Abstract
Objective
The present study aimed to examine the effects of cDMARD and bDMARD therapy on both gene expressions and protein levels of TNF-α, IL-6, IL-10 and fatty acid levels in patients with RA.
Method
Plasma TNF-α, IL-6, and IL-10 levels were examined by the ELISA method, while TNF-α, IL-6, and IL-10 gene expression levels were examined by RT-qPCR, and fatty acid levels were examined by GC/MS.
Results
IL-10 gene expression levels significantly increased in RA patients receiving cDMARD treatment compared to those of the control group. Also, eicosadienoic acid, myristoleic acid and capric acid levels were significantly lower in the patient groups compared to those in the control group.
Conclusion
The drugs used in the treatment of RA had no effect on the fatty acid levels whereas had effects on the mRNA and protein levels of the target cytokines.
Key Points • There are some studies investigating inflammatory cytokines in rheumatoid arthritis but no study includes fatty acid levels besides the cytokines. Therefore, this study has an important contribution for original articles because of including fatty acid levels besides the cytokines on rheumatoid arthritis. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory autoimmune joint disease characterized by cartilage and bone damage as well as extra-articular involvement [1]. The worldwide prevalence of RA is 0.24% based upon the Global Burden of Disease 2010 Study and affect approximately 0.24 to 1% of the population [2]. It has been demonstrated in previous studies that the prevalence of RA disease increases with age. The incidence of RA is higher in northern Europe and North America compared to southern Europe and its incidence is 16.5 cases/100,000 in southern Europe, 29 cases and 38 cases in northern Europe and in North America, respectively. The prevalence and incidence rate of RA is twice as high in women as in men [1, 2].
The exact etiology of RA remains unknown [3, 4]. The most important point in the pathogenesis of RA is joint inflammation and cartilage destruction resulting from the infiltration of immune cells into the synovium and impaired adaptive immune responses [4]. The synovium is infiltrated by innate immune cells including monocytes, dendritic cells, and mast cells, and adaptive immune cells including T-helper-1, T-helper-17, B cells, and plasma cells in the pathogenesis of the disease. Various cytokines and chemokines including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and granulocyte-monocyte colony-stimulating factor regulate the inflammation in the synovium. These cytokines and chemokines activate endothelial cells, attracting immune cells to the synovial region and causing exacerbation of inflammation. The activated fibroblasts and inflammatory cells accumulating in the area of inflammation lead to osteoclast formation, resulting in joint destruction [2]. Also rheumatoid factor (RF) and anticitrullinated protein antibodies (ACPA), which are autoantibodies whose levels increase in the disease, are another feature of RA. Furthermore genetic and environmental factors including smoking, bacteria, and viruses may be other factors in the development of the disease [3, 4].
The increase in oxidative stress and decrease in antioxidant capacity are particularly striking in patients with RA. Previous studies have shown significant increases in mitochondrial ROS production in RA patients [4, 5]. Oxidative stress and chronic inflammation observed in RA patients is associated with an increased dysregulation in lipid metabolism [4]. Inflammatory cytokines, which play a role in the pathogenesis of the disease, contribute not only to oxidative stress but also to the development of dyslipidemia in RA patients [4, 6]. And it has been suggested that changes in lipid metabolism may play a role in the pathogenesis of RA and contribute to the severity of the disease [4].
Conventional disease-modifying anti-rheumatic drugs (cDMARD), biological DMARD drugs (bDMARD), and targeted synthetic DMARD are used in the treatment of RA. The cDMARD group includes methotrexate, leflunomide, hydroxychloroquine, gold salts, and sulfasalazine. TNF inhibitors (adalimumab, etanercept, infliximab, golimumab, and certolizumab) and non-TNF inhibitors (tocilizumab, abatacept, rituximab) are included in the bDMARD group. In addition, JAK inhibitors (tofacitinib) are in the targeted group of synthetic DMARDs [1, 2].
