Abstract
Malathion is an organophosphate pesticide (OP) commonly used in agriculture, industry, and veterinary medicine. Sex is a crucial factor in responding to neurotoxicants, yet the sex-specific effects of OP exposure, particularly neurological impairments following chronic low-level exposure remains limited. Our study aims to evaluate the neurobehavioral and biochemical effects of developmental exposure to Malathion across sexes. Pregnant mice were exposed to a low oral dose of Malathion from gestation up to the weaning of the pups, which were individually gavaged with a similar dose regimen until postnatal day 70. Our results show that Malathion decreased body weight and food intake, reduced locomotor activity and recognition memory. Motor coordination and special memory were only altered in females, whereas we found a male-specific effect of Malathion on social behavior and marble burying. These alterations were accompanied by increased malondialdehyde (MDA), decreased brain acetylcholinesterase activity (AChE), and disrupted brain redox homeostasis. Our findings about the effects of Malathion exposure across sexes may, in part, contribute to understanding the dimorphic susceptibilities observed in neurological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological sex is a crucial factor in responding to neurotoxicants such as organophosphate pesticides (OPs) (Comfort and Re 2017). To this day, there is a considerable amount of evidence elucidating the differences between males and females regarding vulnerability to diseases, xenobiotics, and adverse drug effects (Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences 2001). Those differences are nearly found in every brain area and are fundamental for sexually dimorphic behaviors and brain damage (Ruszkiewicz et al. 2019). OPs neurotoxicity might range from the sexual differentiation of the brain itself to the prevalence of certain neurological disorders that exhibit sex-dependent outcomes (Comfort and Re 2017; Rauh et al. 2012; Mostafalou and Abdollahi 2017). For instance, neurodevelopmental impairments such as autism impact males more than females (Ferri et al. 2018), and female-biased conditions include emotional disorders such as depression (Gobinath et al. 2015; Hodes et al. 2017).
Malathion (MAL) is a broad-spectrum OP insecticide extensively used around the globe in agriculture, industry, and veterinary medicine, leading to excessive exposure from multiple sources (Badr 2020). Like other OP compounds, MAL-induced neurotoxicity is due to AChE inhibition, but alone it cannot explain the range of neurological symptoms and disorders following MAL exposure (Kwong 2002; Venkatesan et al. 2017). Non-cholinergic mechanisms such as oxidative stress also play a role in its toxic effects and appear to be correlated with MAL neurotoxicity (Badr 2020). OPs exposure can begin as early as gestation and traverse the placenta and amniotic fluid to the fetus (Bradman and Whyatt 2005), as well as being present in maternal milk (Sanghi et al. 2003). The fetal and child brains are particularly vulnerable at critical periods of growth and development when a major functional organization is being established; OP toxicity can disrupt many processes leading to alteration in the development of the nervous system (Heyer and Meredith 2017). Moreover, brain sexual differentiation happens during gestation and early adolescence under the instruction of genetic and hormonal factors (Ruszkiewicz et al. 2019). Therefore, this study aims to investigate the sex-specific effects of developmental exposure to MAL, which starts from gestation and lasts until the young adult stage, at a low dose based on a previous study (Ouardi et al. 2019), which mimics the pattern of real-life exposure. All behavioral experiments and biochemical analyses of AChE activity and oxidative stress biomarkers were assessed in male and female mice to answer the recent request posed by the US national institute of health (NIH) to incorporate sex as a biological variable in neuroscience research (McCarthy et al. 2017). Given that most studies in this field have only involved males in their research. The primary reason for scientists to exclude females in animal studies is out of concern for the potential variability introduced by the estrus cycle (Prendergast et al. 2014). In the present study, we only tested females during their non-receptive phase (metestrus and diestrus), thereby minimizing the hormonal factor that may interfere with behavioral phenotypes (Woolley and McEwen 1992; McLean et al. 2012; Moura et al. 2020; Chari et al. 2020). Hence, our study provides a first-time insight into how MAL alters brain biochemicals and behavioral outcomes according to sex while considering the estrus cycle of female mice.
Materials and methods
Chemicals
Commercial-grade Malathion 50 (S-1,2-bis(ethoxycarbonyl)ethyl O,O-dimethyl phosphorodithioate) was purchased. It contains 500 g/liter of Malathion, with the molecular formula C10H19O6PS2 and a molecular weight of 330.4 g/mol. Acetylthiocholine iodide, 5, 5- dithiobis-(2-nitrobenzoic acid) (DTNB) and all other chemicals used in this study were purchased from Sigma (USA).
Animals and treatment
Adult male and female Swiss albino mice were obtained from the laboratory animal house of the Faculty of Science and Technology, Beni Mellal, Morocco. Mice were accustomed to their room and manipulator for at least two weeks before starting the experiment, the animals were housed in Plexiglas cages under standard conditions, 12-h light/12-h dark cycle, 22 ± 2 °C and 50%—70% humidity, with free access to commercial diet (Alf Sahel, Casablanca, Morocco) and water ad libitum.
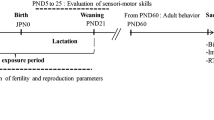
Each virgin female mouse and one male breeder were placed in one cage and the vaginal plug was examined daily, early in the morning. Once the vaginal plug was detected mice were housed individually and randomly divided into two groups of 10 animals each: Group 1 received only the vehicle (5% crone oil, 95% soja oil). Group 2 received MAL dissolved in oil at the dose of 15 mg/kg of body weight per day. The treatment was orally administered by intragastric gavage and lasted throughout gestation and lactation until post-partum day 21, after which weaned pups of each experimental group were submitted to the same regimens of the preliminary protocol. The duration of treatment was extended to the young adult stage. The dose of malathion used in this study, as well as the treatment duration choice, is based on previous work (Ouardi et al. 2019; N’Go et al. 2013a). All experimental procedures performed on animals are in accordance with the scientific procedures of living animals reported by the European Council directive: ACT: 86/609 EEC. The experimental design of our study is summarized in Fig. 1.
Body weight and food intake
The body weight of offspring was recorded once a week from PND 21 until PND 70. At the end of treatment, mice were housed individually per cage and had free access to a normal diet (30 g/cage/day) and water. The experiment lasted 4 weeks, totaling 7 animals/group, the first week serves as the habituation period. At day 0, all animal groups started with an average weight of 19 g (females) and 24 g (males). Every day at 9 AM, the animals’ body weight and water intake were recorded, and the rations per cage that were not consumed were weighed and replaced with new pellets (30 g) (Fig. 2B). Food intake was measured as the difference in pellets weight over 24 h (Ferreira-Paes et al. 2021).
Evaluation of food intake, water intake and body weight in mice offspring exposed to MAL from in-utero until young adult age. A Body weight progression from week 3 of life in male and female offspring. B Food intake protocol. C, D, E Body weight and 24-h food and water intake were measured continuously for 21 days in all male and female mice, n = 7 for each control and treated group. F, G 24-h food intake in males and females were measured daily in all groups and the data are presented in panels F and G, respectively. All results are presented as mean ± SEM. A Comparing body weight as analyzed by three-way repeated measures ANOVA. C, D, E Comparing body weight/food intake/water intake as analyzed by two-way ANOVA. F, G Comparing food intake/body weight in treated and controls as analyzed by student’s paired t test. *p ≤ 0.05; **p ≤ 0.005; ***p ≤ 0.001; ****p ≤ 0.0001
Estrous cycle stage detection
Estrus cycle stage determination was done using vaginal cytology which is a non-invasive method proposed by Ashleigh C. Mclean (McLean et al. 2012). In the morning (between 8:00 AM and 9:00 AM) before behavioral tests, stages of the estrus cycle were detected by examining the vaginal smear under light microscopy. The vaginal cells were flushed with small amounts of distilled water through a sterile pipette and then the droplets of cell suspension were placed in a glass slide to dry on a Hot Plate. For microscopic examination, 0.1% crystal violet was prepared for staining, by adding 0.1 g of crystal violet powder in 100 ml of distilled water. The vaginal secretions are normally composed of three types of cells namely leucocytes, cornified epithelial cells and nucleated epithelial cells. The proportion of cell types present in the vaginal smear will determine which estrous stage is taking place.
