Abstract
The antidiabetic drug empagliflozin is reported to have many cardioprotective effects. However, no studies have investigated the protective effects of empagliflozin (EMPA) in isoprenaline (ISO)-induced cardiac oxidative damage-a model mimicking the harmful effects of excess catecholamines on the heart. Therefore, in this study, we aimed to reveal the protective effect of EMPA in isoproterenol ISO-induced myocardial infarction in rats. We induced myocardial infarction by subcutaneously injecting ISO (100 mg/kg). To determine the protective effects of EMPA on the myocardial damage, we administered two different doses (10 and 20 mg/kg) by gavage for 14 days. Here we have shown that a 20 mg/kg dose of EMPA completely rescues rats from myocardial infarction by normalizing the following: elevated ST-segment, increased heart rate, decreased R amplitude, prolongation of the QT interval, and shortened RR interval. In addition, EMPA (20 mg/kg) ameliorates ISO-induced changes in serum cTnI, CK, ischemia-modified albumin (IMA), LDH, AST, ALT levels, and heart index. It improves serum lipid profile by decreasing cholesterol, triglycerides, LDL, and VLDL levels, and by increasing HDL levels. Moreover, EMPA (20 mg/kg) alleviates increased myocardial oxidative stress and inflammation by decreasing MDA, TNF-α, and IL-6 levels and increasing SOD and GPx levels. Furthermore, 20 mg/kg EMPA leads to reductions in DNA damage and apoptosis by downregulating of 8-OHdG and caspase-3 expressions. Collectively, EMPA exerts its protective effects on myocardial damage by improving oxidative stress, apoptosis, lipid profile and oxidative DNA damage in ISO-induced experimental myocardial infarction in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Myocardial infarction (MI) is a complex phenomenon that affects the mechanical, electrical, structural, and biochemical properties of the heart (Upaganlawar et al., 2010). MI occurs after prolonged ischemia, inducing the loss of the cardiac myocytes. Myocardial ischemia changes the oxidative status by increasing ROS production leading to structural deformation in proteins, lipids, and DNA (Maneewong et al., 2011). Specifically, to ameliorate the effects of oxidative stress on DNA, certain enzymes scavenge 8-hydroxy-2'-deoxyguanosine (8-OHdG), which is formed as a result of oxidative damage in DNA. 8-OHdG triggers apoptosis and has been used as a biomarker of oxidative DNA damage (Norbury and Zhivotovsky, 2004). Other known enzymes that can be used as biomarkers in cellular damage due to MI and stroke are the caspase enzymes (caspase 3 and 9) (Zhang et al., 2017). Besides the oxidative stress, inflammation is also a crucial factor in the ischemic myocardial tissue (Raish, 2017; Woudstra et al., 2017). MI increases the cytokines and inflammatory cells infiltration into the myocardial tissue, what can be lethal for cardiomyocytes (Sharma and Das, 1997).
ECG recording is an important tool and has been used in the diagnosis of MI. Rats with MI have elevated ST-segments and decreased R wave amplitudes, and these changes may reflect a myocardial lesion (El-Gohary and Allam, 2017). On the other hand, creatine kinase-MB (CK-MB), creatinine kinase (CK), lactate dehydrogenase (LDH), and cardiac troponin T (cTnT) or I (cTnI) are conventional sensitive and specific cardiac markers for the detection of myocardial necrosis (Chawla et al., 2006; Gaze, 2009). In addition to ECG, ischemia-modified albumin (IMA), lipid profile including total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL) have been linked to the development of MI (Chawla et al., 2006; Gaze, 2009).
Isoprenaline/Isoproterenol (ISO), a synthetic catecholamine and beta-adrenergic agonist, causes severe stress in the myocardium, resulting in infarction such as heart muscle necrosis in the experimental animal models. ISO-induced MI is a well-standardized model and pathophysiological changes in cardiac muscle are similar to the MI in humans (Panda and N, 2009). ISO injection stimulates auto-oxidation and lipid peroxidation, leading to the generation of free radicals, which cause destruction and damage in the myocardial cell membrane (Loh et al., 2007; Raish, 2017). Furthermore, ISO reduces the activities of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase (Tang et al., 2015). ISO-induced oxidative stress leads to an increase in the production of pro-inflammatory cytokines such as TNF-α and IL-1β (Abdelzaher et al., 2021). ISO also increases the expressions of myocardial pro-apoptotic (e.g. Bax, caspase 3, caspase 9, cytochrome C, and p53) and anti-apoptotic (e.g. Bcl-2) signaling proteins in rats (Radhiga et al., 2012; Othman et al., 2017).
Sodium-glucose cotransporter-2 (SGLT2) reabsorbs approximately 90% of glucose from the blood into the renal tubules. Blockade of SGLT2 prevents the absorption of glucose and Na+ from the kidneys and causes glucose excretion in urine. Recent clinical studies have shown that SGLT2 inhibitors prevent cardiovascular disorders, especially heart failure due to type-2 diabetes (Zinman et al., 2015; Neal et al., 2017). Empagliflozin (EMPA) (trade name Jardiance®) is a new generation of selective SGLT2 inhibitor, which is used for the treatment of type-2 diabetes, and it was approved by the FDA in 2014 (Fala, 2015). Although SGLT2 receptors are not present in cardiac tissue, EMPA directly inhibits the Na+–H+ converters in rabbit myocytes, consequently, increasing mitochondrial calcium concentration and decreasing intracytoplasmic calcium concentration. This ultimately protects myocytes against calcium toxicity that causes heart failure (Baartscheer et al., 2017). The antioxidant, anti-inflammatory, and vasculoprotective effects of EMPA have been demonstrated in a type-1 diabetic rat model (Oelze et al., 2014). Kusaka et al. (2016) reported that EMPA improves cardiac remodeling and alleviates cardiac oxidative stress independently of its effect on blood pressure. However, the exact mechanism is not fully understood. Verma et al. (2018) reported that EMPA improves the energy status of the heart by increasing ATP production in myocardial cells in db/db mice. EMPA also improves myocardial functions, reduces the myocardial infarction area, and alleviates oxidative stress by reducing lipid peroxidation and iNOS expression in type-2 diabetic mice that are subjected to ischemia/reperfusion (Andreadou et al., 2017). After myocardial infarction, induced by coronary ligation, 10 mg/kg dose of EMPA increases the expressions of SOD2, catalase, and the use of ketone bodies in the non-infarct area in rats with type-2 diabetes (Oshima et al., 2019). In addition, EMPA improves the number and size of mitochondria after coronary ligation in diabetic rats (Mizuno et al., 2018).
