Abstract
The objective of this study is to examine the effect of empagliflozin on cardiac function in rats with chronic heart failure and the possible mechanism. Forty 6-week-old male SD rats were randomly divided into the control group, empagliflozin treatment group, and sham-operated group. SD rats in the control group and empagliflozin treatment group were subjected to ligation of the anterior descending coronary artery to induce an acute myocardial infarction model. SD rats in the sham-operated group were only subjected to threading of the anterior descending branch of the coronary artery without ligation. On the second day after surgery, the control group and sham operation group were given physiological saline by gavage, while the empagliflozin treatment group was given empagliflozin (30 mg/kg/day) by gavage. Sixteen weeks later, cardiac function, intracellular reactive oxygen species (ROS) levels, mitochondrial membrane potential (MMP), serum brain natriuretic peptide, hypersensitive C-reactive protein (hs-CRP), iNOS expression levels, and myocardial morphological changes were observed. Compared with that in the control group, heart function in the empagliflozin-treated group was significantly improved, MMP was increased, intracellular ROS levels were decreased, and NT-proBNP and hs-CRP were significantly reduced, and HE staining showed that the cell oedema was less than that in the control group, tissue arrangement was more orderly, and iNOS expression was inhibited. Empagliflozin can improve cardiac function in rats with chronic heart failure, and the mechanism may involve inhibiting inflammation, reducing myocardial oxidative stress, and improving myocardial fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the ageing of the population in China and the increasing incidence of cardiovascular diseases (CVDs) such as coronary heart disease, atrial fibrillation, and hypertension, the number of patients with heart failure (HF) is increasing yearly. Chronic heart failure (CHF) is the final stage of various heart diseases and the final stage for CVD prevention and control. It is estimated that the current number of CVD patients is 290 million (Hu et al. 2019). At present, there are over 13.7 million HF patients (with a prevalence rate of 1.3%) in China (Hao et al. 2019), resulting in a huge burden on the social and economic health of the country. HF has a high incidence rate and mortality. The 1-year mortality of CHF patients is 7.2%, and the 1-year hospitalization rate is 31.9%, while the mortality and hospitalization rates of acute HF patients are as high as 17.4% and 43.9%, respectively (Murphy et al. 2020). Although significant breakthroughs have been made in the treatment of HF in the past decade, the incidence rate and mortality of HF patients are still high (Simpson et al. 2020; Uchmanowicz et al. 2020). Currently, the treatment of HF remains a major challenge in cardiology.
Diabetes is an independent risk factor for HF. Every 1% increase in glycosylated haemoglobin can increase the incidence rate of HF from 8 to 36%. In recent years, the novel hypoglycaemic drug sodium glucose cotransporter 2 inhibitor (SGLT-2i) has shown significant cardiovascular benefits, and SGLT-2i has been included in the 2021 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. The pathogenesis of HF is relatively complex. At present, treatment of HF has shifted from traditional cardiac strengthening, diuresis, and vasodilation by targeting the body’s haemodynamics to treatment based on neurofumoral mechanisms. Treatment options mainly include renin angiotensin aldosterone system (RAAS) inhibitors, β receptor blockers, aldosterone receptor antagonists, positive inotropic drugs, and diuretics (Packer et al. 2020); previous studies have shown that dapagliflozin reduces blood volume, increases haematocrit, and enhances the oxygen carrying capacity of the body (Testani et al. 2010; Kataoka 2019; Ohara et al. 2019). The DAPA-HF test and the latest EMPEROR Reduced test showed that SGLT-2 inhibitors could reduce the risk of cardiovascular death or the composite event endpoint of hospitalization due to HF in patients with HF with reduced ejection fraction (EF%), whether these patients had diabetes or not (Zannad et al. 2020). In addition, CANTOS research has shown that inflammation is an important cardiovascular risk factor, and anti-inflammatory treatment can improve the prognosis of CVDs (Ridker et al. 2017). However, the mechanism by which empagliflozin can treat CHF is not clear. Thus, we established a CHF rat model by ligating the anterior descending branch of the coronary artery in SD rats to study the effect of empagliflozin on heart function during CHF and the possible mechanisms.
