Abstract
Contamination with mercury is a real health issue for humans with physiological consequences. The main objective of the present study was to assess the neurotoxicological effect of inorganic mercury: HgCl2. For this, adult mice were exposed prenatally, postnatally, and during the adult period to a low level of the metal, and their behavior and antioxidant status were analyzed. First, we showed that mercury concentrations in brain tissue of treated animals showed significant bioaccumulation, which resulted in behavioral deficits in adult mice. Thus, the treated mice developed an anxiogenic state, as evidenced by open field and elevated plus maze tests. This anxiety-like behavior was accompanied by a decrease in social behavior. Furthermore, an impairment of memory in these treated mice was detected in the object recognition and Y-maze tests. The enzymatic activity of the antioxidant system was assessed in eight brain structures, including the cerebral cortex, olfactory bulb, hippocampus, hypothalamus, mesencephalon, pons, cerebellum, and medulla oblongata. The results show that chronic exposure to HgCl2 caused alterations in the activity of catalase, thioredoxin reductase, glutathione peroxidase, superoxide dismutase, and glutathione S-transferase, accompanied by peroxidation of membrane lipids, indicating a disturbance in intracellular redox homeostasis with subsequent increased intracellular oxidative stress. These changes in oxidative stress were concomitant with a redistribution of essential heavy metals, i.e., iron, copper, zinc, and magnesium, in the brain as a possible response to homeostatic dysfunction following chronic exposure. The alterations observed in overall oxidative stress could constitute the basis of the anxiety-like state and the neurocognitive disorders observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury occurs naturally in the environment, and most of the mercury found in the environment is in the form of metallic mercury and inorganic mercury compounds. Indeed, mercury is present in three chemical forms, i.e., elementary, organic, and inorganic, and all these forms are harmful to living organisms (Clarkson and Magos 2006). This metal is a highly toxic element, and its potential for toxicity has been demonstrated dramatically in highly contaminated areas, such as the Minamata Bay, Japan, in the 1950s and 1960s. Many experimental studies have reported the adverse effects of this nonessential heavy metal. Although the three chemical forms represent a risk factor for body functioning and, consequently, for health, each form of mercury has its own characteristics and distinctive toxicological profile and clinical symptoms. The adverse toxic effects of the organic form, notably methylmercury mainly present in food, have been abundantly studied and characterized both in experimental laboratory animals and in humans (Ekino et al. 2007; Falluel-Morel et al. 2007). The high lipophilic character of this form of mercury enables easy distribution to the central nervous system and induction of its neurotoxic effects (Ornagh et al. 1993; Vicente et al. 2004; Cernichiari et al. 2007; Stringari et al. 2008; Falluel-Morel et al. 2012). Although information about the toxicity of methylmercury is well-documented, studies concerning inorganic mercury are scarce. Some inorganic mercury compounds are used as fungicides and can enter water or soil. Inorganic salts of mercury, including ammoniated mercuric chloride, have been used in cosmetics (e.g., skin-lightening creams). Mercuric chloride is also used as a topical antiseptic or disinfectant agent. Finally, high levels of exposure to mercury vapor can result from cultural and religious use (Riley et al. 2001). In fact, exposure of the general population comes from dental amalgams and vaccines. Exposure to mercury from dental amalgams has been a concern for decades (Clarkson and Magos 2006). Inorganic mercury causes clinical symptoms that are associated with nervous system dysfunction. Of note, in the general population, brain, blood, and urinary concentrations correlate with the number of amalgam surfaces. Most case studies of neurotoxicity in humans induced by oral exposure to inorganic mercury salts have reported neurotoxic effects as the result of ingestion of therapeutic agents that contain mercurous chloride (e.g., teething powders, ointments, and laxatives) (Kang-Yum and Oransky 1992). Furthermore, concern arises from claims that long-term exposure to low concentrations of inorganic mercury either causes or exacerbates degenerative diseases, such as amyotrophic lateral sclerosis, Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease (for review, see Bernohft 2012). In experimental animals, many studies have addressed the toxicity of chronic exposure to low doses of inorganic mercury on many peripheral organs, such as muscle, kidneys, and liver (Agarwal and Behari 2007; Branco et al. 2012). Immunotoxicity and reproductive toxicity have also been investigated, and significant negative effects of inorganic mercury have been shown (Ryan et al. 1991). Several studies have assessed the effect of inorganic mercury on the central nervous system and reported the presence of behavioral and cognitive dysfunctions (e.g., memory deficits, anxiety, depression, and impairment of social interaction) (Peixoto et al. 2007; Mello-Carpes et al. 2013). Furthermore, it has been shown that mercury is particularly detrimental to brain development with potential alterations and deficits in brain function in adulthood (Huang et al. 2011). These studies used different protocols, notably concerning the route (oral or peritoneal) and the mode (acute, subchronic, or chronic) of HgCl2 administration, the administered dose of the metal (high, medium, or low), and the period of administration (in the prenatal, postnatal, or adult period) (Chehimi et al. 2012; Mello-Carpes et al. 2013; Peixoto et al. 2007). Exposure to excessive, medium or low doses of the metal may cause a variety of neurotoxic effects that involve neurobehavior deficits and/or alterations in brain structure integrity and in biomarkers of oxidative stress (Chehimi et al. 2012; Mello-Carpes et al. 2013; Peixoto et al. 2007). The intensity of the effects and their spectrum depends on at least one of the previously cited factors. The neurotoxic effects of mercury dealing with cognitive and emotional impairments have not received much attention, whereas epidemiological and experimental data have shown neurophysiological impairments following occupational exposure to the metal and in people living in the neighborhood of contaminated places (Azevedo et al. 2012; Bernohft 2012). In animal studies, the three forms of mercury have been shown to induce different types of nervous system damage, including motor activity and anxiety level alterations, learning ability impairments, and neuromuscular and physiologic abnormalities (Azevedo et al. 2012; Mello-Carpes et al. 2013; Abu Bakar et al. 2017). Growing evidence supports a link between these impairments, notably the occurrence of anxiety and the oxidative stress generated by environmental contaminants in general (Bouayed et al. 2009). However, few investigations have analyzed the effects of the continuous exposure to low levels of HgCl2 on general physiology and its long-term outcomes, particularly on the genesis of neuroemotional and neurocognitive disorders, such as anxiety and memory impairment, in relation to the alteration of the antioxidant enzyme activity considered the first defense system against biological macromolecules generated by oxidative stress (Vicente et al. 2004). The majority of antioxidant enzyme activity studies have focused only on the brain as a whole without examining effects on particular brain structures. As a consequence, the mechanisms of the neurotoxicity of inorganic mercury in discrete brain structures have been poorly investigated.
As depicted previously, the long-term outcomes of chronic low-dose HgCl2 exposure (regional, occupational, etc.) are less well understood, and clinical studies are still very limited. Experimental investigations documenting the adverse effects of chronic exposure to low doses of HgCl2 via alimentation from the prenatal period to adult age on brain function are significantly lacking. Therefore, the aim of the present study was to verify the effects of long-term exposure to low HgCl2 doses (typically, not considered harmful) from gestational life to adulthood on anxiety-like and neurocognitive aspects of behavior and mechanisms underlying the eventual dysfunction observed in adult life, in an attempt to approximate the common human situation. To our knowledge, this model of exposure is unique and has not been previously described. Previous studies have given the metal during a precise period of life even if the effects of exposure require long-term evaluation, e.g., in adult rats (Peixoto et al. 2007; Mello-Carpes et al. 2013).