Although the cardioprotective properties of EMPA are known, to the best of our knowledge, no study has experimentally examined its protective efficacy in ISO-induced myocardial infarction in rats. In this study, we aimed to investigate the protective effects of EMPA in the ISO-induced rat myocardial infarction model with regard to oxidative stress, inflammation, apoptosis, biochemical heart function markers, and ECG changes.
MATERIALS AND METHODS
Experimental Design and Animals
The study was approved by Sivas Cumhuriyet University Animal Experiments Local Ethics Committee (approval no. 65202830-050.04.04-293). The experiments were performed under normal experimental settings at the Sivas Cumhuriyet University Faculty of Medicine Experimental Animals Laboratory (light/dark cycle of 12/12 h with 50% humidity, 22°C ambient temperature). Rats received standard rat chow (Bil-Yem, Nükleon®) and tap water ad-libitum. Two-and-a-half-month-old male Wistar Albino rats, weighing 200–250 g, were randomly divided into four equal groups (n = 6) after an adaptation period of one week.
(1) Control: The rats in the control group were administered 0.5% DMSO by oral gavage for 14 days, followed by 1 mL saline subcutaneously on days 13 and 14.
(2) ISO: The rats in this group received 0.5% DMSO by oral gavage for 14 days. In addition to this, isoproterenol hydrochloride (100 mg/kg) (Sigma Aldrich, USA) was given subcutaneously on days 13 and 14 (Panda et al., 2017).
(3) ISO + EMPA10: The rats in this group were administered 10 mg/kg of Empagliflozin (BLD pharm, Shanghai, China) dissolved in 0.5% DMSO by oral gavage for 14 days. Isoproterenol hydrochloride (100 mg/kg) (Sigma Aldrich, USA) was also administered subcutaneously on days 13 and 14.
(4) ISO + EMPA20: The rats in this group were administered 20 mg/kg of Empagliflozin by oral gavage for 14 days. All of the other interventions were done as described in group 3.
ECG Recording
ECG was recorded with alligator clips in Einthoven mode using Televet II ECG equipment (Kruuse, Germany) 24 h after the last isoproterenol hydrochloride administration under Ketamine (60 mg/kg-Alfamine®, Ege Vet, İzmir)/Xylazine (10 mg/kg-Xylazin Bio 2%, Bioveta, Czech Republic) anesthesia. ECG traces were recorded for 5 min. Televet 100 (Version® 7.0.0, Kruuse, Heusenstamm, Germany) was used to evaluate lead II trace the R wave amplitude, ST segment, QT interval, R-R interval, and heart rate (Çetin, 2019; Soraya et al., 2012).
Determination of Serum Myocardial Injury Markers and Lipid Profile
Blood was collected by cardiac puncture under deep anesthesia after the ECG recording. The blood samples were centrifuged at 3500 rpm for 10 min at 4°C and serum was then decanted. In the collected serum samples, cardiac Troponin I (cTnI), lactate dehydrogenase (LDH), and Serum Ischemia Modified Albumin (Serum IMA) were measured with commercial ELISA kits (Bioassay Technology Laboratory, Shanghai, China). Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), triglyceride, cholesterol, LDL, HDL, and VLDL were also measured with an autoanalyzer (Mindray BS 200). Following blood collection, the thoracic cavity was opened, and the heart was removed. Blotting paper was used to remove extra fluids from the heart samples and washed with physiological saline. “Heart weight/Body weight ratio” was calculated with the following formula: Ratio = Heart weight/Body weight × 100.
Determination of Myocardial MDA, SOD, and GPx Levels
Half of the heart was transferred to 10% formaldehyde for immunohistochemistry analysis after the whole heart was weighed. The remaining half-heart samples were weighed and kept at –20°C for further analyses of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx). The collected tissue samples were homogenized using Bead Blaster™ 24 in chilled phosphate-buffered saline (PBS, pH 7.4) for MDA analysis (9 mL of PBS was added to 1 g of tissue). Determination of tissue MDA levels was performed spectrophotometrically as described by Ohkawa et al. (1979). It is based on the 532 nm spectrophotometric analysis of the pink-colored complex produced by MDA, a subsequent product of lipid peroxidation, and TBA following incubation of tissue homogenate in a boiling water bath for one hour under aerobic conditions and pH 3.5.
SOD activity was measured using the ELISA kit (Cayman Chemical Co., Ann Arbor, USA) according to the manufacturer’s protocol. Myocardial tissues were homogenized in 20 mM N-2 hydroxyethyl piperazine-N'-2-ethane sulfonic acid (HEPES buffer), 1 mM ethylene glycol tetra-acetic acid, 210 mM mannitol, and 70 mM sucrose, pH 7.2. The homogenate was centrifuged at 10 000 g for 15 min at 4°C and the supernatant was collected. SOD activity was determined at 450 nm, based on the principle of using tetrazolium salt to detect hypoxanthine and superoxide radicals formed by xanthine oxidase.
For GPx measurement, mycocardial tissues were homogenized in cold buffer at pH 7.5 containing 50 mM Tris-HCl, 5 mM EDTA, and 1 nM dithiothreitol. The homogenate was centrifuged at 10 000 g for 15 min at 4°C. The supernatant was removed. GPx activity was determined ELISA kit (Cayman Chemical Company, USA) according to the manufacturer’s protocol. Total protein determination was determined by an autoanalyzer (Mindray BS 200).
Determination of Myocardial TNF-α and IL-6 Levels
Myocardial tissue was homogenized in chilled PBS (pH 7.4) by Bead Blaster™ 24, centrifuged at 2000–3000 rpm at 4°C for 20 min, and the supernatant was obtained. Tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) levels were determined from the obtained supernatants by ELISA kits (Bioassay Technology Laboratory, Shanghai, China).