Materials and methods
Groupings and processing
The animal experiment was carried out in strict accordance with the “Regulations on the Management of Experimental Animals” issued by the State Council of the People’s Republic of China. This experiment was approved by the Experimental Animal Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. Male SD rats were provided by Chongqing Medical University and were randomly divided into a control group of 15 rats (AMI + NS), an empagliflozin-treated group of 14 rats (AMI + empagliflozin), and a sham-operated group of 11 rats (sham-operated + NS). The ischaemic HF model was established as previously described (Wu et al. 2014) by ligating the left anterior descending artery in the control group and the empagliflozin-treated group. The sham-operated group was only subjected to threading without ligation at the same site. Postoperative intraperitoneal injection of penicillin (4000 U/day * 3 days) was performed to prevent infection. On the second day after surgery, the control group and sham operation group were given physiological saline by gavage, while the treatment group was given empagliflozin (30 mg/kg/day) by gavage for a total of 16 weeks.
Empagliflozin (10 mg/tablet) was purchased from Shanghai Bollinger Ingerhan Pharmaceutical Co., Ltd. The rat hypersensitive C-reactive protein (hs-CRP) enzyme-linked immunosorbent assay (ELISA) kit was purchased from Shenzhen Zike Biotechnology Co., Ltd. The IP lysis buffer was obtained from Shanghai Biyuntian Biotechnology Co., Ltd., and the N-terminal pro-B-type natriuretic peptide (NT-proBNP) ELISA kit for rats was purchased from Shanghai Xitang Biotechnology Co., Ltd. iNOS antibodies (Beijing Boorsen Biotechnology Co., Ltd.), the HX-200 animal ventilator (Chengdu Taimeng Technology Co., Ltd.), and the Vivid Doppler ultrasound instrument (probe frequency 10 MHz, GE company) were used.
Echocardiographic detection of cardiac function and specimen collection
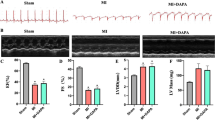
After 16 weeks of gavage, SD rats were intraperitoneally anaesthetized with chloral hydrate, placed on their backs, and fixed on the operating table. The cardiac function of each group of rats was measured by ultrasound. The long axis of the left ventricle was displayed on a two-dimensional ultrasound section, and M-ultrasound was used to measure the left ventricular end diastolic diameter (LVIDd), left ventricular internal diameter (LVIDs), and EF%. After the cardiac function testing, blood was collected from the inferior vena cava, the rats were decapitated, and the heart was removed by thoracotomy. The tissues were washed with physiological saline, and vascular tissue, the atrium, the right ventricle, and fibrotic areas were removed after left ventricular infarction. The remaining noninfarcted myocardium of the left ventricle was prepared into paraffin sections, and the remaining portion was stored in liquid nitrogen for later use. Blood was collected at 950 days, centrifuged for 20 min to extract the supernatant, and stored at − 20 ℃ for future use.
Intracellular reactive oxygen species (ROS) levels and mitochondrial membrane potential (MMP) were measured by flow cytometry
Twenty milligrams of noninfarcted left ventricular myocardial tissue was cut into 1–2 mm fragments with ophthalmic scissors, 2 ml of 0.25% trypsin was added, and the tissue was digested in a 37 °C water bath for 3 min, centrifuged at 500 r for 1 min. Then, 1 ml of foetal bovine serum was added to terminate the digestion. The differential adhesion method was used to separate myocardial cells, and a cell counting plate was used to count the number of myocardial cells. Each group contained 1.0 × 106 resuspended cells suspended in DCFH-DA diluted 1:1000 with serum-free culture medium. A total of 1.0 × 106 resuspended cells were added to 0.5 ml of Rh123 staining solution, and ROS levels and the MMP were detected by flow cytometry.
ELISA analysis of blood BNP levels
The preserved SD rat plasma was tested according to the instructions of the rat BNP kit.
ELISA analysis of blood hs-CRP levels
The preserved SD rat plasma was diluted appropriately and analyzed according to the kit instructions. The absorbance value was measured using ELISA at a wavelength of 450 nm, and a standard curve was drawn using the standard sample provided by the kit. Serum levels of hs-CRP in each group were calculated, and each sample was examined 3 times.