Materials and Methods
Animals, Treatment, and Exposure Modalities
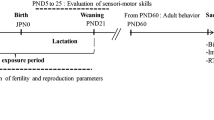
Adult Swiss albino mice were obtained from the animal husbandry of the Faculty of Sciences and Techniques, Beni Mellal, Morocco. The animals were housed in standard Plexiglas cages (30 cm × 15 cm × 12 cm) (4–5 animals per cage) with free access to food and water on a 12 h (light)/12 h (dark) cycle with lights on at 0800. Mice were acclimated for 1 week with the manipulator in a colony room (22° ± 2 °C). After 1 week, two female mice and one male mouse were randomly chosen, housed in a cage, and exposed to water containing HgCl2 (treated group) or fresh water without HgCl2 (control group).
Pregnant mice were then isolated and exposed to HgCl2 through the drinking water (40 ppm) ad libitum, during the entire gestational period (treated group) or not (control group). After parturition, the pups remained with their dams until 21 days of age. The pups of the treated group were supplied with HgCl2 by breastfeeding, and on postnatal day 21, the offspring were separated by sex and randomly housed. The treated group was still exposed to water containing HgCl2 and the control group to fresh water without HgCl2. Adult male mice (9–10 weeks old, 30 g average) were then selected for experiments. All procedures in animal experimentation were performed in conformity with approved institutional protocols and in accordance with the provisions of animal care and use as reported in the Scientific Procedures of Living Animals (European Council directive: ACT: 86/609 EEC).
Water Consumption
The consumption of drinking water was measured on a daily basis. The control mice had access to two bottles: one filled with tap water and the other filled with 1% sucrose. Treated mice had access to a bottle containing HgCl2 solution and the other containing 1% sucrose. The location of the bottles was reversed every 24 h. After every 24 h, the amount of water and sugar solution consumed was recorded. Two days before the start of the exposure in the drinking water, the mice only had access to the sweet solution in the two bottles to avoid any effects of novelty.
Behavioral Tests
Open Field Test
The locomotor activity of treated and control mice was monitored by using an open field device. The mice were 9 weeks old at the beginning of testing in the open field. Each tested mouse was moved from its home cage to the center square (10 × 10 cm) of the apparatus (50 × 50 × 50 cm) that was divided into 25 equal squares. The behavioral measures recorded for 5 min consisted of the number of peripheral and central square crossings, total number of square crossings, and percentage of central square crossings (Goulet et al. 2003).
Elevated Plus Maze Test
The device used for this test was a plus-shaped Plexiglas maze as described by Pellow et al. (1985) with two opposite open arms (50 cm × 10 cm) and two enclosed arms (50 cm × 10 cm × 40 cm), spreading out from a central platform (10 × 10 cm). The apparatus was elevated to a height of 50 cm from the floor, and to minimize accidental falls of the animal from the open arms, these were surrounded by a 1-cm high clear Plexiglas edge. Mice were individually placed in the center of the elevated plus maze (EPM), facing one of the enclosed arms, and were allowed to explore the apparatus for 5 min. All tests were video recorded, and the analysis of videos allowed us to determine the frequency of the open arm entries, the frequency of the enclosed arm entries, the time spent on the open arms, and the time spent in the enclosed arms. An entry was counted whenever the animal placed four paws on a particular arm. The maze was cleaned with ethanol solution (10%, v/v) and dried after each test session. The percentages of open arm entries and of open arm time were calculated according to the following formula: open/total ∗ 100. Anxiogenic effects are defined as a decrease in the proportion of open arm entries divided by the total number of arm entries and the time spent on open arms relative to the total time spent on both arms.
Interactive Sociability
To assess the effect of HgCl2 treatment on the sociability of adult mice, we used the three-chamber test system developed by Crawley (2004). The mice were placed in the test room 30 min before the start of the tests. Each tested mouse was used once a day. A 20-min test was performed for each test mouse. The test was conducted as follows:
-
A.
Adaptation phase:
-
1.
Restricted access to the right and left compartments using removable Plexiglas walls.
-
2.
Placed empty metal containment cups in the middle of the right and left compartments (one for each side).
-
3.
Placed the test mouse in the center of the middle chamber for adaptation.
-
4.
Allowed 5 min for exploration.
-
1.
-
B.
Social interaction (session I):
-
1.
Placed one of the control mice (“stranger 1”) inside a metal containment cup in one of the side chambers. The placement of stranger 1 on the right or left side of the chamber was systematically changed between trials.
-
2.
Removed the walls between the compartments to allow free access for the test mouse to explore each of the three chambers.
-
3.
Immediately started monitoring and recording the following measures:
-
The duration and the number of direct (active) contacts between the test mouse and the cup containing stranger 1 and the empty cup. Direct contact between the test mouse and the confinement cup or the stretching of the mouse body into a 3–5 cm area around the cup was counted as an active contact.
-
The duration and the number of other behaviors by the test mouse in each compartment, including walking, self-grooming, and the absence of body movements for more than 5 s (i.e., “freezing”), as well as unusual behaviors, such as jumping and repetitive behaviors.
-
-
4.
The duration of session I was 10 min.
-
1.
-
C.
Social novelty/preference (session II):
-
1.
Placed a second mouse (“stranger 2”) inside an identical confinement cup in the compartment on the other side (which was empty during session I). Monitor the same parameters described previously, while differentiating behaviors in the presence of stranger 1 versus stranger 2.
-
2.
The duration of session II was 10 min.
-
1.
Y-Maze Spontaneous Alternation
The Y-maze spontaneous alternation paradigm is based on the natural tendency of rodents to explore novel environments. When placed in the Y-maze, normal mice prefer to explore the least recently visited arm and thus tend to alternate visits between the three arms. A mouse with impaired working memory cannot remember which arm it has just visited and thus shows decreased spontaneous alternation (Nagahara and McGaugh 1992). The apparatus was a Y-maze made of Plexiglas with three identical arms (40 × 9 × 16 cm) placed 120° from each other. Each arm had walls with specific motifs allowing it to be distinguished from the others. Each mouse was placed at the end of one of the three arms and was allowed to freely explore the apparatus for 5 min, with the experimenter out of the animal’s sight. Alternation was operationally defined as successive entries into each of three arms as overlapping triplet sets (e.g., ABC, BCA). The percentage of spontaneous alternations was calculated as an index of working memory performance (index of alternation). Total arms entries were also scored as indexes of ambulatory activity.
Object Recognition Task
At the beginning of the test, each animal was allowed to explore the device freely for 5 min in the absence of any object. Twenty-four hours later, the animal explored the chamber with two identical objects (A1 and A2) placed at two adjacent corners of the device 10 cm from the walls for 5 min; this was the “acquisition” session. In this situation, it was considered exploration when the mouse’s nose was directed towards the object and less than 1 cm away, whereas the “nibbling” and the marking of the objects were not considered exploration.
After a delay of 2 h (to evaluate effects on short-term memory), the mouse was placed in the device, but one of the two identical objects was replaced by another, quite distinct object B. The animal explored the two objects for 5 min: There were two duplicate sets of objects, and the objects and chamber were cleaned with 10% ethanol after each session to remove any traces left by the animal in the device (Ennaceur and Meliani 1988).
