Abstract
Methotrexate-induced nephrotoxicity is a medical emergency which is associated with a variety of side effects. Vanillic acid (VA), as an antioxidant, removes free radical oxygen to protect cell defense. Therefore, this study investigated VA’s beneficial effects on nephrotoxicity induced by methotrexate through its anti-apoptosis, antioxidant, and anti-inflammatory properties. Our study included five groups of male Wistar rats (n = 8): sham, MTX (Methotrexate) group: rats receiving methotrexate (20 mg/kg, intraperitoneally) on Day 2. Moreover, the remaining groups consisted of animals that received vanillic acid (25, 50, and 100 mg/kg, orally for seven days) plus MTX on the 2nd day. The rats were deeply anesthetized on the eighth day to obtain blood and renal tissue samples. The results showed that MTX can increase blood urea nitrogen and creatinine. However, VA (50 and 100 mg/kg) improved renal function as approved by histological findings. Compared with MTX-treated rats, VA enhanced the contents of total antioxidant capacity (TAC) and reduced renal malondialdehyde (MDA). Moreover, VA reduced mRNA expressions of caspase-3 and Bcl-2-associated x protein (Bax) and caused mRNA overexpression of the renal B-cell lymphoma-2 (Bcl-2), and Nrf-2 (Nuclear factor erythroid 2-related factor 2) compared to the MTX group. Also, VA administration significantly reduced inflammatory agents. Overall, VA protects the kidneys against methotrexate-induced nephrotoxicity via anti-apoptosis, antioxidant, and anti-inflammatory properties. Our results revealed that the most effective dose of VA was 100 mg/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX) is a chemotherapy agent used for the treatment of a variety of cancers including leukemia, lymphoma, and malignant brain tumors (Devrim et al. 2005; Widemann et al. 2004). Nephrotoxicity induced by MTX is considered a medical emergency because the renal excretion of MTX is distributed and can result in life-threatening conditions (Ramsey et al. 2018). Numerous studies have recently indicated that administration of MTX is limited owing to its side effects including nephrotoxicity (Türk et al. 2022; Yuksel et al. 2017). In addition, high doses of MTX used in chemotherapy have been reported to cause nephrotoxicity due to the accumulation of MTX and metabolite 7-hydroxy-MTX on renal tubules (Türk et al. 2022). Studies have shown that MTX can inhibit cell metabolism (Neradil et al. 2012). MTX can prevent purine bases and protein synthesis for the generation of DNA, RNA, and adenosine triphosphate (Neradil et al. 2012). These alterations in metabolic pathways have essential roles in causing the side effects of MTX (Yuksel et al. 2017). MTX is also known as an inducer of oxidative stress through inhibiting NAD (P) H-dependent dehydrogenases, iso-citrate, malate, and pyruvate dehydrogenases. Therefore, the lack of NADPH is associated with antioxidant capacity deficiency (Neradil et al. 2012; Yuksel et al. 2017). Growing evidence has confirmed that a weakened antioxidant system can lead to an imbalance in oxidant–antioxidant status, and ultimately to oxidative injury (Türk et al. 2022; Yuksel et al. 2017). Nrf-2 (Nuclear factor erythroid 2-related factor 2) is a cytoprotective agent which intensifies antioxidant activity against cell oxidative damage. The overexpression of Nrf-2 could lead to augmentation of antioxidant index and protection of cell defense (Zoja et al. 2014). MTX-induced nephrotoxicity has been shown to increase lipid peroxidation and decrease the antioxidant system in kidney tissue (Devrim et al. 2005). Accumulating evidence suggests that oxidative stress triggers the caspase cascade (Basile et al. 2012) which can make renal epithelial cells prone to injury, dysfunction, and death (Elmore 2007; Havasi and Borkan 2011). Numerous studies have shown that Bax, a well-known agent in apoptosis, is responsible for the initiation of the caspase cascade, whereas B-cell lymphoma protein 2 (Bcl-2) acts as an inhibitor of apoptosis events (Elmore 2007; Havasi and Borkan 2011). Likewise, oxidative stress can exacerbate the inflammation response by generating inflammatory cytokines like TNF-α. Strong evidence has revealed involvement of inflammatory agents in the pathogenesis of nephrotoxicity is followed by acute kidney injury (Kandemir et al. 2017; Sindhu et al. 2015). A preliminary study has indicated that TNF-α, and interleukine-1β (IL-1β) were increased by MTX-nephrotoxicity (Kandemir et al. 2017). TNF-α is a principal key mediator in the inflammatory pathway in cisplatin nephrotoxicity (Amini et al. 2022) and MTX-induced nephrotoxicity (Kandemir et al. 2017). Inhibition of TNF-α prevents the activation of cytokine signaling against MTX-induced nephrotoxicity (Kandemir et al. 2017).