Myocardial Immunohistochemistry Analyses
The heart tissue samples were placed in 10% formaldehyde and fixed for at least 48 hours. The samples were then passed through a serial alcohol series and embedded in paraffin blocks. Streptavidin-biotin-peroxidase complex technique was applied to the sections taken from the tissue blocks. Primary antibodies were diluted with phosphate-buffered saline (PBS, pH 7.4) according to the manufacturer’s recommendations and our preliminary studies. The kit (Zymed, Histostain Plus Kit, California, USA) was used for the immunohistochemistry (IHC) staining based on SABK technique. Accordingly, the sections were placed on slides coated with acetone-3-ethoxypropylamine (Merck, catalog no: 8.21619, 100 mL acetone, 2 mL 3-ethoxypropylamine) and dried in an oven at 58°C for 30 min. The sections were then deparaffinized in xylol and dehydrated in serial alcohols. Antigen retrieval was done by boiling the sections in a citrate-buffered solution in a microwave oven at 600 W for 20 min. Sections were kept in 3% H2O2 prepared in methanol for 10 min to remove endogenous peroxidase activity and kept in protein-blocking serum for 10 min. They were then incubated at 4°C with anti-8-OHdG (1/100, Santa-Cruz, sc-393871) and anti-caspase 3 (1/100, Santa-Cruz, sc-7272) primary antibodies for overnight. After the incubation, the sections were washed and incubated with streptavidin peroxidase enzyme (Histostain-Plus Kits, California, USA) for 30 min by dripping biotin-labeled secondary antibody. After washing twice with PBS for 5 min each, the sections were stained with 3-amino-9-ethyl carbazole (AEC) (Zymed AEC RED substrate kit, USA) chromogen for 10 min. Counterstaining was done with Gill’s hematoxylin. Sections were sealed with water-based adhesive (Shandon Immu-mount) and examined under a light microscope (Nikon, YS 100).
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS for Windows; version® 26.0) package program. The normal distribution of the data was tested using the Shapiro–Wilk tests. The data collected from serum and tissue samples were evaluated using a one-way ANOVA followed by post hoc Tukey HSD test. These data are shown as mean ± standard error of means (sem). The data collected from immunohistochemistry staining were evaluated using Kruskal–Wallis analysis of variance. Post hoc multiple comparisons were done with the Bonferroni-corrected Mann–Whitney U test. The data collected from immunohistochemical analyses are shown as median and interquartile range (IQR). P values less than 0.05 were considered statistically significant.
RESULTS
No mortality was observed in the present study with the applied protocol.
Empagliflozin Rescues Rats from Defective Effects of ISO on ECG Parameters
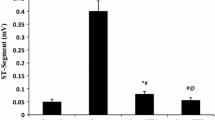
ECG recordings are shown in Fig. 1. ISO injection dramatically elevated the ST segment (P < 0.001) and decreased the R wave amplitude (P < 0.001) which proves that the MI model was successfully established. The ISO administration also increased the heart rate (P < 0.05), QT interval (P < 0.001), and the heart index (P < 0.001), whereas it significantly decreased the RR interval (P < 0.001). A dose of 20 mg/kg EMPA protected rats from ISO-induced disturbances such as elevated ST segments, increased heart rate, decreased R amplitude, increased QT interval, decreased RR interval, and increased heart index (P < 0.05). However, 10 mg/kg dose of EMPA did not cause significant changes (P > 0.05) (Fig. 2).
The effect of empagliflozin on ECG parameters and heart index. Changes in (a) ST segment elevation, (b) R amplitude, (c) Heart rate, (d) QT interval, (e) RR interval, (f) Heart index. Data are expressed as mean ± standard error. * P < 0.05, ** P < 0.01, *** P < 0.001; compared to the control group, # P < 0.05, ## P < 0.01, ### P < 0.001; compared to the ISO group is statistically significant using one-way ANOVA post hoc Tukey HSD test.
Empagliflozin Protects Heart from Myocardial Injury
ISO significantly increased markers associated with serum myocardial injury such as cTnI, LDH, CK, IMA, AST, and ALT (P < 0.05). However, 20 mg/kg dose of EMPA significantly rescued elevated serum cTnI, LDH, CK, IMA, AST, and ALT levels (P < 0.05). The effect of 10 mg/kg EMPA administration was not statistically significant (P > 0.05) (Fig. 3).
The effect of empagliflozin on markers of myocardial damage. Changes in (a) cTnI, (b) CK, (c) IMA, (d) LDH, (e) AST, (f) ALT. Data are expressed as mean ± standard error. * P < 0.05, ** P < 0.01, *** P < 0.001; compared to the control group, # P < 0.05, ## P < 0.01, ### P < 0.001; compared to the ISO group is statistically significant using one-way ANOVA post hoc Tukey HSD test.
Empagliflozin Normalizes the Impaired Lipid Profile
ISO administration markedly changed the serum lipid profile including triglyceride, cholesterol, LDL, and VLDL levels (P < 0.05). Moreover, ISO reduced the serum HDL level (P < 0.01). However, 10 mg/kg dose of EMPA significantly improved serum triglyceride, HDL, and VLDL levels (P < 0.01), but not cholesterol and LDL levels (P > 0.05). Notably, 20 mg/kg dose of EMPA protected the serum lipid profile changes such as increased serum triglyceride, cholesterol, LDL, and VLDL levels (P < 0.05), but decreased HDL levels (P < 0.001) (Fig. 4).
Effect of empagliflozin on serum lipid profile. Changes in (a) Triglyceride, (b) Cholesterol, (c) LDL, (d) HDL, (e) VLDL. Data are expressed as mean ± standard error. * P < 0.05, ** P < 0.01, *** P < 0.001; compared to the control group, # P < 0.05, ## P < 0.01, ### P < 0.001; compared to the ISO group is statistically significant using one-way ANOVA post hoc Tukey HSD test.
