Abstract
Gentamicin (GM) is an aminoglycoside antibiotic used to treat bacterial infections. However, its application is accompanied by renal impairments. Apigenin is a flavonoid found in many edible plants with potent therapeutic values. This study was designed to elucidate the therapeutic effects of apigenin on GM-induced nephrotoxicity. Animals were injected orally with three different doses of apigenin (5 mg kg−1 day−1, 10 mg kg−1 day−1, and 20 mg kg−1 day−1). Apigenin administration abolished the alterations in the kidney index and serum levels of kidney-specific functions markers, namely blood urea nitrogen and creatinine, and KIM-1, NGAL, and cystatin C following GM exposure. Additionally, apigenin increased levels of enzymatic (glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase) and non-enzymatic antioxidant proteins (reduced glutathione) and decreased levels of lipid peroxide, nitric oxide, and downregulated nitric oxide synthase-2 in the kidney tissue following GM administration. At the molecular scope, apigenin administration was found to upregulate the mRNA expression of Nfe2l2 and Hmox1 in the kidney tissue. Moreover, apigenin administration suppressed renal inflammation and apoptosis by decreasing levels of interleukin-1β, tumor necrosis factor-alpha, nuclear factor kappa-B, Bax, and caspase-3, while increasing B-cell lymphoma-2 compared with those in GM-administered group. The recorded data suggests that apigenin treatment could be used to alleviate renal impairments associated with GM administration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gentamicin (GM) is an aminoglycoside antibiotic commonly used for treating severe bacterial infections (Makama et al. 2018). Among aminoglycosides, GM possesses the most adverse effects, including oxidative stress and renal toxicity (Sriram et al. 2012). Another adverse effect of GM is renal dysfunction related to GM’s aggregation in the proximal tubules (Gökce et al. 2016).
Enhanced oxidative stress, inflammatory reaction, necrosis, and apoptosis in tubular cells are the most significant mechanisms in GM-induced kidney dysfunction both in vivo and in cell culture (El Mezayen et al. 2006; Hamdy and Taha 2009). Such complications resulted from the accumulation of GM in cells of the renal tubules and the interaction between GM and cellular organelles (Shehata et al. 2022).
Renal injury may be acute or chronic and is conventionally detected by measuring the serum levels of blood urea nitrogen (BUN) and creatinine, which only increase when renal damage reaches a significant degree and usually rise at least one week after GM treatment initiation (Hamdy and Taha 2009). Although serum creatinine and BUN levels are the main traditional parameters for evaluating kidney function (Xu et al. 2012), they lack sensitivity and specificity. Kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) are recently considered more specific markers for renal injury (Tsiola et al. 2017).
GM binds to phospholipids in the renal cell membranes causing the inactivation of phospholipases, leading to the development of renal disorders, such as toxicity (Kim et al. 2013). Remarkably, flavonoid derivatives were considered nephroprotective agents because they prevent the binding of GM to the phospholipids in the cytoplasmic membranes (Jiménez-Lamana et al. 2014). In the same line, some flavonoids exert nephroprotective activities against GM nephrotoxicity (Hamdy and Taha 2009; Wen et al. 2017).
Apigenin, or 4,5,7-trihydroxyflavone, is a flavonoid naturally found in fruits and vegetables (Su et al. 2014). Apigenin has a nephroprotective effect against cisplatin nephropathy by promoting antioxidant, anti-inflammatory, and anti-apoptotic pathways (Al-Brakati et al. 2020; Tiwari et al. 2011). However, the therapeutic role of apigenin against GM-induced renal damage has not been elucidated. Therefore, this study evaluates the capability of apigenin in attenuating GM-induced nephrotoxicity.
Material and methods
Animals
This study included 42 male rats weighing approximately 200–220 g obtained from VACSERA (Cairo, Egypt). All rats were kept in cages under an appropriate environment with a mean temperature of 25 ± 5 ℃ and mean humidity of 50 ± 10% in a 12-h light/dark cycle. All animals were kept for one week with free water and food before the initiation of experiments that were performed according to the ethical principles of the Institutional Animal Care and Use Committee (IACUC) of Helwan University (approval no. HU2019/Z/AER919-01).
Experimental protocol
Animals were allocated into six groups with seven rats in each, as follows: In the healthy control group, the rats received saline; in the apigenin-treated group, the rats received apigenin (20 mg kg−1 day−1); in the GM-treated group, the rats received an intraperitoneal injection of GM (100 mg kg−1 day−1) (Sigma, St. Louis, MO, USA); in the apigenin-5-GM group, the rats orally received apigenin (5 mg kg−1 day−1) 1 h before GM injection; in the apigenin-10-GM group, the rats orally received apigenin (10 mg kg−1 day−1) 1 h before GM injection; and in the apigenin-20-GM group, the rats orally received apigenin (20 mg kg−1 day−1) 1 h before GM injection. The treatments were repeated daily for seven days at the same time point. Apigenin was first dissolved in dimethyl sulfoxide (DMSO) and further diluted with physiological saline (0.9% sodium chloride). Each rat from the apigenin-treated groups received no more than 0.2% DMSO, corresponding to 10 μl. In the control and GM-treated groups, each rat received physiological saline with 10 μl DMSO. The selected doses for GM and apigenin were based on the studies by Hamdy and Taha (2009) and Al-Brakati et al. (2020), respectively.
Sampling
Twenty-four hours after the experiment, all animals were euthanized by intraperitoneal injection of pentobarbital (300 mg kg−1). The blood samples were collected and centrifuged for 10 min at 3000 × g to separate the serum, which was then stored at − 80 ℃ for biochemical analyses. The kidneys were removed and weighed for calculation of the renal index as follows:
A kidney was divided into two portions: The first portion was fixed in 10% neutral buffered formalin for histopathological examinations; the second portion was retained at − 80 ℃ for biochemical and molecular investigations.
