Abstract
We made an attempt to evaluate the preventive effects of vanillic acid on isoproterenol-induced myocardial infarcted rats. Rats were pretreated with vanillic acid (5 and 10 mg/kg) daily for 10 days. After pretreatment, rats were injected with isoproterenol (100 mg/kg) at an interval of 24 h for 2 days to induce myocardial infarction. Isoproterenol induction increased the activity of serum creatine kinase-MB and increased the levels of serum and heart cholesterol, triglycerides, free fatty acids in rats. It increased the levels of serum low density and very low density lipoprotein cholesterol and decreased the levels of high-density lipoprotein cholesterol. Also, the activity of 3-hydroxy-3methyl glutaryl-coenzyme-A-reductase in the plasma and liver was increased, and lecithin cholesterol acyl transferase activity in the plasma and liver was decreased in isoproterenol-induced rats. Furthermore, isoproterenol-induced rats showed a decrease in myocardial expression of B-cell leukemia/lymphoma-2(bcl-2) gene and an increase in myocardial expression of bcl-2 associated-x (bax)-gene. Vanillic acid pretreated isoproterenol-induced rats positively altered all the above-mentioned biochemical parameters. Vanillic acid pretreatment also reduced myocardial infarct size in myocardial infarcted rats. In vitro study confirmed the potent free radical scavenging effect of vanillic acid. The observed effects are due to free radical scavenging effects of vanillic acid. This study may have a significant impact on myocardial infarcted patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
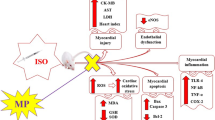
Myocardial infarction (MI) is the most lethal manifestation of cardiovascular disease (CVD) and has been the subject of intense investigation by clinicians and basic medical scientists. MI is the acute condition of necrosis of the myocardium that occurs as a result of imbalance between coronary blood supply and myocardial demand [1]. Isoproterenol (ISO) is a synthetic catecholamine and beta-adrenergic agonist, which causes severe stress in the myocardium, resulting in infarct-like necrosis of the heart muscle [2]. Experimental induction of MI by ISO in animals is a well-established model to study the protective effects of different cardio protective agents. ISO increases lipids such as total cholesterol, triglycerides (TGs), free fatty acids (FFAs), and phospholipids in the circulation [3]. It also increases the levels of low-density lipoprotein (LDL) cholesterol in the blood, which in turn leads to the build up of harmful deposits in the arteries, thus favoring coronary heart disease [4]. MI induced by ISO shows many metabolic and morphologic aberrations in the myocardium of experimental animals similar to those observed in human MI.
In the myocardium, apoptosis has been observed in a number of cardiac pathologies including hypoxia, ischemia followed by reperfusion, MI, myocardial hypertrophy and in patients with end-stage heart failure [5]. Apoptotic processes are regulated by several proteins including Bax and Bcl-2 which both play vital roles. Bcl-2 (an inhibitor of apoptosis) and Bax (an inducer of apoptosis) expression is a critical intracellular checkpoint of apoptosis within a distal common cell death pathway. Over expression of Bcl-2 promotes cell survival in vitro and in vivo [6–8]. When Bax is over expressed, apoptosis death is accelerated. Thus, the ratio of Bcl-2/Bax plays an important role in determining the susceptibility to apoptosis and whether the cell survives or dies [9]. Also, Bax and Bcl-2 play an important role in the pathophysiology of MI and are expressed in human MI. Thus, these are potential targets for the treatment of MI.
Phenolic acids are hydroxylated derivatives of benzoic and cinnamic acids. Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a phenolic derivative of edible plants and fruits. It is also an intermediate in the production of vanillin from ferulic acid [10, 11]. The highest amount of vanillic acid in plants is found in the roots of Angelica sinensis [12]. Previous studies have shown the antifilarial [13], antibacterial [14], and antimicrobial [15] effects of vanillic acid. Vanillic acid also exhibits chemo preventive effect in experimentally induced carcinogenesis in rats [16]. Recently, there has been an upsurge of interest to explore the cardioprotective potential of natural products. Very few scientific reports are available on the effects of phenolic acids on MI. Lipids, lipoproteins, antiapoptotic protein Bcl-2, proapoptotic protein Bax, and free radicals play an important role in the pathology of MI. One of the treatment strategies aims to prevent MI is to maintain lipids, lipoproteins, Bcl-2, Bax, and free radicals in normal levels. Hence, in view of the above facts, we made an attempt to evaluate the preventive effects of vanillic acid on ISO-induced myocardial infarcted rats with reference to lipids, lipoproteins, marker enzymes of lipid metabolism, Bcl-2, Bax, and myocardial infarct size. In vitro study on the effect of vanillic acid on scavenging free radical 1, 1-diphenyl-2-picryl hydrazyl radical (DPPH•) was also carried out.
