responses (Liu et al. 2017). One of these responses is NLRP-3, which is a key sensor of tissue damage and therefore plays a major role in the activation of sterile inflammation (Mezzasoma et al. 2016). After stimulation, the NLRP-3 inflammasome undergoes self-cleavage and activation of many inflammatory precursors like IL-1β and TNF-α (Afonina et al. 2017). Our study showed that GNT targeted the activation of NLRP-3 inflammasome through the stimulation of the TLR-4/NF-kB-p65 pathway. In contrast, UMB treatment exhibited a notable inhibition in NLRP-3 expression. In the same line with our results, a previous study mentioned the role of UMB in the amelioration of myocardial injury through suppression of NLRP-3 inflammasome (Luo et al. 2018).
Additionally, a previous report clarified that the activation of the TLR-4/NF-kB-p65/NLRP-3 signaling pathway controls the expressions of pro-inflammatory cytokines (He et al. 2016). The current study noted that GNT can produce a maximal reduction in NF-κB inhibitor (IkBα) protein level leading to an elevation in renal cytoplasmic protein expression of NF-κB and nuclear NF-κB-DNA binding activity resulting in stimulating TNF-α and IL-1β synthesis. These results are in line with previous data which proved that GNT treatment stimulates macrophage infiltration and elevation of TNF-α and IL-1β level which was produced by renal mesangial and epithelial cells and involved in the regulation of immune and inflammatory responses (Reis et al. 2012). In the current study, co-treatment of GNT rats with UMB significantly attenuated renal damage induced by TNF-α and IL-1β cytokine production. This anti-inflammatory action was demonstrated by the work of Sim et al. (2015) who indicated that UMB possesses anti-inflammatory activity and protects against alcohol-induced liver damage by inhibiting inflammatory cytokine production. Taken together, UMB can attenuate TLR-4/NF-κB-p65/NLRP-3 pathway activation and, in turn, suppress inflammatory cytokine–induced kidney damage, such as TNF-α and IL-1β.
Interestingly, the JAK1/STAT-3 is another pathway that is greatly implicated in the pathogenesis of many inflammatory and immunity diseases including renal disease (Banerjee et al. 2017). The circulating pro-inflammatory cytokines initiate downstream signal transduction through the activation of the JAK/STAT pathway where phosphorylated STAT proteins become a potent transcription factor for specific STAT-target genes (Malemud and Pearlman 2009). Therefore, the current study investigated the effect of GNT, as an induction agent, and UMB, as a suppressor agent, on JAK1/STAT-3 renal expressions. We noted that the GNT administration significantly induced renal expression of JAK1, STAT-3, and p-STAT-3. It is worth mentioning that our study was the first to demonstrate the role of the JAK1/STAT-3 pathway in the GNT-induced nephrotoxicity model. Meanwhile, the treatment with UMB prohibited this activation and opposed the GNT-induced JAK1/STAT-3 stimulation.
The hang-up in all of these data together shed light on the therapeutic role of UMB against GNT-induced nephrotoxicity which may rely on amelioration of certain inflammatory pathways including ERK1/ERK2, TRL-4/NF-κB-p65/NLRP-3, and JAK1/STAT-3 signaling pathways, suggesting that the aberrant GNT-induced nephrotoxicity may contribute to the initiation of these signaling pathways.