Behavioral tests
Twenty-four hours after the final MAL exposure, mice (aged 10 weeks) were subjected to behavioral testing from the least to most stressful ones in the following order: Open Field, Novel Object Recognition, Y-maze, Social Interaction, Marble Burying, Rotarod, Tail Suspension. All mice were acclimated in the testing room for 30 min before each behavioral test, and testing occurred between 9:00 AM and 1:00 PM (first half of the light cycle). After each behavioral test, mice were left to rest for at least one day. Female mice were tested during the metestrus and diestrus phases of the estrous cycle, which are characterized by low levels of estradiol and progesterone, to prevent any hormone-induced effects that may modulate behavioral outcomes (Woolley and McEwen 1992; McLean et al. 2012; Moura et al. 2020; Chari et al. 2020). Male and female mice behavioral tests were conducted on separate days to account for sensitivity to pheromones. The apparatus was cleaned between tests with a solution of ethanol 10% to prevent any bias due to olfactory cues.
Open files test
The open-field test (OF) was used to assess general locomotor activity levels. The apparatus (50 × 50 × 50 cm) is made of black Plexiglas and a black bottom subdivided into 25 squares. Each mouse was placed in the center of the OF and recorded for 5 min using a video camera located above the OF and monitored in another room via a closed-circuit TV camera. The levels of general activity were measured with two parameters, the total distance traveled and average speed. Data were analyzed using ANY-Maze software (Stoelting Co., Wood Dale, IL, USA).
Novel object recognition test
The novel object recognition test is a commonly used test to evaluate general memory function in rodents (Yin et al. 2011). The apparatus used in this test is an open-field-testing arena (50 × 50 × 50 cm) made of black Plexiglas. The test procedure consists of three main sessions conducted over two days. The habituation session (day 1), where each animal was allowed to freely explore the empty arena for 5 min. During the training session (day 2), the animals are exposed to the familiar arena with two identical objects placed at an equal distance of 10 cm away from the walls for 5 min. Two hours later, the test session began by allowing mice to explore the OF for 5 min in the presence of a familiar object and a novel object to test short-term recognition memory. An active investigation was set as when a mouse engages an object with its nose pointed at the object no more than 2 cm away. The time spent exploring each object was recorded and the discrimination index was calculated [((time spent beside the novel object – time spent beside the familiar object) / total duration beside both objects) × 100] (Ennaceur and Delacour 1988).
Y-maze test
Spatial working memory was assessed using the spontaneous alternation Y-maze test as described previously by Malqui (Malqui et al. 2018). The apparatus was made of Plexiglas with three identical arms (40 × 9 × 16 cm) placed 120° from each other. Ten-week-old mice were placed in one of the arms and were allowed five minutes of free exploration in the three arms of the Y-maze. The series of arm entries (e.g., ABC, BCA) and total arm entries were recorded using an overhead camera and analyzed by ANY-Maze software. The percentage of spontaneous alternations was calculated as an index of working memory performance (index of alternation).
Social interaction test
Crawley’s sociability and preference for social novelty test apparatus is comprised of a rectangular, three-chamber box. Each chamber is 20 × 45 cm and the dividing walls are made of clear removable Plexiglas, with an open middle section, which allows free access to each chamber. Both left and right chambers contain an empty wire cup in the center. The test is divided into three different phases (habituation, sociability and social novelty). In the habituation phase, the mouse was placed in the center chamber with no access to both compartments and allowed 5 min of exploration. In the sociability phase, a novel mouse (stranger 1) was enclosed in one of the cups and the test mouse was allowed to explore all three chambers for 10 min. The novel mouse was of the same sex, age, and strain and was not a sibling of the test mouse. In the social novelty phase, a new novel mouse (stranger 2) was placed under the cup of the other side and tested again for 10 min. The time spent in each chamber and the time spent exploring enclosed novel mice or empty cups (novel objects) were recorded in the 10 min sessions. Active contact was defined as when a test mouse stretches toward the cup with a distance of 3–5 cm around the cup or as climbing on the cup (Crawley 2004). Preference for novel mouse 1 was calculated as [(time spent exploring novel mouse)/ (total time spent exploring novel mouse and novel object)] × 100%. And the preference for novel mouse 2 was calculated as [(time spent exploring novel mouse 2)/ (total time spent exploring novel mouse 1 and novel mouse 2)] × 100% (Lo et al. 2016).
Marble burying test
The Marble Burying test was carried out as previously described by Angoa-Pérez (Angoa-Pérez et al. 2013) with minimal modifications. Briefly, mice were individually placed in a mouse cage (47 cm length × 23 cm width × 15 cm height) with 20 glass marbles evenly spaced on a 5 cm deep layer of wood ship bedding lightly pressed to give a flat surface. Each mouse was placed in the corner of the cage containing marbles and allowed 30 min to explore freely; the number of marbles buried by each animal was counted. A Marble was considered buried if 2/3 or more of its volume was submerged.
Rotarod test
The Rotarod test was used to assess motor coordination in mice as described by Dunham and Miya (Dunham and Miya 1957) with some minor modifications. The device used is mouse Rota-Rod NG (Ugo basile, Gemonio, Italy). During testing, when a mouse falls from its cylinder section on a box below, a magnetic switch is activated by pooling the plate box, thus recording the animal’s endurance time in seconds. The test consists of three trials separated by inter-trial intervals of 15 min, with the speed accelerated from 4 to 40 rpm for 300 s. The latency at which each mouse falls from the rod is recorded.
Tail suspension test
The animals were suspended individually, with adhesive tape placed approximately 1 cm from the tip of the tail, on a shelf with a height of 15 cm from the bottom of the testing setup. The duration of immobility was recorded for 6 min. Immobility is defined as the cessation of any bodily movements (Yin et al. 2011).
Biochemical assays
Sample collection
The brains of the control and MAL-treated mice were collected (N = 7/experimental group) by decapitation upon completion of the treatment period. Each of the following brain structures, olfactory bulbs, hippocampus (right and left sides), hypothalamus and cerebellum were rapidly dissected on a plate at 4 °C, weighed, and frozen at − 20 °C until use.
Tissue preparation
Brain structures were homogenized in buffer solution TBS (50 mM Tris, 150 mM NaCl, pH 7.4); and then centrifuged at 10,000 × g for 15 min at 4 °C with centrifuge 5804 R (Eppendorf, Freshwater Blvd Enfield, USA). Supernatants were carefully collected and used for the determination of AChE Activity, antioxidant enzymatic markers (catalase, superoxide dismutase, glutathione peroxidase) and lipid peroxidation marker (malondialdehyde). Protein concentration was assayed by the method of Lowry (Lowry et al. 1951) with bovine serum albumin as the standard.
Acetylcholinesterase activity (AChE)
The AChE specific activity was measured according to the method of Ellman (Ellman et al. 1961) using acetylthiocholine iodide (Sigma-Aldrich, USA) as a substrate. The reaction mixture contained phosphate buffer (0.1 M, pH 8.0), acetylthiocholine iodide (0.075 M), and 5,5- dithiobis-2-nitrobenzoic acid (DTNB; 0.01 M) (Sigma- Aldrich, USA). After the addition of brain structure tissue homogenate (olfactory bulb, cerebral cortex, or brainstem) (30 min at room temperature), the hydrolysis rate of acetylcholine iodide was measured by a spectrophotometer (Selecta, Barcelona, Spain) at 412 nm. The enzyme activity was expressed as μmol Ach hydrolyzed/min/mg of protein.
Malondialdehyde (MDA)
The MDA activity in the brain was measured by the method of Buege and Aust (Buege and Aust 1978). A total of 125 μl of supernatant was homogenized by sonication with 50 μl of PBS and 125 μl of 20% TCA + 1% BHT (TCA-BHT), to precipitate proteins, and centrifuged (1000 × g, 10 min, 4 °C). Afterwards, 200 μl of supernatant was mixed with 40 μl of HCl (0.6 M) and 160 μl of TBA dissolved in Tris (120 mM). The mixture was heated at 80 °C for 10 min, and the absorbance was measured at 530 nm. The amount of thiobarbituric acid reactive substances (TBARS) was calculated using a molar extinction coefficient of 1.56 × 105 M/Cm.
Catalase (CAT)
The CAT activity was measured at 240 nm using a UV/visible spectrophotometer by the variation of the optical density consecutive to the disproportionation of hydrogen peroxide (H2O2). For the enzyme reaction, 20 μl of supernatant was added to 780 μl of phosphate buffer saline (PBS) (0.1 M, pH 7.4) and 200 μl of H2O2 (0.5 M) (Aebi 1974).
Superoxide dismutase (SOD)
The SOD activity was measured as described by Asada (Asada et al. 1974); 0.05 ml of the supernatant was added to 0.1 ml of a mixture containing methionine (13 mM) and Na2EDTA (0.1 mM), 0.8922 ml of phosphate buffer (50 mM, pH = 7.8), 0.95 ml of phosphate buffer, 0.088 ml of NBT (2.64 mM), and 0.0226 ml of riboflavin (0.26 mM). The reduction of NBT was estimated after 20 min at a wavelength of 580 nm against white.