Nowadays, the pharmacological properties of polyphenol compounds used to treat different kinds of chronic and acute diseases have attracted notable scholarly attention (Bai et al. 2021). As a common flavoring agent, VA is identified as a dihydroxybenzoic acid derivative. It is found in a variety of fruits, olive, and wheat (Kiokias et al. 2020). Various studies have demonstrated that VA improves cisplatin-induced kidney damage by increasing antioxidant activity and exerting anti-inflammatory effects (Sindhu et al. 2015). VA has also been shown to protect cardiac tissue in isoproterenol-induced myocardial infarction by reducing apoptosis (Prince et al. 2011).
This was the first report investigating the impact of VA on MTX-induced nephrotoxicity through its anti-apoptosis properties. More particularly, this research explored the beneficial effects of VA on MTX-induced nephrotoxicity with respect to oxidative stress, inflammatory, biochemical, molecular parameters, and histology assay.
Materials and methods
Chemicals
VA and MTX were obtained from Sigma Aldrich (Germany). Inflammatory cytokines kit and TAC assay kit were purchased from the Karmaniaparsgene Co. (Iran). The malondialdehyde (MDA) kit was purchased from Teb Pajouhan Raazi Company (Iran). Specific primers were ordered from Homa Gen, Iran.
Animals
Our study included 40 Wistar male rats weighing 200 ± 50 (provided by the Animal Reproduction Center, Ahvaz Jundishapur University of Medical Sciences, AJUMS). The rats were placed in cages at 22°c temperature, 50% humidity, and 12-h light/dark cycles with free access to water and standard food. The Animal Ethics Committee of AJUMS, Iran (IR. AJUMS.ABHC.REC. 1401.009) approved our study.
Design
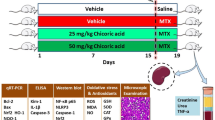
The animals were randomized into five groups (n = 8 each): Group 1: Sham group: the rats received normal saline (NS) by gavage (1 ml) for seven days, and intraperitoneally on the 2nd day. Group 2: MTX (20 mg/kg) was injected as a single dose intraperitoneally on the 2nd day (Öktem et al. 2006). Group 3: MTX + VA (25 mg/kg): The animals were given VA (25 mg/kg, gavage, for one week) and simultaneously MTX (20 mg/kg, single dose, intraperitoneally) on the 2nd day (Sindhu et al. 2015). Group 4: MTX + VA (50 mg/kg): The animals received VA (50 mg/kg, by gavage, for one week) and simultaneously MTX (20 mg/kg, single dose, intraperitoneally) on the 2nd day (Sindhu et al. 2015). Group 5: MTX + VA (100 mg/kg): The animals were given VA (100 mg/kg, by gavage, for one week) and MTX simultaneously (20 mg/kg, single dose, intraperitoneally) on the 2nd day (Sindhu et al. 2015).
On the 8th day, anesthesia was administered using ketamine (100 mg/kg) and xylazine (10 mg/kg) for the purpose of obtaining serum and tissue samples (Amini et al. 2022). The serum levels were obtained after centrifuging at 3000 rpm for 15 min. The kidney tissues were used to determine oxidative stress, analyze histology, and check molecular parameters. The specimens were immediately kept in a refrigerator at -80°c until the tests were performed.
Measurement of kidney function parameters
After centrifuging the blood samples at 3000 rpm for 15 min, we carefully separated the serum to be used by an auto-analyzer for the measurement of BUN and Cr levels.
Measurement of oxidative stress parameters
The kidney tissue was cut into 100-mg pieces, and to homogenize the tissues, cold phosphate-buffered saline (PBS) (1 ml) was used. Then the supernatant was separated, and subsequently for TAC and MDA content, centrifugation was done at 4000 g for 15 min following the guidelines of the manufacturer. Afterward, renal TAC was measured at a wavelength of 593 nm (Karmaniaparsgene Co. Iran), and kidney MDA level was determined at the 535-nm wavelength using colorimetric method (Teb Pajouhan Raazi, Iran).
Measurement of inflammatory factors
To assay the content of TNF-α and IL-1β, as renal inflammatory agents, ELISA assay kit was used following the guidelines of the manufacturer (Karmanya Pars Gene, Iran).