It has been shown that cDMARD and bDMARD therapies used in patients with RA cause changes in gene expressions and protein levels of cytokines which play a role in the inflammatory process [4,5,6]. Additionally, some studies suggested that fatty acid levels are also affected by these drugs. However, there is limited study exploring the treatment effects on fatty acids with conflicting results in RA. The present study aimed to compare the effects of cDMARD and bDMARD therapy on both gene expressions and protein levels of TNF-α, IL-6, IL-10, and fatty acid levels in patients with RA.
Materials and methods
Study groups
Forty patients who admitted to Hatay Mustafa Kemal University Hospital Rheumatology Clinics and were followed up as a result RA diagnosis comprised the study group while 20 patients with matching age and sex attributes to those of the treatment groups were included in the study as the control group. The patients were diagnosed with RA according to the 2010 ACR-EULAR classification criteria [7]. Rheumatoid arthritis patients were divided into two groups as 20 patients receiving cDMARD therapy, and 20 patients receiving bDMARD therapy.
Patients with dyslipidemia, hormone deficiency, and autoimmune disease other than RA were excluded from the study. The control group consisted of non-pregnant healthy individuals over the age of 18 and with no active/chronic infections, rheumatological diseases, hypertension, diabetes, or/and any other chronic diseases known as heart disease. The approval (2020/115) was obtained from the Ethics Committee of Hatay Mustafa Kemal University Faculty of Medicine. Each patient signed a consent form and the present study was performed in accordance with the Helsinki Declaration.
Obtaining plasma and leukocytes from whole blood
Five-milliliter blood samples from the patient and control groups were taken into tubes containing EDTA. The samples brought to the laboratory under the cold chain were centrifuged at + 4 °C for 10 min at 3000 × g. After centrifugation, 1.5-mL plasma from the upper phase was transferred to sterile Eppendorf tubes and kept at − 86 °C until the time of ELISA analysis. The leukocyte layer formed after centrifugation was collected to new nuclease-free tubes for RNA isolation and treated with Red Blood Lysis Buffer [8]. Then, 1 mL of TRIzol Reagent (Thermofisher Scientific, USA) was added to the cell pellets and the samples were homogenized. Following homogenization, the samples were stored at − 86 °C until molecular analysis.
Total RNA isolation
The samples in Trizol at − 86 °C were thawed at room temperature for 20 min and total RNA was isolated according to the modified Trizol method [9]. The samples were passed through chloroform-isopropyl alcohol and ethyl alcohol steps and the obtained total RNAs were diluted with 30–50 µL of nuclease-free water (NFW) according to the pellet size. Following the dilution process, the concentration and purity (A260/280 ratio) of the samples were checked by nucleic acid meter (SMA-1000 Spectrophotometer, Merinton, China). The RNA integrities of samples were checked by electrophoresis with 1% agarose gel (100 V and 30 min).
cDNA synthesis and RT-qPCR application
To eliminate possible genomic DNAs, DNA digestion was performed via genomic DNA digestion kit (DNase I, RNase free, Thermo Scientific, USA, Cat no: EN0525). Then, the cDNA synthesis was performed according to the protocol of the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, USA). Thermal cycler (BioRad T100, USA) protocol for cDNA synthesis was as follows: 10 min at 25 °C, 120 min at 37 °C, and 5 min at 85 °C, respectively. The final volume of the samples was completed with 200 µL nuclease-free water and stored at − 20 °C until the time of RT-qPCR application.
Expression levels of TNF-α, IL-6, and IL-10 genes were determined using the kit containing SYBR Green Dye (Power SYBR® Green PCR Master, ThermoFisher Scientific, USA, Cat no: 4367659). Each sample was run in duplicate and GAPDH was used as the reference gene. The reaction protocol was as follows in real-time PCR (Rotor Gene Q, Qiagen, USA): 10 min at 95 °C, followed by 15 s at 95 °C, 60 s at 60 °C, and 40 cycles. Primer sequences of genes used for the amplification was checked by Primer-Blast (NCBI). In addition, melting curve analysis was performed at the end of the RT-qPCR reaction for studied genes (Table 1).