Glutathione peroxidase (GPx)
For each assay, a mixture containing 200 μl of supernatant, 200 μl of phosphate buffer (100 mM), 200 μl of 4 mM GSH, and 400 μl of H2O2 (5 mM) was incubated for 1 min at 37 °C. After adding 500 μl of 5% TCA, the mixture was then centrifuged for 5 min at 1500 × g. Two hundred microliters of the supernatant was recovered, 500 μl of phosphate buffer and 500 μl of DTNB were added, and the absorbance was measured at 412 nm each min for 5 min according to Flohé and Günzler (Flohé and Günzler 1984) modified method.
Data analysis and statistics
Statistical analysis was performed using parametric tests since all data was normally distributed. The two-way ANOVA was used for all data analysis considering MAL treatment and sex as fixed factors, except for body weight and the time spent in the three-chamber test that was analyzed by three-way ANOVA. Tukey’s Post hoc test was used for all data multiple comparisons used in this study. In addition, data from the daily three-week food intake curves were analyzed by student’s paired t-test using SigmaPlot 11 software (Systat Software Inc, California, US). All data were expressed as the mean ± SEM. Differences were considered statistically significant at p ≤ 0.05. GraphPad Prism 9.0 was used for data analysis and figure generation.
Results
Body weight and food intake
Exposure to 15 mg/kg of MAL did not significantly affect maternal weight compared to controls (data not shown). Offspring’s body weight progression during the seven weeks of treatment was assessed by three-way ANOVA (treatment × sex × Time) with repeated measures. We found a significant effect of treatment (F (1, 323) = 304.2, P < 0.0001), sex (F (1, 323) = 289.1, P < 0.0001), and time (F (7, 323) = 394.3, P < 0.0001) with an interaction between sex × Treatment (F (1, 323) = 18.60, P < 0.0001), but without interaction between all factors (F (7, 323) = 0.45, P = 0.86) in all groups. To further study the weekly development of body weight, a multiple comparison test (Tukey’s post hoc test) was used to assess the weekly differences between groups. MAL-treated males presented a permanent effect of the treatment from week 4 onwards, whereas in MAL-treated females this effect was observed from week 8 onwards (Fig. 2A). These results showed apparent sex- and time-dependent effects of MAL on body weight.
Because the effect on body weight (shown in Fig. 2A) could reflect underlying differences in food intake, we assessed food consumption in mice offspring (aged 10 weeks) exposed to MAL from in-utero until young adult age (Fig. 2B). As shown in Fig. 2F, G, MAL-treated male and female mice had distinct food consumption patterns, specifically female mice that showed a different and fluctuating profile of food intake over the period evaluated. While male-treated mice showed a significant decrease in food intake from day 6 until the end of the experiment (t = 2.822, P = 0.018), females did not exhibit any significant changes in food intake except for a few days throughout the experiment starting from day 8 (t = 2.879, P = 0.015). The Two-way ANOVA showed a main effect of treatment (F (1, 80) = 49.08, P < 0.0001) and sex (F (1, 80) = 199.8, P < 0.0001) with an interaction (F (1, 80) = 4.10, P = 0.046) on food consumption in all groups. The food consumption was higher in males than in females during the period evaluated (Males: 5.110 ± 0.0637 g, Females: 4.197 ± 0.052 g; Two-way ANOVA analysis, mean of three weeks) as males had a higher body weight and therefore a higher energy demand. Moreover, the multiple comparison test also showed a significant decrease in food intake in treated male (P < 0.0001) and female (P = 0.0039) groups compared to controls (Fig. 2C). During this experiment body weight and 24-h water intake were also recorded daily. We found a significant main effect of treatment on body weight (F (1, 80) = 125.6, P < 0.0001), and sex (F (1, 80) = 162.6, P < 0.0001) with an interaction treatment × sex (F (1, 80) = 36.13, P < 0.0001). In addition, the post hoc test also showed a significant decrease in body weight of male (P < 0.0001) and female (P = 0.0024) treated groups compared to controls (Fig. 2D). For water intake, we found a significant difference among the factor treatment (F (1, 80) = 12.47, P = 0.0007), and sex (F (1, 80) = 79.28, P < 0.0001) with the interaction between the two factors (F (1, 80) = 4.450, P = 0.038). However, the post hoc comparison test showed a significant decrease in water intake in treated female mice only (P = 0.0008) compared to controls (Fig. 2E). These data demonstrate that chronic MAL exposure alters feeding behavior in both sexes, with a stronger MAL response on male mice than females, characterized by a stronger decrease of food consumption correlated with a reduction of body weight.
Behavioral tests
Locomotor activity and motor coordination
Both treated male and female mice displayed decreased locomotor activity, as shown by the trajectories and heat plots mapping of the occupied areas in the OF test (Fig. 3A). Indeed, the two-way ANOVA analysis showed that the treatment with MAL had an obvious effect on locomotor activity in both sexes as measured by the total traveled distance (F (1, 28) = 24.23, P < 0.0001) and average speed (F (1, 30) = 25.06, P < 0.0001) in the OF test. However, we found no effect of sex or the interaction between factors in both parameters used to assess locomotor activity. Moreover, the post hoc analysis showed that the traveled distance and average speed significantly decreased in treated female (P = 0.014; P = 0.018; respectively) and male (P = 0.005; P = 0.002; respectively) mice compared to their controls (Fig. 3B, C).
Effect of MAL chronic exposure on locomotor activity and motor coordination. A Trajectories and heat plots mapping the occupied areas in the OF test. B Total distance traveled and. C Average speed in the OF test. D Latency to fall in the rotarod test. A Time and color scales are indicated below heat plots. B, C, D Results are expressed as mean ± SEM. Significant effects were revealed by two-way ANOVA and Tukey’s test. **p ≤ 0.005; ****p ≤ 0.0001
To assess motor coordination, the length of time (second) that the mice remained on the rod was measured in Rotarod test. A two-way ANOVA analysis of this test demonstrated a significant main effect of MAL treatment (F (1, 24) = 11.15, P = 0.0027) on the latency to first fall, as well as a significant treatment × sex interaction (F (1, 24) = 7.905, P = 0.0097). However, the sex factor (F (1, 24) = 2.298, P = 0.14) did not affect the performance in this test. The post hoc multiple comparison analysis showed that MAL treatment significantly decreased the latency to fall off the rod in female mice compared to their controls (P = 0,0012) but not in male mice (Fig. 3D).
Learning and memory
The novel object recognition test indicated that chronic exposure to MAL affected recognition memory in adult mice. The two-way ANOVA analysis revealed a significant main effect of treatment on the discrimination index (F (1, 29) = 41.38, P < 0.0001), but without a sex effect (F (1, 29) = 3.29, P = 0.08) or an interaction between MAL treatment and sex (F (1, 29) = 0.69, P = 0.41). Both treated male and female groups exhibited lower discrimination index in comparison to their control groups as showed by the post hoc analysis (P = 0.004; P < 0.0001; respectively) (Fig. 4A).
Effect of MAL on memory function and depressive-like behavior. A Recognition memory evaluated by the object recognition test. B Spatial working memory evaluated by Y-maze test. C Immobility time recorded in the tail suspension test. B, C, D Results are expressed as mean ± SEM. Significant effects were revealed by two-way ANOVA and Tukey’s test. ****p ≤ 0.0001
Regarding the Y-maze test, chronic exposure to MAL affected short-term spatial working memory in treated mice. Indeed, two-way ANOVA analysis pointed out a significant difference among the factor treatment (F (1, 25) = 28.67, P < 0.0001) and the interaction treatment × sex (F (1, 25) = 4.94, P = 0.03), but not in the sex factor (F (1, 25) = 0.07, P = 0.78). The post hoc test indicated that the treatment significantly decreased the percentage of alternation in females (p < 0.0001) but not in male mice, indicating a short-term memory impairment, especially in the female-treated group (Fig. 4B).
Depression-like behavior
The two-way ANOVA analysis revealed a significant main effect of chronic MAL treatment (F (1, 30) = 30.11, P < 0.0001) on immobility time evaluated in the tail suspension test, with no effect of sex (F (1, 30) = 0.4450, P = 0.5) or the interaction between factors (F (1, 30) = 0.003, P = 0.95). On the other hand, the multiple comparison test showed that MAL treatment increased immobility time more so in females (P = 0.0009) than males (P = 0.0069) compared to their controls (Fig. 4C).