Quantitative real‑time PCR (qRT‑PCR) assay
First, the kidney tissue RNA was extracted using the RNA kit (AnaCell, Iran). Then RNA-to-cDNA conversion was done using the same kit, following the manufacturer’s guidelines (Ana Cell, Iran). After that, the cDNA was analyzed based on the qRT‑PCR method to check the mRNA expression (Bax, Nrf-2, caspase-3, Bcl-2, and GAPDH) level compared to the GAPDH control gene. Table 1 shows the primers. In an ultimate volume of 10 μl, amplification of nucleotides was performed. This volume included 2 μl of cDNA, 2 μl of primers, and 6 μl of SYBR green master mix with SYBR green I, without ROX (Primer design, Denmark). This was done under the following conditions: 95 °C for 15 min for activating DNA Taq polymerase, following 45 cycles at 95 °C for 15 min, 95 °C for 30 s, 52 °C for 30 s, and 72 °C for 30 s. Also, for each PCR, H2O was applied as the negative control. An internal control gene explored the mRNA expression. We used the 2−ΔΔCT method to complete the gene expression.
Histological examination of the kidney
Kidney tissues were separated and washed using NS. Afterward, the kidney tissue specimens were embedded in 10% formaldehyde solution and were placed in paraffin, and a tissue block was prepared. Next, they were cut into pieces of 3–5 µm (μm) by a microtome. Finally, staining was done based on the hematoxylin–eosin (H&E) method.
Statistical analysis
Kolmogorov-Simonov test was used to check normal distribution of data. For multiple comparisons, one-way analysis of variance (ANOVA) and Tukey’s post hoc test were employed. Data analysis was done by Prism 6.0 (San Diego, CA). Findings of the current study were reported as means ± SEM (SEM). Significance level was set at 0.05.
Results
Effect of VA on kidney function
As indicated in Fig. 1, in comparison with the sham group, BUN and Cr levels rose significantly in the MTX receiving animals (p < 0.001, Fig. 1A and B). However, compared to the MTX group, BUN (p < 0.001) and Cr (p < 0.05) levels experienced a significant reduction in rats receiving VA (50 and 100 mg/kg) + MTX (Fig. 1A and B). Moreover, a significant decrease was seen in BUN serum levels in rats receiving VA (100 mg/kg) + MTX as opposed to those receiving VA (25 & 50 mg/kg) + MTX (p < 0.001, p < 0.01, respectively, Fig. 1A). Also, BUN serum levels was significantly decreased in animals receiving VA (50 mg/kg) + MTX as opposed to those treated with VA (25 mg/kg) + MTX (p < 0.001, Fig. 1A).
Effects of VA on serum levels of blood BUN (A), and Cr (B) in methotrexate nephrotoxicity (mean ± SEM n = 8). Sham, methotrexate (20 mg/kg, i.p.), VA (25 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), VA (50 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), and VA (100 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.). One-way ANOVA followed by Tukey’s post hoc test. ###p < 0.001, vs. sham group, *p < 0.05, ***p < 0.001, vs. methotrexate group. $$$p < 0.001, vs. VA (25 mg/kg) + MTX group, &p < 0.05, &&p < 0.01, vs. VA (50 mg/kg) + MTX group. @@@ p < 0.001, vs. VA (25 mg/kg) + MTX group
Also, Cr serum levels noticeably decreased in rats receiving VA (100 mg/kg) + MTX in comparison with animals receiving VA (25 & 50 mg/kg) + MTX (p < 0.001, p < 0.05, respectively, Fig. 1B).
Effect of VA on oxidative stress
As indicated in Fig. 2, renal content of MDA (p < 0.01) rose significantly in MTX nephrotoxicity rats as opposed to sham rats (Fig. 2A). Nevertheless, compared to rats receiving MTX, VA (50 and 100 mg/kg) + MTX could significantly decrease the content of MDA (p < 0.05, p < 0.001, correspondingly) (Fig. 2A). Moreover, VA (100 mg/kg) + MTX led to a significant decline in MDA levels compared with VA at 25 mg/kg + MTX (p < 0.01, Fig. 2A).
Effects of VA on kidney tissue MDA (A), TAC (B) in methotrexate nephrotoxicity (mean ± SEM n = 8). Sham, methotrexate (20 mg/kg, i.p.), VA (25 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), VA (50 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), and VA (100 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.). One way ANOVA followed by Tukey’s post hoc test. ##p < 0.01, ###p < 0.001, vs. sham group, *p < 0.05, ***p < 0.001, vs. methotrexate group. $$p < 0.01, $$$ < p < 0.001, vs. VA (25 mg/kg) + MTX group, &&&p < 0.001, vs. VA (50 mg/kg) + MTX group
Renal content of TAC significantly decreased (p < 0.001) in MTX receiving rats as opposed to the sham group (Fig. 2B). However, VA (100 mg/kg) + MTX significantly enhanced renal TAC content (p < 0.001) in comparison with MTX-exposed rats (Fig. 2B). In addition, VA (100 mg/kg) + MTX remarkably increased renal TAC levels compared to VA (25 and 50 mg/kg) + MTX (P < 0.001, Fig. 2B).
