Abstract
The globe is currently confronting a global fight against the deadliest cancer sickness. Chemotherapy, hormonal therapy, surgery, and radiation therapy are among cancer treatment options. Still, these treatments can induce patient side effects, including recurrence, multidrug resistance, fever, and weakness. As a result, the scientific community is always working on natural phytochemical substances. Numerous phytochemical compounds, including taxol analogues, vinca alkaloids such as vincristine and vinblastine, and podophyllotoxin analogues, are currently undergoing testing and have shown promising results against a number of the deadliest diseases, as well as considerable advantages due to their safety and low cost. According to research, secondary plant metabolites such as myricetin, a flavonoid in berries, herbs, and walnuts, have emerged as valuable bio-agents for cancer prevention. Myricetin and its derivatives have antiinflammatory, anticancer, apoptosis-inducing, and anticarcinogenic properties and can prevent cancer cell proliferation. Multiple studies have found that myricetin has anticancer characteristics in various malignancies, including colon, breast, prostate, bladder, and pancreatic cancers. Current knowledge of the anticancer effects of myricetin reveals its promise as a potentially bioactive chemical produced from plants for the prevention and treatment of cancer. This review aimed to study the numerous bioactivities, mode of action, and modification of several cellular processes that myricetin possesses to impede the spread of cancer cells. This review also addresses the challenges and future prospects of using myricetin as a anticancer drug.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the dawn of civilisation, people have used a variety of natural substances and their derivatives to treat terrible ailments. The use of secondary metabolites derived from plants to treat cancer is becoming more and more popular. Numerous studies have demonstrated the significance of phytochemicals in preventing this condition (Goyal et al. 2022; Khatoon et al. 2020; Shuaib et al. 2021). Therefore, the research community has identified an extensive range of phytochemicals and their mechanism of anticancer activity (Abadi et al. 2021; Yeshi et al. 2022). To understand how phytochemicals can help fight cancer, we need to study how they interact with cellular targets. An effective cancer treatment strategy also requires studying the pharmacokinetics of these compounds. This involves determining the appropriate dose and course of treatment, assessing their acceptability and efficacy in physiological conditions. Additionally, we need to study the remedial index to understand the potential benefits and risks of using these compounds. Finally, it is important to investigate the metabolic mechanism of plant-derived molecules against cancer to develop effective treatments. Among the potent anticancer natural compounds isolated from plant parts, the phytochemical compound myricetin and its derivatives are some of the most promising molecules utilised against various cancer cells (Jan et al. 2022; Siddiqui et al. 2022; Khan et al. 2022). The majority of researchers have found that myricetin promotes apoptosis, the start of ROS-mediated stress, metastatic activity, and DNA damage in various cancer cell lines and animal models. In addition, it controls the expression of inflammatory factors, triggers autophagy, initiates cell cycle arrest, and prevents cell invasion and metastasis (Han et al. 2022a, b, ; Ji et al. 2022). Myricetin is a widely distributed flavonol obtained from various family members of the plants such as Myricaceae, Anacardiaceae, Polygonaceae, Pinaceae, and Primulaceae. Tea, berries, vegetables, fruits, and medicinal plants are all excellent sources of myricetin (Hou et al. 2018; Qu et al. 2020; Gervasi et al. 2022; Chua et al. 2011). Myricetin and its derivatives have shown unique therapeutic effects in vivo and in vitro conditions, including anticancer, antiphotoaging activity, antioxidant activity, antiallergic and analgesic activities, immunomodulatory activity, antihypertensive activity, and cardio-protective and neuro-protective activities (Sharma et al. 2021; Semwal et al. 2016; Hagenacker et al. 2010; Jung et al. 2010; Li et al. 2022a, b). Myricetin causes cancer cell apoptosis that is Bcl-2 family-dependent intrinsically and DR5-dependent extrinsically (Huang et al. 2015; Anwar et al. 2022). Previous research has suggested myricetin’s role in inhibiting cell proliferation by regulating the S6 kinase 2 (RSK2) that increases the expression of Mad-1 and causes cell cycle arrest through ROS-dependent mitochondria-mediated mortality in the cancer cell (Rajendran et al. 2021; Feng et al. 2015). Myricetin increases BAX/BCL-2 and BAK caspase cascade expression in colon cancer, which leads to the induction of apoptosis (Rajabi et al. 2021; Xie et al. 2020; Kim et al. 2014). Myricetin plays a big part in deterring many pathways from occurring, such as PI3K/Akt/mTOR signalling, Akt/mTOR signalling pathway, and suppressing TGF-β1/Smad signalling, and also inhibits human breast cancer cell viability by controlling the PAK1/ ERK/MEK//GSK3β/Bax-caspase-3/β-catenin/cyclin D1/PCNA/surviving signalling (Sharma et al. 2022, Jiao and Zhang 2016). Myricetin reduces breast cancer MMP/2/9 and ST6GALNAC5 mRNA levels (Ci et al. 2018). Myricetin may also be able to block UVB-induced angiogenesis in SKH-1 hairless mouse skin by dramatically decreasing the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-9, and MMP-13 and suppressing factor-1α expression and phosphatidylinositol-3 (PI-3) kinase activity (Jung et al. 2012).