ELISA measurements
The analyses for plasma TNF-α, IL-6, and IL-10 values were carried out by the enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Thermoscientific MultiscanGo; Bioassay Technology, catalog no: E0102Hu, E0090Hu and E0102Hu, respectively). The assay ranges for the TNF-α kit were 3–900 ng/L, sensitivity 1.52 ng/L, and the intra- and interassay coefficients of variance (CV%) were < 8% and < 10%, respectively. The assay ranges for the IL-6 kit were 2–600 ng/L, while sensitivity was 1.03 ng/L, and the intra- and interassay CV% values were determined to be < 8% and < 10%, respectively. The assay ranges for the IL-10 kit were 5–1500 pg/mL, sensitivity was 2.59 pg/mL, and the intra- and interassay CV% values were < 8% and < 10%, respectively. All the findings are given in pg/ mL.
Lipid profile and estradiol measurements
After 12 h of fasting, blood samples were taken from the patients into gel-coated biochemistry tubes. The samples were kept for coagulation for 30 min and then centrifuged at 4000 rpm for 10 min. Total cholesterol, triglyceride, HDL, and LDL levels in obtained serum samples were studied by spectrophotometric method in Advia 1800 autoanalyzer (Siemens, Germany). Serum estradiol levels were measured by Advia Centaur XP Immunoassay autoanalyzer (Siemens, Germany).
Fatty acid analysis
To determine the composition of the fatty acids in the serum phospholipids of the blood samples, firstly, serum was separated and shaken at 180 rpm for 90 min and treating with 10 mL ether. Then, the sample was centrifuged at 1000 rpm and the supernatant was taken into a separate tube. Blood phospholipids were extracted by evaporating ether in a dry block heater at 70 °C. The extracted phospholipids were taken into Teflon-capped tubes, 4 mL of 2% methanolic NaOH was added and kept in a dry block heater at 95 °C for 5 min. Fatty acid methyl esters were formed by adding 5 mL of 14% boron trifluoride in methanol and keeping it in a dry block heater at 95 °C for 6 min. Then, 2 mL n-Heptane (Merck, USA) was added to the samples. After 2 min at 95 °C, the tubes were inverted and saturated using NaCl. In the final step, the tubes containing the samples were centrifuged at 200 rpm for 2 min. The supernatant phase containing the methyl esters was transferred to 1.5-mL volume vials. Fatty acid values of the samples were determined using a gas chromatography device equipped with a flame ionization detector (Shimadzu GC-2025, Japan), an auto-injector (Shimadzu AOC-20i, Japan), and a Restek Rt-2560 column (100 m length, 0.25 mm ID × 0.20 µm film thickness). Injector and detector temperatures were set to 250 °C. Hydrogen was used as the carrier gas and the gas flow was 1.20 mL/min, with a total injection volume of 1 µL. At each sample exchange, the syringe was washed with n-Heptane three times before the run and six times after the run. A gradient program was used for temperature. The initial temperature of the column furnace was 100 °C and the furnace was held at this temperature for 2 min. The temperature was then increased by 4 °C/min up to 250 °C and this temperature remained at 250 °C for 15 min. The total analysis time was 54 min and 30 s. To confirm the fatty acids, the obtained peaks were compared with the internal standard (FAME Mix, Restek, USA).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical data were given as numbers and percentages. The Kolmogorov–Smirnov test or Shapiro–Wilk tests were performed to test the distribution of the data. The one-way ANOVA test was utilized for the normally distributed data while the Kruskal–Wallis Test was applied to analyze non-normally distributed data. Analysis of categorical data was conducted using the chi-square test. We also used covariance analysis (ANCOVA) to assess the differences between the groups after adjusting age for the potential confounder effect. Correlations were assessed using Pearson correlation or Spearman Rho test. The analyses were carried out using the SPSS software version 23.0 (IBM Corporation, Armonk, NY, USA). P value < 0.05 was considered as statistically significant. Also, as previously reported [13], gene expression data were evaluated using the 2−ΔΔCt method and all results were presented as fold-change [13].