Effect of VA on inflammatory factors
Levels of renal TNF-α rose significantly in rats treated with MTX (p < 0.01) comported to rats not receiving MTX (Fig. 3A). However, these levels were significantly decreased in the VA (50, 100 mg/kg) + MTX group (p < 0.05, p < 0.01, respectively) as opposed to the MTX group (Fig. 3A). In comparison with rats receiving VA (25 mg/kg) + MTX, a significant reduction was seen in rats treated with VA (100 mg/kg) + MTX (p < 0.05) (Fig. 3A).
Effects of VA on kidney tissue TNF-α (A), IL-1β (B) in methotrexate nephrotoxicity (mean ± SEM n = 8). Sham, methotrexate (20 mg/kg, i.p.), VA (25 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), VA (50 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), and VA (100 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.). One way ANOVA followed by Tukey’s post hoc test. ##p < 0.01, ###p < 0.001, vs. sham group, *p < 0.05, **p < 0.01, ***p < 0.001, vs. methotrexate group. $p < 0.05, vs. VA (25 mg/kg) + MTX group
In the MTX group, renal IL-1β levels rose significantly as opposed to sham group (p < 0.001) (Fig. 3B). Moreover, VA (50 and 100 mg/kg) + MTX significantly reduced (p < 0.05, p < 0.01, correspondingly) renal IL-1β in comparison with the MTX nephrotoxicity group (Fig. 3B). Also, VA (100 mg/kg) + MTX significantly inhibited renal IL-1β levels as opposed to VA (25 mg/kg) + MTX rats (p < 0.05) (Fig. 3B).
Effect of VA on molecular parameters
According to Fig. 4, mRNA expression of caspase-3 had a significant increase in MTX group as opposed to the sham rats (p < 0.001, Fig. 4A). Yet, administration of VA (50 mg/kg) and VA (100 mg/kg) + MTX significantly reduced the expression of caspase-3 (p < 0.01, p < 0.001, respectively) compared to the MTX receiving rats (Fig. 4A). Moreover, VA (100 mg/kg) + MTX reduced mRNA expression of caspase-3 compared to VA (25 mg/kg) + MTX (p < 0.05) (Fig. 4A). Bax expression had a significant rise in the MTX group in comparison with the sham group (p < 0.001, Fig. 4B). However, VA (50 mg/kg) + MTX and VA (100 mg/kg) + MTX significantly reduced Bax mRNA expression as opposed to the MTX-exposed rats (p < 0.01, p < 0.001, respectively) (Fig. 4B). Likewise, VA (100 mg/kg) + MTX reduced mRNA expression of Bax in comparison with VA (25 mg/kg) + MTX (p < 0.05, Fig. 4B). The mRNA expression of Bcl-2 was significantly decreased in the MTX group compared with the sham rats (p < 0.001, Fig. 4C). However, the mRNA expression of Bcl-2 in the groups of VA (50 mg/kg) + MTX and VA (100 mg/kg) + MTX as opposed to MTX rats rose significantly (p < 0.05, p < 0.01, respectively (Fig. 4C). Interestingly, mRNA expression of Bcl-2 significantly increased in VA (100 mg/kg) + MTX group in comparison with VA (25 mg/kg) + MTX group (p < 0.05, Fig. 4C).
Effect of VA on caspase-3 (A) Bax (B), Bcl-2 (C), and Nrf-2 (D) in methotrexate nephrotoxicity (mean ± SEM n = 6). Sham, methotrexate (20 mg/kg, i.p.), VA (25 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), VA (50 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.), and VA (100 mg/kg, Gavage, seven days) + MTX (20 mg/kg, i.p.). Analysis of the QRT-PCR results showed that the level of caspase-3 and Bax expression in VA groups was significantly lower than that in methotrexate the group. However, mRNA expression of Bcl-2 and Nrf-2 significantly increased in VA groups compared to methotrexate group. One-way ANOVA followed by Tukey’s post hoc test. #p < 0.05, ###p < 0.001, vs. sham, *p < 0.05, **p < 0.01, ***p < 0.001, vs. methotrexate group. $p < 0.05, $$p < 0.01, vs. VA25mg/kg + MTX
Nrf-2 mRNA expression of kidney tissue in MTX-receiving rats had a significant decline as opposed to the sham group (p < 0.05, Fig. 4D). However, administrations of VA (50 mg/kg) + MTX and VA (100 mg/kg) + MTX significantly increased mRNA expression of Nrf-2 as opposed to MTX-rats (p < 0.05, p < 0.01, correspondingly (Fig. 4D). Additionally, mRNA expression of Nrf-2 significantly rose in the VA (100 mg/kg) + MTX group compared to VA (25 mg/kg) + MTX group (p < 0.05, Fig. 4D).