Marble burying behavior
The Marble burying task has been widely applied to asses repetitive and perseverative behaviors for characterizing autism-related behaviors (Thomas 2009; Silverman et al. 2010). The two-way ANOVA analysis demonstrated a significant effect of all three factors, MAL treatment (F (1, 24) = 17.68, P = 0.0003), sex (F (1, 24) = 8.75, P = 0.006) and a significant sex × treatment interaction effect (F (1, 24) = 6.82, P = 0.015), on the number of buried marbles. In addition, the post hoc analysis showed that male-treated mice buried a significant number of marbles (P = 0.0004) than did control males, unlike females displaying no difference between treated and control groups (Fig. 5E). Suggesting a male-specific effect of MAL treatment on marble burying, which elucidate some traits of autistic-like behaviors.
Effect of MAL on sociability and preference for social novelty in the three-chamber test and marble burying test. A, C Sociability test. Graphs show A time spent in each side chamber containing novel mouse or empty wire cup (novel object), or the center chamber; C preference for stranger 1. B, D Social novelty test. Graphs show B time spent in each side chamber containing stranger 1 (now familiar), or stranger 2, or the center chamber; D preference for stranger 2. E Marble burying test. Graphs show results as mean ± SEM. For treatment effect within each sex for C, D, E, by Tukey’s post-hoc tests following two-way ANOVA with the factors of treatment and sex; for chamber effect between novel mouse vs. novel object in (A), and for stranger 1 vs. stranger 2 in (B), by Tukey’s post-hoc test following three-way ANOVA with the factors of treatment, sex and chamber side. *p ≤ 0.05, **p < 0.01, ***p < 0.001
Social interaction and preference to social novelty
In general, autistic individuals are known to have reduced or unusual social behaviors (Gupta et al. 2020). In our study, we used a three-chamber apparatus to asses sociability and social novelty preference in MAL-treated mice. In the sociability test, the time spent in each side chamber containing a novel mouse or empty wire cup (novel object) was recorded. The three-way ANOVA analysis showed that neither the factor treatment nor sex had an effect (P > 0.9999), but pointed a significant difference for the chamber factor (F (2, 60) = 171.0, P < 0.0001) and chamber × treatment × sex interaction (F (2, 60) = 7.94, P = 0.0009) (Fig. 5A). As for the preference for novel mouse, the two-way ANOVA showed a significant main effect of treatment (F (1, 20) = 5.24, P = 0.033) as well as the interaction treatment × sex (F (1, 20) = 4.36, P = 0.049), but not of the sex factor (F (1, 20) = 3.42, P = 0.079). Moreover, male treated mice showed 40% preference for the novel mouse, which is ~ 20% less than control male mice (~ 60%) (P = 0.043 by Tukey’s post hoc test; Fig. 5C). Females, on the other hand, had comparable sociability in both treated and control groups. This data suggests a male-specific effect of MAL treatment on social behavior.
In the social novelty test, the time spent in each chamber containing the novel mouse 1 (familiar mouse), or the novel mouse 2, or the center chamber was recorded. The three-way ANOVA analysis showed no effect of all factors; treatment, sex, chamber × Treatment × sex interaction, except the chamber factor (F (2, 60) = 184.0, P < 0.0001), on the time spent in each chamber (Fig. 5B). As for the preference for stranger 2, the two-way ANOVA analysis also showed no significant difference in all three factors (treatment, sex, and treatment × sex interaction) (Fig. 5D). All experimental animals of both sexes showed a clear preference for investigating novel mouse 2 over the familiar novel mouse 1. Thus, these results suggest that MAL-treated mice, regardless of sex have normal social recognition for familiar versus novel mice, as well as a preference for newer social stimuli.
Biochemical assays
Acetylcholinesterase activity
The data analysis showed that chronic MAL exposure induced a global reduction of AChE activity in almost all brain structures (Fig. 6A). In the cerebellum, the two-way ANOVA showed a significant effect of all factors, treatment (F (1, 8) = 29.62, P = 0.0006), sex (F (1, 8) = 24.40, P = 0.0011), and an interaction sex × treatment (F (1, 8) = 5.75, P = 0.043). The post hoc test revealed a significant decrease in AChE activity in females (P = 0.0024) but with no significance in male groups showing only a small tendency (P = 0.21). The same goes for the hippocampus, where we found an effect of treatment, sex and the interaction sex × treatment (F (1, 8) = 34.92, P = 0.0004; F (1, 8) = 40.26, P = 0.0002; F (1, 8) = 14.22, P = 0.0055, respectively). Also, the post hoc test revealed a significant inhibition of AChE activity in treated females (P = 0.0006) and a slight tendency in treated males (P = 0.47) compared to their sex-matched vehicle controls. For the hypothalamus, we also obtained a significant effect of all factors (F (1, 8) = 302.7, F (1, 8) = 141.9, F (1, 8) = 444.3, respectively and (P < 0.0001) similar in all factors). The multiple comparison test showed a tendency in MAL-exposed females (P = 0.11) and a significant decrease in AChE activity in treated males (P < 0.0001) compared to controls. As for the olfactory bulbs, no statistical differences were observed between groups.
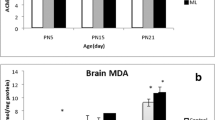
Effect of MAL treatment on AChE and oxidative stress in olfactory bulbs, hypothalamus, cerebellum and hippocampus. (A) AChE activity. (B) MDA activity. (C) SOD activity. (D) CAT activity. (E) GPx activity. Results are presented as mean ± SEM. Significant effects were revealed by Tukey’s test following two-way ANOVA. *p ≤ 0.05; **p ≤ 0.005; ***p ≤ 0.001; ****p ≤ 0.0001
Lipid peroxidation activity
MAL-exposed mice presented a general increase in MDA activity in all brain structures (Fig. 6B). Within the olfactory bulbs, the two-way ANOVA revealed a significant effect of both factors, treatment (F (1, 8) = 34.84, P = 0.0004) and sex (F (1, 8) = 74.64, P < 0.0001) with an interaction sex × treatment (F (1, 8) = 38.23, P = 0.0003). The post hoc test showed a significant increase in MDA activity in female-treated mice (P = 0.0001) but not in male mice. In the cerebellum, we obtained a significant effect of treatment (F (1, 8) = 12.34, P = 0.0079), but with no effect of sex or the interaction between factors. The post hoc test revealed a significant increase in MDA activity only in the male-treated group (P = 0.0299) compared to controls. The hippocampus MDA activity analysis demonstrated a main effect of both factors, treatment (F (1, 8) = 18.38, P = 0.0027) and sex (F (1, 8) = 33.75, P = 0.0004) but without an interaction sex × treatment. The multiple comparison test showed a significant increase in MDA activity in female-treated mice and not in male-treated mice compared to controls. As for the hypothalamus, we obtained a significant effect of treatment (F (1, 8) = 115.6, P < 0.0001) and the interaction between sex × treatment (F (1, 8) = 154.4, P < 0.0001) but with no effect of sex. The post hoc test showed, once again, a significant increase in MDA activity in females but not in male-treated groups compared to controls.
Antioxidant enzyme activity
The data analysis showed that chronic MAL exposure induced biochemical alteration in all brain structures. Concerning the SOD activity (Fig. 6C), the two-way ANOVA revealed a significant effect of treatment, sex, and the interaction treatment × sex in all brain structures (olfactory bulbs: F (1, 8) = 5028, P < 0.0001; F (1, 8) = 1178, P < 0.0001; F (1, 8) = 714.1, P < 0,0001; hypothalamus: F (1, 8) = 1382, P < 0.0001; F (1, 8) = 5185, P < 0.0001; F (1, 8) = 1662, P < 0.0001; cerebellum: F (1, 8) = 1258, P < 0.0001; F (1, 8) = 446.3, P < 0.0001; F (1, 8) = 1336, P < 0.0001; hippocampus: F (1, 8) = 56.85, P < 0.0001; F (1, 8) = 515.1, P < 0.0001; with no interaction between factors). Overall, the post hoc analysis revealed a significant increase in SOD activity in the hippocampus (males: P = 0.0012, females P = 0.0090) and olfactory bulbs (P < 0.0001) of both sexes. However, a significant increase in SOD activity was only found in the cerebellum of male-treated mice (P < 0.0001) and the hypothalamus of female-treated mice compared to controls (P < 0.0001).
Also, there was an increase of CAT activity in the olfactory bulbs and hypothalamus, but no differences were found in the hippocampus and cerebellum (Fig. 6D). As revealed by the two-way ANOVA there was a significant effect of treatment, sex, and interaction (olfactory bulbs: F (1, 8) = 5123, P < 0.0001; F (1, 8) = 552.3, P < 0.0001; F (1, 8) = 298.7, P < 0.0001; hypothalamus: F (1, 8) = 157.8, P < 0.0001; F (1, 8) = 14.53, P = 0.0051; F (1, 8) = 223.4, P < 0.0001). The post hoc test confirmed the increase of CAT activity in both males and females relative to their sex-matched vehicle controls (P < 0.0001).