Measurement of BUN, creatinine, sodium, and potassium
The serum levels of creatinine, BUN, sodium, and potassium were measured using colorimetric kits following the methods performed by Biodiagnostics (Giza, Egypt).
Determination of KIM-1, NGAL, and cystatin C levels
The serum levels of KIM-1, NGAL, and cystatin C were measured using an enzyme-linked immunosorbent assay (ELISA) according to the instructions by Abcam (Cambridge, UK).
Measurement of oxidative stress parameters
The kidneys were minced and homogenized in a phosphate buffer (10 mM; pH 7.4). The homogenate was centrifuged at 3000 × g for 10 min. Lipid peroxide (LPO) levels were detected according to the method used by Ohkawa et al. (1975). Nitric oxide (NO) levels were investigated following the technique used by Green et al. (1982). Glutathione (GSH) level reductions were estimated using the procedure used by Ellman (1959). The activities of GSH peroxidase (GPx), GSH reductase (GR), superoxide dismutase (SOD), and catalase were analyzed using the protocol described by Paglia and Valentine (1967), De Vega et al. (2002), Nishikimi et al. (1972), and Aebi (1984), respectively.
Determination of inflammatory biomarkers
The renal levels of nuclear factor kappa B p65 subunit (NF-κB-p65), tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-1β were assayed using ELISA kits produced by CUSABIO Life Sciences (Wuhan, China) according to the protocols of the manufacturer.
Assessment of apoptotic protein markers
The protein levels of B-cell lymphoma 2 (Bcl-2), Bax, and caspase-3 in the kidneys were measured using ELISA kits bought from CUSABIO Life Sciences (Wuhan, China).
Quantitative real-time polymerase chain reaction (PCR) technique
Total RNA was extracted from renal tissues using a TRIzol reagent kit (Qiagen, Germantown, MD, USA); then, their concentrations were measured in nanodrops. cDNA was obtained from isolated RNA using the reverse-transcription method according to the RevertAid™ H Minus Reverse Transcriptase kit provided by Fermentas (Thermo Fisher Scientific Inc., Canada). mRNA levels of Nos2, Nfe212, and Hmox1 were quantitatively measured using the ViiA™ 7 PCR system (Applied Biosystems, USA) using the SYBR Green PCR kit (Qiagen, Germany). The fold changes of all mRNAs were calculated using the 2−ΔΔCt method, where they were normalized to the Actb acting as the internal control. Primer sequences of the selected genes are presented in Table 1.
Histological procedures
Freshly isolated kidneys were cut into small pieces and placed in 10% neutral buffered formalin. Specimens were passed through a standard alcohol dehydration–xylene sequence, embedded in paraffin, and cut into thin Sects. (5 μm in thickness). Tissue sections were stained with hematoxylin and eosin to record any histological lesions (Zhang et al. 2016). A light microscope (Nikon Eclipse, E200-LED, Tokyo, Japan) with various magnifications was used for this purpose.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (IBM Corp., Armonk, NY, USA), and all data were presented as mean ± standard deviation. One-way analysis of variance was used to assess the significant difference between different groups; p values less than 0.05 were used to denote statistical significance.
Results
Effects of apigenin on renal index and kidney function markers
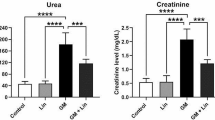
A significant increase in the renal index was detected in the GM-treated group (p < 0.05) compared with the control group (Fig. 1). Conversely, apigenin-treated rats had significantly decreased renal index compared with nephrotoxic rats (p < 0.05); however, the renal index of rats treated with low-dose apigenin was still significantly higher than that of healthy control rats. Remarkably, the two doses (10 mg kg−1 and 20 mg kg−1) of apigenin significantly altered the renal index, suggesting that apigenin restrains the increment in the renal index triggered by GM in a dose-dependent manner.
The renal index and blood urea nitrogen, creatinine, sodium, potassium, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and cystatin C levels following apigenin treatment (5 mg kg−1, 10 mg kg−1, and 20 mg kg−1) in rats with gentamicin (GM)-induced nephrotoxicity. Data are expressed as mean ± standard deviation (n = 7). aIt represents the statistical significance relative to that of the control group at p < 0.05. bIt represents the statistical significance relative to that of the GM-treated group at p < 0.05
Furthermore, the levels of kidney function markers, BUN, and creatinine in different animal groups are shown in Fig. 1. The GM-injected group showed a significant increase in BUN and creatinine levels compared with the control group (p < 0.05). Remarkably, a significant decrease (p < 0.05) in BUN and creatinine levels was detected in the three groups pretreated with apigenin (doses: 5 mg kg−1, 10 mg kg−1, and 20 mg kg−1) compared with the GM-treated group. Additionally, a non-significant change in the sodium and potassium levels was observed in the GM- and apigenin-treated groups compared with the control group.
Figure 1 also displays the concentrations of KIM-1, NGAL, and cystatin C in the different groups. Serum KIM-1, NGAL, and cystatin C concentrations significantly increased in the GM-treated group compared with the control group. However, treatment with the three doses of apigenin resulted in a significant decrease in the serum concentrations of KIM-1, NGAL, and cystatin compared with the GM-treated group.
Apigenin stimulates renal antioxidant mechanisms in GM-treated animals
The injection of GM-induced oxidative stress in renal tissues is evidenced by the rising levels of NO and LPO and decreasing levels of SOD, CAT, GPx, GR, and GSH (Fig. 2). However, apigenin (5 mg kg−l, 10 mg kg−l, and 20 mg kg−l) possessed antioxidant properties, as shown by the elevated protein levels of SOD, CAT, GPx, GR, and GSH, along with a significant reduction in the levels of LPO, NO, and Nos2 compared with the GM-injected group.