Empagliflozin Protects Myocardium from Lipid Peroxidation and Inflammation
Figure 5 shows the effect of EMPA on myocardial lipid peroxidation and inflammation in ISO-induced MI. The ISO administration dramatically increased MDA, TNF-α, and IL-1β levels in myocardial tissue, and considerably reduced SOD and GPx levels (P < 0.05). However, MDA levels were significantly improved by 10 mg/kg dose of EMPA (P < 0.05). In addition, 20 mg/kg dose of EMPA rescued rats from the decreased levels of SOD, GPx, TNF-α, and IL-1β (P < 0.05), while 10 mg/kg EMPA did not cause statistically significant changes (P > 0.05).
Effect of empagliflozin on oxidative stress and inflammation. Changes in myocardial tissue levels of (a) MDA, (b) SOD, (c) GPx, (d) TNF-α, and (e) IL-6. Data are expressed as mean ± standard error. * P < 0.05, ** P < 0.01, *** P < 0.001; compared to the control group, # P < 0.05, ## P < 0.01, ### P < 0.001; compared to the ISO group is statistically significant using one-way ANOVA post hoc Tukey HSD test.
Empagliflozin Improves DNA Damage and Apoptosis
The control rats showed no 8-OHdG expression (Fig. 6a), but the rats received ISO had abundant 8‑OHdG expression (Fig. 6b). In addition, the intensity of 8-OHdG IHC-staining in rats received 10 mg/kg EMPA was similar to the ISO group (Fig. 6c). However, the 8-OHdG IHC-staining intensity was significantly reduced in rats that received 20 mg/kg EMPA compared to the rats that received ISO only (P < 0.05) (Figs. 6c, 6d, Table 1). No caspase 3 expression was observed in the control rats (Fig. 7a) while intense caspase 3 expression was detected in the myocardial tissues of ISO-treated rats (Fig. 7b). The expression of caspase 3 in myocardial tissue of rats that received 10 mg/kg EMPA was similar to that of the rats in ISO group (Fig. 7c). However, the 20 mg/kg EMPA dose significantly reduced caspase 3 expression compared to the rats in ISO group (P < 0.01) (Fig. 7d, Table 1).
DISCUSSION
In experimental rodent studies, isoproterenol is frequently used to induce myocardial infarction in order to investigate different cardioprotective agents. High doses of isoproterenol cause myocardial necrosis, ischemia, hypoxia, and hyperplasia and these findings are similar to the clinical findings of myocardial infarction seen in humans (Panda and N, 2009). Empagliflozin, a new generation selective sodium-glucose co-transporter 2 (SGLT2) inhibitor, was found to exhibit preventive effects in a rat myocardial infarction model induced by isoproterenol. Empagliflozin (20 mg/kg) significantly protected myocardial infarction by reducing oxidative stress, DNA damage, inflammation, and apoptosis, and improving myocardial markers.
Myocardial lesions induced by isoproterenol have been well-characterized in rats for over 50 years (Rona et al., 1959; Hasan et al., 2020). The literature has recently focused on the isoproterenol model recapitulating some salient features of Type 2 MI (T2MI) (Forte et al., 2021). The harmful effects of excess catecholamines on the heart are caused by impaired diastolic function, tachycardia, and tachyarrhythmia, resulting in an imbalance between myocardial oxygen supply and demand and dysregulation of ryanodine receptor 2 (RyR2) and calcium utilization (Ellison et al., 2007). These are all contributing factors to cardiomyocyte necrosis and acute myocardial injury, and the resulting degree and pattern of myocardial necrosis, inflammatory infiltration, and replacement fibrosis are reminiscent of the myocardial injury induced by T2MI in humans (Forte et al., 2021). Recent clinical studies have shown that SGLT2 inhibitors prevent cardiovascular disorders, especially heart failure due to Type-2 diabetes (Zinman et al., 2015; Neal et al., 2017). It is clear that the effect of EMPA on outcomes used prophylactically in ISO-induced MI in rats is consistent with its curative effect on T2MI-induced cardiovascular complications in humans and may reveal possible mechanisms (Zinman et al., 2015).
It has been shown that cTnI is a powerful biomarker for the sensitive and specific detection of heart damage caused by various reasons in laboratory animals (O’Brien et al., 2006; Frobert et al., 2015). cTnI, which is specific for the heart muscle, is released into the circulation 6–8 h after myocardial damage, peaks at 12–24 h, and remains elevated for 7–10 days in humans (Tucker et al., 1997). High troponin levels and increase in the activities of CK, LDH, AST, ALT enzymes indicate the risk of cardiac damage and subsequent infarction (Lobo Filho et al., 2011; Li et al., 2012) which is consistent with our study as evidenced by the fact that rats with ISO-induced myocardial infarction have higher cTnI levels than those of other groups. Importantly, the administration of 20 mg/kg EMPA protected rats from increased serum cTnI levels. EMPA (20 mg/kg) also protected other important markers of myocardial infarction (CK, AST, ALT, and LDH) from ISO’s negative effects on myocardium. Overall, the data suggest that EMPA (20 mg/kg) has important preventive action on myocardial tissue by restoring cellular integrity in rats with ISO-induced myocardial infarction.
The IMA test can reliably detect the presence of ischemia before necrosis develops (Uygun et al., 2011) as serum IMA levels have been found to increase in acute ischemia conditions such as myocardial infarction (Ertekin et al., 2013; Toker et al., 2013), indicating that IMA might be used as a diagnostic marker. Indeed, the increase in ISO-induced serum IMA levels confirms this (Fig. 3). Notably, 20 mg/kg dose of EMPA prevents rats from high IMA levels (Fig. 3), suggesting that EPMA (20 mg/kg) restores the low binding capacity of cobalt ions to albumin (Gurumurthy et al., 2014).
Lipid parameters may be useful in determining risk factors for cardiovascular diseases. Hyperlipidemia and hypercholesterolemia are risk factors for developing heart failure (Nagoor Meeran et al., 2012). It has previously been reported that ISO administration results in high lipid profile in an experimental model of ISO-induced myocardial infarction in rats (Farvin et al., 2006). The mechanism for this is that ISO leads to an increase in cAMP formation by activating adenylate cyclase. cAMP induces lipolytic activity resulting in the hydrolysis of stored triacylglycerol, thus causing hyperlipidemia (Li et al., 2021). Consistent with studies mentioned previously, we found that ISO treatment increased blood levels of cholesterol, triglyceride, LDL, and VLDL, but reduced HDL levels, suggesting that EMPA (20 mg/kg) protects heart from the development of myocardial infarction by improving high lipid profile which further improves the membrane integrity in the myocardial tissue.