Biochemical Assays
Procedures for Brain and Tissue Dissection
Mice were first deeply anesthetized with pentobarbital and then decapitated. Then, the brains of the control and HgCl2-treated mice were removed from the skull. For each brain, the two hemispheres of the cerebral cortex, olfactory bulbs, hippocampus (right and left sides), hypothalamus, mesencephalon, cerebellum, and midbrain were rapidly dissected on a plate at 4 °C, weighed, and frozen at − 20 °C for 48 h until use. For the biochemical assays, the dissected brain regions were then homogenized according to the protocol reaction.
Determination of Acetylcholinesterase Activity
Acetylcholinesterase (AchE)-specific activity was measured according to the method of Ellman et al. (1961) using acetylthiocholine iodide (Sigma-Aldrich, USA) as a substrate. The reaction mixture contained phosphate buffer (0.1 M, pH 8.0), acetylthiocholine iodide (0.075 M), and 5,5-dithiobis-2-nitrobenzoic acid (DTNB; 0.01 M) (Sigma-Aldrich, USA). After the addition of the brain structure tissue homogenate (olfactory bulb, cerebral cortex, or brainstem) (30 min at room temperature), the hydrolysis rate of acetylcholine iodide was measured by a spectrophotometer (Selecta, Spain) at 412 nm. The enzyme activity was expressed as μmol Ach hydrolyzed/min/mg of protein.
Determination of Lipid Peroxidation and Antioxidant Enzymatic Activity
Tissue Preparation
Brain structures were homogenized in buffer solution TBS (50 mM Tris, 150 mM NaCl, pH 7.4); homogenates were centrifuged at 10,000 ×g for 15 min at 4 °C. The resulting supernatant was used for the determination of the following antioxidant enzymatic activities: catalase, superoxide dismutase, glutathione S-transferase, glutathione peroxidase (GPx), and thioredoxin reductase (ThxR).
Determination of Lipid Peroxidation
The lipid peroxidation (LPO) level in the brain was measured by the method of Buege and Aust (1984). A total of 125 μl of supernatant was homogenized by sonication with 50 μl of PBS and 125 μl of 20% TCA + 1% BHT (TCA-BHT), to precipitate proteins, and centrifuged (1000 ×g, 10 min, 4 °C). Afterwards, 200 μl of supernatant was mixed with 40 μl of HCl (0.6 M) and 160 μl of TBA dissolved in Tris (120 mM). The mixture was heated at 80 °C for 10 min, and the absorbance was measured at 530 nm. The amount of thiobarbituric acid reactive substances (TBARS) was calculated using a molar extinction coefficient of 1.56 × 105 M/Cm.
Catalase
The activity of catalase (CAT) was measured at 240 nm using a UV/visible spectrophotometer by the variation of the optical density consecutive to the disproportionation of hydrogen peroxide (H2O2). For the enzyme reaction, 20 μl of supernatant was added to 780 μl of phosphate buffer saline (PBS) (0.1 M, pH 7.4) and 200 μl of H2O2 (0.5 M).
Superoxide Dismutase
Superoxide dismutase (SOD) activity was determined by the method of Asada et al. (1974); 0.05 ml of the supernatant was added to 0.1 ml of a mixture containing methionine (13 mM) and Na2EDTA (0.1 mM), 0.8922 ml of phosphate buffer (50 mM, pH = 7.8), 0.95 ml of phosphate buffer, 0.088 ml of NBT (2.64 mM), and 0.0226 ml of riboflavin (0.26 mM). The reduction of NBT was estimated after 20 min at a wavelength of 580 nm against white.
Glutathione Peroxidase
For each assay, a mixture containing 200 μl of supernatant, 200 μl of phosphate buffer (100 mM), 200 μl of 4 mM GSH, and 400 μl of H2O2 (5 mM) was incubated for 1 min at 37 °C. After adding 500 μl of 5% TCA, the mix was then centrifuged for 5 min at 1500 ×g. Two hundred microliters of the supernatant was recovered, 500 μl of phosphate buffer and 500 μl of DTNB were added, and the absorbance was measured at 412 nm each min for 5 min according to a modified method of Flohe and Gunzler (1984).
Thioredoxin Reductase
The activity of thioredoxin reductase (TrxR) was determined following the instructions of Cayman’s colorimetric assay kit for detecting mammalian TrxR activity in tissue homogenate. It is based on the reduction of DTNB with NADPH to TNB. For an assay, NADPH and DTNB were added to a sample obtained from the brain supernatant (0.1 g of tissue/100 ml of TBS buffer pH 7.4) to initiate the reaction, which produces a yellow product that is measured at 412 nm.
Protein Measurement
Protein concentration was assayed by the method of Lowry et al. (1951) with bovine serum albumin as the standard.
Determination of Essential Heavy Metals and Minerals
Dissected brain structures were dried for 48 h. For the digestion of the tissue, the dried samples were weighed and placed in 10 ml conical flasks with polypropylene lids containing 3 ml of HNO3 at room temperature until the solution became clear. Then, 1 ml of 30% H2O2 was added to the samples. After the effervescence ceased, the samples were heated at 80 °C until the HNO3 evaporated. The samples were then cooled to room temperature. The final volume was increased to 10 ml with 2% HNO3. The samples were brought to a constant volume. Brain mercury (Hg), iron (Fe), copper (Cu), magnesium (Mg), zinc (Zn), and calcium (Ca) levels were determined with an Inductive Coupled Plasma: Optical Emission Spectrometry (ICP-AES; Optima 8000-PerkinElmer, USA).
Statistics
We used Student’s t-test for the comparison of the two experimental groups (control and treated). Statistical significance was set at p < 0.05. Sigma Plot 11.0 software was used for statistical analysis.
Results
Water Consumption
Due to the particular taste of mercury, we have first assessed its impact on water consumption by experimental group given HgCl2 in water and we noticed no statistically difference regarding water daily intake between treated and control mice during pregnancy (7.73 ± 0.56 ml vs. 7.39 ± 0.49 ml; t = 0.451, p = 0.655). In another experimental group, in male adults, we measured the amount of consumption of sweet solution (water containing 1% of sucrose) and compared it to standard fresh water consumption (controls) or to HgCl2 solution (treated). The amount of consumption of sweet solution was 4.91 ± 0.242 vs. 3.423 ± 0.432 of fresh water, in controls and 8.037 ± 0.245 vs. 1.22 ± 0.192 of HgCl2 solution in treated mice (Table 1).
Open Field
Analysis of open field test sessions revealed an effect of HgCl2 treatment on time and the number of crossed squares. Thus, the time spent in the periphery was significantly higher in the treated group than in the control group (272.461 ± 2.64 vs 259.129 ± 3.766; p = 0.009) (Fig. 1a). Furthermore, the number of peripheral squares crossed was higher in the treated animals compared to that in the controls (211.71 ± 7.831 vs. 147.286 ± 13.016; p = 0.004) (Fig. 1b), whereas the percentage of central squares crossed by the controls was higher compared to that by the treated mice (Fig. 1c). Regarding total locomotor activity, we noticed no significant differences between the treated and control mice.