The renal levels of lipid peroxidation, nitric oxide (NO), NO synthase 2 (Nos2) expression, glutathione, superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase following apigenin treatment (5 mg kg−1, 10 mg kg−1, and 20 mg kg−1) in rats with gentamicin (GM)-induced nephrotoxicity. Data are expressed as mean ± standard deviation (SD) (n = 7). mRNA expression results are expressed as mean ± SD of three assays in duplicate references to Actb and represented as fold changes (log2 scale) compared with the mRNA levels of the control group. aIt represents the statistical significance relative to that of the control group at p < 0.05. bIt represents the statistical significance relative to that of the GM-treated group at p < 0.05
Furthermore, to test the effect of apigenin on the Nrf2/HO-1 pathway in the renal tissues of GM-treated rats, we examined the mRNA expression of Nfe2l2 and Hmox1in the renal tissues of the different animal groups. GM induced a significant downregulation in the mRNA expression of Nfe2l2 and Hmox1 (Fig. 3). Apigenin per se caused a significant upregulation in Nfe2l2 expression. Moreover, pretreatment with apigenin restrained the harmful effects of GM, and their expressions were upregulated after apigenin administration.
Renal mRNA expression of nuclear factor-erythroid-2-related factor 2 and heme oxygenase 1 following apigenin treatment (5 mg kg−1, 10 mg kg−1, and 20 mg kg−1) in rats with gentamicin (GM)-induced nephrotoxicity. mRNA expression results are expressed as mean ± standard deviation of three assays in duplicate references to Actb and represented as fold changes (log2 scale) compared with the mRNA levels of the control group. aIt represents the statistical significance relative to that of the control group at p < 0.05. bIt represents the statistical significance relative to that of the GM-treated group at p < 0.05
Apigenin administration mitigates GM-induced renal inflammation and apoptosis
GM induced a pro-inflammatory response evidenced by a significant increase (p < 0.05) in the protein levels of NFκB-p65, IL-1β, and TNF-α in the kidneys compared with those of the control group (Fig. 4). However, apigenin treatment diminished renal inflammation, as shown by a significant decrease in the protein levels of IL-1β, NFκB-p65, and TNF-α compared with those in the GM-treated group (p < 0.05).
The renal levels of nuclear factor kappa B p65 subunit, tumor necrosis factor-alpha, interleukin-1β, and apoptotic-related protein levels (Bcl-2, Bax, and caspase-3) following apigenin treatment (5 mg kg−1, 10 mg kg−1, and 20 mg kg−1) in rats with gentamicin (GM)-induced nephrotoxicity. Data are expressed as mean ± standard deviation (n = 7). aIt represents the statistical significance relative to that of the control group at p < 0.05. bIt represents the statistical significance relative to that of the GM-treated group at p < 0.05
In relation to the results in the control group, GM mediated apoptosis in the kidneys of rats, as evidenced by a significant rise (p < 0.05) in the protein levels of Bax and caspase-3 along with a decline in Bcl-2 level (Fig. 4). Conversely, in the three apigenin-treated groups, apigenin could promote anti-apoptotic mechanisms by increasing Bcl-2 and decreasing Bax and caspase-3 protein levels compared with the GM-treated group.
Apigenin protects renal tissue following GM administration
To evaluate the nephroprotective effect of apigenin on the renal histological alternations, the renal tissues were examined using hematoxylin and eosin (H&E) staining. As a result, the GM-treated group showed edema in the renal corpuscles with congested glomeruli, severe infiltration of inflammatory leukocytes, cytoplasmic vacuolation, and tubular epithelial injury (Fig. 5C). Pretreatment with apigenin attenuated glomerular and tubular injuries in rats with GM-induced nephrotoxicity (Fig. 5D-F). The nephroprotective effects of apigenin were supported by a decrease in inflammatory cell infiltration and preservation of the glomerulus. The control and rats treated with apigenin alone showed normal morphology of the glomeruli and tubular cells (Fig. 5A, B).
Photomicrographs of kidney tissues from the control and apigenin-treated groups (A and B, respectively) show a normal kidney structure. In the gentamicin (GM)-treated rats (C), severe inflammation, cytoplasmic vacuolation, severe tubular necrosis and apoptosis, and congested glomeruli are shown. Pretreatment with apigenin (5 mg kg−1, 10 mg kg−1, and 20 mg kg−1) (D, E, and F, respectively) markedly attenuated all renal damages caused by GM. Hematoxylin and eosin, scale bar = 20 μm
Discussion
GM-induced renal injury has been considered the most well-defined model for studying drug-associated nephrotoxicity (Hamdy and Taha 2009). A significant increase in the renal index was observed in the current study after GM treatment, which conforms to Abdelrahman and Abdelmageed (2020) and Feyzi et al. (2020). This increase may be related to histopathological alterations, as Udupa and Prakash (2019) reported. Moreover, Karadeniz et al. (2008) have attributed this increase to the generation of inflammatory mediators and interstitial infiltration of immune cells, followed by edema development in GM-treated animals. Interestingly, apigenin administration reduced the increased renal index in response to GM exposure.
Creatinine and BUN are considered the main traditional parameters for evaluating kidney function (Kim et al. 2010). In this study, a significant increase in creatinine and BUN serum levels was observed in the GM-treated group compared with the control group. These results conform to many studies that have demonstrated that over-discharge of creatinine and urea into the serum could indicate reduced glomerular filtration rate (GFR) and impaired renal function, especially proximal tubule function (Abd-Elbaset et al. 2017; Liu et al. 2020; Talib and AbuKhader 2013). Moreover, elevated creatinine and BUN levels were considered the most important hallmarks of GM-induced renal toxicity (Al-Malki and Sayed 2014; Shehata et al. 2022). Upraised creatinine and BUN levels in this study may be explained by efficient reabsorption of GM in the proximal renal tubule, and GM’s accumulation in tubular cells disturbed the renal circulation, reducing the GFR and subsequently raising serum creatinine and BUN levels (Al-Malki and Sayed 2014). On the other hand, apigenin administration caused a noticeable reduction in the serum levels of creatinine and BUN and restored their normal levels as compared to GM-treated rats. The renoprotective effect of apigenin was shown by a reduction in the serum levels of BUN, and creatinine could be due to its antioxidant capabilities, as reactive oxygen species (ROS) might be responsible for reducing GFR (Mansour 2000).