Hence, the review article has emphasised exploring the mechanism of action of myricetin bioactive compound that helps us understand the biology of neoplastic diseases and their regulation and cellular progression. Furthermore, it provides a detailed study of the molecules in cancer treatment, their chemical structure, chemopreventive properties, antioxidant and antiinflammatory activities, inhibitory role in angiogenesis and metastasis, and interactions of myricetin with other drugs. There has been discussion over the formulation of myricetin to include a comprehensive strategy for myricetin as a potential medicinal molecule.
Chemistry of myricetin
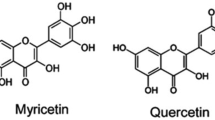
Myricetin is a naturally occurring flavone in fruits, vegetables, teas, and plant wines. Both the free and glycosidic bond forms of myricetin exist with hexahydroxyl substitutions at the 3,3′, 4′, 5, 5′, and 7 positions (Zhou et al. 2022; Sun et al. 2012). It is less soluble in water but readily dissolves in organic solvents such as acetone, dimethylformamide, dimethylacetamide, tetrahydrofuran, and several primary aqueous media. The degradation of the compound is pH and temperature-dependent, and it is highly stable at pH2 (Yan et al. 2021; De Leo et al. 2006; Kong et al. 2014). The researchers initially isolated myricetin in light yellow crystal form from the bark of the plant Myrica nagi Thunb. (Myricaceae) hundreds of years ago in India. It was also isolated from the aerial part of the Polygonumbellardii All. Strawberry, spinach, Euphorbia tirucalli L., Cyperusrotundus L. rhizomes, and Trigonella foenum-graecum seed extract had the most amazing myricetin content among the Polygonaceae in methanol extract (Sultana and Anwar 2008; Yang et al. 2021; Jahan et al. 2013). It contains pyrogallol B-ring and hydroxylated structure, which is highly responsible for its various biological activities compared to other flavonols (Sato et al. 2013; Mendes et al. 2015; Kenouche et al. 2022; ONO et al. 1990). Myricetin has a linkage structurally to several phenolic chemicals, including quercetin, morin, kaempferol, and fisetin, and sometimes it is also called hydroxyquercetin due to its structural similarity with quercetin molecule (Lin 2012; Parvez et al. 2020). The dietary consumption of myricetin decreases the risk of cancer because of its numerous antitumour properties like antiproliferative, proapoptotic, and antimetastatic activities in various cancers (Micek et al. 2021; Geybels et al. 2013; Marrero et al. 2022). The hexane/ethyl acetate/methanol/water extract of Davillaelliptica St. Hill. (Dilleniaceae) analysed by column chromatography and thin layer chromatography resulted in the isolation of myricetin and quercetin-3-O-a-L-rhamnopyranosid (Rinaldo et al. 2006). In another study, the initial extraction of myricetin compound comprises column chromatography over Sephadex LH-20 using a methanol fraction of Davillaelliptica St.-Hil. and finally characterised by preparative RP-HPLC (Campos et al. 2013). In one approach, a low-cost extraction process for myricetin, quercetin, luteolin, and kaemferol has been developed through a complex cap espresso machine using ethanol and water, and liquid chromatography determined chemical compounds (Corell et al. 2018) (Fig. 1).