Behavior in the open field during the 5 min of recording. Each histogram represents the mean ± S.E.M. a Time spent in center and periphery. b Number of square crossings. c Percentage of central square crossings. Each value represents the mean ± S.E.M. (10 animals/group). Student’s t-test: *p < 0.05 compared to the control group
Elevated Plus Maze
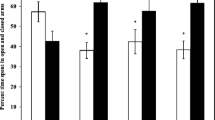
Analysis of the EPM test sessions showed a significant effect of HgCl2. In this experiment, the treated group showed a higher anxiety index compared to the control group (t = − 2.445, p = 0.050) and a lower spent time in the open arms (t = 2.573, p = 0.030). We also observed a reduced percentage of entries into open arms following HgCl2 treatment in this behavioral test (t = 2.637, p = 0.030). (Fig. 2).
Anxiety-related behavior of mice that received mercury during the development of the CNS and evaluated in the EPM test. a Anxiety index. b The percentage of open arm time. c The number of open arm entries. Each value represents the mean ± S.E.M. (10 animals/group). Student’s t-test: *p < 0.05 compared to the control group
Social Interaction
The social behavior of mice treated with mercury chloride was assessed in the “three-chamber mouse social behavior testing” apparatus. For this procedure, we used mice with matched ages, and the time spent in each compartment (with or without a “stranger mouse”) and grooming were measured. The obtained results showed that a behavioral deficit was present in the mice treated with mercury chloride. Thus, the control mice spent significantly more time exploring the new strange mouse (stranger 2) than the first strange mouse (stranger 1) (t = − 3.134, p = 0.014), while the treated mice did not show any difference between the different strangers (t = − 1.235, p = 0.263). Concerning grooming, a significant effect of HgCl2 treatment on repetitive self-grooming was observed in the two sessions analyzed (t = − 3.081, p = 0.027 and t = − 2.736, p = 0.034) (Fig. 3).
Social behavior of mice treated with inorganic mercury. Three-chamber mouse social behavior testing was performed. Mice of the same age were used as stimulus mice, and the following behaviors were recorded: (a) grooming in the first session; (b) grooming in the second session; (c) duration in the boxes where the tested mice are present in the second session; and (d) duration near the stranger mice in the second session. Each value represents the mean ± S.E.M. (10 animals/group). Student’s t-test: *p < 0.05 compared to the control group
Y-Maze
In this test, we measured the effect of mercury chloride on spontaneous alternation of mice in the Y-maze. Our results showed that the % alternation (t = 3.054, p = 0.01) and the number of entries (t = 2.435, p = 0.026) in the three arms were significantly lower after chronic exposure to mercury chloride compared to no exposure. Furthermore, mercury chloride reduced locomotor activity and the alternating performance in mice using the Y-maze (Fig. 4).
Object Recognition Test
In this test, we assessed the effects of mercury on the exploration time of two objects, A and B, and on the recognition index in mercury-treated mice and their controls. The results obtained in these tests showed that the time spent exploring the objects (familiar and novel) differed significantly between the two groups (familiar object, t = − 2.375, p = 0.032, and novel object, t = 2.947, p = 0.026). Indeed, mice treated with mercury chloride had a worse performance in the exploration of the familiar object compared to controls. Moreover, the treated group showed a significantly lower object recognition index compared to the control group (t = 2.156, p = 0.047) (Fig. 5).
Biochemical Assays
Acetylcholinesterase Activity
Among the eight brain structures analyzed in the HgCl2-treated mice and corresponding controls, a significant decrease in AchE activity of 25 to 42% was observed in the olfactory bulb, hypothalamus, hippocampus, mesencephalon, and cerebellum. A marked decrease was observed in medulla oblongata (up to 80%); however, Student’s t-test revealed that the inhibition was only significant in the cerebellum (t = 3.157, p = 0.034) and medulla oblongata (t = 3.915, p = 0.017) after treatment compared to control conditions (Fig. 6). In the cerebral cortex and pons of the HgCl2-exposed mice, the activity of AChE was, in contrast, higher than in controls (t = − 3.333, p = 0.029) and (t = − 2.372, p = 0.077), respectively.
Estimation of Lipid Peroxidation
To estimate lipid peroxidation, MDA activity was measured in eight brain structures, namely, the cerebral cortex, olfactory bulb, hypothalamus, hippocampus, mesencephalon, pons, cerebellum, and medulla oblongata of mice treated with HgCl2 and their corresponding controls. The activity was significantly increased in the treated group in many structures, such as the cerebral cortex (35.096 ± 1.019 vs. 5.609 ± 4.435) (t = − 6.480, p = 0.003), hippocampus (3.33 ± 0.848 vs. 1.154 ± 0.225) (t = − 2.478, p = 0.048), pons (12.691 ± 3.473 vs. 2.117 ± 0.398) (t = − 3.025, p = 0.039), and cerebellum (13.335 ± 4.772 vs. 2.163 ± 0.433) (t = − 2.332, p = 0.050), compared to the control group. The activity was also increased in the mesencephalon but did not reach a significant level (Fig. 7).
Superoxide Dismutase Activity
A significant decrease in SOD activity, a 76% effect, was observed in the hypothalamus (t = 15.130, p < 0.001) and pons (t = 2.362, p = 0.047) of the HgCl2-exposed mice compared with the control mice (Fig. 8a). The other brain structures did not show any significant differences in SOD activity between the control and treated mice.
Glutathione S-Transferase Activity
The activity of glutathione S-transferase (GST) was decreased in all the brain structures analyzed. The largest decreases were observed in the hypothalamus (t = 2.770, p = 0.050) and mesencephalon (t = 2.576, p = 0.042) (with 90 and 80% decreases, respectively), whereas the other structures, notably cerebellum (t = 3.688, p = 0.021), pons (t = 2.548, p = 0.043), and medulla oblongata (t = 2.279, p = 0.045), displayed a maximal decrease of 75% (Fig. 8b). The decreases observed in the hippocampus and cerebral cortex were not statistically significant.
Glutathione Peroxidase Activity
The activity of GPx was characterized by contrasting effects in the different brain structures analyzed. Thus, we noticed a significant increase in the mesencephalon (36%) (t = − 4.446, p = 0.004) and medulla oblongata (17%) (t = − 3.460, p = 0.013), whereas a slight but significant decrease was observed in the pons (19%) (t = 2.484, p = 0.048) and a significant and important decrease was observed in the hypothalamus (64%) (t = 14.251, p < 0.001) (Fig. 8c).
Catalase
The activity of catalase (CAT) showed a dramatic decrease in the majority of brain structures analyzed, e.g., in the hypothalamus and brainstem structures. The highest decrease was observed in the mesencephalon (t = 4.188, p = 0.009), medulla oblongata (t = 4.117, p = 0.015), and hypothalamus (t = 13.092, p = 0.006), with decreases of 90 and 80%, respectively, whereas other structures, i.e., the cerebellum (t = 3.375, p = 0.028) and pons (t = 4.117, p = 0.015), displayed maximal decreases of 75% (Fig. 8d).
Thioredoxin Reductase
After exposure to HgCl2, thioredoxin reductase activity was significantly decreased in the olfactory bulb and hippocampus (t = 7.864, p = 0.01) and strongly downregulated in the hypothalamus (t = 17.536, p < 0.001) (Fig. 8e).