Results
Demographic, clinical, and laboratory data of the study groups are shown in Table 2. The mean age of the bDMARD group was significantly higher than those of the control and cDMARD groups. In the cDMARD group, patients were treated with leflunomide (n = 4), sulfasalazine (n = 2), and methotrexate (n = 14). In the bDMARD group, 8 patients were treated with adalimumab, 8 patients with etanercept, 2 patients with infliximab, and 2 patients with certolizumab. The standard daily steroid dose used by the patients was below 15 mg prednisone. Comorbidities including hypertension in 4 patients and asthma in one patient in the cDMARD group and also hypertension and diabetes mellitus in 3 patients, hypertension in 2 patients, and asthma in one patient in the bDMARD group were observed. No significant difference was observed in lipid profile levels (total cholesterol, HDL, LDL, and triglyceride) and in estradiol levels between the study and control groups.
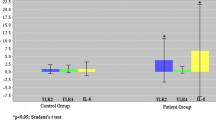
Purity (1.76 ± 0.01) and concentration (260.08 ± 25.44 ng/µL) values of isolated RNA were suitable for gene expression studies. Fold-change results of the target genes are given in Fig. 1 and protein results of patient and control groups are shown in Fig. 2. There was no significant difference in IL-10 protein levels between all three groups, whereas IL-10 gene expression levels were significantly higher in the cDMARD group compared to bDMARD and healthy controls. There was no significant difference in gene expression and protein levels of TNF-α and IL-6 between study groups and healthy controls.
Gene expression levels of patient and control groups. TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, IL-10 interleukin-10, Cont control, cDMARD conventional synthetic disease-modifying anti-rheumatic drugs, bDMARD biological disease-modifying anti-rheumatic drugs. *Comparison between control and cDMARD group (p = 0.04)
Fatty acid analysis of study groups are given in Table 3. C10:0 (capric acid), C14:1 (myristoleic acid), and C20:2n6 (eicosadienoic acid) fatty acid levels were significantly decreased in the cDMARD and bDMARD groups compared to the control group. In addition, C14:1 (myristoleic acid) levels in the bDMARD group were found significantly lower than those of the cDMARD group.
The correlation analyses of the protein levels of TNF-α, IL-6, and IL-10 and fatty acids are shown in Table 4. A positive significant correlation was observed between TNF-α and IL-6 (r = 0.328, p < 0.05), TNF-α and IL-10 (r = 0.597, p < 0.001), and IL-6 and IL-10 (r = 0.336, p < 0.001); however, no significant correlation was observed between inflammatory markers and fatty acids.
Discussion
For the first time in the literature, the protein levels and gene expression levels of inflammatory markers and fatty acid analysis were investigated in patients who received bDMARD and cDMARD treatment. IL-10 gene expression levels significantly increased in RA patients receiving cDMARD treatment compared to those in the control group. Also, eicosadienoic acid, myristoleic acid and capric acid levels were significantly lower in the patient groups compared to those of the control group.