Effect of VA on kidney tissue
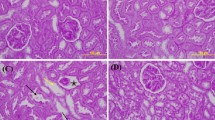
The results of histology indicated normal structure of renal tissue in glomeruli and proximal tubules in sham animals (Fig. 5A). Nonetheless, MTX at the dose of 20 mg/kg could result in contracted glomeruli, infiltration of inflammatory cells, and congestion in kidney tissue (5B, C). Administration of VA (25 mg/kg) + MTX was not effective in improving tissue damage induced by MTX (Fig. 5D). However, VA (50 mg/kg) + MTX decreased inflammatory cell infiltration and congestion (Fig. 5D). Moreover, VA (100 mg/kg) + MTX completely ameliorated renal histology changes (Fig. 5E). The histopathological alterations are demonstrated in Table 2.
Effects of VA on histological evaluation of kidney tissue in different groups. A Sham group: Indicating regular structure of the proximal convoluted tubules (10 ×). B, C MTX group: Indicating contracted glomerular tufts (Black arrows), congestion (Green arrows), tubular necrosis (asterisk), and inflammatory cell infiltrations (arrowhead) (20 ×). D Rats receiving VA (25 mg/kg) + MTX: indicating contracted glomerular tufts (Black arrows), congestion (Green arrows), and vacuolization (Yellow arrows) (20X). E Rats receiving VA (50 mg/kg) + MTX: indicating contracted glomerular tufts (Black arrow) (20 ×). F Rats receiving VA (100 mg/kg) + MTX: indicating normal structure. Tissue samples were stained with H&E dyes
Discussion
Limited experimental studies have explored the protective effects of VA on nephrotoxicity, especially MTX-induced nephrotoxicity. Hence, the current research was done to determine if administration of VA could alleviate kidney dysfunction through its anti-apoptosis properties.
As expected, MTX at the dose of 20 mg/kg led to kidney injury, as confirmed by alterations of kidney tissue and a rise in BUN and Cr. Additionally, MTX increased MDA content, reduced TAC, and enhanced inflammatory cytokines in kidney tissue. Another prominent finding of this study was the effect of VA on MTX nephrotoxicity via Bax/Bcl-2/Caspase-3. As the results of molecular parameters showed, MTX intensified the expressions of apoptosis genes and could reduce Nrf-2 levels in kidney tissue. However, VA could reverse these changes. Moreover, the results of this study revealed that VA could protect against kidney dysfunction in MTX-induced nephrotoxicity. Interestingly, the most effective dose of VA was 100 mg/mg in this model. According to preliminary studies, nephrotoxicity is the most common side effect of MTX treatment. MTX-induced nephrotoxicity depends on its high doses, which may result in acute kidney injury (Widemann et al. 2004). Growing evidence has confirmed that oxidative stress, inflammation, and apoptosis are involved in nephrotoxicity (Pabla and Dong 2008). Numerous studies have also indicated that oxidative stress is linked to progression of different pathological processes alongside the adverse effects of MTX in kidneys, which causes histological and functional alterations (Yuksel et al. 2017). Recently, studies have confirmed that oxidative stress leads to cellular injury due to MTX-nephrotoxicity (Yuksel et al. 2017). Lipid peroxidation and apoptotic cell death contribute to renal toxicity induced by MTX (Neradil et al. 2012; Yuksel et al. 2017). They are accompanied by morphological and biochemical findings (Yuksel et al. 2017). Simultaneously, because of the disturbed balance of the antioxidant –oxidant system and switching to the oxidant situation, alterations in MDA and TAC can be detected (Yuksel et al. 2017). MDA acts as a lipid peroxidation index which changes membrane permeability, following oxidative stress (Dalle-Donne et al. 2006). Our study confirmed that MTX (20 mg/kg) elevated the MDA level and reduced TAC content, which corroborates the results of an earlier study (Yuksel et al. 2017). Based on the findings of another study, increased kidney MDA level after MTX administration (20 mg/kg) could lead to diminished antioxidant content (Mahmoud et al. 2019). In line with this study, elevated renal MDA was observed in the MTX-treated rats, which was dose-dependently inhibited by VA administration.