Conclusion
Conclusively, the present study suggested that renal toxicity induced by GNT may contribute to activation of each ERK1/ERK2, TRL4/NF-κB-p65/NLRP-3, and JAK1/STAT-3 signaling pathway along with their massive cytokine production. Furthermore, UMB treatment showed a prospective therapeutic impact against these pathways. These findings may provide new insights into the molecular mechanisms underlying the action of UMB in the treatment of GNT-induced nephrotoxicity. However, further studies are warranted before clinical application.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CMC:
-
Carboxymethyl cellulose
- ERK:
-
Extracellular signal-regulated protein kinases
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GNT:
-
Gentamicin
- IkBα:
-
Nuclear factor of kappa light polypeptide gene enhancer in B cell inhibitor-alpha
- IL-1β:
-
Interleukin-1 beta
- JAK:
-
Janus kinase
- KIM-1:
-
Kidney injury molecule-1
- NF-κB-p65:
-
Nuclear factor-kappa B-p65
- NLRP-3:
-
Nucleotide-binding domain (NOD)-like receptor protein-3
- P38 MAPK:
-
p38 mitogen-activated protein kinase
- STAT-3:
-
Signal transducers and activators of transcription-3
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor-alpha
- UMB:
-
Umbelliferone
References
Abd-Elhamid TH, Elgamal DA, Ali SS, Ali FEM, Hassanein EHM, El-Shoura EAM, Hemeida RAM (2018) Reno-protective effects of ursodeoxycholic acid against gentamicin-induced nephrotoxicity through modulation of NF-κB, eNOS and caspase-3 expressions. Cell Tissue Res 374:367–387. https://doi.org/10.1007/s00441-018-2886-y
Abdeen A, Sonoda H, El-Shawarby R, Takahashi S, Ikeda M (2014) Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am J Physiol Ren Physiol 307:F1227–F1237. https://doi.org/10.1152/ajprenal.00140.2014
Afonina IS, Zhong Z, Karin M, Beyaert R (2017) Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol 18:861–869. https://doi.org/10.1038/ni.3772
Ali FEM, Azouz AA, Bakr AG, Abo-Youssef AM, Hemeida RAM (2018a) Hepatoprotective effects of diosmin and/or sildenafil against cholestatic liver cirrhosis: the role of Keap-1/Nrf-2 and P(38)-MAPK/NF-κB/iNOS signaling pathway. Food Chem Toxicol 120:294–304. https://doi.org/10.1016/j.fct.2018.07.027
Ali FEM, Bakr AG, Abo-Youssef AM, Azouz AA, Hemeida RAM (2018b) Targeting Keap-1/Nrf-2 pathway and cytoglobin as a potential protective mechanism of diosmin and pentoxifylline against cholestatic liver cirrhosis. Life Sci 207:50–60. https://doi.org/10.1016/j.lfs.2018.05.048
Ali FEM, Hassanein EHM, Bakr AG, El-Shoura EAM, El-Gamal DA, Mahmoud AR, Abd-Elhamid TH (2020) Ursodeoxycholic acid abrogates gentamicin-induced hepatotoxicity in rats: Role of NF-κB-p65/TNF-α, Bax/Bcl-xl/Caspase-3, and eNOS/iNOS pathways. Life Sci 254:117760. https://doi.org/10.1016/j.lfs.2020.117760
Balakumar P, Rohilla A, Thangathirupathi A (2010) Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacol Res 62:179–186. https://doi.org/10.1016/j.phrs.2010.04.004
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM (2017) JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 77:521–546. https://doi.org/10.1007/s40265-017-0701-9
Bartels H, Böhmer M, Heierli C (1972) Serum creatinine determination without protein precipitation. Clin Chim Acta 37:193–197. https://doi.org/10.1016/0009-8981(72)90432-9
Blunston MA, Yonovitz A, Woodahl EL, Smolensky MH (2015) Gentamicin-induced ototoxicity and nephrotoxicity vary with circadian time of treatment and entail separate mechanisms. Chronobiol Int 32:1223–1232. https://doi.org/10.3109/07420528.2015.1082483
Chuang PY, He JC (2010) JAK/STAT signaling in renal diseases. Kidney Int 78:231–234. https://doi.org/10.1038/ki.2010.158