HE staining and immunohistochemical analysis of iNOS expression
Rat myocardial tissue was fixed with 4% paraformaldehyde for 24 h and embedded in paraffin (5 μm) for HE staining. The immunohistochemical steps were carried out according to the kit instructions (Beijing Zhongshan Jinqiao Company). The paraffin sections were dewaxed with xylene and rehydrated through a gradient, and antigen repair was performed with citric acid solution. Goat serum sealing was performed at room temperature for 1 h, and diluted rabbit-derived iNOS polyclonal antibodies (1:250) were added dropwise. The samples were incubated overnight at 4 °C. Goat anti-rabbit secondary antibodies were added the next day. The horseradish enzyme-labelled albumin working solution (S-A/HRP) was incubated at room temperature for 30 min, followed by DAB staining and haematoxylin staining. The film was sealed, and myocardial morphology and iNOS expression were observed under a microscope. The integrated optical density (IOD) of iNOS-positive myocardial cells in each group was measured using Image-Pro Plus (IPP) software to determine the relative protein expression of iNOS.
Statistical analysis
The experimental data were analyzed with SPSS 21.0 statistical software, and the experimental results were subjected to a normal distribution. Indicators with a normal distribution and homogeneity of variance between groups were compared by ANOVA; otherwise, the rank sum test was used. The difference was statistically significant at P < 0.05.
Results
Feeding situation
Eight SD rats in the model group died, and one rat in the sham surgery group died. The causes of death were infection, acute left HF, and malignant arrhythmia. The remaining 31 SD rats reached the experimental endpoint.
Cardiac function test results
At 16 weeks after ligation of the left anterior descending artery, the control group showed a significant increase in LVIDd and LVIDs (P < 0.05) compared with the sham-operated group, while LVEF decreased significantly (P < 0.05). Compared with the control group, the empagliflozin treatment group showed a significant reduction in LVIDd and LVIDs (P < 0.05) and a significant increase in LVEF (P < 0.05), as shown in Table 1 and Fig. 1.
Myocardial ROS and MMP
Compared with the sham surgery group (107.14 ± 4.79), the control group (196.85 ± 12.25) and empagliflozin-treated group (133.16 ± 12.25) showed significant increases in intracellular ROS in myocardial cells (P < 0.05). The empagliflozin treatment group showed a significant decrease compared to the control group (P < 0.05). Compared with that in the sham operation group (104.73 ± 4.31), the MMP in the control group (32.76 ± 7.7) and empagliflozin treatment group (80.21 ± 14.98) decreased. The empagliflozin treatment group showed an increase compared to the control group, as shown in Fig. 2.
Serum BNP levels
Compared to those in the sham surgery group (8.76 ± 0.46 μg/L), serum BNP levels were significantly increased in the control group (22.7 ± 1.23 μg/L) and empagliflozin treatment group (13.5 ± 0.59 μg/L) (P < 0.05). The empagliflozin treatment group showed a significant decrease compared to the control group (P < 0.05).
Serum levels of hs-CRP
Compared to those in the sham surgery group (10.1 ± 0.37 μg/L), serum BNP levels were significantly increased in the control group (29.4 ± 1.02 μg/L) and empagliflozin treatment group (14.3 ± 0.53 μg/L) (P < 0.05). The empagliflozin treatment group showed a significant decrease compared to the control group (P < 0.05).
HE staining and iNOS expression in the myocardium of rats in each group
HE staining showed that in the sham surgery group [percentage of oedematous cells among total cells: (12 ± 0.13)%], the myocardial fibres were arranged neatly, the cytoplasm was rich and uniform, and the nucleus was intact. The control group showed significant myocardial tissue oedema compared to the sham surgery group [percentage: (79 ± 0.53)%] (P < 0.05), and the myocardial fibres were broken and disordered, there was nuclear disappearance, and a large amount of fibrous tissue formed around the infarcted area. Compared with that in the control group, myocardial tissue oedema in the empagliflozin treatment group was significantly reduced [percentage: (31 ± 0.29)%] (P < 0.05). The arrangement was relatively neat, the nucleus was relatively intact, and fibrosis around the infarction was reduced, as shown in Fig. 3.