On the other hand, MAL exposure induced a general decrease in GPx activity in almost all brain structures, especially in females (Fig. 6E). In the hippocampus, the two-way ANOVA revealed a significant effect of treatment (F (1, 8) = 6.67, P = 0.032) and the interaction treatment × sex (F (1, 8) = 19.06, P = 0.024). While Tukey’s test showed a significant decrease only in female treated mice (P = 0.051). In the cerebellum, there was a clear tendency of the effect of treatment (F (1, 8) = 3.68, P = 0.091) and the interaction treatment × sex (F (1, 8) = 18.95, P = 0.0024). The post hoc test also revealed a significant decrease in GPx activity in only female-treated mice (P = 0.0094). As for the hypothalamus, there was an effect of both factors’ treatment (F (1, 8) = 41.41, P = 0.0002) and sex (F (1, 8) = 30.09, P = 0.0006). The post hoc test demonstrated that GPx activity presented a clear tendency to decrease in treated females (P = 0.058), whereas it significantly decreased in treated males (P = 0.0014).
Discussion
In neurotoxicology, up to this day, there is so little known about the neurotoxicity of OPs and sex differences (Comfort and Re 2017). The purpose of this study is to investigate how MAL, an OP alters brain biochemicals and behavioral outcomes according to sex. The data presented here demonstrate that MAL chronic low-level exposure during a critical window of development induced sex-dependent neurotoxic effects, highlighted by a decrease in body weight and feeding behavior and several neurobehavioral deficits, accompanied by neurochemical alterations in different brain regions.
In our experiments, and because our focus is to study chronic low-dose exposure that represents the case of the majority of the world’s population, no overt toxicity signs were observed in any of the treated groups. To assess pesticide toxicity, body weight is often used as a morphological marker. Several in vivo studies have found that organophosphate insecticides induce decreased body weight (Djekkoun et al. 2022; Lasram et al. 2014). In the present study, the body weight of male and female treated groups was significantly lower than their sex-matched vehicle controls. Besides, treated males presented a more drastic decrease in body weight (4 weeks onwards) than females (8 weeks onwards), suggesting sex- and time-dependent effects of MAL on body weight. Our findings are consistent with the results presented by other studies with a similar period of exposure to chlorpyrifos (CPF), which is another OP (Djekkoun et al. 2022; Reygner et al. 2016). It has been suggested by many researchers that a decrease in body weight may be due to increased oxidative stress and enhanced catabolism of lipids and proteins (Djekkoun et al. 2022; Gupta et al. 2020). Decreased body weight might also be associated with decreased food consumption, which is the case in our study where both sexes demonstrated altered feeding behavior with a strong MAL response in male mice than in females, thus explaining their dramatic loss of body weight compared to females. Similar disturbances were declared in other experimental animals (Lasram et al. 2014; Seif et al. 2015). During the period evaluated both body weight and food intake in male mice were higher than that in females; these gender differences are knowingly explained by sex hormones (e.g., estrogens and testosterone) and sex chromosomes (Palmer and Clegg 2015; Chen et al. 2012). In addition to that, a recent study found that in the hypothalamus, female mice pro-opiomelanocortin (POMC) neurons display higher neural activities, compared to male counterparts, and further identified the transcription factor, TAp63, as one key molecule that contributes to the enhanced POMC neurons functions in females that allows them a certain protection from gaining weight (Wang et al. 2018). Indeed, the regulation of food intake requires the coordinated response of several central and peripheral factors. As mentioned earlier, the most studied central factor for the past two decades is the hypothalamic arcuate nucleus, where two neuronal populations are expressed, either POMC or neuropeptide Y and agouti-related peptide (NPY/AgRP). The peripheral factors are hormones including insulin, ghrelin, etc. (Williams and Elmquist 2012). In fact, it has been found that MAL increased plasma insulin levels in rats, and insulin is known to reduce food consumption by acting locally to inhibit hypothalamic NPY mRNA expression (Lasram et al. 2014; Schwartz et al. 2000). Moreover, research suggests that increased extracellular acetylcholine (ACh) in the nucleus accumbens (NAc) can inhibit feeding behavior. The cholinergic influence in the hypothalamus also plays a role in food intake regulation (Avena and Rada 2012). Actually, our results demonstrate decreased levels of AChE activity in the hypothalamus of treated mice, and highly more so in males than females, which promotes ACh levels in the hypothalamic synapses, thus food intake is attenuated especially in males. Furthermore, in the three weeks of food intake evaluation, both sexes displayed distinct food consumption patterns, where females showed an unstable and fluctuating profile and males presented a slightly steady curve throughout the experiment. Estrogen is well known to exert an inhibitory effect on feeding behavior (Olofsson et al. 2009). In female mammals, food intake is lower during the periovulatory period of the ovarian cycle, which coincides with a rise in serum estrogen levels (Asarian and Geary 2002). For instance, in rats, food intake decreases during estrus compared to metestrus and diestrus (Alonso-Caraballo and Ferrario 2019); this may explain the fluctuations found in the food intake curve of female mice.
Following developmental exposures to OP insecticides, rodents have been found to exhibit a range of neurobehavioral impairments and oxidative stress, that are often sexually dimorphic, and depend on the dose and the exposure duration (Todd et al. 2020). In our study, long-term exposure to MAL extending from gestation to adulthood induced a decrease in locomotor activity in both sexes in the OF test, the same was reported in a previous study on rats (N’Go et al. 2013a). However, motor coordination was only altered in treated female mice in the Rotarod test, indicating a sexually dimorphic response. Another study also found that motor coordination was only impaired in female rats following a developmental exposure to CPF correlated with an increase of extracellular GABA in their cerebellum (Gómez-Giménez et al. 2018). The same was reported in a cohort study of infants showing signs of prenatal exposure to OPs (Silver et al. 2017). Moreover, It is well known that for brain development, ACh and cholinergic projection play a central role in synaptogenesis and the development of normal neural cytoarchitecture (Hohmann 2003). So, any interference of MAL with the cholinergic and dopaminergic system due to AChE inhibition would lead to several behavioral impairments, including motor development or motor coordination deficits (Ramos et al. 2006; Fereidounni and Dhawan 2018). The cerebellum is traditionally viewed as the structure that computes how to make movements smooth and coordinated (Manto et al. 2012). Hence, the significant decrease in AChE activity in the cerebellum of treated females and not in males is correlated with their decreased rotarod latency.
The present study also revealed that chronic exposure to MAL disrupts recognition memory in both sexes, as shown in the object recognition task where the treated groups exhibited a lower discrimination index compared to their control groups. On the other hand, short-term spatial working memory was significantly altered in treated female mice only, once again displaying a sexually dimorphic neurobehavioral impairment. Several studies have shown that MAL interferes with learning and memory function (N’Go et al. 2013b; Santos et al. 2016; N’go et al. 2021). Also, behavioral studies have long suggested that the hippocampus plays a crucial role in memory and learning (Squire 1992). However, unlike special memory, which relies heavily on hippocampal substrates, object recognition memory appears to rely on a variety of brain regions and the involvement of the hippocampus is unsettled (Denninger et al. 2018). Thus, in this study, we note that chronic low-level exposure to MAL was sufficient to cause a significant decrease of AChE in the hippocampus of female-treated mice only, which subsequently resulted in their marked learning and spatial memory impairments. Another plausible explanation for the general memory dysfunction, is the mitochondrial dysfunction, astrogliosis, and activation of proapoptotic proteins in the hippocampus, as reported by Santos et al. (Santos et al. 2016) following repeated low-dose MAL exposure. There is growing evidence suggesting that Male rodents outperform females on a range of spatial learning tasks (Hawley et al. 2012; Leary et al. 2022; Yagi and Galea 2019). One of the reasons behind females’ lower ability in spatial learning and memory is their lower magnitude of long-term potentiation (LTP), which is the main hippocampal mechanism that modulates spatial learning and memory (Bliss and Collingridge 1993; Morris and Frey 1997; Monfort et al. 2015; Safari et al. 2021). Taken together, these findings might further explain the sex difference found in spatial memory performance following MAL exposure.