Coutinho AGG, Biscaia SMP, Fernandez R, Tararthuch AL (2018) The aminoglycoside antibiotic gentamicin is able to alter metabolic activity and morphology of MDCK-C11 cells: a cell model of intercalated cells Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 51:e7417 https://doi.org/10.1590/1414-431x20187417
Delanghe JR, Speeckaert MMJNDTP (2011) Creatinine determination according to Jaffe—what does it stand for? 4:83-86
Derelanko MJ (2017) The toxicologist's pocket handbook. CRC Press
Dou W et al (2013) Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br J Nutr 110:599–608. https://doi.org/10.1017/s0007114512005594
Doumas BT, Watson WA, Biggs HGJCca (1971) Albumin standards and the measurement of serum albumin with bromcresol green 31:87-96
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159. https://doi.org/10.1136/jcp.13.2.156
Fossati P, Prencipe L, Berti G (1980) Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26:227–231
Garud MS, Kulkarni YA (2017) Attenuation of renal damage in type I diabetic rats by umbelliferone - a coumarin derivative Pharmacological reports : PR 69:1263-1269 https://doi.org/10.1016/j.pharep.2017.06.014
Germoush MO et al (2018) Umbelliferone prevents oxidative stress, inflammation and hematological alterations, and modulates glutamate-nitric oxide-cGMP signaling in hyperammonemic rats. Biomed Pharmacother 102:392–402. https://doi.org/10.1016/j.biopha.2018.03.104
Gibson-Corley KN, Olivier AK, Meyerholz DK (2013) Principles for valid histopathologic scoring in research Veterinary pathology 50:1007-1015 https://doi.org/10.1177/0300985813485099
González-Guerrero C, Cannata-Ortiz P, Guerri C, Egido J, Ortiz A, Ramos AM (2017) TLR4-mediated inflammation is a key pathogenic event leading to kidney damage and fibrosis in cyclosporine nephrotoxicity. Arch Toxicol 91:1925–1939. https://doi.org/10.1007/s00204-016-1830-8
Hassanein EHM, Mohamed WR, Shalkami AS, Khalaf MM, Hemeida RAM (2018) Renoprotective effects of umbelliferone on methotrexate-induced renal injury through regulation of Nrf-2/Keap-1, P(38)MAPK/NF-κB, and apoptosis signaling pathways. Food Chem Toxicol 116:152–160. https://doi.org/10.1016/j.fct.2018.03.041
Hassanein EHM, Shalkami AS, Khalaf MM, Mohamed WR, Hemeida RAM (2019) The impact of Keap1/Nrf2, P(38)MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed Pharmacother 109:47–56. https://doi.org/10.1016/j.biopha.2018.10.088
He X, Wei Z, Wang J, Kou J, Liu W, Fu Y, Yang Z (2016) Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis. Sci Rep 6:28370. https://doi.org/10.1038/srep28370
Huang YC et al (2017) Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol Appl Pharmacol 329:128–139. https://doi.org/10.1016/j.taap.2017.05.034
Katary M, Salahuddin A (2017) Ameliorative effect of gossypin against gentamicin-induced nephrotoxicity in rats. Life Sci 176:75–81. https://doi.org/10.1016/j.lfs.2017.03.009
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Front Immunol 5:461. https://doi.org/10.3389/fimmu.2014.00461
Kim SY, Moon A (2012) Drug-induced nephrotoxicity and its biomarkers. Biomol Ther 20:268–272. https://doi.org/10.4062/biomolther.2012.20.3.268
Kohda Y, Hiramatsu J, Gemba M (2003) Involvement of MEK/ERK pathway in cephaloridine-induced injury in rat renal cortical slices. Toxicol Lett 143:185–194. https://doi.org/10.1016/s0378-4274(03)00174-7
Kohda Y, Kawai Y, Iwamoto N, Matsunaga Y, Aiga H, Awaya A, Gemba M (2005) Serum thymic factor, FTS, attenuates cisplatin nephrotoxicity by suppressing cisplatin-induced ERK activation. Biochem Pharmacol 70:1408–1416. https://doi.org/10.1016/j.bcp.2005.08.002