Materials and Methods
Experimental Animals
Male albino Wistar rats (Rattus norvegicus) weighing 180–200 g, obtained from the Central Animal House, Rajah Muthiah Institute of Health Sciences, Annamalai University, Tamil Nadu, India were used in this study. They were housed (3 rats/cage) in polypropylene cages (47 × 34 × 20 cm) lined with husk, renewed every 24 h under a 12:12 h light and dark cycle at around 22°C. The rats had free access to tap water and food. The rats were fed on a standard pelleted diet (Pranav Agro Industries Ltd., Maharashtra, India). The experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi, India and approved by the Animal Ethical Committee of Annamalai University (Proposal No: 699; Approval date: 11-01-2010).
Drug and Chemicals
Vanillic acid, isoproterenol hydrochloride, and 1, 1-diphenyl-2-picryl hydrazyl radical were purchased from Sigma Chemical Co., St. Louis, MO, USA. Thiobarbituric acid, trichloro acetic acid, ferrous ammonium sulphate, phosphotungstic acid, digitonin, and hydroxylamine hydrochloride were obtained from Himedia, Mumbai, India. All the other chemicals used in the study were of analytical grade.
Induction of Experimental Myocardial Infarction and Experimental Design
Isoproterenol (100 mg/kg) dissolved in saline was subcutaneously injected to rats at an interval of 24 h for 2 days. ISO-induced MI was confirmed by elevated activity of serum creatine kinase-MB (CK-MB) in rats. The rats were randomly divided into six groups of six rats each. Group I: Normal control rats; Group II: Rats were orally treated with vanillic acid (5 mg/kg) daily for a period of 10 days by an intragastric tube; Group III: Rats were orally treated with vanillic acid (10 mg/kg) daily for a period of 10 days by an intragastric tube; Group IV: Rats were subcutaneously injected with ISO alone (100 mg/kg) at an interval of 24 h for 2 days (on 11th and 12th day); Group V: Rats were orally pretreated with vanillic acid (5 mg/kg) by an intragastric tube daily for a period of 10 days and then subcutaneously injected with ISO (100 mg/kg) at an interval of 24 h for 2 days (on 11th and 12th day); Group VI: Rats were pretreated with vanillic acid (10 mg/kg) orally by an intragastric tube daily for a period of 10 days and then subcutaneously injected with ISO (100 mg/kg)) at an interval of 24 h for 2 days (on 11th and 12th day); normal control and ISO control rats were received saline alone daily for a period of 10 days. Vanillic acid was dissolved in saline and administered to rats one milliliter each.
At the end of the experimental period, after 12 h of second ISO injection (i.e. on 13th day), all the rats were anesthetized and then killed by cervical decapitation. Blood was collected; plasma and serum were separated by centrifugation. Heart and liver tissues were excised immediately and rinsed in ice-chilled saline. A portion of the heart was used for reverse transcription polymerase chain reaction (RT–PCR) study and determination of myocardial infarct size.
Biochemical Analysis
Serum CK-MB activity was assayed by a commercial kit obtained from Agappe Diagnostics, Kerala, India. Lipids were extracted from the heart tissues by the method of Folch et al. [17] using chloroform/methanol mixture (2:1 v/v). The levels of total cholesterol, TGs, and FFAs in the serum and heart were estimated [18–20]. Also, the activity of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase and lecithin cholesterol acyl transferase (LCAT) in the plasma and liver was assayed by standard methods [21, 22]. Cholesterol in the lipoprotein fraction was also determined by the method of Zlatkis et al. [18]. High-density lipoprotein (HDL)-cholesterol was estimated by a standard commercial kit obtained from Agappe Diagnostics, Kerala, India. LDL-cholesterol and very low-density lipoprotein (VLDL)-cholesterol were calculated. VLDL-C = TGs/5; LDL-C = total cholesterol−(HDL-C + VLDL-C). The content of protein in the heart and liver tissue homogenates was estimated by the method of Lowry et al. [23].