The expression level of iNOS [IOD (15.67 ± 2.41)] in the sham operation group was very low, while the expression level of iNOS in the control group [IOD (1254 ± 23.18)] and empagliflozin treatment group [IOD 109.83 ± 15.09)] was significantly higher than that in the sham operation group (P < 0.05); the expression level of iNOS in the empagliflozin treatment group was significantly lower than that in the control group (P < 0.05), as shown in Fig. 3.
Discussion
In this study, it was found that empagliflozin had anti-inflammatory effects, reduced myocardial oxidative stress, stabilized cell membrane potential, and inhibited myocardial fibrosis, thereby improving heart function in rats with HF.
Empagliflozin is a novel oral hypoglycaemic drug that is an SGLT2i. SGLT2i drugs mainly inhibit the reabsorption of sodium and glucose by SGLT2 in the renal tubules. They can promote the excretion of sodium in urine while excreting sugar and have an osmotic diuretic effect, thus causing a mild antihypertensive effect (Kalluri et al. 2021). In the reanalysis of the subjects in the EMPEROR Reduced study, the researchers found that when blood pressure was less than 110 mmHg, treatment with empagliflozin did not reduce blood pressure but rather slightly increased blood pressure (Böhm et al. 2021). In this experimental study, I observed that empagliflozin has a mild antihypertensive effect on heart failure rats. However, even if the baseline blood pressure level of heart failure rats is low, empagliflozin treatment is still safe and effective.
According to the previous EMPEROR series of studies, regardless of the eGFR of patients (as low as 20 mL/min/1.73 m2), empagliflozin can reduce cardiovascular events and delay the progression of renal function deterioration, which has shown clear benefits in heart failure and CKD (Böhm et al. 2022).The EMPEROR series studies analyzed the relationship between heart rate (HR) and the outcome of heart failure in patients with LVEF > 40%, as well as the impact of empagliflozin on patients. It is well known that an increased HR means increased sympathetic nervous system excitability and catecholamine levels. There is currently evidence that resting HR is an important predictive factor for cardiovascular complications and mortality, especially in patients with chronic heart failure.The EMPEROR Preserved analysis showed that HR could predict the outcome of heart failure in HFpEF and HFmrEF patients with sinus rhythm (non-atrial fibrillation). While empagliflozin reduced the main outcomes (cardiovascular death and the first incidence of HF), the time of the first occurrence of HF, and the treatment effect of recurrent HF in HFpEF and HFmrEF patients, the treatment had no effect on HR, which indicates that empagliflozin does not activate the sympathetic nervous system while improving the prognosis of heart failure, resulting in a faster HR (Habal et al. 2014).
It has been reported that the blood ketone body concentration is significantly elevated regardless of the presence or absence of diabetes. The mechanism by which SGLT2 inhibitors increase blood ketone body concentrations is related to the following factors: (1) SGLT2 inhibitors cause an increase in glucagon/insulin, leading to the accumulation of ketone bodies in the body. SGLT2 inhibitors directly or indirectly regulate glucagon expression, resulting in the accumulation of ketone bodies. (2) SGLT2 inhibitors promote fatty acid oxidation to produce ketones. (3) SGLT2 inhibitors inhibit renal clearance of ketone bodies. The clearance rate of ketones decreases, and ketones accumulate. (4) The osmotic diuretic effect of SGLT2 inhibitors leads to hypovolemia, and dehydration leads to increased concentrations of lipolytic hormone and increases in the production of ketone bodies (Ferrannini et al. 2014; Bonner et al. 2015; Perry et al. 2019; Yokono et al. 2014; Mende 2022). However, Tang et al. included 10 RCTs involving 13,134 patients and 14 DKA events. Compared with the control group, the risk of DKA did not significantly increase in the SGLT2i group (OR = 1.71, 95% CI 0.56–5.20) (Tang et al. 2016). Therefore, SGLT2i drugs have good safety.