The elevation in ST-segment is the best reflection of ECG characteristics in the diagnosis of acute myocardial infarction in humans and animals (Çetin, 2019). In addition, the reduced R wave amplitude together with ST-segment elevation may be attributable to ISO-induced myocardial necrosis because the elevated ST-segment reflects the potential difference at the border between ischemic and non-ischemic regions and the consequent loss of cell membrane function, while the decrease in R wave amplitude might be related to the onset of myocardial edema associated with oxidative damage (Patel et al., 2010). Our data show that pretreatment of 20 mg/kg EMPA markedly reduces the ST-segment elevation and increases the R wave amplitude in rats with ISO-induced myocardial infarction, suggesting that EMPA exerts cell membrane protective effects by scavenging free radicals. ISO exerts its activity through both adrenoceptors 1 and 2 which leads to positive inotropic and chronotropic effects (Prince, 2011). We found that the administration of ISO significantly increased heart rate which is consistent with a previous report (Patel et al., 2010). The increase in heart rate may be related to the positive chronotropic effects of ISO and insufficient blood flow to the heart (Patel et al., 2010; Prince, 2011). EMPA (20 mg/kg) administration protected the heart from tachycardia likely via its action on adrenoreceptors (Prince, 2011).
Oxidative stress and reactive oxygen species (ROS) play an important role in the pathogenesis and development of myocardial infarction (Dhalla et al., 2010). During the oxidation of catecholamines, oxidant radicals are formed, which produce oxidative stress and cardiotoxic effects. The overproduction of ROS significantly disrupts the balance between the antioxidant defense system and ROS. Superoxide radicals are produced in the damaged area of the myocardium causing a reduction in the activity of antioxidant enzymes and accumulation of superoxide anion, which further damages myocardial cells (Searle and Willson, 1980). GPx, one of the antioxidant enzymes linked to GSH, protects the cell and subcellular membranes from peroxidative damage by eliminating hydrogen peroxide and lipid peroxides. Inhibition of this enzyme leads to the accumulation of these oxidants and makes myocardial cell membranes more susceptible to oxidative damage (Wattanapitayakul and Bauer, 2001). The activities of SOD and CAT are decreased by ISO administration. The decrease in the activities of these enzymes in ISO-induced myocardial damage may be due to the increased production of reactive oxygen radicals such as superoxide and hydrogen peroxide (Karthikeyan et al., 2007; Li et al., 2012). ISO also increases the MDA level in the heart tissue and decreases the levels of antioxidant enzymes (Oktar et al., 2010). In a previous experimental study, it was revealed that EMPA improves the increased MDA levels in damaged kidney tissue and the decreased SOD and GPx activities (Ashrafi Jigheh et al., 2019). Consistent with these studies, our data indicate that EMPA (20 mg/kg) administration increases the levels of antioxidant enzymes (SOD and GPx) and decreases MDA levels which suggests EMPA protects heart from the harmful effects of free radicals in ISO-induced rats.
Previous studies have reported that inflammatory responses together with oxidative stress play an important role in myocardial infarction (Kumar et al., 2016; Thangaiyan et al., 2020). It has been shown that excess proinflammatory cytokines such as TNF-α and IL-6 are responsible for myocardial damage, which is critically dependent on NF-kB activation. In normal cells, NF-κB activation is inhibited due to tight control of the inhibitory kappa B (κB) family. During pathophysiological conditions, IκB-α is phosphorylated and degraded by IjB kinase (IKK) enzymes. Thus, IκB loses control over NF-κB, which allows for NF-κB activation and translocation from the cytosol to the nucleus. NF-κB binds to the promoter region of target genes and causes the translation of inflammatory cytokines (Karin and Delhase, 2000). In ISO-induced rat myocardial infarction, TNF-α, IL-6, and IL-1β levels are increased by ISO (Li et al., 2021) which is consistent with our results. In this study, EMPA significantly decreased TNF-α and IL-6 levels in heart tissue and exerted its protective effects on the heart in rats with ISO-induced myocardial infarction which might be due to its anti-inflammatory effect on the NF-κB pathway (Zhang et al., 2020).
It has been suggested that the increased heart weight seen in ISO-induced rats may be related to increased water content, edematous intramuscular space, and severe necrosis of cardiac muscle fibers, followed by inflammatory cell invasion of injured tissues (Patel et al., 2010). In our study, ISO significantly increased the heart index in line with previous studies (Patel et al. 2010; Li et al., 2012). Apoptosis has been proposed as the cause of cardiac tissue destruction in myocardial infarction. Furthermore, oxidative stress increases myocardial infarction, beginning the apoptosis process by activating the mitochondrial apoptosis pathway via Bax protein and caspase enzyme such as caspase 3 (Zhang et al., 2017). Caspase 3 expression was shown to be higher in ISO-induced MI in a previous study (Zhang et al., 2017). Consistent with these findings, ISO enhanced myocardial caspase 3 expression in this study. It has been reported that EMPA reduces caspase 3 expression in heart tissue in ISO-induced MI model (Widyaningsih et al., 2017). Similar to this, we also found that administration of 20 mg/kg EMPA protects myocardial cells from apoptosis by decreasing the expression of caspase 3.
8-OHdG is a very specific and sensitive indicator of the degree of oxidative stress-related DNA damage (Fiordaliso et al., 2006). Patients with heart failure have higher levels of 8-OHdG in serum and myocardium biopsy samples (Kono et al., 2006). In addition, a study (Rani et al., 2015) reported that serum 8‑OHdG level increases in the ISO-induced rat MI model. In rats with ISO-induced MI showed increased myocardial 8-OHdG expression that indicates high DNA damage in the myocardium. However, EMPA (20 mg/kg) administration improved myocardial 8-OHdG expression. This is consistent with a previous study showing that EMPA alleviates the increased cerebral 8-OHdG level in obese and type 2 diabetic mice (Lin et al., 2014).