Moreover, MAL-treated animals showed enhanced immobility time, an indication of depressant-like behavior in both sexes, but highly more so in females than males. Indeed, there is a positive association between depression and MAL exposure in male workers frequently exposed to this pesticide (Beard et al. 2014; Harrison and Mackenzie 2016), and despite females presenting over 40% of the global agricultural workforce, most studies are solely focused on male participants (Raney et al. 2011; Malekirad et al. 2013). Furthermore, several behavioral studies have also revealed depressive-like phenotypes following acute and chronic exposures to MAL, correlated with decreased AChE activity in some brain areas associated with depression (Saeedi Saravi et al. 2016; Savall et al. 2020; Ardebili Dorri et al. 2015; Pinto Savall et al. 2021). Our results are in line with these findings, and also reveal decreased levels of AChE activity, especially in the hippocampus of female treated mice, which is known to play a major role in depression (MacQueen and Frodl 2011). Interestingly, a study found that nitric oxide (NO) plays a role in the sex difference of depression-like behaviors in rodents, where female mice presented lower NO production in the hippocampus and were significantly more likely to display depressive behaviors than their male littermates, these behavioral sex gaps were mended when differences in the hippocampus NO levels were abolished between male and female mice (Hu et al. 2012).
Furthermore, our study demonstrates that MAL treatment results in a male-specific reduction in sociability, whereas females showed comparable sociability in the three-chamber test. Notably, autistic individuals are primarily characterized by deficits in social interaction and communication (Happé and Ronald 2008). Hence, the male-dependent effect of treatment on this social behavior is reminiscent of the strong male bias found in human autism spectrum disorder (ASD) prevalence (Dworzynski et al. 2012). On top of that, gestational OP exposure was associated with social deficits that were more prominent among boys than girls (Furlong et al. 2014). Similar to our results Lan et al. (Lan et al. 2019) also showed sex-selective social deficits, where only males were impaired in the social preference test, following gestational exposure (GD 12-15) to a subtoxic dose of CPF (5 mg/kg). However, one of the limitations of this study is not taking the hormonal state of tested females into consideration, since sexual receptivity may be a factor that differentially regulates sociability (Jeon et al. 2018). Indeed, a recent study found that sexually-receptive (proestrus and estrus) mice showed no preferential interest in a novel female mouse compared with an empty chamber, while non-receptive (metestrus and diestrus) females showed normal preference similar to males (Chari et al. 2020). Nevertheless, our study is the first to carefully examine MAL exposure effect on males versus females in a non-receptive state and revealed sex-dependent social deficits. Additionally, our results showed that MAL treatment induced a male-specific increase in marble burying, which is a measure of repetitive and perseverative behaviors that represents some of the core behaviors in ASDs (Thomas 2009; Silverman et al. 2010). Raising the possibility that MAL exposure, one of the most used OPs around the world, might be a pervasive risk factor for ASD as von Ehrenstein et al. (Ehrenstein et al. 2019) also reported; thus more in-depth investigations are required.
Other than inhibiting AChE activity, OPs are also known to induce their neurotoxicity via oxidative stress. In light of this, we assessed the degree of oxidative damage imposed by MAL chronic exposure by evaluating the level of antioxidant enzyme activities (SOD, CAT, GPx) in parallel with MDA as the biomarker of lipid peroxidation (LPO) in all brain areas investigated. Several studies have found that MAL induces oxidative stress by showing increased levels of MDA and depletion of GSH, SOD, and CAT activities (Coban et al. 2014; Hazarika et al. 2003). Contrariwise, other MAL dosing regimens triggered the increase of CAT and SOD activities (Fortunato et al. 2006). Likewise, we found a general increase of SOD activity in all brain structures of both sexes, except the cerebellum of females and the hypothalamus of male treated groups. As for CAT activity, we also found increased levels in olfactory bulbs and hypothalamus of both treated animals, but with no differences in the hippocampus and the cerebellum. However, we found a general decrease in GPx activity in almost all brain structures, especially in females, which is consistent with the results of Maris et al. (Maris et al. 2010) where a trend of GPx activity inhibition was observed especially in females upon an acute MAL treatment. Therefore, since both increase and/or decrease in antioxidant enzyme activities are indicators of oxidative stress (Khan et al. 2017), the disrupted levels of oxidative stress markers in this study suggest a potential effect of reactive oxygen species (ROS) in neuronal damage which is expressed by the behavioral alterations induced by MAL exposure. Moreover, MAL induced an overall increase in MDA activity in all brain areas, especially in females. Similarly, Delgado et al. (Delgado et al. 2006) reported that MAL administration for 28 days resulted in increased MDA levels in the brain tissues of rats. In fact, the lipophilic nature of MAL may allow it to interfere with the cellular plasma membrane and cause increased LPO (Hazarika et al. 2003). Coupled with the greater vulnerability of the brain to oxidative stress due to its poor antioxidant defense system and high metabolic rate (Delgado et al. 2006), it may partly explain the rise of MDA production in the brain of MAL-treated mice.
Conclusion
To conclude, our work demonstrates that chronic low-level exposure to MAL during a critical period of development not only induced a range of neurobehavioral impairments that are often sex-dependent but also provoked oxidative stress and altered AChE activity at varying degrees between sexes in almost all brain structures. While there is a multitude of studies covering the adverse toxicity of OPs, limited research is available on their effects across sex. Our findings showed that treated females were more vulnerable regarding their special memory, motor coordination and were significantly more depressed, while only treated males’ sociability was impaired and their food intake has highly plummeted. Nonetheless, more research is required to understand the mechanisms behind these sexually dimorphic responses in animal models, which may explain some of the patterns underlying the dimorphic susceptibilities noticed in neurological disorders.
Data availability
Available on request.
References
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie/Academic Press Inc., Weinheim/NewYork, p 673–680. https://doi.org/10.1016/b978-0-12-091302-2.50032-3
Alonso-Caraballo Y, Ferrario CR (2019) Effects of the estrous cycle and ovarian hormones on cue-triggered motivation and intrinsic excitability of medium spiny neurons in the Nucleus Accumbens core of female rats. Horm Behav 116:104583. https://doi.org/10.1016/j.yhbeh.2019.104583
Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM (2013) Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp 82:50978. https://doi.org/10.3791/50978
Ardebili Dorri S, Hosseinzadeh H, Abnous K, Vahdati Hasani F, Yazdian Robati R, Razavi BM (2015) Involvement of brain‐derived neurotrophic factor (BDNF) on malathion induced depressive‐like behavior in subacute exposure and protective effects of crocin. Iran J Basic Med Sci 18(10):958–966
Asada K, Takahashi MA, Nagate M (1974) Assay and inhibitors of spinach superoxide dismutase. Agric Biol Chem 38(2):471–473. https://doi.org/10.1080/00021369.1974.10861178
Asarian L, Geary N (2002) Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42(4):461–471. https://doi.org/10.1006/hbeh.2002.1835
Avena NM, Rada PV (2012) Cholinergic modulation of food and drug satiety and withdrawal. Physiol Behav 106(3):332–336. https://doi.org/10.1016/j.physbeh.2012.03.020
Badr AM (2020) Organophosphate toxicity: updates of malathion potential toxic effects in mammals and potential treatments. Environ Sci Pollut Res 27(21):26036–26057. https://doi.org/10.1007/s11356-020-08937-4
Beard JD et al (2014) Pesticide exposure and depression among male private pesticide applicators in the agricultural health study. Environ Health Perspect 122(9):984–991. https://doi.org/10.1289/ehp.1307450
Bliss TVP, Collingridge GL (1993) A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361(6407):31–39. https://doi.org/10.1038/361031a0
Bradman A, Whyatt RM (2005) Characterizing exposures to nonpersistent pesticides during pregnancy and early childhood in the National Children’s Study: a review of monitoring and measurement methodologies. Environ Health Perspect 113(8):1092–1099. https://doi.org/10.1289/ehp.7769
Buege JA, Aust SD (1978) Biomembranes - Part C: Biological Oxidations. Methods Enzymol 52:302–310
Chari T, Griswold S, Andrews NA, Fagiolini M (2020) The stage of the Estrus cycle is critical for interpretation of female mouse social interaction behavior. Front Behav Neurosci 14:113. https://doi.org/10.3389/fnbeh.2020.00113
Chen X, McClusky R, Chen J, Beaven S. W, Tontonoz P, Arnold A. P, & Reue K (2012) The number of X chromosomes causes sex differences in adiposity in mice. PLoS genetics 8(5). https://doi.org/10.1371/journal.pgen.1002709
Coban FK, Ince S, Kucukkurt I, Demirel HH, Hazman O (2014) “Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug and chemical toxicology 38(4):391–399. https://doi.org/10.3109/01480545.2014.974109
Comfort N, Re DB (2017) Sex-specific neurotoxic effects of organophosphate pesticides across the life course. Curr Env Heal Rep 4(4):392–404. https://doi.org/10.1007/s40572-017-0171-y
Crawley JN (2004) Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev 10(4):248–258. https://doi.org/10.1002/mrdd.20039
Delgado EHB, Streck EL, Quevedo JL, Dal-Pizzol F (2006) Mitochondrial respiratory dysfunction and oxidative stress after chronic malathion exposure. Neurochem Res 31(8):1021–1025. https://doi.org/10.1007/s11064-006-9111-1
Denninger JK, Smith BM, Kirby ED (2018) Novel object recognition and object location behavioral testing in mice on a budget. J vis Exp 2018(141):1–10. https://doi.org/10.3791/58593
Djekkoun N, Depeint F, Guibourdenche M, El Khayat El Sabbouri H, Corona A, Rhazi L, Gay-Queheillard J, Rouabah L, Hamdad F, Bach V, Benkhalifa M, & Khorsi-Cauet H (2022) Chronic perigestational exposure to chlorpyrifos induces perturbations in gut bacteria and glucose and lipid markers in female rats and their offspring. Toxics 10(3):138. https://doi.org/10.3390/toxics10030138
dos Santos AA et al (2016) Long-term and low-dose malathion exposure causes cognitive impairment in adult mice: evidence of hippocampal mitochondrial dysfunction, astrogliosis and apoptotic events. Arch Toxicol 90(3):647–660. https://doi.org/10.1007/s00204-015-1466-0
Dunham NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 46(3):208–209
Dworzynski K, Ronald A, Bolton P, Happé F (2012) How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry 51(8):788–797. https://doi.org/10.1016/j.jaac.2012.05.018
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31(1):47–59. https://doi.org/10.1016/0166-4328(88)90157-X
Fereidounni S, Dhawan DK (2018) Understanding the role of quercetin during neurotoxicity induced by Chlorpyrifos. J Phytopharm 7(1):33–39.