The antioxidant system is an outstanding defense state whose function is to protect cells from detrimental effects including lipid peroxidation, by removing oxygen free radicals (Maiorino et al. 2018), which is verified by results of earlier research where improved antioxidant levels could ameliorate renal dysfunction induced by oxidative stress (Mahmoud et al. 2017b). The present study showed that VA could enrich TAC content in the kidney tissue of rats. A similar preliminary study validated that VA could restore antioxidant agents in rat cisplatin nephrotoxicity (Sindhu et al. 2015). According to our results, VA could be considered as a potent scavenger of free radicals, preserving the kidney from oxidative damage induced by MTX. These results showed that VA exerted remarkably protective effects against MTX-induced nephrotoxicity. A pervious study investigated the possible improving activity of VA on mitomycin C –induced genotoxic damage, and the results revealed that the hydroxyl group is possible factor contributing to this activity, which locates VA in the classification of phenolic compounds (Yetiştirme et al. 2012). These conclusions support our assumption that the underlying mechanisms of nephrotoxicity involve free radical production and that the ameliorative effect of VA is owing to the antioxidant system.
Nrf-2 has been reported to protect cells against oxidative stress and adjust the transcription of cellular protective genes such as the antioxidant enzymes (Zoja et al. 2014). Available evidence suggests that Nrf-2 can act as a cytoprotective factor in several diseases including renal ischemia–reperfusion injury, nephrotoxicity (Liu et al. 2009), and cerebral ischemia–reperfusion injury (Shih et al. 2005). Nrf-2 is activated by overproduction of ROS, which results in its separation from Keap1 and relocation into the nucleus, and this will in turn increase the expression of cytoprotective proteins (Jaiswal 2004). According to our results, the reduced mRNA of Nrf-2 expression was caused by a single dose of MTX. Similarly, an in vitro study demonstrated the effect of reduced Nrf-2 expression on deteriorating kidney damage after oxidative stress, leading to increased mortality (Liu et al. 2009). In addition, VA can elevate renal TAC content by increasing Nrf-2 expression. Our results indicated that the potential effects of VA might be correlated with its ability to activate Nrf-2, which confirms the findings of a recent study (Ramadan et al. 2022).
Dysregulation of apoptosis signaling can cause renal cell death and ultimately kidney injury (Hassanein et al. 2019). Caspase-3 is recognized as an inducer in the apoptosis pathway. Accordingly, its activation can reduce Bcl-2 and increase Bax protein (Hassanein et al. 2019). According to our qRT-PCR results, MTX could significantly increase mRNA expression of caspase-3 and Bax, and this could significantly reduce Bcl-2 in renal tissue, which corroborates the results of a previous study (Hassanein et al. 2019). VA inhibited apoptosis by decreasing mRNA expression of both caspase-3 and Bax expression and elevating the mRNA expression of Bcl-2. According to these findings, suppression of apoptosis in kidney tissue by VA may potentially ameliorate kidney injury, and the caspase-3/Bax/Bcl-2 pathway may be considered the target of VA’s nephroprotective activity. The current findings lend support to previous research indicating that the beneficial effect of VA prevented apoptosis through regulating apoptotic factors (Prince et al. 2011; Vishnu et al. 2018). In line with the present study, other experimental works have confirmed the nephroprotective effect of antioxidant agents on drug-induced nephrotoxicity through adjustment of apoptosis signaling (Gur et al. 2022; Joardar et al. 2019).
Accumulating evidence has revealed that oxidative stress is a well-known nuclear factor-κB (NF-κβ) stimulator, which also stimulates generation of inflammatory agents (Chowdhury et al. 2016; Mahmoud et al. 2017a). In the present study, a single dose of MTX was found to elevate renal TNF-α and IL-1β content. This is confirmed in previous studies where administration of toxic doses of nephrotoxic drugs including cisplatin and MTX accounted for increased TNF-α and IL-1β levels (Sindhu et al. 2015; Oguz et al. 2015). Interestingly, oral VA administration reduced inflammatory cytokines in kidney tissue against oxidative damage induced by MTX. Inconsistent with the current study, however, administration of VA was found to improve myocardial injury through anti-inflammatory and anti-oxidative properties (Prince et al. 2011) (Prince et al. 2015). Another significant finding of this study was that the removal of free radical by VA resulted in decreased oxidative stress level, thereby suppressing the renal pro-inflammatory cytokines and preventing inflammation induced by MTX. These properties could be attributed to the anti-inflammatory effect of VA.