KIM-1 and NGAL are recently considered more specific and sensitive renal injury markers (Tsiola et al. 2017; Yin et al. 2019). KIM-1 elevation may indicate renal damage, as demonstrated by Hansen et al. (2001), who reported that KIM-1 is a membranous glycoprotein unnoticeable in normal renal tissue or the urine but is overexpressed in the proximal renal tubules after renal injury. Besides, KIM-1 is used for the early sensitive and specific detection of nephrotoxicity induced by chemicals (Al-Brakati et al. 2021a; Albrahim 2020). NGAL is a glycoprotein found at low concentrations in the proximal tubule and neutrophils (Wang et al. 2015). A recent study detected a significant increase in NGAL in the GM-treated group compared with the healthy control group. Similar to our results, a highly expressed NGAL was observed within the thick ascending loop of Henle, distal renal tubule, and collecting tubule of rats and mice with renal toxicity (Zhang and Sun 2015). Likewise, in vivo studies have shown that the levels of KIM-1 and NGAL altered before the tubular renal injury, and these changes are coupled with the severe histopathological changes in the renal tubules proposing their efficiency in predicting GM-induced acute renal damage (Shehata et al. 2022).
Cystatin C is a lysosomal enzyme that constrains the degradation of specific proteins within and outside a cell. This enzyme is expressed by all cells, except for nucleated ones, and is formed and carried into the plasma at a stable level in healthy conditions (Choi et al. 2010). In this study, a substantial upregulation of cystatin C was observed in GM-treated rats compared with the healthy control group. These observations conform to the study of Udupa and Prakash (2019), who have attributed this increase to the cellular degradation of the proximal tubules and diminished reabsorptive ability. Furthermore, cystatin C was used for detecting acute damage in the renal tubules and glomeruli in experimental animals, and it was considered a highly sensitive biomarker (Park et al. 2010).
It was indicated that treatment of experimental animals with GM elicited renal dysfunction depending on its accumulation in cells of the renal tubules (Gökce et al. 2016). Several studies have proposed that ROS is known to be the master mediator of GM-induced acute renal injury (Hamdy and Taha 2009). GM induces nephrotoxicity by overproducing ROS, triggering lipid peroxidation of cytoplasmic membranes and protein degradation. These severe changes consequently resulted in improper enzymatic activation, mitochondrial function, and cellular damage (Al-Malki and Sayed 2014; Shehata et al. 2022; Wen et al. 2017).
NO acts as a signal substance and guardian of cell functions as it plays an important role in maintaining the normal physiology of the kidney by controlling both blood flow in the renal cortex and the function of the renal tubules (Kassab et al. 2021; Korani et al. 2013). In addition, NO regulates signal transduction pathways, cells energetics, host immunoreactions, and the pathology of kidney failure (Gaiser et al. 2013). In this study, the level of NO increased in the kidneys of GM-treated rats, and these results conform to other studies (Darakhshan et al. 2015). In this study, the overproduction of NO may be due to a hyperactive inducible NO synthase (iNOS) that induces NO production (Piao et al. 2011). NO overproduction can induce cell damage by reacting with superoxide anion O2− generating cytotoxic peroxynitrite. In other words, NO and peroxynitrite act as key mediators of oxidative stress and pathophysiology in GM-induced nephrotoxicity (Akter et al. 2018). They cause several adverse effects, such as protein degradation, enzymatic inactivation, peroxidation of lipids, disruption of the respiratory chain in cells, and impairment of DNA repair systems (Piao et al. 2011). Our data showed that NO levels decreased after apigenin treatment. This observation was confirmed by Al-Brakati et al. (2021b), who have reported that flavonoid-derived compounds prevent the expression of Nos2 and its isoforms, which are responsible for the production of NO and pro-inflammatory cytokines.
The formation of renal LPO in this study conforms to previous studies (Sethi et al. 2008; Wen et al. 2017). LPO increases cytoplasmic membranes’ permeability, resulting in ion-exchange imbalance (Sethi et al. 2008). In normal conditions, antioxidant enzymes, such as SOD, CAT, GPx, and GR, can reduce renal damage by scavenging free radicals or ROS and preventing GM-induced apoptosis (El-Ghany et al. 2009; Sethi et al. 2008). In this study, the decreased levels of SOD, CAT, GR, and GPx observed in GM-treated rats may be attributed to GM-induced ROS generation that inhibits the defense mechanisms elicited by these enzymes (El-Ghany et al. 2009; Sethi et al. 2008). GM-induced oxidative injury in this study conforms to those indicated by several studies (Al-Quraishy et al. 2020; Dkhil et al. 2019), which have clarified that disruption between massive free radicals and deficient degradation of these radicals by antioxidant defenders might be the reason for GM-induced renal injury.
Remarkably, the increased LPO levels and decreased antioxidants concentrations were noticeably retained by apigenin treatment. These results conform to several studies (Tiwari et al. 2011; Wang et al. 2014), which have indicated that apigenin exerts a nephroprotective effect by presenting antioxidant properties, allowing it to scavenge several free radicals, preventing LPO formation, and restoring antioxidant enzyme levels to normal.
Heme oxygenase 1 (HO-1) is a rate-limiting protein in the catabolic pathway of heme, which converts into bilirubin by liberating iron and carbon monoxide. HO-1, with other antioxidant enzymes, limits redox imbalance; thus, it is activated to respond to oxidative stress (Abraham and Kappas 2008). Hmox1 is a target gene of nuclear factor-erythroid-2-related factor 2 (Nfe2l2 or Nrf2) that regulates its expression (Piao et al. 2011). This regulation can be explained as follows: Nrf2 is considered a cell defender factor that is enhanced in response to ROS through translocation into the nucleus and binding to an antioxidant response element (ARE) that in turn triggers the transcription of Hmox1 and other antioxidant genes (Mills et al. 2018; Dkhil et al. 2019; Al-Quraishy et al. 2020).