Biosynthesis of myricetin
In myricetin biosynthesis, the plant typically follows the phenylpropanoid biosynthetic mechanism. The mechanism begins with converting phenylalanine to cinnamic acid, catalysed by the phenylalanine ammonia-lyase (PAL). The cinnamic acid was further catalysed by an enzyme cinnamate 4-hydroxylase (C4H) to generate p-coumaric acid and then 4-coumaroly-CoA. Natural phenylpropanoids, such as cumarins, stilbenes, and flavonoids, are formed by condensing three molecules of malonyl-CoA and one molecule of p-coumaroyl CoA that further changed into naringenin chalcone with the help of chalcone synthase (CHS). This enzyme is regarded as the initial enzyme in flavonoid biosynthesis. The chalcone isomerase (CHI) enzyme further converts the intermediate molecule, naringenin in chalcone, into naringenin. In the next step of myricetin biosynthesis, the enzyme flavone 3-hydroxylase (F3H) converts naringenin to pentahydroxyflavanone and dihydromyricetin. In the last stage of the biosynthesis of this compound, the flavonol synthase (FLS), an enzyme, finally transformed the dihydromyricetin into myricetin (Martens et al. 2010; Fogelman et al. 2015; Javed et al. 2022; Arafah et al. 2022) (Fig. 2).
Chemical synthesis of myricetin
For the chemical synthesis of myricetin, the first step was taken in 1925 by Dean and Nierenstein (Kalff and Robinson 1925) by using Kostanecki and Auwer’s approach but failed to gain any success. However, one of Kalff and Robinson’s other research groups synthesised myricetin from ω-methoxyphloroacetophenone that same year. The first stage of this process involves heating the starting material with trimethylgallic anhydride and sodium trimethylgallate, following the product’s hydrolysis results in the formation of an intermediate known as 5,7-dihydroxy-3,31,41,51-tetramethoxyflavone, and following the demethylation of the intermediate, which results in the formation of myricetin. A series of myricetin analogues having a 1,3,4-thiadiazole scaffold was also synthesised chemically and observed the antibacterial activity against Xoo and Rs and the antiviral activity against the TMV (Zhong et al. 2017) (Fig. 3).
In a different method, myricetin was synthesised from quercetin by Rao and Seshadri (Rao and Seshadri 1948) by an ortho-oxidation reaction that transformed 3,5,7,3′-tetra-O-methylquercetin into 5′-aldehyde. In the next phase, 5′-aldehyde is transformed into 3,5,7,31-tetra-O-methylmyricetin, which yields 5-methoxykanugin followed by cyclisation at 41 and 51 positions. The subsequent methylation of 5-methoxykanugin produced hexamethylmyricetin that, after demethylation, failed myricetin (Tranchimand et al. 2006). One study designed a variety of myricetin analogues with a quinazolinone moiety and found the compound’s in vitro antibacterial and in vivo antiviral activities (Liu et al. 2021).
Derivatives of myricetin
The derivatives of myricetin are also widely synthesised, designed, extracted, and tested for their anticancer properties against various cancer cell lines. In one study, different types of myricetin derivatives were synthesised by altering the original compounds’ structures and observing their antitumour activity against human non-small cell lung cancer (NSCLC) A549 cells (Li et al. 2021). One of the myriceitn derivatives known as S4-2–2 (5,7-dimethoxy-3-(4-(methyl(1-(naphthalen-2-ylsulfonyl)piperidin-4-yl)amino)butoxy)-2-(3,4,5-trimethoxyphenyl)-4H- chromen-4-one) has strongly inhibited the migration and invasion but induced the apoptosis in of non-small cell lung cancer A549 (Zhou et al. 2023). From all of the myricetin derivatives, the S4-10 has shown the maximum activity in cell migration, proliferation, invasion, induced apoptosis, and cell cycle arrest in A549 Cells. Myricetin derivatives, such as 2-(2′,6′-dimethyl-3′,4′,5′-alkyl or hydroxy alkyl substituted phenyl)-3-oxy-(alkyl or hydoxy alkyl)-5,7-dihydroxy-chromen-4-one, were isolated and characterised from the Mimosa pudica plant. These derivatives were tested for in vitro anticancer activity against human lung adenocarcinoma cell lines (A549) and human erythroleukaemic cells (K562). The tests utilised 3-(4,5-dimethylthiazol-2-yl)MTT assay-2,5-diphenyl tetrazolium bromide (Jose et al. 2016; Xianghui et al. 2018). Oral treatment of one of myricetin derivatives, M10, inhibited ulcerative colitis (UC) and colorectal tumours in the murine azoxymethane/dextran sodium sulfate model. The treatment was administered at 50–100 mg/kg daily for 12 weeks. The study discovered that M10 myricetin derivatives enhanced CD8 + T and CD4 + T cells in colorectal tissues while attenuating chronic inflammation and inhibiting the invasion of myeloid-derived suppressor cells. The M10 derivative of myricetin significantly decreased the pro-inflammatory factors IL-6, TNF-, and granulocyte–macrophage colony-stimulating factor, as well as the NF-B/IL-6/STAT3 pathways and colorectal carcinogenesis. (Wang et al. 2018). Two myricetin derivatives, 3,7,4,5-tetramethyl ether of myricetin and 3,5-diacetyl derivative, were also cytotoxic against human leukaemic cell lines when isolated from Cistus monspeliensis in hexane extract (Dimas et al. 2000).