Assessment of Heavy Metal and Mineral Concentrations
Hg, Fe, Cu, Mg, and Zn concentrations in brain tissue were determined by ICP-AES, and the results are shown in Fig. 9a–e. Assessment of mercury (Hg) showed the presence of significantly higher levels in many brain structures of the treated animals compared to the controls (Fig. 9a). HgCl2 ingestion via drinking water resulted in a significant elevation of iron (Fe) in the hypothalamus (t = − 3.321, p = 0.021) and hippocampus (t = − 2.039, p = 0.097) and of copper (Cu) in the hypothalamus (t = − 2.756, p = 0.033), mesencephalon (t = − 2.803, p = 0.031), cerebellum (t = − 2.769, p = 0.032), and medulla oblongata (t = − 2.769, p = 0.096) (Fig. 9b,c). A general increase was also observed for Mg and Zn concentrations in the treated mice, but statistical significance was only observed in the olfactory bulb for Zn (t = − 2.492, p = 0.047) (Fig. 9d,e). Finally, calcium concentrations measured in different brain structures showed a marked tendency to decrease but did not reach statistical significance in the treated mice compared to the control mice (Fig. 10).
Discussion
The aim of the present study was to investigate in adult male mice, the effects of exposure to low doses of HgCl2, given in the drinking water from the perinatal period to adulthood, on behavior and cognition and to explore the mechanistic actions underlying its neurotoxicity by assessing oxidative stress status in multiple major brain structures. Taken as a whole, our results clearly indicated that low HgCl2 exposure throughout life led to functional abnormalities. In a sense, the current investigation attempted to fill a gap in the literature and extend previous findings dealing with neurotoxicological consequences caused by continuous exposure to low doses of inorganic mercury. Mercury neurotoxicity studies and those concerning inorganic mercury, in particular, have used different exposure models with regard to the dose given and the period of treatment (Chehimi et al. 2012; Oliveira et al. 2012). Although highly interesting, those studies only describe a limited period of exposure and do not represent what often occurs in reality. The protocol we used with the continuous administration of low inorganic mercury via alimentation during a great period of adult life reflects common human environmental conditions. The dose we used in our study is not high and is below the lowest observed effect dose (LOEL) (Institóris et al. 2001). From another experimental point, it is interesting to note that the comparison of daily average water intake in both control and treated mice during the experimental period did not show any significant difference, in contrast to previous studies in rats (Chehimi et al. 2012; Mello-Carpes et al. 2013; Peixoto et al. 2007; Oliveira et al. 2012). This suggests the possibility that there was no metallic taste associated with the inorganic mercury at the dose we used, which could have affected the palatability of the HgCl2 solution. This ensures that under our experimental conditions, an appropriate amount of HgCl2 was supplied to the mice. Furthermore, it has been reported that when inorganic mercury is swallowed, up to 40% may enter the body through the stomach and intestines. Immediately after its entry into the body, it enters the bloodstream and moves to many different tissues, including the central nervous system, even though it does not easily pass from the blood into the brain, in contrast to MeHg and metallic mercury vapors, and only long-term oral exposure to inorganic mercury salts causes nervous system damage (Peixoto et al. 2007; Chehimi et al. 2012). Our results strongly indicate that HgCl2 is able to pass the blood brain barrier despite its low lipophilic capacity, as the assessment of the metal concentrations in the brain showed its presence with considerable amounts in different structures. Furthermore, the presence of marked alterations in enzymatic activities as well as in the behavioral tests favors the presence of HgCl2 in the brain. However, it is possible that HgCl2 could reach the CNS after a methylation step. Indeed, previous studies suggested that inorganic mercury can be methylated in the gut lumen prior to absorption (Rudd et al. 1980) and cross the placental barrier (Yang et al. 1996; Chehimi et al. 2012) and are therefore able to act in the CNS.
Our biochemical investigations revealed the presence of significantly higher levels of lipid peroxidation in brain structures, as confirmed by the high concentrations of MDA in the treated mice compared to the controls. Lipid peroxidation is known to affect membrane organelles, particularly mitochondria (Gstraunthaler et al. 1983). MeHg has been reported to increase lipid peroxidation biomarkers, altering mitochondrial permeability and calcium (Ca2+) homeostasis (Yin et al. 2007). The high Ca2+ amounts released by mitochondria promote the release of acetylcholine (Ach) by MeHg, whereas the inhibition of Ca2+ release diminishes the effectiveness of the metal (Yin et al. 2007). It is noteworthy that lipid peroxidation has been reported to be elevated in tissues exposed to anticholinesterase (Milatovic et al. 2006). Given that we found a clear effect of HgCl2 treatment on brain lipid peroxidation and inhibition of AchE activity, we speculate that the low amounts of Ca2+ observed, although the difference between the treated and control mice did not reach statistical significance, would negatively modulate the effect of low dose HgCl2 on ACh release. Our results indicated that exposure to low HgCl2 levels decreased the activity of AChE involved in the degradation of ACh at muscarinic and nicotinic synapses. These data are in line with previous studies documenting the negative modulation of brain AchE activity by hazardous components, such as pesticides, and nonessential heavy metals, such as manganese, lead, and mercury (Goel et al. 2007; Liapi et al. 2008; Franciscato et al. 2009; Santos et al. 2012; Basha and Reddy 2015). This indicates that such a parameter is sensitively affected by low doses of HgCl2. Although no hypercholinergic sign was noticed (i.e., hyperstimulation due to increased acetylcholine), this reduction in AChE activity could be related to abnormal locomotor activity measured in the treated animals. Indeed, previous results also revealed increased locomotor activity in adults exposed to low HgCl2 doses during perinatal and weaning stages (Huang et al. 2011). Our behavioral tests in the open field did not show abnormal locomotor activity, whereas AChE activity was significantly inhibited in the cerebellum, the brain structure involved in reflex adjustments and motor control (Glickstein et al. 2009). These results suggest that chronic exposure to low doses of HgCl2 leads to significant bioaccumulation of the metal in the brain, notably in the cerebellum, at a concentration that is able to alter the enzymatic activity of AChE but is still not sufficient to alter motor function. The assessment of AChE activity has also revealed that this activity could be increased in other brain structures after HgCl2 treatment. This duality of response to HgCl2 has been reported in the brains of zebrafish following the administration of ethanol (Rico et al. 2007). The authors reported that the increase in AChE activity is not directly related to a higher AChE gene expression, and it could be due to an effect of the toxicant on the AChE post-translational modulation (possible changes in phosphorylation state) that in turn regulates its own expression. Taken together, these results suggest that mouse brain AChE activity is differentially altered after chronic exposure to low HgCl2 treatment, which could reveal different molecular mechanisms related to cholinergic signaling in the toxicity of mercury.
The results obtained in the OF also show that mercury induces anxiety, as we measured an increase in the number of crossed squares and time spent in the periphery compared with activity and time in the center. Indeed, a higher time spent in the periphery of the open field is associated with a higher level of anxiety. This demonstrated that the HgCl2-treated mice were more anxious than the control animals in the open field. In this study, in addition to the OF test, we examined the anxiogenic effect of HgCl2 in adult mice by other behavioral tests, such as the EPM test, which is undoubtedly one of the most widely used animal models in contemporary preclinical research on anxiety (Rodgers and Cole 1994; Hogg 1996). Thus, in the EPM test, the treated mice showed significantly higher anxiety-related behavior as the percentages of entries and spent time in the anxiogenic open arms of the maze were significantly higher compared to those in the control group. This anxiogenic effect of HgCl2, even given at low doses, seemed to be specific, as the percentage of entries in the enclosed arms was not different between the controls and treated mice. Equivalent effects have been reported for MeHg and ethanol (Maia et al. 2010). Finally, the fact that the level of grooming, considered an anxiety-related behavior, was significantly higher in treated mice also supports the role of HgCl2 in the genesis of anxiety and therefore confirmed the increase in anxiety level of mercury-exposed animals in the elevated plus maze. Of interest, the degree of social interaction inhibition may be used as an indirect index of defensiveness or anxiety (Blanchard et al. 1998; Bramley and Wass 2001; Mastripieri et al. 1992). Our results showed that mercury contamination decreased social behavior as the treated mice spent significantly less time exploring the second strange mouse compared to the first strange one in the second session of the test.