In the same line, Nrf2 is involved in various cellular protective mechanisms, such as mediating antioxidative mechanisms in various renal diseases (Nezu and Suzuki 2020). The upregulation of Hmox1 and Nfe212 mRNAs after apigenin treatment observed in this study was also observed in rat primary hepatocytes, as shown by Huang et al. (2013), who have reported that the overexpression of these genes could inhibit oxidative stress induced by tert-butyl hydroperoxide. Additionally, Yang et al. (2018a) have indicated that treatment of high-fructose diet-fed mice with apigenin enabled the translocation of Nfe212 into the nucleus and the subsequent rise of Hmox1 expression led to the alleviation of oxidative stress. Similar results were observed in ischemic male rats treated with apigenin in a study by Zhang et al. (2019), who concluded that apigenin acts as a powerful antioxidant.
Apoptosis plays a key role in the normal physiology of renal functions and in drug-induced nephrotoxicity, where dysregulation of apoptotic mechanisms results in various renal disorders (Li et al. 2018). Apoptosis is initiated by a cascade of caspase-1, caspase-8, and caspase-9 that trigger caspase-3, whose activation is the key mediator of GM-induced renal cell death (Chen et al. 2011). Bcl-2 is the primary apoptotic inhibitor that is considerably underexpressed in injured renal tissues (Meier et al. 2000).
Currently, the GM-treated group displayed an elevation in caspase-3 and Bax levels and a decrease in Bcl-2 levels; our data conform to other studies (Liu et al. 2020; Yang et al. 2018b). Apoptosis detected in GM-treated rats in this study may be due to several mechanisms, including the accumulated GM within the endoplasmic reticulum preventing RNA translation into proteins and constrained the posttranslational processing of proteins (Horibe et al. 2004). These mysterious events put the endoplasmic reticulum under stress and stimulated cell death (Peyrou et al. 2007). Additionally, GM may interfere with the transport function of some transmembrane proteins, such as the sodium–potassium pump (Sassen et al. 2006), resulting in improper tubular reabsorption, cell swelling, and substantial necrosis or programmed cell death (Sriram et al. 2012).
In addition, apoptosis observed after GM administration in this study may be due to the overproduction of ROS, as was indicated in other studies (Bustos et al. 2016; Kim et al. 2010) that recorded a significant relationship between oxidative stress and the induction and progression of renal cell death in many experimental animals. This relationship can be explained as follows: GM-induced ROS can activate intrinsic pathways of mitochondria by disturbing cellular respiration machinery and reducing ATP formation and trigger the liberation of cytochrome C and the overexpression of other pro-apoptotic proteins, such as Bax, causing cleavage of key proteins, nuclear envelope, and DNA, resulting in apoptosis (Bustos et al. 2016). Importantly, in this study, apigenin could attenuate GM-induced apoptosis by decreasing renal caspase-3 and Bax levels and increasing Bcl-2 levels. Similarly, in vivo studies, apigenin treatment could reverse renal alterations caused by apoptosis in animals with cisplatin-induced nephrotoxicity and renal ischemia–reperfusion injury (Liu et al. 2017; Tiwari et al. 2011).
Cytokines are inflammatory mediators that regulate normal cellular physiology and are associated with tissue damage and repair (Ramesh and Reeves 2004). GM-induced nephrotoxicity has also been associated with the activation and excessive release of inflammatory cytokines, predominantly IL-1β and TNF-α (Mahmoud 2017; Salama et al. 2018). In this study, the significant increase in the renal levels of NFκB-p65 and TNF-α observed in the GM-treated group was also indicated in other studies revealing that NFκB-p65 overstimulation is accompanied by an aggregation of TNF-α (Darakhshan et al. 2015; Sahu et al. 2014). Additionally, NF-κB is considered a principal initiator of inflammatory reactions, especially renal inflammation, in GM-induced renal toxicity (Bae et al. 2014). Furthermore, TNF-α is the main regulator of the renal immune response triggered by many drugs by amplifying renal pathophysiological mechanisms elicited by drugs (Fredriksson et al. 2011).
The increased level of IL-1β recorded in this study was also observed by Bae et al. (2008), who have elucidated that GM activates NFκB-p65, which subsequently resulted in the overproduction of pro-inflammatory cytokines. The reduction of the protein levels of NFκB-p65, IL-1β, and TNF-α after apigenin treatment recorded in this study could be attributed to the anti-inflammatory ability of apigenin, which was also observed in cisplatin-induced nephrotoxicity models in a study by Funakoshi-Tago et al. (2011), who have observed that apigenin could overwhelm the inflammatory response immunomodulation in the kidneys by diminishing NFκB-p65, IL-1β, and TNF-α levels.
The anti-inflammatory effect exerted in the apigenin-treated groups may be due to apigenin’s capability of interfering with the NF-κB signaling pathway through several molecular mechanisms, such as direct inhibition of signal transducer and activator of transcription 3, blockage of the phosphorylation and degradation of inhibitor of NF-κB, and inactivation of inhibitor of NF-κB kinase (Shukla et al. 2015). These adverse events, in turn, resulted in the inactivation of NF-κB (Qin et al. 2016), improper translocations to the nucleus, and loss of DNA-binding activity (Wang et al. 2014).
Although TQ administration mitigated AgNPs-mediated renal injury significantly, further mechanistic studies are required to investigate its anti-apoptotic, antioxidant, and anti-inflammatory activities in other organs.
Conclusion
Collectively, apigenin administration normalized kidney index and kidney function markers following GM exposure. Additionally, apigenin prevented renal oxidative damage by decreasing pro-oxidant and enhancing Nrf2 and its downstream antioxidant proteins. Moreover, apigenin suppressed the inflammatory and apoptotic cascades induced by GM. These results suggest that apigenin could use to alleviate the renal impairments associated with GM application.
Data availability
All relevant data are within the paper.