Ferreira-Paes T, Seixas-Costa P, Almeida-Amaral EE (2021) Validation of a feed protocol in a mouse model that mimics marasmic malnutrition. Front Vet Sci 8(November):1–10. https://doi.org/10.3389/fvets.2021.757136
Ferri SL, Abdel T, Brodkin ES (2018) Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep 20(2):69–86. https://doi.org/10.4324/9780429454646
Flohé L, Günzler WA (1984) Assays of gluthathione peroxidase. Methods Enzymol 105:114–120
Fortunato JJ, Feier G, Vitali AM, Petronilho FC, Dal-Pizzol F, Quevedo J (2006) Malathion-induced oxidative stress in rat brain regions. Neurochem Res 31(5):671–678. https://doi.org/10.1007/s11064-006-9065-3
Furlong MA, Engel SM, Barr DB, Mary SW (2014) Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Env Int 70:125–131. https://doi.org/10.1016/j.envint.2014.05.011
Gobinath AR, Mahmoud R, Galea LA (2015) Influence of sex and stress exposure across the lifespan on endophenotypes of depression: Focus on behavior, glucocorticoids, and hippocampus. Front Neurosci 8:420. https://doi.org/10.3389/fnins.2014.00420
Gómez-Giménez B, Felipo V, Cabrera-Pastor A, Agustí A, Hernández-Rabaza V, Llansola M (2018) Developmental exposure to pesticides alters motor activity and coordination in rats: sex differences and underlying mechanisms. Neurotox Res 33(2):247–258. https://doi.org/10.1007/s12640-017-9823-9
Gupta VK, Kumar A, de L. Pereira M, Siddiqi NJ, Sharma B (2020) Anti-inflammatory and antioxidative potential of aloe vera on the cartap and malathion mediated toxicity in wistar rats. Int J Environ Res Public Health 17(14):1–19. https://doi.org/10.3390/ijerph17145177
Happé F, Ronald A (2008) The ‘ Fractionable Autism Triad’:a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychology review, 18(4):287–304. https://doi.org/10.1007/s11065-008-9076-8
Harrison V, Mackenzie S (2016) Anxiety and depression following cumulative low-level exposure to organophosphate pesticides. Environ Res 151:528–536. https://doi.org/10.1016/j.envres.2016.08.020
Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP (2012) The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav 105(4):1014–1020. https://doi.org/10.1016/j.physbeh.2011.11.021
Hazarika A, Sarkar SN, Hajare S, Kataria M (2003) Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology 185(1–2):1–8. https://doi.org/10.1016/s0300-483x(02)00574-7
Heyer DB, Meredith RM (2017) Environmental toxicology: sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 58:23–41. https://doi.org/10.1016/j.neuro.2016.10.017
Hodes GE, Walker DM, Labonté B, Nestler EJ, Russo SJ (2017) Understanding the epigenetic basis of sex differences in depression. J Neurosci Res 95(1–2):692–702. https://doi.org/10.1002/jnr.23876
Hohmann CF (2003) A morphogenetic role for acetylcholine in mouse cerebral neocortex. Neurosci Biobehav Rev 27(4):351–363. https://doi.org/10.1016/S0149-7634(03)00066-6
Hu Y et al (2012) Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc Natl Acad Sci U S A 109(35):14224–14229. https://doi.org/10.1073/pnas.1207461109
Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences (2001) Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academies Press (US), Washington (DC)
Jeon SJ et al (2018) Sex-specific behavioral features of rodent models of autism spectrum disorder. Exp Neurobiol 27(5):321–343. https://doi.org/10.5607/en.2018.27.5.321
Khan AM, Raina R, Dubey N, Verma PK (2017) Effect of deltamethrin and fluoride co-exposure on the brain antioxidant status and cholinesterase activity in Wistar rats. Drug Chem Toxicol 41(2):123–127. https://doi.org/10.1080/01480545.2017.1321009
Kwong TC (2002) Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit 24(1):144–149. https://doi.org/10.1097/00007691-200202000-00022
Lan A, Stein D, Portillo M, Toiber D, Kofman O (2019) Impaired innate and conditioned social behavior in adult C57Bl6/J mice prenatally exposed to chlorpyrifos. Behav Brain Funct 15(1):1–9. https://doi.org/10.1186/s12993-019-0153-3
Lasram MM, El-Golli N, Lamine A. J, Douib I. B, Bouzid K, Annabi A, El Fazaa S, Abdelmoula J, & Gharbi N (2014) Changes in glucose metabolism and reversion of genes expression in the liver of insulin-resistant rats exposed to malathion. The protective effects of N-acetylcysteine. Gen Comp Endocrinol 215:88–97. https://doi.org/10.1016/j.ygcen.2014.10.002
Leary TPO, Askari B, Lee BH, Darby K, Knudson C, Ash AM, Seib DR, Espinueva DF, Snyder JS (2022) Sex differences in the spatial behavior functions of adult-born neurons in rats. eNeuro. 9(3). https://doi.org/10.1523/ENEURO.0054-22.2022
Lo SC, Scearce-Levie K, Sheng M (2016) Characterization of social behaviors in caspase-3 deficient mice. Sci Rep 6(January):1–9. https://doi.org/10.1038/srep18335
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
MacQueen G, Frodl T (2011) The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research. Mol Psychiatry 16(3):252–264. https://doi.org/10.1038/mp.2010.80
Malekirad AA, Faghih M, Mirabdollahi M, Kiani M, Fathi A, Abdollahi M (2013) Neurocognitive, mental health, and glucose disorders in farmers exposed to organophosphorus pesticides. Arh Hig Rada Toksikol 64(1):1–8. https://doi.org/10.2478/10004-1254-64-2013-2296
Malqui H, Anarghou H, Ouardi FZ, Ouasmi N, Najimi M, Chigr F (2018) Continuous exposure to inorganic mercury affects neurobehavioral and physiological parameters in mice. J Mol Neurosci 66(2):291–305. https://doi.org/10.1007/s12031-018-1176-1
Manto M et al (2012) Consensus paper : roles of the cerebellum in motor control — the diversity of ideas on cerebellar involvement in movement. Cerebellum 11(2):457–487. https://doi.org/10.1007/s12311-011-0331-9
Maris AF et al (2010) Gender effects of acute malathion or zinc exposure on the antioxidant response of rat hippocampus and cerebral cortex. Basic Clin Pharmacol Toxicol 107:965–970. https://doi.org/10.1111/j.1742-7843.2010.00614.x
McCarthy MM, Woolley CS, Arnold AP (2017) Incorporating sex as a biological variable in neuroscience: what do we gain? Nat Rev Neurosci 18(12):707–708. https://doi.org/10.1038/nrn.2017.137
McLean AC, Valenzuela N, Fai S, Bennett SAL (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J vis Exp 67:4–9. https://doi.org/10.3791/4389
Monfort P, Gomez-Gimenez B, Llansola M, Felipo V (2015) Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: molecular mechanisms. ACS Chem Neurosci 6(8):1420–1427. https://doi.org/10.1021/acschemneuro.5b00096
Morris RGM, Frey U (1997) Hippocampal synaptic plasticity: Role in spatial learning or the automatic recording of attended experience? Philos Trans r Soc B Biol Sci 352(1360):1489–1503. https://doi.org/10.1098/rstb.1997.0136
Mostafalou S, Abdollahi M (2017) Pesticides: an update of human exposure and toxicity. Arch Toxicol 91(2):549–599. https://doi.org/10.1007/s00204-016-1849-x
Moura CA, Oliveira MC, Costa LF, Tiago PRF, Holanda VAD, Lima RH, Cagni FC, Lobão-Soares B, Bolaños-Jiménezet F, Gaviolial EC (2020) Prenatal restraint stress impairs recognition memory in adult male and female offspring. Acta Neuropsychiatr 29:1–6. https://doi.org/10.1017/neu.2020.3