Ribonucleic Acid (RNA) Isolation and RT–PCR Analysis of Cardiac Gene Expression of Bax and Bcl-2
Reverse transcription polymerase chain reaction was performed to validate the differential expression of genes. Heart samples were immediately taken from each group after killing animals, and left ventricular area was removed and chopped into small pieces. To measure myocardial gene expression, total RNA was extracted from the heart tissues by Medox-Easy TM spin column total minipreps kit purchased from Medox Biotech India Private Limited, Chennai, India, and the isolated RNA was treated with RNase-free DNase I (Medox) at 37°C for 30 min for the removal of DNA. Samples were incubated at 30°C for 60 min. Reactions were stopped by heating at 95°C for 10 min. Reverse transcription was carried out using 2 μg of total RNA and 200 Units of M-MuLV Reverse Transcriptase (Medox Biotech). Amplification was performed by Medox PCR Master Mix in a volume of 25 μl. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for RT–PCR study.
Polymerase chain reaction was carried out using a hot start method by adding 2 μl of cDNA product to 18 μl of PCR buffer containing 67 mM Tris (pH 8.8), 1.5 mM magnesium chloride, 16.6 mM ammonium sulphate, 200 μM mixed dNTPs, 125 U/ml Taq polymerase (Gibco/BRL), sense and anti-sense primers. The primers used were (a) rat GAPDH sense primer: TTCTTGTGCAGTGCCAGCCTCGTC and anti-sense primer: TAGGAACACGGAA GGCCATGCCAG (461 bp product size), (b) rat bax sense primer: TTCATCCAGGATCGAGCAGA and anti-sense primer: GCAAAGTAGAAGGCAACG (263 bp product size), (c) rat bcl-2 sense primer: CTGGTGGACAACATCGCTCTG and anti-sense primer: GGTCTGCTGACCTCACTTGTG (560 bp product size). After denaturing at 94°C for 2 min, the reaction was conducted for 30 cycles at 94°C for 1 min, 60°C (bax and bcl-2) for 1 min, and 72°C for 1 min. Products were electrophoresed on 3% agarose gel and visualized by staining with ethidium bromide.
Macroscopic Enzyme Mapping of Infarcted Myocardium (Triphenyl Tetrazolium Chloride Test, TTC)
A section of the heart tissue from each group was used for the TTC test. The macroscopic enzyme mapping assay (TTC test) of the infarcted myocardium was done according to the method of Lie et al. [24]. A freshly prepared solution of 1% TTC in phosphate buffer was prewarmed at 37–40°C for 30 min in a darkened glass. To remove excess blood, the heart tissues were washed rapidly in cold water without macerating the tissue. After removing epicardial fat, the left ventricle was taken separately. The heart was transversely cut across the left ventricle to obtain slices not more than 0.1–0.2 mm in thickness. Then, the heart tissue slices were kept in a covered, darkened glass dish containing prewarmed solution of TTC, and the dish was kept in an incubator and heated to 37–40°C for 45 min. The heart slices were turned over thrice and made certain that it remains fully immersed in the TTC solution. At the end of the incubation period, the heart slices were kept in fixing solution to fix the tissue. Colour photographs of heart slices were obtained by a camera with macrolens. The expected reaction of the TTC test was as follows: normal myocardium (Lactate dehydrogenase (LDH) enzyme active) turned to bright red, infarcted myocardium (LDH-enzyme deficient) turned to pale gray or uncoloured and fibrous scars turned to white.
In Vitro Free Radical (DPPH•) Scavenging Test
The ability to scavenge the stable free radical, DPPH• by vanillic acid was determined by the method of Mensor et al. [25].
Statistical Analysis
Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using Software Package for the Social Science software package version 12.00. Results were expressed as mean ± SD for six rats in each group. P values <0.05 were considered significant.
Results
Effect of Vanillic Acid on Serum Cardiac Marker Enzyme, CK-MB
Rats treated with vanillic acid (5 and 10 mg/kg) (70.1 ± 6.5a and 69.7 ± 5.7a respectively) did not show any significant (P < 0.05) effect in serum CK-MB. Rats induced with ISO showed significant (P < 0.05) increased activity of serum CK-MB (158.8 ± 13.5b) compared to normal control rats (70.3 ± 6.1a). Pretreatment with vanillic acid (5 and 10 mg/kg) significantly (P < 0.05) decreased the activity of this enzyme in the serum of ISO-induced rats (122.5 ± 11.8c and 93.4 ± 8.7d, respectively) compared to ISO alone induced rats. The values are expressed in IU/L.