Conclusions
Overall, the results of the current work indicate that VA could ameliorate MTX-induced kidney injury by enhancing TAC, inhibiting apoptosis, and preventing inflammatory response. In addition, VA could improve the kidney tissue injury caused by MTX-nephrotoxicity. Hence, VA could be considered an efficient candidate for the reduction of complications and the treatment of MTX-nephrotoxicity after the clinical trial phases are completed. The results of the current research confirmed that the most effective dose of VA is 100 mg/kg.
Data availability
All data generated or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- VA:
-
Vanillic acid
- MTX:
-
Methotrexate
- TAC:
-
Total antioxidant capacity
- MDA:
-
Malondialdehyde
- BAX:
-
Bcl-2-associated x protein
- Bcl-2:
-
B-cell lymphoma-2
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukine-1β
- NS:
-
Normal saline
- BUN:
-
Blood urea nitrogen
- Cr:
-
Creatinine
- qRT‑PCR:
-
Quantitative real‑time PCR
- H&E:
-
Hematoxylin-eosin
- ANOVA:
-
One-way analysis of variance
- NF-κβ:
-
Nuclear factor-κB
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- Nrf-2:
-
Nuclear factor erythroid 2-related factor 2
References
Amini N, Badavi M, Mard SA, Dianat M, Moghadam MT (2022) The renoprotective effects of gallic acid on cisplatin-induced nephrotoxicity through anti-apoptosis, anti-inflammatory effects, and downregulation of lncRNA TUG1. Naunyn-Schmiedeberg’s Arch Pharmacol 395(6):691–701
Bai J, Zhang Y, Tang C, Hou Y, Ai X, Chen X, Zhang Y, Wang X, Meng X (2021) Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed Pharmacother 133:110985. https://doi.org/10.1016/j.biopha.2020.110985
Basile DP, Anderson MD, Sutton TA (2012) Pathophysiology of acute kidney injury. Compr Physiol 2(2):1303–1353
Chowdhury S, Sinha K, Banerjee S, Sil PC (2016) Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. BioFactors 42(6):647–664
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52(4):601–623
Devrim E, Çetin R, Kılıçoğlu B, Imge Ergüder B, Avcı A, Durak İ (2005) Methotrexate causes oxidative stress in rat kidney tissues. Ren Fail 27:771–773
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Gur C, Kandemir FM, Caglayan C, Satıcı E (2022) Chemopreventive effects of hesperidin against paclitaxel-induced hepatotoxicity and nephrotoxicity via amendment of Nrf2/HO-1 and caspase-3/Bax/Bcl-2 signaling pathways. Chem Biol Interact 365:110073. https://doi.org/10.1016/j.cbi.2022.110073. (Epub 2022 Jul 31)
Hassanein EHM, Shalkami AS, Khalaf MM, Mohamed WR, Hemeida RAM (2019) The impact of Keap1/Nrf2, P(38)MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed Pharmacother 109:47–56
Havasi A, Borkan SC (2011) Apoptosis and acute kidney injury. Kidney Int 80:29–40
Jaiswal AK (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–1207
Joardar S, Dewanjee S, Bhowmick S, Dua TK, Das S, Saha A, De Feo V (2019) Rosmarinic acid attenuates cadmium-induced nephrotoxicity via inhibition of oxidative stress, apoptosis, inflammation and fibrosis. Int J Mol Sci 20:2027
Kandemir FM, Kucukler S, Caglayan C, Gur C, Batil AA, Gülçin İ (2017) Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: Biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem 41:e12398. https://doi.org/10.1111/jfbc.12398
Kiokias S, Proestos C, Oreopoulou V (2020) Phenolic acids of plant origin—A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods 9:534. https://doi.org/10.3390/foods9040534
Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H (2009) Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76:277–285
Mahmoud AM, Hussein OE, Hozayen WG, Abd el-Twab SM, (2017a) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: Protective effect of 18β-Glycyrrhetinic acid. Chem Biol Interact 270:59–72
Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR (2017b) Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact 277:146–158
Mahmoud AM, Hussein OE, Abd El-Twab SM, Hozayen WG (2019) Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct 10:4593–4607
Maiorino M, Conrad M, Ursini F (2018) GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid Redox Signal 29:61–74
Neradil J, Pavlasova G, Veselská R (2012) New mechanisms for an old drug; DHFR-and non-DHFR-mediated effects of methotrexate in cancer cells. Klin Onkol 25:2S87-82S92