Kostova I, Bhatia S, Grigorov P, Balkansky S, Parmar VS, Prasad AK, Saso L (2011) Coumarins as antioxidants. Curr Med Chem 18:3929–3951. https://doi.org/10.2174/092986711803414395
Kumar D, Singla SK, Puri V, Puri S (2015a) The restrained expression of NF-kB in renal tissue ameliorates folic acid induced acute kidney injury in mice. PLoS One 10:e115947. https://doi.org/10.1371/journal.pone.0115947
Kumar V, Ahmed D, Anwar F, Ali M, Mujeeb M (2013) Enhanced glycemic control, pancreas protective, antioxidant and hepatoprotective effects by umbelliferon-α-D-glucopyranosyl-(2(I) → 1(II))-α-D-glucopyranoside in streptozotocin induced diabetic rats. SpringerPlus 2:639. https://doi.org/10.1186/2193-1801-2-639
Kumar V, Anwar F, Verma A, Mujeeb M (2015b) Therapeutic effect of umbelliferon-α-D-glucopyranosyl-(2(I) → 1(II))-α-D-glucopyranoside on adjuvant-induced arthritic rats. J Food Sci Technol 52:3402–3411. https://doi.org/10.1007/s13197-014-1403-x
Lee JW et al (2014) Renoprotective effect of paricalcitol via a modulation of the TLR4-NF-κB pathway in ischemia/reperfusion-induced acute kidney injury. Biochem Biophys Res Commun 444:121–127. https://doi.org/10.1016/j.bbrc.2014.01.005
Lisi S, Sisto M, Lofrumento DD, D’Amore M (2012) Altered IkBα expression promotes NF-kB activation in monocytes from primary Sjögren’s syndrome patients Pathology 44:557-561 https://doi.org/10.1097/PAT.0b013e3283580388
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method Methods (San Diego, Calif) 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ (2011) New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 79:33–45. https://doi.org/10.1038/ki.2010.337
Luo H, Fan Z, Xiang D, Jiang Z, Zhang W, Gao L, Feng C (2018) The protective effect of umbelliferone ameliorates myocardial injury following ischemia-reperfusion in the rat through suppression NLRP3 inflammasome and upregulating the PPAR-γ Molecular medicine reports 17:3404-3410 https://doi.org/10.3892/mmr.2017.8208
Mahmoud AM, Germoush MO, Alotaibi MF, Hussein OE (2017) Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed Pharmacother 86:297–306. https://doi.org/10.1016/j.biopha.2016.12.047
Mahmoud AM, Hussein OE, Abd El-Twab SM, Hozayen WG (2019) Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct 10:4593–4607. https://doi.org/10.1039/c9fo00114j
Malemud CJ, Pearlman EJCSTT (2009) Targeting JAK/STAT signaling pathway in inflammatory diseases 4:201-221
McWilliam SJ, Antoine DJ, Smyth RL, Pirmohamed M (2017) Aminoglycoside-induced nephrotoxicity in children. Pediatric Nephrology (Berlin, Germany) 32:2015–2025. https://doi.org/10.1007/s00467-016-3533-z
Mezzasoma L, Antognelli C, Talesa VN (2016) Atrial natriuretic peptide down-regulates LPS/ATP-mediated IL-1β release by inhibiting NF-kB, NLRP3 inflammasome and caspase-1 activation in THP-1 cells. Immunol Res 64:303–312. https://doi.org/10.1007/s12026-015-8751-0
Mingeot-Leclercq MP, Tulkens PM (1999) Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother 43:1003–1012
Moresco EM, LaVine D, Beutler B (2011) Toll-like receptors Current biology : CB 21:R488-R493 doi:https://doi.org/10.1016/j.cub.2011.05.039
Ouyang L et al (2019) Effect of umbelliferone on adjuvant-induced arthritis in rats by MAPK/NF-κB pathway. Drug Des Devel Ther 13:1163–1170. https://doi.org/10.2147/dddt.S190155
Perazella MA, Moeckel GW (2010) Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol 30:570–581. https://doi.org/10.1016/j.semnephrol.2010.09.005
Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N (2012) Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One 7:e44092. https://doi.org/10.1371/journal.pone.0044092
Sahu BD et al (2014) Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats: possible mechanism of nephroprotection. Toxicol Appl Pharmacol 277:8–20. https://doi.org/10.1016/j.taap.2014.02.022
Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L, Liu C, Wang J, Yang X, Vohra A, Ma D (2013) Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med 11:141. https://doi.org/10.1186/1479-5876-11-141
Sim MO, Lee HI, Ham JR, Seo KI, Kim MJ, Lee MK (2015) Anti-inflammatory and antioxidant effects of umbelliferone in chronic alcohol-fed rats. Nutr Res Pract 9:364–369. https://doi.org/10.4162/nrp.2015.9.4.364
Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S (1997) The allergen Der p1 induces NF-kappaB activation through interference with IkappaB alpha function in asthmatic bronchial epithelial cells. Biochem Biophys Res Commun 236:522–526. https://doi.org/10.1006/bbrc.1997.6997
Stojiljkovic N, Stoiljkovic M, Randjelovic P, Veljkovic S, Mihailovic D (2012) Cytoprotective effect of vitamin C against gentamicin-induced acute kidney injury in rats. Exp Toxicol Pathol 64:69–74. https://doi.org/10.1016/j.etp.2010.06.008
Van Weemen BK, Schuurs AH (1971) Immunoassay using antigen-enzyme conjugates FEBS letters 15:232-236 doi:https://doi.org/10.1016/0014-5793(71)80319-8
Vijayalakshmi A, Sindhu G (2017) Umbelliferone arrest cell cycle at G0/G1 phase and induces apoptosis in human oral carcinoma (KB) cells possibly via oxidative DNA damage. Biomed Pharmacother 92:661–671. https://doi.org/10.1016/j.biopha.2017.05.128
Wang HQ, Wang SS, Chiufai K, Wang Q, Cheng XL (2019) Umbelliferone ameliorates renal function in diabetic nephropathy rats through regulating inflammation and TLR/NF-κB pathway. Chin J Nat Med 17:346–354. https://doi.org/10.1016/s1875-5364(19)30040-8
Wang X, Li R, Wang X, Fu Q, Ma S (2015) Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci Lett 600:182–187. https://doi.org/10.1016/j.neulet.2015.06.016
Wargo KA, Edwards JD (2014) Aminoglycoside-induced nephrotoxicity. J Pharm Pract 27:573–577. https://doi.org/10.1177/0897190014546836
Woods JS, Dieguez-Acuña FJ, Ellis ME, Kushleika J, Simmonds PL (2002) Attenuation of nuclear factor kappa B (NF-kappaB) promotes apoptosis of kidney epithelial cells: a potential mechanism of mercury-induced nephrotoxicity Environ Health Perspect 110 Suppl 5:819-822 doi:https://doi.org/10.1289/ehp.02110s5819
Zambon M, Vincent JL (2008) Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133:1120–1127. https://doi.org/10.1378/chest.07-2134
Acknowledgments
The authors are greatly indebted to Dr. Mohamed Abdelrazik, Lecturer of Veterinary Cytology & Histology, Faculty of Veterinary Medicine, Cairo University, Egypt, for his kind help in the histopathological investigations.
Author information
Authors and Affiliations
Contributions
Conception and experimental design: FEMA and EHMH. Statistical analysis: MRK. Writing of the manuscript: EHMH and OAMA. The final version of the manuscript has been approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of the Faculty of Medicine, Assiut University (No. IRB17300463), and followed the guidelines for the care and use of laboratory animals declared by the National Institutes of Health Guide and Laboratory Animals Use (NIH Publications No. 8023, revised 1978).
Consent for publication
The authors declared that the final version of the manuscript has been reviewed, approved, and consented for publication.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassanein, E.H.M., Ali, F.E.M., Kozman, M.R. et al. Umbelliferone attenuates gentamicin-induced renal toxicity by suppression of TLR-4/NF-κB-p65/NLRP-3 and JAK1/STAT-3 signaling pathways. Environ Sci Pollut Res 28, 11558–11571 (2021). https://doi.org/10.1007/s11356-020-11416-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11416-5