Effect of Vanillic Acid on Serum, Heart Lipids and Serum Lipoproteins
Rats induced with ISO showed significant (P < 0.05) increased levels of serum total cholesterol, TGs and FFAs (Fig. 1). ISO-induced rats also showed significant (P < 0.05) increased levels of heart total cholesterol, TGs, and FFAs (Fig. 2). Vanillic acid (5 and 10 mg/kg) pretreated ISO-induced rats significantly (P < 0.05) minimized the alterations of lipids. Also, rats induced with ISO showed significant (P < 0.05) increased LDL and VLDL-cholesterol levels with significant (P < 0.05) decreased HDL-cholesterol levels and vanillic acid (5 and 10 mg/kg) pretreated ISO-induced rats showed significant (P < 0.05) decreased levels of LDL-cholesterol, VLDL-cholesterol and significant (P < 0.05) increased levels of HDL-cholesterol (Fig. 3).
Levels of serum total cholesterol, TGs, and FFAs. Group I: Normal control; Group II: Vanillic acid (5 mg/kg); Group III: Vanillic acid (10 mg/kg); Group IV: ISO control (100 mg/kg); Group V: Vanillic acid (5 mg/kg) + ISO (100 mg/kg); Group VI: Vanillic acid (10 mg/kg) + ISO (100 mg/kg). Each column is mean ± SD for six rats in each group; Columns not sharing a common letter (a, b, c, d) differ significantly with each other (P < 0.05; DMRT). Group I is compared with Group II, Group III, and Group IV; Group V and Group VI are compared with Group IV
Levels of heart total cholesterol, TGs, and FFAs. Group I: Normal control; Group II: Vanillic acid (5 mg/kg); Group III: Vanillic acid (10 mg/kg); Group IV: ISO control (100 mg/kg); Group V: Vanillic acid (5 mg/kg) + ISO (100 mg/kg); Group VI: Vanillic acid (10 mg/kg) + ISO (100 mg/kg). Each column is mean ± SD for six rats in each group; Columns not sharing a common letter (a, b, c, d) differ significantly with each other (P < 0.05; DMRT). Group I is compared with Group II, Group III, and Group IV; Group V and Group VI are compared with Group IV
Levels of serum low-density lipoprotein (LDL), very low-density lipoprotein (VLDL) and high-density lipoprotein (HDL)-cholesterol. Group I: Normal control; Group II: Vanillic acid (5 mg/kg); Group III: Vanillic acid (10 mg/kg); Group IV: ISO control (100 mg/kg); Group V: Vanillic acid (5 mg/kg) + ISO (100 mg/kg); Group VI: Vanillic acid (10 mg/kg) + ISO (100 mg/kg). Each column is mean ± SD for six rats in each group; Columns not sharing a common letter (a, b, c, d) differ significantly with each other (P < 0.05; DMRT). Group I is compared with Group II, Group III and Group IV; Group V and Group VI are compared with Group IV
Effect of Vanillic Acid on Plasma and Liver Lipid Metabolizing Enzymes
Rats induced with ISO showed significant (P < 0.05) increased activity of HMG-CoA reductase in the plasma and liver. Vanillic acid (5 and 10 mg/kg) pretreated ISO-induced rats significantly (P < 0.05) decreased the activity of HMG-CoA reductase in the plasma and liver compared to ISO-induced rats. Lower ratio indicates higher enzyme activity and vice versa. In Group-IV, lower ratio of HMG-Co-A/mevalonate indicates higher enzyme activity and in Groups-V, VI higher ratio indicates lower enzyme activity (Fig. 4). Also, rats induced with ISO showed significant (P < 0.05) decreased activity of LCAT in the plasma and liver and vanillic acid (5 and 10 mg/kg) pretreated ISO-induced rats significantly (P < 0.05) increased the activity of this enzyme in the plasma and liver (Fig. 5).