In addition to these neurobehavioral abnormalities, chronic exposure to HgCl2 has been shown to alter biomarkers of oxidative stress in many organs and in many species (Agarwal and Behari 2007; Branco et al. 2012). Indeed, oxidative stress is strongly linked to the neurobehavioral effects induced by HgCl2 (Mello-Carpes et al. 2013; Teixeira et al. 2014). This is of high interest in the case of the anxiety state observed in the treated mice, as previous data demonstrated the presence of a link between oxidative stress and high anxiety-related behavior (for review, see Bouayed et al. 2009). Moreover, some of these studies provide direct and indirect evidence for a causal link between oxidative stress and anxiety-related behavior (Desrumaux et al. 2005; Hovatta et al. 2005; Souza et al. 2007; Berry et al. 2007). Here, we report on the inhibition of all the activities of the enzymes assessed (SOD, CAT, GPx, ThR, and GST), and among the brain regions analyzed, hypothalamus and, to a lesser degree, brainstem structures, were found to be most affected. The observed inhibition is generally due to the binding of mercury to the active site of each enzyme or to the high concentration of free radicals occasioned by the action of the metal. These observations strongly indicated that HgCl2, administered at low doses but in a continuous manner, was able to alter the balance between oxidative and antioxidant systems. Concerning SOD, it is interesting to note that this enzyme catalyzes the conversion of superoxide radicals to hydrogen peroxide, which is decomposed to water and molecular oxygen, thus preventing the formation of hydroxyl radicals by CAT and GPx. SOD activity, in this study, was generally downregulated in the brain structures analyzed, indicating that the first enzymatic antioxidant defense system was seriously damaged, which can consequently initiate lipid peroxidation as demonstrated by the elevation of MDA levels. This mechanism could result in an elevation of nitric oxide, which is known to damage CNS structures (Songur et al. 2004). On the other hand, Hg exposure is associated with significant alterations of the thioredoxin (ThR) and glutathione-glutaredoxin (GPx) systems. Indeed, it has been reported that Hg disturbs intracellular redox homeostasis with subsequent increased intracellular oxidative stress and dysregulation of vital antioxidant enzymes, including selenoproteins, such as TrxR and GPx. Indeed, via its binding to the selenium (Se) site of these enzymes, Hg inhibits their function (see Spiller 2018, for review). Here, we show for the first time that Hg impairment of antioxidant enzyme activity assessed at the brain level is region-specific, which is probably because these brain regions show different biochemical characteristics (Das et al. 1982). As a complement of these investigations, we also assessed the status of essential heavy metals, such as Fe, Cu, Mg, and Zn. These trace elements are able to bind to mercuric ions or to antioxidant enzymes, playing a protective role against mercury toxicity (Feng et al. 2004). In this study, we found an increase in the concentrations of all the essential metals we analyzed, although to different extents. Fe, Cu, and Mg showed significantly high levels, particularly in the hypothalamus, whereas significantly higher levels of Zn are present principally in the olfactory bulb. The presence of such heterogeneous and specific distributions could reflect the differential action of Hg on the homeostasis of these essential metals. Furthermore, these results are of high interest as, at least of some of them, e.g., Cu and Zn, form a part of metallothionein (MT) that antagonizes heavy metal toxicity (Girardi and Elías 1995), and SOD has a vital role against the lipid peroxidation caused by HgCl2. Taken together, the results concerning enzymatic activities and trace elements show that while a differential modulation of ThxR and GPx was noticed in the different analyzed brain areas, Hg seems to induce a drastic inhibition of their activities (as well as of CAT, SOD, and GST, the other markers of oxidative stress) as well as upregulate the levels of essential trace elements at the hypothalamus level. This striking decrease in the hypothalamus could be related to the fact that Hg accumulated in the hypothalamus after exogenous supplementation and is not rapidly eliminated compared to other brain regions. Supplementation of Se, which antagonizes mercury toxicity with antioxidant effects, failed to reduce inorganic mercury accumulation, whereas it decreased the accumulation of the organic form, MeHg (Branco et al. 2012). This finding reveals that the hypothalamus is more sensitive to Hg compared to other brain regions, which is in line with a subsequent eventual impairment of homeostasis and neuroendocrine/endocrine functions in relation to anxiety-related disorders (Zeng et al. 2007). Thus, previous studies in humans have indicated that exposure to mercury causes enlargement of the thyroid, accompanied by elevated triiodothyronine and thyroxine and low thyroid-stimulating hormone levels (Karpathios et al. 1991; Barregård et al. 1994). Furthermore, Simon et al. (2002) reported an increased prevalence of thyroid disorders in patients with general anxiety disorder and panic disorder. Fischer and Ehlert (2018), in their exhaustive review of the literature, found that the comorbidity between anxiety and thyroid disorders was significant. This prompted them to claim that this is supportive of the recommendation to routinely screen for thyroid disorders when treating patients with anxiety. Finally, they reported that half of the studies supported the notion of subclinical thyroid dysfunction as TSH responses to TRH administration were blunted and an inverse relationship was observed between self-reported anxiety levels and TSH. Interestingly, exaggerated free radical production and oxidative stress levels have been reported in patients with thyroid disorders (Komosinska-Vassev et al. 2000; Bhimte et al. 2012), which could explain the anxiety-related disorder observed, due to impairment of the hypothalamo-pituitary-thyroid axis. Finally, these findings of oxidant stress status at the level of the hypothalamus could explain at least partially the memory impairment observed. Indeed, we noticed a significant decrease in the spatial memory tasks, i.e., the object recognition task and Y-maze test, expressed by a decreased recognition index and percent alternation in the Y-maze. Generally, the results obtained were in concordance with those obtained in adult rats (Mello-Carpes et al. 2013). Thus, it was suggested that mercury indirectly disrupts endocrine function by altering the HPT axis, which can mediate the cognitive impairments caused by mercury (Schantz and Widholm 2001).
In conclusion, our results provide new evidence that continuous exposure to low doses of inorganic mercury throughout life induces lipid peroxidation of organelle membranes and significant changes in the enzymatic activities of oxidative stress that could disturb neuronal cell physiology leading eventually by consequent to neurofunctional abnormalities in adult mice (Fig. 11).