References
Abd-Elbaset M, Arafa E-SA, El Sherbiny GA, Abdel-Bakky MS, Elgendy ANA (2017) Thymoquinone mitigate ischemia-reperfusion-induced liver injury in rats: a pivotal role of nitric oxide signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 390:69–76
Abdelrahman RS, Abdelmageed ME (2020) Renoprotective effect of celecoxib against gentamicin-induced nephrotoxicity through suppressing NFκB and caspase-3 signaling pathways in rats. Chemico-Biological Interactions 315:108863
Abraham NG, Kappas A (2008) Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60:79–127
Abugomaa A, Elbadawy M (2020) Olive leaf extract modulates glycerol-induced kidney and liver damage in rats. Environ Sci Pollut Res 27:22100–22111
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–6. https://doi.org/10.1016/s0076-6879(84)05016-3
Akter M, Sikder MT, Rahman MM, Ullah AA, Hossain KFB, Banik S, Hosokawa T, Saito T, Kurasaki M (2018) A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res 9:1–16
Albrahim T (2020) Silver nanoparticles-induced nephrotoxicity in rats: the protective role of red beetroot (Beta vulgaris) juice. Environ Sci Pollut Res 27:38871–38880
Al-Brakati A, Albarakati AJA, Daabo H, Baty RS, Salem FEH, Habotta OA, Elmahallawy EK, Abdel-Mohsen DM, Taha H, Akabawy A (2020) Neuromodulatory effects of green coffee bean extract against brain damage in male albino rats with experimentally induced diabetes. Metab Brain Dis 35:1175–1187
Al-Brakati A, Alsharif KF, Alzahrani KJ, Kabrah S, Al-Amer O, Oyouni AA, Habotta OA, Lokman MS, Bauomy AA, Kassab RB (2021a) Using Green biosynthesized lycopene-coated selenium nanoparticles to rescue renal damage in glycerol-induced acute kidney injury in rats. Int J Nanomed 16:4335
Al-Brakati A, Albarakati AJA, Lokman MS, Theyab A, Algahtani M, Menshawi S, AlAmri OD, Al Omairi NE, Essawy EA, Kassab RB, Abdel Moneim AE (2021b) Possible role of kaempferol in reversing oxidative damage inflammation and apoptosis-mediated cortical injury following cadmium exposure. Neurotoxicity Research 39(2):198–209. https://doi.org/10.1007/s12640-020-00300-2
Al-Malki AL, Sayed AAR (2014) Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC Complement Altern Med 14:1–8
Al-Quraishy S, Dkhil MA, Abdel-Gaber R, Zrieq R, Hafez TA, Mubaraki MA, Abdel Moneim AE (2020) Myristica fragrans seed extract reverses scopolamine-induced cortical injury via stimulation of HO-1 expression in male rats. Environ Sci Pollut Res 27:12395–12404
Bae WK, Lee J, Park JW, Bae EH, Ma SK, Kim SH, Kim SW (2008) Decreased expression of Na+/K+-ATPase, NHE3, NBC1, AQP1 and OAT in gentamicin-induced nephropathy. Korean J Physiol Pharmacol 12:331–336
Bae EH, Kim IJ, Joo SY, Kim EY, Choi JS, Kim CS, Ma SK, Lee J, Kim SW (2014) Renoprotective effects of the direct renin inhibitor aliskiren on gentamicin-induced nephrotoxicity in rats. J Renin-Angiotensin-Aldosterone Syst 15:348–361
Bustos PS, Deza-Ponzio R, Páez PL, Albesa I, Cabrera JL, Virgolini MB, Ortega MG (2016) Protective effect of quercetin in gentamicin-induced oxidative stress in vitro and in vivo in blood cells. Effect on gentamicin antimicrobial activity. Environ Toxicol Pharmacol 48:253–264
Chen Y-C, Chen C-H, Hsu Y-H, Chen T-H, Sue Y-M, Cheng C-Y, Chen T-W (2011) Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. Eur J Pharmacol 658:213–218
Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu D-Y (2010) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100:151–159
Darakhshan S, Pour AB, Colagar AH, Sisakhtnezhad S (2015) Thymoquinone and its therapeutic potentials. Pharmacol Res 95:138–158
De Vega L, Fernández RP, Martin Mateo M, Bustamante JB, Herrero AM, Munguira EB (2002) Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail 24:421–432
Dkhil MA, Abdel Moneim AE, Hafez TA, Mubaraki MA, Mohamed WF, Thagfan FA, Al-Quraishy S (2019) Myristica fragrans kernels prevent paracetamol-induced hepatotoxicity by inducing anti-apoptotic genes and Nrf2/HO-1 pathway. Int J Mol Sci 20:993
El Mezayen R, El Gazzar M, Nicolls MR, Marecki JC, Dreskin SC, Nomiyama H (2006) Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol Lett 106:72–81
El-Ghany A, Ragwa M, Sharaf NM, Kassem LA, Mahran LG, Heikal OA (2009) Thymoquinone triggers anti-apoptotic signaling targeting death ligand and apoptotic regulators in a model of hepatic ischemia reperfusion injury. Drug Discov Ther 3
Ellman GL (1959) Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics 82:70–77
Feyzi R, Yari S, Karamian R, Hasanein P (2020) Preventive effect of Stachys lavandulifolia against gentamicin-induced oxidative stress and nephrotoxicity in rats. Comparative Clinical Pathology 29:1127–1135
Fredriksson L, Herpers B, Benedetti G, Matadin Q, Puigvert JC, de Bont H, Dragovic S, Vermeulen NP, Commandeur JN, Danen E (2011) Diclofenac inhibits tumor necrosis factor-α-induced nuclear factor-κB activation causing synergistic hepatocyte apoptosis. Hepatology 53:2027–2041
Funakoshi-Tago M, Nakamura K, Tago K, Mashino T, Kasahara T (2011) Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int Immunopharmacol 11:1150–1159
Gaiser BK, Hirn S, Kermanizadeh A, Kanase N, Fytianos K, Wenk A, Haberl N, Brunelli A, Kreyling WG, Stone V (2013) Effects of silver nanoparticles on the liver and hepatocytes in vitro. Toxicol Sci 131:537–547