Activity of plasma and liver 3-hydroxyl-3-methyl glutaryl coenzyme A(HMG-CoA)-reductase. Group I: Normal control; Group II: Vanillic acid (5 mg/kg); Group III: Vanillic acid (10 mg/kg); Group IV: ISO control (100 mg/kg); Group V: Vanillic acid (5 mg/kg) + ISO (100 mg/kg); Group VI: Vanillic acid (10 mg/kg) + ISO (100 mg/kg). In Group IV, lower ratio of HMG-CoA/mevalonate indicates higher enzyme activity and in Groups V and VI higher ratio of HMG-CoA/mevalonate indicates lower enzyme activity. Each column is mean ± SD for six rats in each group; columns not sharing a common letter (a, b, c, d) differ significantly with each other (P < 0.05; DMRT). Group I is compared with Group II, Group III and Group IV; Group V and Group VI are compared with Group IV
Activity of lecithin cholesterol acyl transferase(LCAT)in the plasma and liver. Group I: Normal control; Group II: Vanillic acid (5 mg/kg); Group III: Vanillic acid (10 mg/kg); Group IV: ISO control (100 mg/kg); Group V: Vanillic acid (5 mg/kg) + ISO (100 mg/kg); Group VI: Vanillic acid (10 mg/kg) + ISO (100 mg/kg). Each column is mean ± SD for six rats in each group; Columns not sharing a common letter (a, b, c, d) differ significantly with each other (P < 0.05; DMRT). Group I is compared with Group II, Group III and Group IV; Group V and Group VI are compared with Group IV
Effect of Vanillic Acid on Myocardial Gene Expression
An increased myocardial expression of bax gene and decreased myocardial expression of bcl-2 gene was observed in ISO-induced rats compared to normal control rats. Pretreatment with vanillic acid (5 and 10 mg/kg) decreased the expression of bax and increased the expression of bcl-2 in ISO-induced myocardium (Fig. 6).
Effect of Vanillic Acid on Myocardial Infarct Size
The size of the myocardial infarct as determined by TTC test is shown in Fig. 7a–d. Figure 7a indicates a section of heart from normal control rats with completely viable myocardial tissue stained with TTC to indicate the presence of LDH (bright red) and intact myocardial tissue. Figure 7b, c indicates a section of heart from rats treated with vanillic acid (5 and 10 mg/kg) shows results similar to that of normal control rats. Figure 7d shows a section of heart from ISO-induced group. Infarcted tissues are clearly visible, pale gray, or colorless. Infarcted tissues did not stain with TTC because of the leakage of LDH in that area. Figure 7e, f shows a section of heart tissue of vanillic acid (5 and 10 mg/kg) pretreated rats administered with ISO. A major portion of the heart tissue stained positively for viability (mild LDH-enzyme leakage) and much reduced myocardial infarct size. The results of Fig. 7f clearly revealed that prior oral administration of vanillic acid (10 mg/kg) might have prevented the membrane damage caused by ISO, thereby reducing myocardial infarct size and maintaining near normal myocardial membrane structural and functional integrity.
Macroscopic enzyme mapping assay of heart (Triphenyl Tetrazolium Chloride, TTC). a Normal control rat’s heart showing completely viable myocardial tissue stained with TTC. b Rats treated with vanillic acid (5 mg/kg) showing completely viable myocardial tissue stained with TTC. c Rats treated with vanillic acid (10 mg/kg) showing completely viable myocardial tissue stained with TTC. d ISO-induced rat’s heart tissue did not stain with TTC and infarcted tissue showing pale grey or colourless. e Vanillic acid (5 mg/kg) pretreated ISO-induced rat’s heart tissue stained positively for viability (reduced infarct size). f Vanillic acid (10 mg/kg) pretreated ISO-induced rat’s heart tissue stained highly positive for viability (much reduced myocardial infarct size)
Effect of Vanillic Acid on In Vitro Free Radical (DPPH•) Scavenging
Figure 8 shows the percentage scavenging effects of vanillic acid on the free radical, DPPH•. Vanillic acid scavenges these free radicals in vitro in a dose dependent manner (15, 30, 45, 60 μM). The percentage scavenging effect of vanillic acid on DPPH• increases with increasing concentration. The percentage scavenging effects of vanillic acid on DPPH• at different concentrations (15, 30, 45, 60 μM) were found to be 17.92, 35.82, 53.73 and 71.64%, respectively. Moreover, vanillic acid at the concentration of 60 μM showed the highest percentage scavenging effect on DPPH• (71.64%).
For all the biochemical parameters studied, vanillic acid (5 and 10 mg/kg) pretreated ISO-induced rats showed significant effects. But, vanillic acid (10 mg/kg) showed the highest significant effect than the lower dose (5 mg/kg). Rats treated with vanillic acid (5 and 10 mg/kg) daily for a period of 10 days did not show any significant effect on all the biochemical parameters studied.