Study Limitations
There are several limitations in this study. First, inhibition of SGLT2 was not proved. This is the main limitation of the study even though previous studies have shown that inhibition of SGLT2 and the beneficial effects of SGLT2 inhibitors on heart failure in patient with or without diabetes (Verma et al., 2018; Savarimuthu and Harky, 2022). Second, echocardiography and hemodynamic parameters could have been considered as other important variables for the heart function. Third, mitochondrial functions might have been analyzed to monitor high-energy phosphate production and increased ROS production.
CONCLUSIONS
EMPA has important protective effects on heart by improving myocardial cell contractility, lipid profile, cardiovascular markers, IMA levels, oxidative stress, inflammation, apoptosis, and DNA damage.
REFERENCES
Abdelzaher, W.Y., Ahmed, S.M., Welson, N.N., Alsharif, K.F., Batiha, G.E.S., and Labib, D.A.A., Dapsone ameliorates isoproterenol-induced myocardial infarction via Nrf2/ HO-1; TLR4/TNF-α signaling pathways and the suppression of oxidative stress, inflammation, and apoptosis in rats, Front. Pharmacol., 2021, vol. 12, p. 669679.
Andreadou, I., Efentakis, P., Balafas, E., Togliatto, G., Davos, C.H., Varela, A., Dimitriou, C.A., Nikolaou, P.-E., Maratou, E., Lambadiari, V., Ikonomidis, I., Kostomitsopoulos, N., Brizzi, M.F., Dimitriadis, G., and Iliodromitis, E.K., Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects, Front. Physiol., 2017, vol. 8, p. 1077.
Ashrafi Jigheh, Z., Ghorbani Haghjo, A., Argani, H., Roshangar, L., Rashtchizadeh, N., Sanajou, D., Nazari Soltan Ahmad, S., Rashedi, J., Dastmalchi, S., and Mesgari Abbasi, M., Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis, Iran. J. Basic Med. Sci., 2019, vol. 22, pp. 384–390.
Baartscheer, A., Schumacher, C.A., Wüst, R.C.I., Fiolet, J.W.T., Stienen, G.J.M., Coronel, R., and Zuurbier, C.J., Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits, Diabetologia, 2017, vol. 60, pp. 568–573.
Çetin, E., Pretreatment with β-glucan attenuates isoprenaline-induced myocardial injury in rats, Exp. Physiol., 2019, vol. 104, pp. 505–513.
Chawla, R., Goyal, N., Calton, R., and Goyal, S., Ischemia modified albumin: a novel marker for acute coronary syndrome, Indian J. Clin. Biochem., 2006, vol. 21, pp. 77–82.
Dhalla, N.S., Adameova, A., and Kaur, M., Role of catecholamine oxidation in sudden cardiac death, Fundam. Clin. Pharmacol., 2010, vol. 24, pp. 539–546.
El-Gohary, O.A. and Allam, M.M., Effect of vitamin D on isoprenaline-induced myocardial infarction in rats: possible role of peroxisome proliferator-activated receptor-γ, Can. J. Physiol. Pharmacol., 2017, vol. 95, pp. 641–646.
Ellison, G.M., Torella, D., Karakikes, I., Purushothaman, S., Curcio, A., Gasparri, C., Indolfi, C., Cable, N.T., Goldspink, D.F., and Nadal-Ginard, B., Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells, J. Biol. Chem., 2007, vol. 282, pp. 11397–11409.
Ertekin, B., Kocak, S., Defne Dundar, Z., Girisgin, S., Cander, B., Gul, M., Doseyici, S., Mehmetoglu, I., and Kemal Sahin, T., Diagnostic value of ischemia-modified albumin in acute coronary syndrome and acute ischemic stroke, Pak. J. Med. Sci., 2013, vol. 29, pp. 1003–1007.
Fala, L., Jardiance (Empagliflozin), an SGLT2 inhibitor, receives FDA approval for the treatment of patients with type 2 diabetes, Am. Health Drug Benefits, 2015, vol. 8, pp. 92–95.
Farvin, K.H.S., Anandan, R., Kumar, S.H.S., Shiny, K.S., Mathew, S., Sankar, T. V, and Nair, P.G.V., Cardioprotective effect of squalene on lipid profile in isoprenaline-induced myocardial infarction in rats, J. Med. Food, 2006, vol. 9, pp. 531–536.
Fiordaliso, F., Cuccovillo, I., Bianchi, R., Bai, A., Doni, M., Salio, M., De Angelis, N., Ghezzi, P., Latini, R., and Masson, S., Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes, Life Sci., 2006, vol. 79, pp. 121–129.
Forte, E., Panahi, M., Baxan, N., Ng, F.S., Boyle, J.J., Branca, J., Bedard, O., Hasham, M.G., Benson, L., Harding, S.E., Rosenthal, N., and Sattler, S., Type 2 MI induced by a single high dose of isoproterenol in C57BL/6J mice triggers a persistent adaptive immune response against the heart, J. Cell Mol. Med., 2021, vol. 25, pp. 229–243.
Frobert, A., Valentin, J., Magnin, J.L., Riedo, E., Cook, S., and Giraud, M.N., Prognostic value of troponin I for infarct size to improve preclinical myocardial infarction small animal models, Front. Physiol., 2015, vol. 6, p. 353.
Gaze, D.C., Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia, Drug Metab. Pharmacokinet., 2009, vol. 24, pp. 333–341.
Gurumurthy, P., Borra, S.K., Yeruva, R.K.R., Victor, D., Babu, S., and Cherian, K.M., Estimation of Ischemia Modified Albumin (IMA) levels in patients with acute coronary syndrome, Indian J. Clin. Biochem., 2014, vol. 29, pp. 367–371.
Hasan, R., Lasker, S., Hasan, A., Zerin, F., Zamila, M., Chowdhury, F.I., Nayan, S.I., Rahman, M.M., Khan, F., Subhan, N., and Alam, M.A., Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways, Sci. Rep., 2020, vol. 10, p. 14459.
Karin, M. and Delhase, M., The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signaling, Semin. Immunol., 2000, vol. 12, pp. 85–98.
Karthikeyan, K., Bai, B.R.S., and Devaraj, S.N., Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats, Int. J. Cardiol., 2007, vol. 115, pp. 326–333.