Pharmacological utilisation of myricetin
Anticancer properties of myricetin
One of the crucial dietary components present in foods and beverages is the bioactive substance myricetin. Myricetin has potential antioxidant, antiinflammatory, and anticancer effects, according to numerous studies, as shown in Fig. 4. According to myricetin’s biological characteristics, it is highly effective against many cancer cell lines, including those found in the liver, skin, bladder, pancreas, breast, and colon. It is also known to inhibit the activity of several molecular enzymes, such as DNA polymerases, RNA polymerases, reverse transcriptases, telomerase, kinases, and helicases (Awadelkareem et al. 2022; Jain et al. 2021). Myricetin and dihydromyricetin inhibited fibroblast proliferation in lung and breast cancer (MCF-7 and A549) via inducing apoptosis, inhibiting cell proliferation, and downregulating PDGFRβ signalling pathway and extracellular signal-regulated kinase (Erk) 1/2 and Akt expression (Fan et al. 2017). Myricetin inhibited the expression of MMP-2 and MMP-9 proteins in cancer cells. It also inhibited the phosphorylation of the FAK (focal adhesion kinase) signalling pathway and changed the F-actin/G-actin ratio in A549-IR cells (Kang et al. 2020). Another report involving the inhibition of cytokine-induced invasion and migration of KKU-100 cells treated with myricetin consists of the downregulation of STAT3, matrix metalloproteinase-9, inducible nitric oxide synthase, intercellular adhesion molecule-1, and cyclooxygenase 2 (COX-2). Similarly, treating myricetin on mouse skin epidermal JB6 P + cells indicated the role of UVB-induced cyclooxygenase (COX-2) expression (Senggunprai et al. 2018). Myricetin strongly suppressed UVB-induced start of activator protein-1, NF-κβ and Fyn kinase activity, MEK1 kinase activity, and transformation of JB6 P + mouse epidermal cells, as reported by the author (Jung et al. 2008). The combination of myricetin (MYR), methyl eugenol (MEG), and cisplatin (CP) significantly inhibited cancer cell growth, induction of cell apoptosis, loss of mitochondrial potential, and upregulation of caspase-3 activity, as well as increased the number of cells in the Go/G1 phase in human cervical cancer (Yi et al. 2015). One study reported that the myricetin induced apoptosis by serum deprivation in PCL-12 cell dose-dependently by expressing the tumour suppressor gene p53 and proapoptotic and antiapoptotic Bcl-2 family proteins Bax and Bcl-2 and induced the expression of caspase 3 and caspase 9 cascades (Tan et al. 2018). Mitogen-activated protein kinase (MAPK) and PI3/AKT signalling pathways are crucial for myricetin’s ability to inhibit cell proliferation, regulate the cell cycle, and invade and promote angiogenesis [68]. Myricetin has also responsible for the initiation of apoptosis in breast cancer SK-BR3 cells via upregulation of PARP, Bax protein, expression of phosphorylated c-Jun N-terminal kinase (p-JNK) and phosphorylated mitogen-activated protein kinases (p-p38), and downregulation of Bcl2 and phosphorylated extracellular-regulated kinase (p-ERK) (Han et al. 2022a, b, ). Another study found that myricetin caused breast cancer cells to undergo apoptosis through both intrinsic and extrinsic pathways, most notably the BRCA1-GADD45 pathway, which increased the expression of caspase-3, caspase-8 and caspase-9 as well as the proportion of BAX/Bcl-2, P53, BRCA1, GADD45, and annexin (Soleimani and Sajedi 2020). Myricetin caused apoptotic cell death in A431 human cancer cells in a dose-dependent manner by increasing the rate of reactive oxygen species (ROS) that leads to the disruption of outer mitochondria potential caused by discharged apoptotic triggering proteins and is also responsible for the alteration of Bcl and Bax expressions (Sun et al. 2018). Another study found that myricetin inhibits HCC cell growth by causing cell cycle arrest and autophagy by downregulating MARCH 1 mRNA but upregulating MARCH 1 mRNA in Hep3B cells. Furthermore, it suppressed p38 MAPK and Stat3 signalling by reducing MARCH 1 to suppress HCC proliferation in vitro and in vivo (Yang et al. 2021).