Discussion
The diagnostic marker enzyme, CK-MB, is increased in the serum of ISO-induced myocardial infarcted rats. The observed increase in the activity of this enzyme is due to ISO-induced cardiac damage. Oral pretreatment with vanillic acid (5 and 10 mg/kg) to ISO-induced rats decreased the activity of serum CK-MB. This effect revealed the cardio protective effect of vanillic acid in ISO-induced rats.
Excess lipids in the blood are considered to be the major risk factor in the development of MI. ISO-induced MI is associated with enhanced levels of circulatory lipids. It has been reported that the increased myocardial cholesterol content in ISO-induced rats is due to increased uptake of LDL-cholesterol from the blood by myocardial membranes [26]. Oral pretreatment with vanillic acid decreased the levels of total cholesterol in the serum and heart of myocardial infarcted rats. The decreased level of total cholesterol observed in this study is due to decreased activity of HMG-CoA reductase in vanillic acid pretreated ISO-induced rats.
Increased level of TGs is one of the risk factors of MI. Increased level of TGs is associated with cardiovascular disturbances, and ISO promotes lipolysis in the myocardium [27]. Enhancement in lipolysis and subsequent elevation of plasma FFAs levels may lead to increased hepatic TGs synthesis and secretion of elevated plasma TGs concentration. The mechanism of observed increase in the synthesis of TGs in the heart tissue could be due to accumulation of acyl-CoA and an augmented production of glycerol by increased glycolytic flux [28]. Vanillic acid pretreatment decreased the levels of TGs in ISO-induced rats.
We observed an increase in the level of FFAs in the serum and heart of ISO-induced rats. It has been reported that increased levels of blood FFAs may depress cardiac function, promote arrhythmias, and further increase the extent of myocardial damage [29]. The heart derives a significant portion of its fatty acid substrates as FFAs derived by lipolysis from adipose tissue. Although lipid availability is important for the heart, excess levels of fatty acid in myocytes can be deleterious. Prior treatment with vanillic acid lowered the levels of FFAs in myocardial infarcted rats.
Lipoproteins are closely associated with MI. Increased levels of serum LDL and VLDL-cholesterol fractions along with decreased levels of HDL-cholesterol were observed in ISO-induced rats. Increased levels of LDL-cholesterol show a positive correlation with MI, whereas HDL-cholesterol levels show a negative correlation. Pretreatment with vanillic acid to ISO-induced rats minimized the alterations in serum lipoprotein levels by increasing HDL-cholesterol and decreasing LDL and VLDL-cholesterol levels.
HMG-CoA reductase plays a major role in the regulation of cholesterol metabolism and a rate-limiting enzyme in the pathway of cholesterol biosynthesis. The observed increase in HMG-CoA reductase activity leads to the excessive production and accumulation of cholesterol resulting in the formation of foam cell, a pre-requisite step in the development of atherosclerosis [30]. Prior treatment with vanillic acid lowered the activity of HMG-CoA reductase in ISO-induced rats. Thus, decreased activity of HMG-CoA reductase resulted in decreased levels of cholesterol observed in vanillic acid pretreated rats.
Decreased activity of LCAT inhibits the esterification of cholesterol in ISO-induced rats. This leads to increased levels of lipids and lipoproteins in the circulation, which are at high risk of MI. Vanillic acid pretreatment enhanced the activity of LCAT in ISO-induced rats. Thus, vanillic acid increased the HDL-cholesterol levels as discussed earlier is further confirmed by its modulation of LCAT activity, which increases the concentration of HDL-cholesterol in ISO-induced rats.
Cellular cholesterol is an important factor in the prevention of MI. One of the possible mechanisms for the antihypercholesterolemic effects of vanillic acid may be its regulation of cholesterol biosynthesis by decreasing the activity of HMG-CoA reductase in ISO-induced rats. Also, enhancement of turnover of HDL-cholesterol by increased LCAT activity may be another possible mechanism of vanillic acid in ISO-induced rats. The observed effects clearly revealed the lipid-lowering effect of vanillic acid in ISO-induced rats.
In our study, decreased expression of myocardial bcl-2 gene and over expression of myocardial bax gene was observed in ISO-induced rats. Thus, decreased expression of bcl-2 slows myocardial cell survival, and over expression of bax accelerates myocardial cell death (apoptosis). Pretreatment with vanillic acid increased the expression of bcl-2 and decreased the expression of bax in the myocardium, thereby preventing myocardial apoptosis which leads to increased survival of rats.