Various studies have shown that cDMARD and bDMARD treatments decrease serum levels and gene levels of proinflammatory cytokines IL-6 and TNF-α, which play a very important role in the pathogenesis of the RA disease [14,15,16,17,18]. The administration of bDMARD group drugs was initiated in the treatment of RA after cDMARD group drugs. The exact action mechanism of cDMARD group drugs remains unknown. On the other hand, bDMARD group drugs act on soluble extracellular and cell membrane-associated proteins [3]. In the present, inflammatory markers were examined in patients who received cDMARD and bDMARD treatment in RA patients, and it was found that there were no statistically significant differences between the groups and the control group. Similarly, examining the effects of inflammatory markers at the gene level in patients receiving cDMARD and bDMARD treatment, it was observed that there were no significant differences between both the patient groups and the control group. Both cDMARD and bDMARD drugs caused suppression at the gene level. The long-term effects of adalimumab plus methotrexate in comparison with MTX in patients with early RA were examined in different clinical studies. Smolen et al. reported a higher proportion of patients treated with adalimumab plus methotrexate achieved the low disease activity target compared with methotrexate alone. On the other hand, in patients who responded to adalimumab plus methotrexate, outcomes were almost the same regardless of whether adalimumab was continued or discontinued [19]. In another study Detert et al. showed that there was no clinically significant difference between the groups at week 48 [20]. According to the results of the studies mentioned, it can be said that cDMARD and bDMARD treatments are similarly effective in RA patients [19, 20]. In light of all these results, it can be argued that the anti-inflammatory effects of both cDMARD and bDMARD, albeit with different mechanisms, have similar anti-inflammatory activities.
IL-10 is a cytokine with strong anti-inflammatory and immunosuppressive effects [21]. Qu et al. have reported that serum IL-10 levels decreased significantly in RA patients compared to those in healthy individuals [22]. Osiri et al. reported that there were no significant differences between the serum IL-10 levels in patients receiving bDMARD-cDMARD-combined treatment and the patients receiving cDMARD treatment [23]. In another study, no significant differences were found between the RA patients and the control group in terms of serum IL-10 levels; however, it has been stated that IL-10 expression was significantly higher in RA patients [24]. In the present study, serum IL-10 levels and gene expression levels were investigated in RA patients receiving cDMARD and bDMARD treatment and in healthy individuals. Also, it was found that, although the gene expression levels of IL-10 had a tendency to increase in the bDMARD group compared to that in the control group, there were no significant changes (p > 0.05). Moreover, gene expression levels of IL-10 were upregulated approximately four-fold in the cDMARD group compared to that in the control group (p < 0.05). However, the level of the protein encoded by this gene was found to be similar in all groups. Although the treatment applied in the cDMARD group increased the activity of IL-10 at the level of mRNA in leukocytes, it was thought that post-transcriptional factors such as some microRNAs might be effective in finding similar plasma protein levels [25, 26]. Because, some studies have reported that some miRNAs, such as miR-98 and miR-155, play a role in suppressing IL-10 protein levels [27, 28]. It has been known that the regulation of molecular mechanisms in the organism is affected by numerous factors including miRNAs. It was thought that further studies are needed on the effects of the drugs and applications preferred in the treatment of RA on the mRNA and protein levels of the target cytokines.
Inflammation is one of the mechanisms that play a major role in the pathogenesis of RA. Studies have shown that, in addition to cytokines, fatty acids may also play a role in inflammation [29,30,31]. Studies on the changes in fatty acid levels with drug treatment in RA patients have shown varying results. In one study, it was observed that arachidonic acid levels decreased significantly in RA patients who were treated with cDMARD (TNF-α inhibitor) in the 3-month period following the treatment compared to those in healthy individuals [32]. In the present study, fatty acid levels were investigated in patients receiving cDMARD and bDMARD treatment. Capric acid and eicosadienoic acid levels significantly decreased in cDMARD and bDMARD groups compared to those in the control group. However, myristoleic acid levels significantly decreased in the bDMARD group compared to those in the control group and the cDMARD group, and also in the cDMARD group compared to those in the control group. However, it was observed that drug treatments did not affect short-, medium-, and long-chain fatty acids in RA patients. Accordingly, the present study found that both cDMARD and bDMARD applications had no effect on fatty acid levels in the treatment of RA.