Kono, Y., Nakamura, K., Kimura, H., Nishii, N., Watanabe, A., Banba, K., Miura, A., Nagase, S., Sakuragi, S., Kusano, K.F., Matsubara, H., and Ohe, T., Elevated levels of oxidative DNA damage in serum and myocardium of patients with heart failure, Circ. J., 2006, vol. 70, pp. 1001–1005.
Kumar, M., Kasala, E.R., Bodduluru, L.N., Dahiya, V., and Lahkar, M., Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation, Inflamm. Res., 2016, vol. 65, pp. 613–622.
Kusaka, H., Koibuchi, N., Hasegawa, Y., Ogawa, H., and Kim-Mitsuyama, S., Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome, Cardiovasc. Diabetol., 2016, vol. 15, p. 157.
Li, H., Xie, Y.H., Yang, Q., Wang, S.W., Zhang, B.L., Wang, J.B., Cao, W., Bi, L.L., Sun, J.Y., Miao, S., Hu, J., Zhou, X.X., and Qiu, P.C., Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats, PLoS One, 2012, vol. 7, p. e48872.
Li, J., Thangaiyan, R., Govindasamy, K., and Wei, J., Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals, Hum. Exp. Toxicol., 2021, vol. 40, pp. 915–927.
Lin, B., Koibuchi, N., Hasegawa, Y., Sueta, D., Toyama, K., Uekawa, K., Ma, M., Nakagawa, T., Kusaka, H., and Kim-Mitsuyama, S., Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice, Cardiovasc. Diabetol., 2014, vol. 13, p. 148.
Lobo Filho, H.G., Ferreira, N.L., Sousa, R.B. de, Carvalho, E.R. de, Lobo, P.L.D., and Lobo Filho, J.G., Experimental model of myocardial infarction induced by isoproterenol in rats. Rev. Bras. Cir. Cardiovasc., 2011, vol. 26, pp. 469–476.
Loh, H.K., Sahoo, K.C., Kishore, K., Ray, R., Nag, T.C., Kumari, S., and Arya, D.S., Effects of thalidomide on isoprenaline-induced acute myocardial injury: a haemodynamic, histopathological and ultrastructural study, Basic Clin. Pharmacol. Toxicol., 2007, vol. 100, pp. 233–239.
Maneewong, K., Mekrungruangwong, T., Luangaram, S., Thongsri, T., and Kumphune, S., Combinatorial determination of ischemia modified albumin and protein carbonyl in the diagnosis of nonST-elevation myocardial infarction, Indian J. Clin. Biochem., 2011, vol. 26, pp. 389–395.
Mizuno, M., Kuno, A., Yano, T., Miki, T., Oshima, H., Sato, T., Nakata, K., Kimura, Y., Tanno, M., and Miura, T., Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts, Physiol. Rep., 2018, vol. 6, p. e13741.
Nagoor Meeran, M.F., Stanely Mainzen Prince, P., and Hidhayath Basha, R., Preventive effects of N-acetyl cysteine on lipids, lipoproteins and myocardial infarct size in isoproterenol induced myocardial infarcted rats: an in vivo and in vitro study, Eur. J. Pharmacol., 2012, vol. 677, pp. 116–122.
Neal, B., Perkovic, V., Mahaffey, K.W., de Zeeuw, D., Fulcher, G., Erondu, N., Shaw, W., Law, G., Desai, M., and Matthews, D.R., Canagliflozin and cardiovascular and renal events in type 2 diabetes, N. Engl. J. Med., 2017, vol. 377, pp. 644–657.
Norbury, C.J. and Zhivotovsky, B., DNA damage-induced apoptosis, Oncogene, 2004, vol. 23, pp. 2797–2808.
O’Brien, P.J., Smith, D.E.C., Knechtel, T.J., Marchak, M.A., Pruimboom-Brees, I., Brees, D.J., Spratt, D.P., Archer, F.J., Butler, P., Potter, A.N., Provost, J.P., Richard, J., Snyder, P.A., and Reagan, W.J., Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals, Lab. Anim., 2006, vol. 40, pp. 153–171.
Oelze, M., Kröller-Schön, S., Welschof, P., Jansen, T., Hausding, M., Mikhed, Y., Stamm, P., Mader, M., Zinßius, E., Agdauletova, S., Gottschlich, A., Steven, S., Schulz, E., Bottari, S.P., Mayoux, E., Münzel, T., and Daiber, A., The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity, PLoS One, 2014, vol. 9, p. e112394.
Oktar, S., Aydin, M., Yönden, Z., Alçin, E., Ilhan, S., and Nacar, A., Effects of caffeic acid phenethyl ester on isoproterenol-induced myocardial infarction in rats, Anadolu Kardiyol. Derg., 2010, vol. 10, pp. 298–302.
Oshima, H., Miki, T., Kuno, A., Mizuno, M., Sato, T., Tanno, M., Yano, T., Nakata, K., Kimura, Y., Abe, K., Ohwada, W., and Miura, T., Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats, J. Pharmacol. Exp. Ther., 2019, vol. 368, pp. 524–534.
Othman, A.I., Elkomy, M.M., El-Missiry, M.A., and Dardor, M., Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction, Eur. J. Pharmacol., 2017, vol. 794, pp. 27–36.
Panda, S., Kar, A., and Biswas, S., Preventive effect of agnucastoside C against isoproterenol-induced myocardial injury, Sci. Rep., 2017, vol. 7, p. 16146.
Panda, V.S. and N, S.R., Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents, Altern. Med. Rev., 2009, vol. 14, pp. 161–171.
Patel, V., Upaganlawar, A., Zalawadia, R., and Balaraman, R., Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation, Eur. J. Pharmacol., 2010, vol. 644, pp. 160–168.
Prince, P.S.M., A biochemical, electrocardiographic, electrophoretic, histopathological and in vitro study on the protective effects of (–)epicatechin in isoproterenol-induced myocardial infarcted rats, Eur. J. Pharmacol., 2011, vol. 671, pp. 95–101.