Triphenyl tetrazolium chloride staining is a well-accepted method to determine myocardial infarct size which provides a reliable index of necrosis [31]. Myocardial necrosis can be detected by direct staining using TTC dye, which forms a red formazan precipitate with LDH of the viable myocardial tissue, whereas the infarcted myocardium fails to stain with TTC [32]. ISO-induced rat’s heart showed increase in myocardial infarct size with less TTC absorbing capacity, thus indicating significant leakage of LDH when compared to normal control rats. Vanillic acid (5 and 10 mg/kg) pretreatment decreased myocardial infarct size with increased TTC absorbing capacity, thus indicating a mild leakage of LDH when compared to normal control rats. The effect of 10 mg/kg vanillic acid was highly effective in reducing myocardial infarct size. Thus, vanillic acid prevented membrane damage and decreased myocardial infarct size and protected the heart from ISO-induced MI.
In this study, free radical scavenging activity of vanillic acid in vitro was determined by DPPH• method. It has been shown that DPPH• is widely used to evaluate the free radical scavenging effects of various antioxidant substances [33]. In the DPPH• method, the antioxidants are able to reduce the stable radical, DPPH• to diphenyl-picryl hydrazine. In this study, vanillic acid scavenges DPPH• dose dependently. The highest percentage DPPH• scavenging effect of vanillic acid at the concentration of 60 μM was found to be 71.64%. Increased free radical production is the major mechanism of ISO-induced MI. ISO metabolism produces excessive free radicals such as superoxide anion and hydroxyl radicals. The free radical scavenging effect of vanillic acid scavenges these free radicals and protects the myocardium against ISO-induced rats. Thus, vanillic acid is a potent free radical scavenger.
The possible mechanism for the observed cardio protective effects of vanillic acid is due to its free radical scavenging action. The free radical scavenging effect of vanillic acid scavenges free radicals thereby decreasing lipids, maintaining lipoproteins, preventing apoptosis by maintaining bax and bcl-2 genes and reducing myocardial infarct size. Our study also revealed that 10 mg/kg of vanillic acid was highly effective than 5 mg/kg. Thus, the present study revealed the cardio protective potential of vanillic acid. This study may have a significant impact on the treatment of myocardial infarcted patients.
References
De Bono, D. P., & Boon, N. A. (1992). Diseases of the cardiovascular system. In C. R. W. Edwards & I. A. D. Boucheir (Eds.), Davidson’s principles and practice of medicine (pp. 249–340). Hong Kong: Churchill Livingstone.
Sushama Kumari, S., Jayadeep, A., Kumar, J. S., & Menon, V. P. (1989). Effect of carnitine on malondialdehyde, taurine and glutathione levels in heart of rats subjected to myocardial stress by isoproterenol. Indian Journal of Experimental Biology, 27, 134–137.
Prabhu, S. N., & Shyamala Devi, C. S. (2006). Efficacy of mangiferin on serum and heart tissue lipids in rats subjected to isoproterenol induced cardiotoxicity. Toxicology, 228, 135–139.
Goldstein, J. L., & Brown, M. S. (1984). Progress in understanding the LDL receptor and HMG-CoA reductase, two membrane proteins that regulate the plasma cholesterol. Journal of Lipid Research, 25, 1450–1461.
Kunapuli, S., Rosanio, S., & Schwarz, E. R. (2006). How do cardiomyocytes die? Apoptosis and autophagic cell death in cardiac myocytes. Journal of Cardiac Failure, 12, 381–391.
Garcia, L., Martinou, I., Tsujimoto, Y., & Martinou, J. C. (1992). Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science, 258, 302–304.
Allsopp, T. E., Wyatt, S., Paterson, H. F., & Davies, A. M. (1993). The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell, 73, 295–307.
Pinon, L. G. P., Middleton, G., & Davies, A. M. (1997). Bcl-2 is required for cranial sensory neuron survival at defined stages of embryonic development. Development, 124, 4173–4178.
Chao, D. T., & Korsmeyer, S. J. (1998). Bcl-2 family: Regulators of cell death. Annual Review of Immunology, 16, 395–419.
Lesage-Meessen, L., Delattre, M., Haon, M., Thibault, J. F., Ceccaldi, B. C., Brunerie, P., et al. (1996). A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus. Journal of Biotechnology, 50, 107–113.
Civolani, C., Barghini, P., Roncetti, A. R., Ruzzi, M., & Schiesser, A. (2000). Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13. Applied and Environmental Microbiology, 66, 2311–2317.
Duke, J. A. (1992). Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton: CRC Press.