Myristoleic acid is an omega-5 monounsaturated fatty acid. It is found in human adipose tissue and also in various foods. In animal experiments and cell culture studies, myristoleic acid has been reported to have various effects including osteoclast inhibition [33] anti-obesity, and anti-cancer effects on prostate cancer [34]. On the other hand, in the study conducted by Romanowicz et al., myristoleic acid levels in the umbilical cord structure were significantly higher in preeclampsia patients compared to those in the control group [35]. There are a limited number of studies on myristoleic acid in the literature. Varying results were obtained in these studies. To the best of our knowledge, the present study is the first that evaluated myristoleic acid levels in RA patients.
G protein-coupled receptors (GPCR) consist of seven transmembrane receptors that activate the G protein. Some orphan receptors which are activated by free fatty acids and their derivatives were identified in different studies. Free fatty acid receptors (FFAR), FFAR1 (GPR40), and FFAR4 (GPR120) are activated by medium- and long-chain fatty acids. Of these pathways, GPR40/FFAR1 inhibits osteoclastogenesis and stimulates bone formation, while GPR120/FFAR4 causes osteoblastic bone formation by inhibiting osteoclastic development and bone resorption [36]. Studies have suggested that GPCR-signaling pathways play a role in the inflammatory and immune response of RA [37] These decreases in eicosadienoic acid, myristoleic acid, and capric acid levels in patients with RA may play a role in the pathogenesis of the disease by leading to a decrease in the effects of the GPR40/FFAR1 and GPR120/FFAR4 pathways.
In the present study, fatty acid profiles of RA patients who were treated with different treatment regimens were investigated. Different results have been reported in previous studies comparing the changes in fatty acid profiles in RA patients to those in healthy individuals. These differences may be due to different experimental approaches, because some studies evaluated plasma fatty acid levels [32] while others evaluated phosphatidylcholine or total phospholipid [4]. The present research assessed the composition of fatty acids in serum phospholipids and the results were interpreted accordingly.
The current study has some limitations. Lipid metabolism is affected by various factors such as lifestyle, physical activity, and diet. However, information on the factors mentioned was not collected in the current study.
It was found that IL-10 gene expression levels significantly increased in RA patients receiving cDMARD treatment compared to those of healthy individuals. Also, eicosadienoic acid, myristoleic acid, and capric acid fatty acid levels significantly decreased in RA patients receiving cDMARD treatment compared to those of the control group. It is thought that future studies can investigate the effects of drugs used in the treatment of RA on the mRNA and protein levels of target cytokines.
References
Chauhan K, Jandu JS, Goyal A, Bansal P, Al-Dhahir MA (2020) Rheumatoid arthritis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK441999/
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L (2014) The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73(7):1323–1330. https://doi.org/10.1136/annrheumdis-2013-204763
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K (2018) Rheumatoid arthritis Nat Rev Dis Primers 8(4):18001. https://doi.org/10.1038/nrdp.2018.1
Ferreira HB, Melo T, Paiva A, Domingues MDR (2021) Insights in the role of lipids, oxidative stress and inflammation in rheumatoid arthritis unveiled by new trends in lipidomic investigations. Antioxidants (Basel) 2 10(1):45. https://doi.org/10.3390/antiox10010045
Quiñonez-Flores CM, González-Chávez SA, Del Río ND, Pacheco-Tena C (2016) Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res Int 2016:6097417. https://doi.org/10.1155/2016/6097417