Gökce EC, Kahveci R, Gökce A, Cemil B, Aksoy N, Sargon MF, Kısa Ü, Erdoğan B, Güvenç Y, Alagöz F (2016) Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine 24:949–959
Green LC, Wagner DA, Glogowski J et al (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Analytical Biochemistry 126:131–138
Hamdy NM, Taha RA (2009) Effects of Nigella sativa oil and thymoquinone on oxidative stress and neuropathy in streptozotocin-induced diabetic rats. Pharmacology 84:127–134
Hansen M, Christrup L, Jarløv J et al (2001) Gentamicin dosing in critically ill patients. Acta Anaesthesiologica Scandinavica 45:734–740
Horibe T, Matsui H, Tanaka M, Nagai H, Yamaguchi Y, Kato K, Kikuchi M (2004) Gentamicin binds to the lectin site of calreticulin and inhibits its chaperone activity. Biochem Biophys Res Commun 323:281–287
Huang C-S, Lii C-K, Lin A-H et al (2013) Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Archives of Toxicology 87:167–178
Jiménez-Lamana J, Laborda F, Bolea E, Abad-Álvaro I, Castillo JR, Bianga J, He M, Bierla K, Mounicou S, Ouerdane L (2014) An insight into silver nanoparticles bioavailability in rats. Metallomics 6:2242–2249
Karadeniz A, Yildirim A, Simsek N, Kalkan Y, Celebi F (2008) Spirulina platensis protects against gentamicin‐induced nephrotoxicity in rats. Phytotherapy Research 22:1506–1510
Kassab RB, Theyab A, Al-Ghamdy AO, Algahtani M, Mufti AH, Alsharif KF, Abdella EM, Habotta OA, Omran MM, Lokman MS (2021) Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ Sci Pollut Res 1–14
Kim J, Cha Y-N, Surh Y-J (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res/Fundam Mol Mech Mutagen 690:12–23
Kim HR, Park YJ, Da Young Shin SMO, Chung KH (2013) Appropriate in vitro methods for genotoxicity testing of silver nanoparticles. Environ Health Toxicol 28
Korani M, Rezayat SM, Bidgoli SA (2013) Sub-chronic dermal toxicity of silver nanoparticles in guinea pig: special emphasis to heart, bone and kidney toxicities. Iran J Pharm Res: IJPR 12:511
Li L, Cui J, Liu Z, Zhou X, Li Z, Yu Y, Jia Y, Zuo D, Wu Y (2018) Silver nanoparticles induce SH-SY5Y cell apoptosis via endoplasmic reticulum-and mitochondrial pathways that lengthen endoplasmic reticulum-mitochondria contact sites and alter inositol-3-phosphate receptor function. Toxicol Lett 285:156–167
Liu Y, Liu X, Wang L, Du Y, Chen Z, Chen H, Guo J, Weng X, Wang X, Wang M (2017) Effects of apigenin on the expression levels of B-cell lymphoma-2, Fas and Fas ligand in renal ischemia-reperfusion injury in rats. Exp Ther Med 14:5345–5354
Liu W, Worms I, Slaveykova VI (2020) Interaction of silver nanoparticles with antioxidant enzymes. Environ Sci Nano 7:1507–1517
Lokman MS, Zaafar D, Althagafi HA, Abdel Daim MM, Theyab A, Hasan Mufti A, Algahtani M, Habotta OA, Alghamdi AA, Alsharif KF (2022) Antiulcer activity of proanthocyanidins is mediated via suppression of oxidative, inflammatory, and apoptotic machineries. J Food Biochem e14070
Mahmoud YI (2017) Kiwi fruit (Actinidia deliciosa) ameliorates gentamicin-induced nephrotoxicity in albino mice via the activation of Nrf2 and the inhibition of NF-κB (Kiwi & gentamicin-induced nephrotoxicity). Biomed Pharmacother 94:206–218
Makama S, Kloet SK, Piella J, van den Berg H, de Ruijter NC, Puntes VF, Rietjens IM, van den Brink NW (2018) Effects of systematic variation in size and surface coating of silver nanoparticles on their in vitro toxicity to macrophage RAW 264.7 cells. Toxicol Sci 162:79–88
Mansour MA (2000) Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci 66:2583–2591
Meier P, Finch A, Evan G (2000) Apoptosis in development. Nature 407:796–801
Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa AS, Higgins M, Hams E (2018) Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556:113–117
Mori A, Hiramatsu M, Namba S, Nishimoto A, Ohmoto T, Mayanagi Y, Asakura T (1987) Decreased dopamine level in the epileptic focus. Res Commun Chem Pathol Pharmacol 56:157–164
Nezu M, Suzuki N (2020) Roles of Nrf2 in protecting the kidney from oxidative damage. Int J Mol Sci 21:2951
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Ohkawa H, Ohishi N, Yagi K (1975) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine 70:158–169
Park E-J, Bae E, Yi J, Kim Y, Choi K, Lee SH, Yoon J, Lee BC, Park K (2010) Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol 30:162–168
Patlolla AK, Hackett D, Tchounwou PB (2015) Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem 399:257–268
Peyrou M, Hanna PE, Cribb AE (2007) Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci 99:346–353
Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW (2011) Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett 201:92–100
Qin Y, Zhao D, Zhou H-g, Wang X-h, Zhong W-l, Chen S, Gu W-g, Wang W, Zhang C-h, Liu Y-r (2016) Apigenin inhibits NF-κB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget 7:41421
Ramesh G, Reeves WB (2004) Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-α. Kidney Int 65:490–498
Sahu BD, Tatireddy S, Koneru M, Borkar RM, Kumar JM, Kuncha M, Srinivas R, Sistla R (2014) Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats: possible mechanism of nephroprotection. Toxicol Appl Pharmacol 277:8–20
Salama SA, Arab HH, Maghrabi IA (2018) Troxerutin down-regulates KIM-1, modulates p38 MAPK signaling, and enhances renal regenerative capacity in a rat model of gentamycin-induced acute kidney injury. Food Funct 9:6632–6642