Varma, R. S., Shukla, A., & Chatterjee, R. K. (1993). Evaluation of vanillic acid analogues as a new class of antifilarial agents. Indian Journal of Experimental Biology, 31, 819–821.
Rai, R. P., & Maurya, M. S. (1966). Synthesis and evaluation of antibacterial activity of vanillin derivatives. Journal of Science and Technology (Peshawar), 4, 275–276.
Delaquis, P., Stanich, K., & Toivonen, P. (2005). Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. Journal of Food Protection, 68, 1472–1476.
Tsuda, H., Uehara, N., Iwahori, Y., Asamoto, M., Ligo, M., Nagao, M., et al. (1994). Chemopreventive effects of beta-carotene, alpha-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo [4, 5] quinoline in the rat. Japanese Journal of Cancer Research, 85, 1214–1219.
Folch, J., Lees, M., & Sloane, S. G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
Zlatkis, A., Zak, B., & Boyle, A. J. (1953). A new method for the direct determination of serum cholesterol. Journal of Laboratory and Clinical Medicine, 41, 486–492.
Fossati, P., & Prencipe, L. (1982). Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry, 28, 2077–2080.
Falholt, K., Lund, B., & Falholt, W. (1973). An easy colorimetric micro method for routine determination of free fatty acids in plasma. Clinica Chimica Acta, 46, 105–111.
Rao, A. V., & Ramakrishnan, S. (1975). Indirect assessment of hydroxy methyl glutaryl CoA reductase activity in liver tissue. Clinical Chemistry, 21, 1523–1525.
Hitz, J., Steinmetz, J., & Siest, G. (1983). Plasma lecithin: Cholesterol acyltransferase-reference values and effects of xenobiotics. Clinica Chimica Acta, 133, 85–96.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with Folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Lie, J. T., Pairolero, P. C., Holley, K. E., & Titus, J. L. (1975). Macroscopic enzyme mapping verification of large, homogenous, experimental myocardial infarcts of predictable size and location in dogs. Journal of Thoracic and Cardiovascular Surgery, 69, 599–605.
Mensor, L. L., Menezes, F. S., Leitao, G. G., Reis, A. S., dos Santos, T. C., Coube, C. S., et al. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research, 15, 127–130.
Anandan, R., Mathew, S., Sankar, T. V., & Viswanathan Nair, P. G. (2007). Protective effect of n-3 polyunsaturated fatty acids concentrate on isoproterenol induced myocardial infarction in rats. Prostaglandins Leukotrienes and Essential Fatty Acids, 76, 153–158.
Sushama Kumari, S., Varghese, A., Muraleedharan, D., & Menon, V. P. (1990). Protective action of aspirin in experimental myocardial infarction induced by isoproterenol in rats and its effect on lipid peroxidation. Indian Journal of Experimental Biology, 28, 480–485.
Subramanian, R., Ramaswamy, M., & Wasan, K. M. (2003). Role of lipid and lipoprotein metabolizing enzymes in the development of atherosclerosis. Indian Journal of Experimental Biology, 41, 14–25.
Jacksen, G. (1998). Metabolic agents for stable angina. Heart and Metabolism, 1, 10–11.
Esterbauer, H., Gebicki, J., Puhl, H., & Jurgens, G. (1992). The role of lipid peroxidation and antioxidants in oxidative modifications of LDL. Free Radical Biology and Medicine, 13, 341–390.
Prabu, S., Jainu, M., Sabitha, K. E., & Devi, C. S. (2006). Role of mangiferin on biochemical alterations and antioxidant status in isoproterenol-induced myocardial infarction in rats. Journal of Ethnopharmacology, 107, 126–133.
Donnelly, T. J., Sievers, R. E., Vissern, F. L., Welch, W. J., & Wolfe, C. L. (1992). Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion. Circulation, 85, 769–778.
Nishizawa, M., Kohno, M., Nishimura, M., Kitagawa, A., & Niwano, Y. (2005). Non-reductive scavenging of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) by peroxyradical: A useful method for quantitative analysis of peroxyradical. Chemical and Pharmaceutical Bulletin, 53, 714–716.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prince, P.S.M., Dhanasekar, K. & Rajakumar, S. Preventive Effects of Vanillic Acid on Lipids, Bax, Bcl-2 and Myocardial Infarct Size on Isoproterenol-Induced Myocardial Infarcted Rats: A Biochemical and In Vitro Study. Cardiovasc Toxicol 11, 58–66 (2011). https://doi.org/10.1007/s12012-010-9098-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-010-9098-3