Radhiga, T., Rajamanickam, C., Sundaresan, A., Ezhumalai, M., and Pugalendi, K.V., Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction, Biochimie, 2012, vol. 94, pp. 1135–1142.
Raish, M., Momordica charantia polysaccharides ameliorate oxidative stress, hyperlipidemia, inflammation, and apoptosis during myocardial infarction by inhibiting the NF-κB signaling pathway, Int. J. Biol. Macromol., 2017, vol. 97, pp. 544–551.
Rani, N., Bharti, S., Bhatia, J., Tomar, A., Nag, T.C., Ray, R., and Arya, D.S., Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism, Nutr. Metab., 2015, vol. 12, p. 11.
Rona, G., Chappel, C.I., Balazs, T., and Gaudry, R., An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat, A.M.A. Arch. Pathol., 1959, vol. 67, pp. 443–455.
Savarimuthu, S. and Harky, A., The role of sodium-glucose co-transporter 2 protein inhibitors in heart failure: more than an antidiabetic drug?, Expert. Opin. Pharmacother., 2022, vol. 23, pp. 377–386.
Searle, A.J. and Willson, R.L., Glutathione peroxidase: effect of superoxide, hydroxyl and bromine free radicals on enzyme activity, Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 1980, vol. 37, pp. 213–217.
Sharma, H.S. and Das, D.K., Role of cytokines in myocardial ischemia and reperfusion, Mediators Inflamm., 1997, vol. 6, pp. 175–183.
Soraya, H., Khorrami, A., Garjani, Afagh, Maleki-Dizaji, N., and Garjani, A., Acute treatment with metformin improves cardiac function following isoproterenol induced myocardial infarction in rats, Pharmacol. Rep., 2012, vol. 64, pp. 1476–1484.
Tang, Y.-N., He, X.-C., Ye, M., Huang, H., Chen, H.-L., Peng, W.-L., Zhao, Z.-Z., Yi, T., and Chen, H.-B., Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia, J. Ethnopharmacol., 2015, vol. 175, pp. 451–455.
Thangaiyan, R., Arjunan, S., Govindasamy, K., Khan, H.A., Alhomida, A.S., and Prasad, N.R., Galangin attenuates isoproterenol-induced inflammation and fibrosis in the cardiac tissue of albino Wistar rats, Front. Pharmacol., 2020, vol. 11, p. 585163.
Toker, A., Aribas, A., Yerlikaya, F.H., Tasyurek, E., and Akbuğa, K., Serum and saliva levels of ischemia-modified albumin in patients with acute myocardial infarction, J. Clin. Lab. Anal., 2013, vol. 27, pp. 99–104.
Tucker, J.F., Collins, R.A., Anderson, A.J., Hauser, J., Kalas, J., and Apple, F.S., Early diagnostic efficiency of cardiac troponin I and Troponin T for acute myocardial infarction. Acad. Emerg. Med., 1997, vol. 4, pp. 13–21.
Upaganlawar, A., Gandhi, H., and Balaraman, R., Effect of vitamin E alone and in combination with lycopene on biochemical and histopathological alterations in isoproterenol-induced myocardial infarction in rats, J. Pharmacol. Pharmacother., 2010, vol. 1, pp. 24–31.
Uygun, M., Yilmaz, S., Pekdemir, M., Duman, C., and Gürbüz, Y.S., The diagnostic value of ischemia-modified albumin in a rat model of acute mesenteric ischemia, Acad. Emerg. Med., 2011, vol. 18, pp. 355–359.
Verma, S., Rawat, S., Ho, K.L., Wagg, C.S., Zhang, L., Teoh, H., Dyck, J.E., Uddin, G.M., Oudit, G.Y., Mayoux, E., Lehrke, M., Marx, N., and Lopaschuk, G.D., Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors, JACC Basic Transl. Sci., 2018, vol. 3, pp. 575–587.
Wattanapitayakul, S.K. and Bauer, J.A., Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications, Pharmacol. Ther., 2001, vol. 89, pp. 187–206.
Widyaningsih, W., Pramono, S., Zulaela, Sugiyanto, and Widyarini, S., Protection by ethanolic extract from Ulva lactuca L. against acute myocardial infarction: antioxidant and antiapoptotic activities, Malays. J. Med. Sci., 2017, vol. 24, pp. 39–49.
Woudstra, L., Biesbroek, P.S., Emmens, R.W., Heymans, S., Juffermans, L.J., van Rossum, A.C., Niessen, H.W.M., and Krijnen, P.A.J., Lymphocytic myocarditis occurs with myocardial infarction and coincides with increased inflammation, hemorrhage and instability in coronary artery atherosclerotic plaques, Int. J. Cardiol., 2017, vol. 232, pp. 53–62.
Zhang, B., Wang, H., Yang, Z., Cao, M., Wang, K., Wang, G., and Zhao, Y., Protective effect of alpha-pinene against isoproterenol-induced myocardial infarction through NF-κB signaling pathway, Hum. Exp. Toxicol., 2020, vol. 39, pp. 1596–1606.
Zhang, W., Li, Y., and Ge, Z., Cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model, Biomed. Pharmacother., 2017, vol. 93, pp. 376–382.
Zinman, B., Wanner, C., Lachin, J.M., Fitchett, D., Bluhmki, E., Hantel, S., Mattheus, M., Devins, T., Johansen, O.E., Woerle, H.J., Broedl, U.C., and Inzucchi, S.E., Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes, N. Engl. J. Med., 2015, vol. 373, pp. 2117–2128.
ACKNOWLEDGMENTS
We thank Amanda Chilaka (microbiologist at the Whitehead Institute in Boston) for language editing of the manuscript.
Funding
This research was supported by Sivas Cumhuriyet University Scientific Research Projects Commission as research project no. V-092.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Mehmet Ekici, Güngör, H., Karayığıt, M.Ö. et al. Cardioprotective Effect of Empagliflozin in Rats with Isoproterenol-Induced Myocardial Infarction: Evaluation of Lipid Profile, Oxidative Stress, Inflammation, DNA Damage, and Apoptosis. Biol Bull Russ Acad Sci 49 (Suppl 1), S159–S172 (2022). https://doi.org/10.1134/S1062359022130039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022130039