Myricetin’s proapoptotic activities in human hepatocarcinoma HepG2 cells are enhanced by reduced mitochondrial fragmentation and transmembrane potential. Furthermore, the author proposed that myricetin promoted apoptosis in HepG2 cells via the mitochondrial apoptotic route and the Akt/p70s6k1/Bad signalling pathways, which resulted in the production of proapoptotic proteins Bax and Bad in the mitochondria and the downregulation of Bcl-2 expression. Furthermore, the study discovered that myricetin boosted caspase-3 proteolytic activation and PARP protein degradation, which was followed by cytochrome C release in the cytoplasm (ZHAO et al. 2012). Similar to this report, another research reported that the induction of ERK1/2 and JNK signalling pathways supported the generation of ROS, increased apoptotic DNA fragmentation, lipid peroxidation, phosphorylation of AKT, p70S6K, and depolarisation of MMP in D-17 and DSN canine osteosarcoma cells (Li et al. 2019). Myricetin administration increased apoptosis-related genes caspase-3, caspase-8, and caspase-9, and the BAX/Bcl-2 ratio, as well as p53, BRCA1, and GADD45 in MCF-7 breast cancer cells (Park et al. 2018) (Fig. 5).
Myricetin affects the invasion and migration of radioresistant lung cancer cell A549IR. Similarly, dose- and time-dependent inhibitions of adhesion, migration, and invasion by matrix metalloproteinase-2 and urokinase plasminogen activator properties in A549 cells were also seen in specific investigations with myricetin. Myricetin-treated A549 cells inhibited nuclear factor kappa B c-Fos and c-Jun activation and phosphorylated extracellular signal-regulated 1 and 2 (Shih et al. 2009). Moreover, myricetin reduced the expression of Yes-associated protein (YAP) by promoting its phosphorylation and subsequent degradation via stimulating the LATS1/2 pathway that induced apoptosis and cell proliferation inhibition in hepatocellular carcinoma HepG2 and Huh-7 cells (Li et al. 2019).
Another study discovered that myricetin’s role in the breakdown of mitochondrial membrane potential leads to the release of cytochrome-C in the cytosol, which increases the proteolytic activation of caspase-3 and the degradation of PARP protein. The author hypothesised that myricetin caused the inactivation of Bcl-2 expression, the activation of proapoptotic protein Bad, and the translocation of Bax-induced death in HepG2 cells via the mitochondrial apoptotic route and the Akt/p70s6k1/Bad signalling pathway (Knickle et al. 2018).
Myricetin treatment of PC-3 and DU 145 prostate cancer cells reduced tumour potential by upregulating caspase-3 and caspase-9 activities and decreasing phosphorylation of ERK1/2 and Akt (Ma et al. 2019; Ye et al. 2018). Researchers evaluated myricetin’s toxicity on a non-tumour cell and observed its effects on the growth, migration, and invasion of the SKVO3 cancer cell in one study. The data revealed that myricetin concentration from 0 to 40 μM increases apoptosis that inhibits oxidative stress reduces ROS levels and suppresses cancer cell growth by activating the p38/Sapla signalling mechanism (Li et al. 2022a, b). Myricetin has also induced apoptosis and autophagy in human colon cancer cells such asHT-29, HCT116, SW480, and SW620 by inhibiting the PI3K/Akt/mTOR signalling pathway (Zhu et al. 2020) (Fig. 6).