References
Abu Bakar N, Mohd Sata NS, Ramlan NF, Wan Ibrahim WN, Zulkifli SZ, Che Abdullah CA, Ahmad S, Amal MN (2017) Evaluation of the neurotoxic effects of chronic embryonic exposure with inorganic mercury on motor and anxiety-like responses in zebrafish (Danio rerio) larvae. Neurotoxicol Teratol 59:53–61. https://doi.org/10.1016/j.ntt.2016.11.008
Agarwal JR, Behari (2007) Role of selenium in mercury intoxication in mice. Ind Health 45(3):388–395. https://doi.org/10.2486/indhealth.45.388
Asada K, Takahashi M, Nagate M (1974) Assay and inhibitors of spinach superoxide dismutase. Agric Biol Chem 38:471–473. https://doi.org/10.1080/00021369.1974.10861178
Azevedo FB, Barros FL, Peçanha FM, Wiggers GA, Frizera VP, Ronacher SM, Fiorim J, Rossi de Batista P, Fioresi M, Rossoni L, Stefanon I, Alonso MJ, Salaices M, ValentimVassallo D (2012) Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol 949048:1–11. https://doi.org/10.1155/2012/949048
Barregård L, Lindstedt G, Schütz A, Sällsten G (1994) Endocrine function in mercury exposed chloralkali workers. Occup Environ Med 51(8):536–540
Basha CD, Reddy RG (2015) Long-term changes in brain cholinergic system and behavior in rats following gestational exposure to lead: protective effect of calcium supplement. Interdiscip Toxicol 8:159–168. https://doi.org/10.1515/intox-2015-0025
Bernohft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Public Health 460508:1–10. https://doi.org/10.1155/2012/460508
Berry A, Capone F, Giorgio M, Pelicci PG, de Kloet ER, Alleva E, Minghetti L, Cirulli F (2007) Deletion of the life span determinant p66Shc prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp Gerontol 42:37–45. https://doi.org/10.1016/j.exger.2006.05.018
Bhimte B, Agrawal BK, Sharma VK, Chauhan SS (2012) Oxidative stress status in hypothyroid patients. Biomed Res 23:286–288
Blanchard RJ, Hebert MA, Ferrari PF, Palanza P, Figueira R, Blanchard DC, Parmigiani S (1998) Defensive behaviors in wild and laboratory (Swiss) mice: the mouse defense test battery. Physiol Behav 65:201–209
Bouayed J, Rammal H, Soulimani R (2009) Oxidative stress and anxiety. Oxidative Med Cell Longev 2:63–67 http://www.landesbioscience.com/journals/oximed/article/7944
Bramley GN, Wass JR (2001) Laboratory and field evaluation of predator odors as repellents for kiore (Rattus exulans) and ship rats (R. rattus). J Chem Ecol 27:1029–1047
Branco V, Canário J, Lu J, Holmgren A, Carvalho C (2012) Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radic Biol Med 52(4):781–793. https://doi.org/10.1016/j.freeradbiomed.2011.12.002
Buege JA, Aust SD (1984) Microsomal lipid peroxidation. Methods Enzymol 105:302–310
Cernichiari E, Myers GJ, Ballatori N, Zareba G, Vyas J, Clarkson T (2007) The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology 28:1015–1022. https://doi.org/10.1016/j.neuro.2007.02.009
Chehimi L, Roy V, Jeljeli M, Sakly M (2012) Chronic exposure to mercuric chloride during gestation affects sensorimotor development and later behaviour in rats. Behav Brain Res 234:43–50. https://doi.org/10.1016/j.bbr.2012.06.005
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662. https://doi.org/10.1080/10408440600845619
Crawley JN (2004) Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev 10:248–258
Das M, Mukhtar H, Seth PK (1982) Aryl hydrocarbon hydroxylase and glutathione-S-transferase activities in discrete regions of rat brain. Toxicol Lett 13(1–2):125–128
Desrumaux C, Risold PY, Schroeder H, Deckert V, Masson D, Athias A, Laplanche H, Le Guern N, Blache D, Jiang XC, Tall AR, Desor D, Lagrost L (2005) Phospholipid transfer protein (PLTP) deficiency reduces brain vitamin E content and increases anxiety in mice. FASEB J 19:296–297. https://doi.org/10.1096/fj.04-2400fje
Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T (2007) Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci 262:131–144. https://doi.org/10.1016/j.jns.2007.06.036
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ennaceur A, Meliani K (1988) A new one-trial test for neurobiological studies of memory in rats III. Spatial vs. non-spatial working memory. Behav Brain Res 51(1):83–92
Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E (2007) Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem 103(5):1968–1981. https://doi.org/10.1111/j.1471-4159.2007.04882.x
Falluel-Morel A, Lin L, Sokolowski K, McCandlish E, Buckley B, Dicicco-Bloom E (2012) N-acetyl cysteine (NAC) treatment reduces mercury-induced neurotoxicity in the developing rat hippocampus. J Neurosci Res 90:743–750. https://doi.org/10.1002/jnr.22819
Feng W, Wang M, Li B, Liu J, Chai Z, Zhao J, Deng G (2004) Mercury and trace element distribution in organic tissues and regional brain of fetal rat after in utero and weaning exposure to low dose of inorganic mercury. Toxicol Lett 152(3):223–234. https://doi.org/10.1016/j.toxlet.2004.05.001
Fischer S, Ehlert U (2018) Hypothalamic–pituitary–thyroid (HPT) axis functioning in anxiety disorders. A systematic review. Depress Anxiety 35(1):98–110. https://doi.org/10.1002/da.22692
Flohe L, Gunzler WA (1984) Analysis of glutathione peroxidase. Methods Enzymol 105:114–121
Franciscato C, Goulart FR, Lovatto NM, Duarte FA, Flores EM, Dressler VL, Peixoto NC, Pereira ME (2009) ZnCl2 exposure protects against behavioral and acetylcholinesterase changes induced by HgCl2. Int J Dev Neurosci 27:459–468. https://doi.org/10.1016/j.ijdevneu.2009.05.002
Girardi G, Elías MM (1995) Mercuric chloride effects on rat renal redox enzymes activities: SOD protection. Free Radic Biol Med 18:61–66
Glickstein M, Strata P, Voog DJ (2009) Cerebellum: history. Neuroscience 162:549–559
Goel A, Dani V, Dhawan DK (2007) Zinc mediates normalization of hepatic drug metabolizing enzymes in chlorpyrifos-induced toxicity. Toxicol Lett 169:26–33. https://doi.org/10.1016/j.toxlet.2006.07.342
Goulet S, Dore FY, Mirault ME (2003) Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol 25:335–347. https://doi.org/10.1016/S0892-0362(03)00007-2
Gstraunthaler G, Pfaller W, Kotanko P (1983) Glutathione depletion and in vitro lipid peroxidation in mercury or maleate induced acute renal failure. Biochem Pharmacol 32:2969–2972
Hogg S (1996) A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 54:21–30
Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C (2005) Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature 438:662–666
Huang CF, Liu SH, Hsu CJ, Lin-Shiau SY (2011) Neurotoxicological effects of low-dose methylmercury and mercuric chloride in developing offspring mice. Toxicol Lett 201:196–204. https://doi.org/10.1016/j.toxlet.2010.12.016
Institóris L, Siroki O, Undeger U, Basaran N, Dési I (2001) Immunotoxicological investigations on rats treated subacutely with dimethoate, As3+ and Hg2+ in combination. Hum Exp Toxicol 20:329–336