N’Go PK et al (2013b) Developmental effects of malathion exposure on recognition memory and spatial learning in males wistar rats. J Behav Brain Sci 03(03):331–340. https://doi.org/10.4236/jbbs.2013.33033
N’Go PK, Azzaoui F-Z, Ahami AOT, Soro PR, Najimi M, Chigr F (2013) Developmental effects of Malathion exposure on locomotor activity and anxiety-like behavior in Wistar rat. Health (Irvine. Calif) 05(03): 603–611. https://doi.org/10.4236/health.2013.53a080
Olofsson LE, Pierce AA, Xu AW (2009) Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A 106(37):15932–15937. https://doi.org/10.1073/pnas.0904747106
Ouardi FZ et al (2019) Gestational and lactational exposure to malathion affects antioxidant status and neurobehavior in mice pups and offspring. J Mol Neurosci 69(1):17–27. https://doi.org/10.1007/s12031-018-1252-6
N’go PK et al (2021) Comparison of the effects of argan and nigella oils on malathion-induced cognitive-behavioral alterations and brain histopathology in male Wistar rats. Curr Top Toxicol 17:131–142. https://doi.org/10.31300/cttx.17.2021.131-142
Palmer BF, Clegg DJ (2015) The sexual dimorphism of obesity. Mol Cell Endocrinol 402:113–119. https://doi.org/10.1016/j.mce.2014.11.029
Pinto Savall AS, Fidelis EM, Quines CB, Bresolin L, Gervini V, Pinton S (2021) Potential role of a newly AChE reactivator in the depressive-like behavior induced by malathion. Neuroscience letters 749:135697. https://doi.org/10.1016/j.neulet.2021.135697
Prendergast BJ, Onishi KG, Zucker I (2014) Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40:1–5. https://doi.org/10.1016/j.neubiorev.2014.01.001
Ramos ZR, Fortunato JJ, Agostinho FR, Martins MR, Correa M, Schetinger M. R, Dal-Pizzol F, & Quevedo J (2006) Influence of malathion on acetylcholinesterase activity in rats submitted to a forced swimming test. Neurotox Res 9(4):285–290. https://doi.org/10.1007/BF03033318
Raney T, Croppenstedt A, Anriquez G, Lowder S (2011) The state of food and agriculture 2010-11: Women in Agriculture: Closing the Gender Gap for Development. 2.
Rauh VA et al (2012) Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci U S A 109(20):7871–7876. https://doi.org/10.1073/pnas.1203396109
Reygner J et al (2016) Inulin supplementation lowered the metabolic defects of prolonged exposure to chlorpyrifos from gestation to young adult stage in offspring rats. PLoS ONE 11(10):1–17. https://doi.org/10.1371/journal.pone.0164614
Ruszkiewicz JA et al (2019) Sex-specific differences in redox homeostasis in brain norm and disease. J Mol Neurosci 67(2):312–342. https://doi.org/10.1007/s12031-018-1241-9
Saeedi Saravi SS et al (2016) On the effect of minocycline on the depressive-like behavior of mice repeatedly exposed to malathion: interaction between nitric oxide and cholinergic system. Metab Brain Dis 31(3):549–561. https://doi.org/10.1007/s11011-015-9764-z
Safari S et al (2021) Sex differences in spatial learning and memory and hippocampal long-term potentiation at perforant pathway-dentate gyrus (PP-DG) synapses in Wistar rats. Behav Brain Funct 17(1):1–11. https://doi.org/10.1186/s12993-021-00184-y
Sanghi R, Pillai MKK, Jayalekshmi TR, Nair A (2003) Organochlorine and organophosphorus pesticide residues in breast milk from Bhopal, Madhya Pradesh, India. Hum Exp Toxicol 22(2):73–76. https://doi.org/10.1191/0960327103ht321oa
Savall ASP et al (2020) Antidepressant-like effect of (3Z)-5-Chloro-3-(hydroxyimino)indolin-2-one in rats exposed to malathion: involvement of BDNF-Trkβ pathway and AChE. Life Sci 256:117892. https://doi.org/10.1016/j.lfs.2020.117892
Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404(6778):661–671. https://doi.org/10.1038/35007534
Seif MM, Khalil FA, Abou Arab AA, Donia AMA, El-Sherbiny AM, Mohamed SR (2015) Ameliorative role of Melissa officinal is against hepatorenal toxicities of organophosphorus malathion in male rats. MOJ Toxicolology 1(3). https://doi.org/10.15406/mojt.2015.01.00014
Silver MK, Shao J, Zhu B, Chen M, Xia Y, Kaciroti N, Lozoff B, Meeker JD (2017) Prenatal naled and chlorpyrifos exposure is associated with deficits in infant motor function in a cohort of Chinese infants. Environ Int 106:148-256. https://doi.org/10.1016/j.envint.2017.05.015
Silverman JL, Yang M, Lord C, Crawley JN (2010) Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11 (7):490–502. https://doi.org/10.1038/nrn2851
Squire LR (1992) Memory and the hippocampuss: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99(2):195–231. https://doi.org/10.1037/0033-295X.99.2.195
Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R (2009) Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 204(2):361–73. https://doi.org/10.1007/s00213-009-1466-y.Marble
Todd SW, Lumsden EW, Aracava Y, Mamczarz J, Albuquerque EX, Pereira EFR (2020) Gestational exposures to organophosphorus insecticides: from acute poisoning to developmental neurotoxicity. Neuropharmacology 180:108271. https://doi.org/10.1016/j.neuropharm.2020.108271
Venkatesan R, Park YU, Ji E, Yeo EJ, Kim SY (2017) Malathion increases apoptotic cell death by inducing lysosomal membrane permeabilization in N2a neuroblastoma cells: a model for neurodegeneration in Alzheimer’s disease. Cell Death Discov 3: 17007. https://doi.org/10.1038/cddiscovery.2017.7
Von Ehrenstein OS et al (2019) Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ 364:1–10. https://doi.org/10.1136/bmj.l962
Wang C et al (2018) TAp63 contributes to sexual dimorphism in POMC neuron functions and energy homeostasis. Nat Commun 9(1):1–11. https://doi.org/10.1038/s41467-018-03796-7
Williams KW, Elmquist JK (2012) From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15(10):1350–1355. https://doi.org/10.1038/nn.3217
Woolley CS, McEwen BS (1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12(7):2549–2554. https://doi.org/10.1523/JNEUROSCI.12-07-02549.1992
Yagi S, Galea LAM (2019) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44(1):200–213. https://doi.org/10.1038/s41386-018-0208-4
Yin C et al (2011) Antidepressant-like effects of L-theanine in the forced swim and tail suspension tests in mice. Phyther Res 25(11):1636–1639. https://doi.org/10.1002/ptr.3456
Funding
This research was funded by Project CNRST Ibn Khaldoune 2018-18.
Author information
Authors and Affiliations
Contributions
L.B. performed the experiments and wrote the manuscript with support from all authors. M.L. performed the statistical analysis. L.B., M.L. and O.E. processed the experimental data and designed the figures. H.M. and H.A. along with L.B., M.L. and O.E. contributed to sample preparation and biochemical analysis. F.C., M.N. and A.C. supervised the project. All authors have read and agreed to the published version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
All experimental procedures performed on animals are in accordance with the European Council directive: ACT: 86/609 EEC.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berroug, L., Laaroussi, M., Essaidi, O. et al. Sex-specific neurobehavioral and biochemical effects of developmental exposure to Malathion in offspring mice. Naunyn-Schmiedeberg's Arch Pharmacol 397, 2215–2231 (2024). https://doi.org/10.1007/s00210-023-02749-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02749-2