Myricetin has also been observed for its cytotoxicity, cell cycle inhibition, and DNA damage through a dose-dependent way in human papillary thyroid cancer (HPTC) cells by upregulating the caspase cascade and Bax/Bcl2 ratio and also initiated the apoptosis-inducing factor by changing the potential of mitochondria membrane (Ha et al. 2017). Apart from the above anticancer activities, the bio-molecules have diverse anticancer effects on the cell line as given in Table 1.
Antiinflammatory and antioxidant activities of myricetin
Myricetin, a natural bioactive component, controls numerous molecules involved in inflammatory reactions, such as cytokines and enzymes. The scientific community has done several investigations and observations to discover myricetin’s antiinflammatory action. It can obstruct the production of pro-inflammatory mediators by initiating Nrf2 mediated HO-1 expression and suppressing the NF-κB and STAT1 in LPS-stimulated RAW264.7 macrophages (Oh et al. 2020; Gupta et al. 2020).
In one study, myricetin decreased the synthesis of interleukin-12 via lowering the macrophase’s binding capabilities to nuclear kappa-B and preventing interleukin-1 (IL-β1) production in SW982 human synovial sarcoma cells (Lee and Choi 2010; Kang et al. 2005). By altering the gut microbiota associated with faecal butyric acid and preserving the integrity of the gut barrier, myricetin also aids in the reduction of hepatic lipid production and inflammation (Sun et al. 2021). Treating rat wounds with myricetin helps elevate the pro-inflammatory factors such as cytokines, IL-1β, TNF-α, and macrophage CD68, which are essential in promoting lesion healing (Elshamy et al. 2020).
Similarly, in TNF-α-activated ECV304 cells, the myricetin molecule inhibited the expression of TNF-α-mediated NF-κB by downregulating the inhibitor-κB kinase (IKK) (Tsai 1999). Myricetin treatment of HepG2 cells results in the downregulation of several inflammatory molecules such asiNOS, COX-2, IL-2 and IL-6, TNF-α, and IFN-γ in a dose-dependent manner (Zhou et al. 2019). In vivo research using mice treated with myricetin revealed that the severity of inflammatory lesions and tumourigenesis, which were accountable for tumour inhibition in various ways, were less severe (Zhang et al. 2017). Myricetin suppresses acute and chronic inflammation in vivo in models of xylene-induced ear oedema, acetic acid–induced vascular permeability, carrageenan-induced paw oedema, and cotton pellet granuloma. Myricetin has dramatically elevated the serum level of SOD, decreased the serum level of MDA leukocyte count, and inhibited the formation of antiinflammatory granuloma tissue (Wang et al. 2010a, b). Myricetin can inhibit oxidative stress by enhancing the activities of SOD, glutathione peroxidase GPX, CAT, malondialdehyde, GSH, and hydrogen peroxide enzymes, reducing inflammation. Similarly, myricetin also decreases inflammation by regulating the production of oxidase-dependent ROS, NADPH, by inhibiting the JAK/STAT1 and NOX2/p47(phox) mechanism (Mao and Huang 2018; Hassan et al. 2017; Qi et al. 2017). Myricetin administration in lipopolysaccharide-stimulated RAW 264.7 cells and a lipopolysaccharide-induced lung damage model resulted in the downregulation of NF-κB p65 and the elevation of NF-κB pathway, JNK, p-ERK, and p38 in MAPK signalling pathway (Bai et al. 2021). Therefore, the initial results suggest that myricetin has the potential as an antiinflammatory chemical and could be employed as a medication in future in vivo trials.