Sassen MC, Kim S, Kwon T-H, Knepper MA, Miller RT, Frøkiær J, Nielsen S (2006) Dysregulation of renal sodium transporters in gentamicin-treated rats. Kidney Int 70:1026–1037
Sethi G, Ahn KS, Aggarwal BB (2008) Targeting nuclear factor-κB activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res 6:1059–1070
Shehata AM, Salem F, El-Saied EM, El-Rahman A, Sahar S, Mahmoud MY, Noshy PA (2022) Evaluation of the ameliorative effect of zinc nanoparticles against silver nanoparticle–induced toxicity in liver and kidney of rats. Biol Trace Elem Res 200:1201–1211
Shukla S, Shankar E, Fu P, MacLennan GT, Gupta S (2015) Suppression of NF-κB and NF-κB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice. Plos one 10:e0138710
Sriram MI, Kalishwaralal K, Barathmanikanth S, Gurunathani S (2012) Size-based cytotoxicity of silver nanoparticles in bovine retinal endothelial cells. Nanosci Meth 1:56–77
Su C-K, Liu H-T, Hsia S-C, Sun Y-C (2014) Quantitatively profiling the dissolution and redistribution of silver nanoparticles in living rats using a knotted reactor-based differentiation scheme. Anal Chem 86:8267–8274
Sun X-j, Zhao X, Xie J-n, Wan H (2020) Crocin alleviates schizophrenia-like symptoms in rats by upregulating silent information regulator-1 and brain derived neurotrophic factor. Compr Psychiatr 103:152209
Talib WH, AbuKhader MM (2013) Combinatorial effects of thymoquinone on the anticancer activity and hepatotoxicity of the prodrug CB 1954. Sci Pharm 81:519–530
Tiwari DK, Jin T, Behari J (2011) Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol Mech Methods 21:13–24
Tsiola A, Pitta P, Callol AJ, Kagiorgi M, Kalantzi I, Mylona K, Santi I, Toncelli C, Pergantis S, Tsapakis M (2017) The impact of silver nanoparticles on marine plankton dynamics: dependence on coating, size and concentration. Sci Total Environ 601:1838–1848
Udupa V, Prakash V (2019) Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicology Reports 6:91–99
Wang J, Liu Y-T, Xiao L, Zhu L, Wang Q, Yan T (2014) Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37:2085–2090
Wang Z, Xia T, Liu S (2015) Mechanisms of nanosilver-induced toxicological effects: more attention should be paid to its sublethal effects. Nanoscale 7:7470–7481
Wen H, Dan M, Yang Y, Lyu J, Shao A, Cheng X, Chen L, Xu L (2017) Acute toxicity and genotoxicity of silver nanoparticle in rats. PLOS ONE 12:e0185554
Xu L, Li X, Takemura T, Hanagata N, Wu G, Chou LL (2012) Genotoxicity and molecular response of silver nanoparticle (NP)-based hydrogel. J Nanobiotechnol 10:1–11
Yang M, Jiang Z-h, Li C-g, Zhu Y-j, Li Z, Tang Y-z, Ni C-l (2018) Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed Pharmacother 105:1283–1290
Yang Q, Zhou Y, Yin H, Li H, Zhou M, Sun G, Cao Z, Man R, Wang H, Li J (2018) PINK1 protects against gentamicin-induced sensory hair cell damage: possible relation to induction of autophagy and inhibition of p53 signal pathway. Front Mol Neurosci 11:403
Yin M, Jiang N, Guo L, Ni Z, Al-Brakati AY, Othman MS, Moneim AEA, Kassab RB (2019) Oleuropein suppresses oxidative, inflammatory, and apoptotic responses following glycerol-induced acute kidney injury in rats. Life Sci 232:116634
Zhang H, Sun S-C (2015) NF-κB in inflammation and renal diseases. Cell Biosci 5:1–12
Zhang X-F, Shen W, Gurunathan S (2016) Silver nanoparticle-mediated cellular responses in various cell lines: an in vitro model. Int J Mol Sci 17:1603
Zhang S, Xu S, Duan H et al (2019) A novel, highly-water-soluble apigenin derivative provides neuroprotection following ischemia in male rats by regulating the ERK/Nrf2/HO-1 pathway. European Journal of Pharmacology 855:208–215
Zhou Y-T, He W, Lo YM, Hu X, Wu X, Yin J-J (2013) Effect of silver nanomaterials on the activity of thiol-containing antioxidants. J Agric Food Chem 61:7855–7862
Acknowledgements
The authors thank the Taif University Researchers Supporting Program (Project number: TURSP-2020/151), Taif University, Saudi Arabia, for supporting this study.
Author information
Authors and Affiliations
Contributions
Animal treatments, molecular and biochemical methodologies were performed by M.M.H., R.B.K., A.E.A., and M.S.L.; histological methodology and investigation were performed by A.A. and A.H.M.; data analysis, software, data curation, and visualization were performed by H.A.A., F.A., K.F.A., and A.T.; writing – reviewing and editing manuscript was performed by M.A., R.S.B., R.B.K., and M.M.H. All authors participated in the design and interpretation of the study and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were performed according to the ethical principles of the Institutional Animal Care and Use Committee (IACUC) of Helwan University (approval no. HU2019/Z/AER919-01).
Consent to participate
Not applicable.
Consent for publication
Consented.
Conflict of interest
The author declares no competing interest.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussein, M.M., Althagafi, H.A., Alharthi, F. et al. Apigenin attenuates molecular, biochemical, and histopathological changes associated with renal impairments induced by gentamicin exposure in rats. Environ Sci Pollut Res 29, 65276–65288 (2022). https://doi.org/10.1007/s11356-022-20235-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20235-9