Phull AR, Nasir B, Haq IU, Kim SJ (2018) Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact 1(281):121–136. https://doi.org/10.1016/j.cbi.2017.12.024
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581. https://doi.org/10.1002/art.27584
Lim JH, Goh LPW, Chong ETJ, Lee PC (2016) High integrity total RNA isolation from human peripheral blood that is as competitive to commercialize kits. Res J Pharm Biol Chem Sci 7(4):1937–1942
Rio DC, Ares M Jr, Hannon GJ, Nilsen TW (2010) Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc 2010(6):pdb.prot5439. https://doi.org/10.1101/pdb.prot5439
Hruz T, Wyss M, Docquier M, Pfaffl MW, Masanetz S, Borghi L, Descombes P (2011) RefGenes: identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genomics 12(1):156. https://doi.org/10.1186/1471-2164-12-156
Keller C, Keller P, Marshal S, Pedersen BK (2003) IL-6 gene expression in human adipose tissue in response to exercise–effect of carbohydrate ingestion. J Physiol 1 550(3):927–931. https://doi.org/10.1113/jphysiol.2003.044883
Vors C, Allaire J, Marin J, Lépine MC, Charest A, Tchernof A, Lamarche B (2017) Inflammatory gene expression in whole blood cells after EPA vs. DHA supplementation: Results from the ComparED study. Atherosclerosis 257:116–122. https://doi.org/10.1016/j.atherosclerosis.2017.01.025
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Noack M, Miossec P (2017) Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol 39(4):365–383. https://doi.org/10.1007/s00281-017-0619-z
Radner H, Aletaha D (2015) Anti-TNF in rheumatoid arthritis: an overview. Wien Med Wochenschr 165(1–2):3–9. https://doi.org/10.1007/s10354-015-0344-y
Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, Jiang YF (2014) Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One 1 9(5):e95346. https://doi.org/10.1371/journal.pone.0095346
Musacchio E, Valvason C, Botsios C, Ostuni F, Furlan A, Ramonda R, Modesti V, Sartori L, Punzi L (2009) The tumor necrosis factor-{alpha}-blocking agent infliximab inhibits interleukin 1beta (IL-1beta) and IL-6 gene expression in human osteoblastic cells. J Rheumatol 36(8):1575–1579. https://doi.org/10.3899/jrheum.081321
Wielińska J, Dratwa M, Świerkot J, Korman L, Iwaszko M, Wysoczańska B, Bogunia-Kubik K (2018) Interleukin 6 gene polymorphism is associated with protein serum level and disease activity in Polish patients with rheumatoid arthritis. HLA 92(2):38–41. https://doi.org/10.1111/tan.13355
Smolen JS, Emery P, Fleischmann R, van Vollenhoven RF, Pavelka K, Durez P, Guérette B, Kupper H, Redden L, Arora V, Kavanaugh A (2014) Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 383(9914):321–332. https://doi.org/10.1016/S0140-6736(13)61751-1
Detert J, Bastian H, Listing J, Weiß A, Wassenberg S, Liebhaber A, Rockwitz K, Alten R, Krüger K, Rau R, Simon C, Gremmelsbacher E, Braun T, Marsmann B, Höhne-Zimmer V, Egerer K, Buttgereit F, Burmester GR (2013) Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 72(6):844–850. https://doi.org/10.1136/annrheumdis-2012-201612
Chen Z, Bozec A, Ramming A et al (2019) Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 15:9–17. https://doi.org/10.1038/s41584-018-0109-2
Qu CH, Hou Y, Bi YF, Han QR, Jiao CH, Zou QF (2019) Diagnostic values of serum IL-10 and IL-17 in rheumatoid arthritis and their correlation with serum 14–3–3η protein. Eur Rev Med Pharmacol Sci 23(5):1899–1906. https://doi.org/10.26355/eurrev_201903_17227
Osiri M, Wongpiyabovorn J, Sattayasomboon Y, Thammacharoenrach N (2016) Inflammatory cytokine levels, disease activity, and function of patients with rheumatoid arthritis treated with combined conventional disease-modifying antirheumatic drugs or biologics. Clin Rheumatol 35(7):1673–1681. https://doi.org/10.1007/s10067-016-3306-x