Kang-Yum E, Oransky SH (1992) Chinese patent medicine as a potential source of mercury poisoning. Vet Hum Toxicol 34(3):235–238
Karpathios T, Zervoudakis A, Theodoridis C, Vlachos P, Apostolopoulou E, Fretzayas A (1991) Mercury vapor poisoning associated with hyperthyroidism in a child. Acta Peadiatrica Scand 80:551–552. https://doi.org/10.1111/j.1651-2227.1991.tb11903.x
Komosinska-Vassev K, Olczyk K, Kucharz EJ, Marcisz C, Winsz-Szczotka K, Kotulska A (2000) Free radical activity and antioxidant defense mechanisms in patients with hyperthyroidism due to Graves' disease during therapy. Clin Chim Acta 300:107–117
Liapi C, Zarros A, Galanopoulou P, Theocharis S, Skandali N, Al-Humadi H, Anifantaki F, Gkrouzman E, Mellios Z, Tsakiris S (2008) Effects of short-term exposure to manganese on the adult rat brain antioxidant status and the activities of acetylcholinesterase, (Na,K)-ATPase and Mg-ATPase: modulation by L-cysteine. Basic Clin Pharmacol Toxicol 103:171–175. https://doi.org/10.1111/j.1742-7843.2008.00281.x
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maia C d S, Ferreira VM, Diniz JS, Carneiro FP, de Sousa JB, da Costa ET, Tomaz C (2010) Inhibitory avoidance acquisition in adult rats exposed to a combination of ethanol and methylmercury during central nervous system development. Behav Brain Res 211(2):191–197. https://doi.org/10.1016/j.bbr.2010.03.032
Mastripieri D, Martel FL, Nevison CM, Simpson MJA, Keverne EB (1992) Anxiety in rhesus monkey infants in relation to interactions with their mother and other social companions. Dev Psychobiol 24:571–581
Mello-Carpes PB, Barros W, Borges S, Alves N, Rizzetti D, Peçanha FM, Vassallo DV, Wiggers GA, Izquierdo I (2013) Chronic exposure to low mercury chloride concentration induces object recognition and aversive memories deficits in rats. Int J Dev Neurosci 31:468–472. https://doi.org/10.1016/j.ijdevneu.2013.05.009
Milatovic D, Gupta RC, Aschner M (2006) Anticholinesterase toxicity and oxidative stress. Sci World J 6:295–310. https://doi.org/10.1100/tsw.2006.38
Nagahara AH, McGaugh JL (1992) Muscicimol infused into the medial septal area impairs long term memory but not short-term memory in inhibitory avoidance, water maze place learning and rewarded alternation tasks. Brain Res 591:54–61
Oliveira CS, Oliveira VA, Ineu RP, Moraes-Silva L, Pereira ME (2012) Biochemical parameters of pregnant rats and their offspring exposed to different doses of inorganic mercury in drinking water. Food Chem Toxicol 50:2382–2387. https://doi.org/10.1016/j.fct.2012.04.046
Ornagh IF, Ferrini S, Prati M, Giavini E (1993) The protective effects of N-acetyl-L-cysteine against methylmercury embryotoxicity in mice. Fundam Appl Toxicol 20:437–445
Peixoto NC, Roza T, Morsch VM, Pereira ME (2007) Behavioral alterations induced by HgCl2 depend on the postnatal period of exposure. Int J Dev Neurosci 25:39–46. https://doi.org/10.1016/j.ijdevneu.2006.11.002
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Rico EP, Rosemberg DB, Dias RD, Bogo MR, Bonan CD (2007) Ethanol alters acetylcholinesterase activity and gene expression in zebrafish brain. Toxicol Lett 174(1–3):25–30. https://doi.org/10.1016/j.toxlet.2007.08.005
Riley DM, Newby CA, Leal-Almeraz TO, Thomas VM (2001) Assessing elemental mercury vapor exposure from cultural and religious practices. Environ Health Perspect 109:779–784. https://doi.org/10.1289/ehp.8410
Rodgers RJ, Cole JC (1994) The elevated plus-maze: pharmacology, methodology and ethology. In: Cooper SJ, Hendrie CA (eds) Ethology and psychopharmacology. Wiley, Chichester, pp 9–44
Rudd JW, Furutani A, Turner MA (1980) Mercury methylation by fish intestinal contents. Appl Environ Microbiol 40:777–782
Ryan DM, Sin YM, Wong MK (1991) Uptake distribution and immunotoxicological effects of mercury in mice. Environ Monit Assess 19:507–517
Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Marreilha dos Santos AP (2012) The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 292:90–98. https://doi.org/10.1016/j.tox.2011.11.017
Schantz SL, Widholm JJ (2001) Cognitive effects of endocrine-disrupting chemicals in animals. Environ Health Perspect 109:1197–1206
Simon NM, Blacker D, Korbly NB, Sharma SG, Worthington JJ, Otto MW, Pollack MH (2002) Hypothyroidism and hyperthyroidism in anxiety disorders revisited: new data and literature review. J Affect Disord 69:209–217
Songur A, Sarsilmaz M, Sogut S, Ozyurt B, Ozyurt H, Zararsiz I, Turkoglu AO (2004) Hypothalamic superoxide dismutase, xanthine oxidase, nitric oxide, and malondialdehyde in rats fed with fish omega-3 fatty acids. Prog Neuro-Psychopharmacol Biol Psychiatry 28:693–698. https://doi.org/10.1016/j.pnpbp.2004.05.006
Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, Souza TM, Portela LV, Perry ML (2007) Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci 81:198–203. https://doi.org/10.1016/j.lfs.2007.05.001
Spiller HA (2018) Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin Toxicol (Phila) 56:313–326. https://doi.org/10.1080/15563650.2017.1400555
Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M (2008) Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol 227:147–154. https://doi.org/10.1016/j.taap.2007.10.010
Teixeira FB, Fernandes RM, Farias-Junior PM, Costa NM, Fernandes LM, Santana LN, Silva-Junior AF, Silva MC, Maia CS, Lima RR (2014) Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int J Environ Res Public Health 11:9171–9185. https://doi.org/10.3390/ijerph110909171
Vicente E, Boer M, Netto C, Fochesatto C, Dalmaz C, Rodrigues SI, Gonçalves CA (2004) Hippocampal antioxidant system in neonates from methylmercury-intoxicated rats. Neurotoxicol Teratol 26:817–823. https://doi.org/10.1016/j.ntt.2004.08.003
Yang JM, Jiang XZ, Chen QY, Li PJ, Zhou YF, Wang YL (1996) The distribution of HgCl2 in rat body and its effects on fetus. Biomed Environ Sci 9:437–442
Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, Sidoryk M, Albrecht J, Aschner M (2007) Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res 1131:1–10. https://doi.org/10.1016/j.brainres.2006.10.070
Zeng H, Schimpf BA, Rohde AD, Pavlova MN, Gragerov A, Bergmann JE (2007) Thyrotropin-releasing hormone receptor 1-deficient mice display increased depression and anxiety-like behavior. Mol Endocrinol 21:2795–2804. https://doi.org/10.1210/me.2007-0048
Author information
Authors and Affiliations
Contributions
HM, FC, and MN designed the experiments and performed the analysis of the data; HM, HA, NO, and FZO performed the experiments and assembled the figures. All the authors wrote or edited and validated the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Malqui, H., Anarghou, H., Ouardi, F.Z. et al. Continuous Exposure to Inorganic Mercury Affects Neurobehavioral and Physiological Parameters in Mice. J Mol Neurosci 66, 291–305 (2018). https://doi.org/10.1007/s12031-018-1176-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1176-1