Oguz E, Kocarslan S, Tabur S, Sezen H, Yilmaz Z, Aksoy N (2015) Effects of lycopene alone or combined with melatonin on methotrexate-induced nephrotoxicity in rats. Asian Pac J Cancer Prev 16:6061–6066
Öktem F, Yilmaz HR, Ozguner F, Olgar S, Ayata A, Uzar E, Uz E (2006) Methotrexate-induced renal oxidative stress in rats: the role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health 22:241–247
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994–1007
Prince PS, Dhanasekar K, Rajakumar S (2011) Preventive effects of vanillic acid on lipids, bax, bcl-2 and myocardial infarct size on isoproterenol-induced myocardial infarcted rats: a biochemical and in vitro study. Cardiovasc Toxicol 11:58–66
Prince PSM, Dhanasekar K, Rajakumar S (2015) Vanillic acid prevents altered ion pumps, ions, inhibits Fas-receptor and caspase mediated apoptosis-signaling pathway and cardiomyocyte death in myocardial infarcted rats. Chem Biol Interact 232:68–76
Ramadan SS, Almeer R, Albasher G, Abdel Moneim AE (2022) Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin Reviews 41:446–456
Ramsey LB, Balis FM, O’Brien MM, Schmiegelow K, Pauley JL, Bleyer A, Widemann BC, Askenazi D, Bergeron S, Shirali A (2018) Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 23:52–61
Shih AY, Li P, Murphy TH (2005) A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. Journal Neursci 25:10321–10335
Sindhu G, Nishanthi E, Sharmila R (2015) Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: a biochemical and molecular study. Environ Toxicol Pharmacol 39:392–404
Türk E, Güvenç M, Cellat M, Uyar A, Kuzu M, Ağgül AG, Kırbaş A (2022) Zingerone protects liver and kidney tissues by preventing oxidative stress, inflammation, and apoptosis in methotrexate-treated rats. Drug Chem Toxicol 45:1054–1065
Vishnu KV, Ajeesh Kumar KK, Chatterjee NS, Lekshmi RGK, Sreerekha PR, Mathew S, Ravishankar CN (2018) Sardine oil loaded vanillic acid grafted chitosan microparticles, a new functional food ingredient: attenuates myocardial oxidative stress and apoptosis in cardiomyoblast cell lines (H9c2). Cell Stress Chaperones 23:213–222
Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, Bacci G, Craft AW, Adamson PC (2004) High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma: incidence, treatment, and outcome. Cancer 100:2222–2232
Yetiştirme DH, Erdem MG, Cinkılıç N, Vatan Ö, Yılmaz D, Bağdaş D, Bilaloğlu R (2012) Genotoxic and anti-genotoxic effects of vanillic acid against mitomycin C-induced genomic damage in human lymphocytes in vitro. Asian Pac J Cancer Prev 13:4993–4998
Yuksel Y, Yuksel R, Yagmurca M, Haltas H, Erdamar H, Toktas M, Ozcan O (2017) Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum Exp Toxicol 36:51–61
Zoja C, Benigni A, Remuzzi G (2014) The Nrf2 pathway in the progression of renal disease. Nephrol Dial Transplant 29:i19–i24
Acknowledgements
This paper was extracted from an MD thesis written by Ms. Mahla Hasanzadeh Shoshtari, a student of medicine at Jundishapur University of Medical Sciences, Ahvaz, Iran. The authors would like to express their gratitude for the assistance of the Persian Gulf Physiology Research Center, Ahvaz Jundishapur University of Medical Sciences.
Funding
This work was supported financially by the Persian Gulf Physiology Research Center (APRC-0102) funded by the Vice Chancellor of Research, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author information
Authors and Affiliations
Contributions
NA designed the study and wrote the manuscript. MH contributed to the data collection. MB performed the data analysis and interpreted the results. FN performed molecular analysis and the histology. Mahin Dianat contributed to the data analysis. All authors read and approved the final version of the manuscript.The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical considerations
The current research was confirmed by the Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Iran (IR.AJUMS.ABHC.REC.1401.009).
Consent to participate
This is an animal study.
Consent for publication
All authors agree to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amini, N., Shoshtari, M.H., Nejaddehbashi, F. et al. Dose-dependent renoprotective effect of vanillic acid on methotrexate-induced nephrotoxicity via its anti-apoptosis, antioxidant, and anti-inflammatory properties. Naunyn-Schmiedeberg's Arch Pharmacol 397, 4195–4204 (2024). https://doi.org/10.1007/s00210-023-02866-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02866-y