Hernández-Bello J, Oregón-Romero E, Vázquez-Villamar M, García-Arellano S, Valle Y, Padilla-Gutiérrez JR, Román-Fernández IV, Palafox-Sánchez CA, Martínez-Bonilla GE, Muñoz-Valle JF (2017) Aberrant expression of interleukin-10 in rheumatoid arthritis: relationship with IL10 haplotypes and autoantibodies. Cytokine 95:88–96. https://doi.org/10.1016/j.cyto.2017.02.022
Farazi TA, Spitzer JI, Morozov P, Tuschl T (2011) miRNAs in human cancer. J Pathol 223(2):102–115. https://doi.org/10.1002/path.2806
Friedman JM, Jones PA (2009) MicroRNAs: critical mediators of differentiation, development and disease. Swiss Med Wkly 139(33–34):466–472
Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu H, Cao X, Wang Q (2011) MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett 585(12):1963–8. https://doi.org/10.1016/j.febslet.2011.05.029
Billeter AT, Hellmann J, Roberts H, Druen D, Gardner SA, Sarojini H, Galandiuk S, Chien S, Bhatnagar A, Spite M, Polk HC Jr (2014) MicroRNA-155 potentiates the inflammatory response in hypothermia by suppressing IL-10 production. FASEB J 28(12):5322–5336. https://doi.org/10.1096/fj.14-258335
Brouwers H, von Hegedus J, Toes R, Kloppenburg M, Ioan-Facsinay A (2015) Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best Pract Res Clin Rheumatol 29(6):741–755. https://doi.org/10.1016/j.berh.2016.02.003
Frommer KW, Schäffler A, Rehart S, Lehr A, Müller-Ladner U, Neumann E (2015) Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Ann Rheum Dis 74(1):303–310. https://doi.org/10.1136/annrheumdis-2013-203755
Frommer KW, Hasseli R, Schäffler A, Lange U, Rehart S, Steinmeyer J, Rickert M, Sarter K, Zaiss MM, Culmsee C, Ganjam G, Michels S, Müller-Ladner U, Neumann E (2019) Free fatty acids in bone pathophysiology of rheumatic diseases. Front Immunol 3(10):2757. https://doi.org/10.3389/fimmu.2019.02757
Rodríguez-Carrio J, Alperi-López M, López P, Ballina-García FJ, Suárez A (2016) Non-esterified fatty acids profiling in rheumatoid arthritis: associations with clinical features and Th1 response. PLoS One 11(8):e0159573. https://doi.org/10.1371/journal.pone.0159573
Kwon J-O, Jin WJ, Kim B, Kim H-H, Lee ZH (2015) Myristoleic acid inhibits osteoclast formation and bone resorption by suppressing the RANKL activation of Src and Pyk2. Eur J Pharmacol 768:189–198. https://doi.org/10.1016/j.ejphar.2015.10.053
Choi YK, Kang JI, Hyun JW, Koh YS, Kang JH, Hyun CG, Yoon KS, Lee KS, Lee CM, Kim TY, Yoo ES, Kang HK (2021) Myristoleic acid promotes anagen signaling by autophagy through activating Wnt/β-catenin and ERK pathways in dermal papilla cells. Biomol Ther (Seoul) 29(2):211–219. https://doi.org/10.4062/biomolther.2020.169
Romanowicz L, Bańkowski E (2009) Lipid compounds of the umbilical cord vein and their alterations in preeclampsia. Biochimie 91(2):288–294. https://doi.org/10.1016/j.biochi.2008.10.004
Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M (2020) Free fatty acid receptors in health and disease. Physiol Rev 100(1):171–210. https://doi.org/10.1152/physrev.00041.2018
Shu J, Zhang F, Zhang L, Wei W (2017) G protein coupled receptors signaling pathways implicate in inflammatory and immune response of rheumatoid arthritis. Inflamm Res 66(5):379–387. https://doi.org/10.1007/s00011-016-1011-5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The approval (2020/115) was obtained from the Ethics Committee of Hatay Mustafa Kemal University Faculty of Medicine. Each patient signed a consent form and the present study was performed in accordance with the Helsinki Declaration.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dogan, S., Kimyon, G., Ozkan, H. et al. TNF-alpha, IL-6, IL-10 and fatty acids in rheumatoid arthritis patients receiving cDMARD and bDMARD therapy. Clin Rheumatol 41, 2341–2349 (2022). https://doi.org/10.1007/s10067-022-06180-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06180-5