Regarding antioxidant characteristics, the low concentration of myricetin inhibits the synthesis of reactive oxygen species and shields cells from the cytotoxic action of peroxide molecules. (Barzegar 2016; Taheri et al. 2020). In a dose-dependent manner, myricetin suppressed the activity of XOD up to 50% at a concentration of (8.66 ± 0.03) × 10–6 molL-1 showed that myricetin could inhibit the synthesis of superoxide anion (Zhang et al. 2017). Myricetin can also control hydrogen peroxide-induced DNA damage at a concentration of 100 µM in human lymphocytes (Duthie et al. 1997). Myricetin also inhibits oxidative stress-induced apoptosis via modulating PI3K/Akt and MAPK signalling pathways. It also increases the production of SOD, catalase (CAT), and glutathione peroxidase (GPx), which are all lowered by H2O2 treatment (Wang et al. 2010b). Myricetin treatment caused triple-negative breast cancer (TNBC) cells to undergo early and late apoptosis and necrosis caused by oxidative stress. H2O2 and myricetin autooxidation produce oxidative stress. In human colonocyte Caco-2 cells, myricetin also prevented the oxidative effect of H2O2 and protected DNA strand breaks (Duthie and Dobson 1999) (Fig. 7).
Angiogenesis and metastasis effects of myricetin
Angiogenesis is the process by which new blood cells are formed from preexisting vessels, and it plays an essential role in cancer cell proliferation in cells and organs. Therefore, considerable work is going on the natural bioactive compound that can help inhibit the angiogenesis of the cancer cell (Huang et al. 2015; Marrero et al. 2022; Birbrair et al. 2014). In human umbilical vascular endothelial cells, myricetin activated reactive oxygen species, causing apoptosis and the cleavage of procaspase-3, which reduced cell migration, PI3K/Akt/mTOR, and tube formation (Kim 2017). In the case of hepatocellular carcinoma cell lines (HCC), myricetin plays a substantial role in the reversion of PAR1-mediated EET that inhibited the invasion, migration, vasculogenic mimicry (VM) formation, and angiogenesis by targeting Leu258 and Thr261 of PAR1 involved in VM and angiogenesis (Wang et al. 2022) (Fig. 8).
In the case of SKH-1 hairless mouse skin carcinogenesis, myricetin is critical in suppressing UV-induced B-angiogenesis. It inhibited the expression of MMP-9, MMP-13, and vascular endothelial growth factor and the activity of phosphatidylinositol-3 (PI-3) kinase (Jung et al. 2010). Similarly, myricetin downregulated the tumour promoter-induced cancer cell formation by inhibiting the direct activity of MEK, JAK1, Akt, and MKK4 kinases in skin carcinogenesis. Myricetin dramatically attenuated the ultraviolet B-induced COX-2 expression and skin tumour formation by controlling the Fyn (Kang et al. 2011). Myricetin also helps to reduce cell proliferation in JAR and JEG-3 choriocarcinoma cells by increasing apoptosis and trophoblast cell attenuation via the MAPK and PI3/AKT signalling pathways. Myricetin also increases reactive oxygen species (ROS), lipid peroxidation, glutathione depletion, and mitochondrial membrane potential loss (Yang et al. 2021). A nucleoside diphosphate kinase encoded by NM23 is essential in suppressing metastasis. As a result, myricetin reduced the expression of Bcle-2, Parp, and caspase-related proteins in a human colon cancer cell line while increasing the expression of nucleoside diphosphate kinase, PARPs, caspase-3, and caspase-9 cleavage (Attwood and Muimo 2017; Tan and Chang 2018). Myricetin also decreased the activity of the epithelial-mesenchymal transition (EMT), which is essential for metastasis, by increasing E-cadherin and decreasing vimentin (Ye et al. 2018). In the 4T1 mouse lung metastasis model, myricetin molecules at 50 mg/kg concentration reduce the size and number of tumour nodules compared to vehicles (Lee et al. 2012) (Fig. 9).
Effect of myricetin on miRNA and mRNA
Myricetin’s interaction with RNAs opens new avenues for targeting various cancer cells. Micro-RNAs (miRNA) play a wide range of roles in human biological processes, illnesses, and metabolic disorders. A dysregulated miRNA impacts various signalling pathways (Lee et al. 2015). By downregulating miR-29a-3p, myricetin can provide an antiinflammatory impact against ox-LDL-induced HUVEC (Bai et al. 2021). In one study, it was observed that myricetin significantly downregulated the level of IL-1β mRNA. However, no effect was observed in the synthesis of IL-1β protein in RAW 264.7 macrophages through inhibiting gene transcription (Blonska et al. 2003). Similarly, in one of the investigations, myricetin interacted with telomere G-quadruplex TTAGGG 3 DNA in the MCF-7 human breast cancer cell line in a concentration-dependent way. It inhibited the activity of human telomerase reverse transcriptase mRNA and telomerase (Mondal et al. 2016).