Previous studies have shown that SGLT-2i can significantly reduce the risk of cardiovascular death and hospitalization rate for HF, but the mechanism of action is still not fully understood. Research has shown that oxidative stress is present in almost all forms of CVD and plays a crucial role in energy regulation within myocardial cells. Oxidative stress is defined as a state in which cells and/or the body produce excessive ROS that exceed endogenous antioxidant defence capabilities, thereby damaging proteins, lipids, and DNA. Active oxygen includes superoxide (O-2 ·), hypochlorite (HOCl), and hydrogen peroxide (H2O2). Under normal physiological conditions, the generation of ROS in the heart is minimal, and there is an antioxidant defence system that clears ROS to maintain metabolic balance. However, in response to certain harmful stimuli, cardiac oxidative antioxidant homeostasis is disrupted, and the accumulated O-2 · is highly diffused and damages myocardial cells (Halliwell 2006; Palmieri et al. 2015). In addition, ROS can reduce myocardial cell contraction in a concentration-dependent manner (Kwon et al. 2003; Sabri et al. 1998), leading to a certain degree of cardiac dysfunction (Wu et al. 2019; Canton et al. 2004). This study showed a significant increase in myocardial ROS levels in rats with CHF, confirming that CHF rats were in a state of oxidative stress at this time, while myocardial ROS levels in rats treated with empagliflozin were significantly lower than those in the control group. Oxidative stress plays an important role in the occurrence and development of HF; it can mediate cell proliferation, myocardial remodelling, and myocardial apoptosis by activating various signalling pathways, thereby causing further deterioration of cardiac function. Moreover, the role of mitochondrial dysfunction in CVDs has been fully confirmed. A decrease in the MMP is a sign of early apoptosis. This study showed that the MMP of CHF rats was lower than that of sham rats, the MMP of CHF rats was higher than that of control rats after treatment with empagliflozin, and intracellular ROS levels were lower than those in the control group. These results suggested that empagliflozin could stabilize MMP, improve mitochondrial function, and increase left ventricular EF% in rats with HF by reducing myocardial ROS production. Empagliflozin can reduce myocardial cell apoptosis and improve cardiac function in HF rats. These results are consistent with previous studies showing that empagliflozin reduces oxidative stress and improves cardiac function (Lee et al. 2017; Tanajak et al. 2018).
Inflammation is an important factor in the severity of CHF. The increase in proinflammatory biomarkers in patients with HF is related to disease severity. Inflammatory cytokines not only lead to endothelial dysfunction but also increase the development of myocardial fibrosis (Briasoulis et al. 2016). Previous studies have shown that inflammation plays a crucial role in ischaemic myocardial damage, leading to the deterioration of cardiac structure and function, thereby promoting structural changes and functional decline in ischaemic heart disease (Wilhelmi et al. 2005). Inflammatory reactions are present during the entire occurrence and development of HF in patients. hs-CRP, which is a biomarker of inflammation, was significantly elevated in the serum of CHF rats compared to sham-operated rats. The serum levels of hs-CRP in the empagliflozin-treated group were decreased compared to those in the control group, indicating that empagliflozin has anti-inflammatory effects. Furthermore, this study showed that myocardial fibres in HF rats were disrupted and arranged in a disordered manner, which was accompanied by the disappearance of cell nuclei and the formation of a large amount of fibrous tissue around the infarcted area. After treatment with empagliflozin, myocardial cell tissue oedema in HF rats was significantly reduced, and fibrosis around the infarcted area was reduced, indicating that empagliflozin could inhibit myocardial fibrosis. In addition, studies have shown a significant increase in iNOS levels in the myocardial cells of patients with HF (Umar and Laarse 2010; Li and Olshansky 2011). INOS can catalyze the synthesis of excess NO from L-arginine, which in turn reacts with O2 to form the strong oxidant peroxynitrite ion (ONOO-), which can cause tissue damage and participate in the development of CHF (Giordano 2005). The results of this study showed a significant increase in iNOS levels in the myocardial tissue of HF rats compared to sham-operated rats, which is consistent with the significant increase in iNOS levels in the myocardium of HF patients reported by Umar (Umar and Laarse 2010). However, iNOS levels in the myocardial tissue of HF rats were significantly decreased after treatment with empagliflozin. This finding suggests that empagliflozin can alleviate oxidative stress in CHF rats by downregulating iNOS expression, thereby improving cardiac function in CHF rats.
In summary, empagliflozin ameliorated cardiovascular risk factors and played a role in cardiovascular protection by exerting anti-inflammatory effects, reducing oxidative stress, stabilizing cell membrane potential, inhibiting myocardial fibrosis, and increasing the EF% and other mechanisms. However, its mechanism of action is still not fully understood. We believe that with further research, the mechanism by which empagliflozin affects CHF will be revealed, providing new therapeutic targets for CHF.
Conclusion
This study demonstrated that empagliflozin improves cardiac function in the context of CHF. Its mechanism may be related to inhibiting inflammation, reducing myocardial oxidative stress, and improving myocardial fibrosis.
Data availability
All source data for this study are available upon reasonable request from the authors.
References
Böhm M, Anker SD, Butler J et al (2021) Empagliflozin improves cardiovascular and renal outcomes in heart failure irrespective of systolic blood pressure. J Am Coll Cardiol 78:1337–1348. https://doi.org/10.1016/j.jacc.2021.07.049
Böhm M, Butler J, Filippatos G et al (2022) Empagliflozin improves outcomes in patients with heart failure and preserved ejection fraction irrespective of age. J Am Coll Cardiol 80:1–18. https://doi.org/10.1016/j.jacc.2022.04.040
Bonner C, Kerr-Conte J, Gmyr V et al (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21:512–7. https://doi.org/10.1038/nm.3828
Briasoulis A, Androulakis E, Christophides T et al (2016) The role of inflmmation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev 21:169–176. https://doi.org/10.1007/s10741-016-9533-z
Canton M, Neverova I, Menabo R et al (2004) Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 286:H870–H877. https://doi.org/10.1152/ajpheart.00714.2003
Ferrannini E, Muscelli E, Frascerra S et al (2014) Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124:499–508. https://doi.org/10.1172/JCI72227
Giordano FJ (2005) Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115:500–508. https://doi.org/10.1172/JCI24408
Habal MV, Liu PP, Austin PC et al (2014) Association of heart rate at hospital discharge with mortality and hospitalizations in patients with heart failure. Circ Heart Fail 7:12–20. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000429
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322. https://doi.org/10.1104/pp.106.077073
Hao G, Wang X, Chen Z et al (2019) Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012–2015. Eur J Heart Fail 21:1329–1337. https://doi.org/10.1002/ejhf.1629
Hu ST, Gao RL, Liu LS et al (2019) Summary of the 2018 report on cardiovascular diseases in China. Chin Circ J 34:209–220
Kalluri SR, Bhutta TH, Hannoodee H et al (2021) Do SGLT2 inhibitors improve cardio-renal outcomes in patients with type II diabetes mellitus: a systematic review. Cureus 13:e17668. https://doi.org/10.7759/cureus.17668
Kataoka H (2019) Biochemical determinants of changes in plasma volume after decongestion therapy for worsening heart failure. J Card Fail 25:213–217. https://doi.org/10.1016/j.cardfail.2018.09.014
Kwon SH, Pimentel DR, Remondino A et al (2003) H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol 35:615–621. https://doi.org/10.1016/s0022-2828(03)00084-1
Lee TM, Chang NC, Lin SZ (2017) Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med 104:298–310. https://doi.org/10.1016/j.freeradbiomed.2017.01.035
Li WW, Olshansky B (2011) Inflammatory cytokines and nitric oxide in heart failure and potential modulation by vagus nerve stimulation. Heart Fail Rev 16:137–45. https://doi.org/10.1007/s10741-010-9184-4
Mende CW (2022) Chronic kidney disease and SGLT2 inhibitors: a review of the evolving treatment landscape. Adv Ther 39:148–164. https://doi.org/10.1007/s12325-021-01994-2
Murphy SP, Ibrahim NE, Januzzi JL Jr (2020) Heart failure with reduced ejection fraction: a review. JAMA 324:488–504. https://doi.org/10.1001/jama.2020.10262
Ohara K, Masuda T, Murakami T et al (2019) Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflflozin on flfluid distribution: a comparison study with furosemide and tolvaptan. Nephrology (Carlton) 24:904–911. https://doi.org/10.1111/nep.13552
Packer M, Anker SD, Butler J et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383:1413–1424. https://doi.org/10.1056/NEJMoa2022190
Palmieri V, Innocenti F, Guzzo A et al (2015) Left ventricular systolic longitudinal function as predictor of outcome in patients with sepsis. Circ Cardiovasc Imaging 8:e003865. https://doi.org/10.1161/CIRCIMAGING.115.003865
Perry RJ, Rabin-Court A, Song JD et al (2019) Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor-treated rats. Nat Commun 10(1):548. https://doi.org/10.1038/s41467-019-08466-w
Ridker PM, Everett BM, Thuren T et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131. https://doi.org/10.1056/NEJMoa1707914
Sabri A, ByronK L, Samarel AM et al (1998) Hydrogen peroxide activates mitogen-activated protein kinases and Na+-H+ exchange in neonatal rat cardiac myocytes. Circ Res 82:1053–1062. https://doi.org/10.1161/01.res.82.10.1053
Simpson J, Jhund PS, Lund LH et al (2020) Prognostic models derived in PARADIGM-HF and validated in AT-MOSPHERE and the Swedish Heart Failure Registry to predict mortality and morbidity in chronic heart failure. JAMA Cardiol 5:432–441. https://doi.org/10.1001/jamacardio.2019.5850
Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S et al (2018) Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J Endocrinol 236:69–84. https://doi.org/10.1530/JOE-17-0457
Tang H, Li D, Wang TS et al (2016) Effect of sodium-glucose cotransporter 2 inhibitors on diabetic ketoacidosis among patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 39(8):e123–124. https://doi.org/10.2337/dc16-0885
Testani JM, Chen J, McCauley BD et al (2010) Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 122:265–272. https://doi.org/10.1161/CIRCULATIONAHA.109.933275
Uchmanowicz I, Lee CS, Vitale C et al (2020) Frailty and the risk of all-cause mortality and hospitalization in chronic heart failure:a meta-analysis. ESC Heart Fail 7:3427–3437. https://doi.org/10.1002/ehf2.12827
Umar S, van der Laarse A (2010) Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem 333:191–201. https://doi.org/10.1007/s11010-009-0219-x
Wilhelmi MH, Leyh RG, Wilhelmi M et al (2005) Upregulation of endothelial adhesion molecules in hearts with congestive and ischemic cardiomyopathy: immunohistochemical evaluation of inflammatory endothelial cell activation. Eur J Cardiothorac Surg 27:122–127. https://doi.org/10.1016/j.ejcts.2004.09.027
Wu HQ, Zhu Q, Cai M et al (2014) Effect of inhibiting malonyl-CoA decarboxylase on cardiac remodeling after myocardial infarction in rats. Cardiology 127:236–244. https://doi.org/10.1159/000356471
Wu Y, Yao YM, Lu ZQ (2019) Mitochondrial quality control mechanisms as potential therapeutic targets in sepsis-induced multiple organ failure. J Mol Med (Berl) 97:451–462. https://doi.org/10.1007/s00109-019-01756-2
Yokono M, Takasu T, Hayashizaki Y et al (2014) SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol 727:66–74. https://doi.org/10.1016/j.ejphar.2014.01.040
Zannad F, Ferreira JP, Pocock SJ et al (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396:819–829. https://doi.org/10.1016/S0140-6736(20)31824-9
Funding
This work was supported by Shandong Province Medical and Health Technology Development Plan Project.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Zhenzhen Wang, QianLiu, Xiaofang Wang, Pengpeng Wang, Fenglei Zhang, and Zhuwen Wang. The first draft of the manuscript was written by Zhenzhen Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
The animal experiment was carried out in strict accordance with the “Regulations on the Management of Experimental Animals” issued by the State Council of the People’s Republic of China. This experiment was approved by the Experimental Animal Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Liu, Q., Wang, X. et al. Empagliflozin improves cardiac function in rats with chronic heart failure. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1037–1044 (2024). https://doi.org/10.1007/s00210-023-02655-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02655-7