Effect of myricetin on autophagy
Myricetin has a significant role in the induction of apoptosis and autophagy in the various cancer cells alone or in adjuvant form. In colon cancer cells, myricetin initiates apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling pathway (Zhu et al. 2020). Similarly to the previous observation, myricetin affected autophagy-related proteins. It enhanced the stimulation of microtubule-associated protein 1A/1B light chain 3 (LC 3) and Beclin 1. This stimulation occurred in human breast cancer SK BR 3 cells. The apoptosis rate increased when myricetin and 3 methyladenine (3 MA) were administered simultaneously to cancer cells. The subsequent treatment of a JNK inhibitor to the cells reduced cell viability, triggered the expression of Bax, and decreased the expression of p-JNK, Bcl 2, and LC 3 II/I. Hence, these actions revealed that myricetin triggered and controlled apoptosis and autophagy in SK BR 3 cells via the MAPK pathway and JNK-mediated autophagy (Han et al. 2022a, b, ). A research group observed the protective autophagy of myricetin on hepatocellular carcinoma (HCC) cells. The molecule directly linked the activation of endoplasmic reticulum stress, which boosted autophagy, as shown by the result indicating that it induced apoptosis. AGS gastric cancer cell myricetin induced apoptosis and autophagy by inhibiting the PI3K/Akt/mTOR pathway that leads to cell-protective autophagy and inhibition of cancer cell proliferation (Han et al. 2022a, b, ). Researchers investigated myricetin's antihepatocellular carcinoma (anti-HCC) mechanism in one study. The researchers observed that inhibiting the expression of MARCH 1 induced autophagy and cell cycle arrest in the G2/M phase. They utilised this effect to inhibit the growth of HCC cells. In Hep3B cells, myricetin increased MARCH1 mRNA levels, whereas, in HepG2 cells, it lowered them. Thus, knocking down MARCH1 by siRNAs (small interfering RNAs) downregulates phosphorylated p38 MAPK (p-p38 MAPK) and Stat3 (p-Stat3). This downregulation reduces the viability of HCC cells. Myricetin and the autophagy inhibitor bafilomycin A1 (BafA1) significantly reduced the development of HCC cells (Yang et al. 2021). Cucurbitacin E (CuE) and myricetin (Myr) of Citrullus colocynthis (L.) Schrad are essential in inhibiting cell proliferation and colony formation but increases apoptosis and cell cycle arrest in the G0/G1 phase. Moreover, the CuMy-12 combination leads to the inhibition of autophagy and commencement of the PI3K/AKT/mTOR signalling mechanism that was differentiated by a reduction in Beclin 1, AKT, and phospho-AKT, exhibiting a synergistic effect. Furthermore, CuMy-12 caused a decrease in Beclin 1, AKT, and phospho-AKT proteins, indicating inhibition of autophagy and activation of the PI3K/AKT/mTOR signalling pathway (Zhang et al. 2023).
Synergistic effect of myricetin
Myricetin is essential in various human foods, including vegetables, beverages, fruits, and other natural foods. Myricetin is well-known for its antioxidant, antiinflammatory, and antitumour properties. Numerous studies have found that cancer cells generally resist the anticancer drug cisplatin, which causes degeneration. However, myricetin exhibits more significant cell toxicity than cisplatin in cisplatin-resistant cancer cell lines, such as OVCAR-3 and A2780/CP70, while exhibiting minimal toxicity effects on the normal cell IOSE-364. Myricetin promoted intrinsic and extrinsic apoptosis and Bcl-2 family protein pathways in the standard cell line but did not initiate the cell cycle arrest. The combination of 5-fluorouracil and myricetin inhibited cell proliferation and function, initiated apoptosis, increased caspase-3 and P53 expression levels, and decreased survivin, cyclin D, and Bcl-2 expression levels, according to research on the chemosensitisation activity of the two drugs in the EC9706 cancer cell line (Wang et al. 2014).
One study found that cervical cancer cells were impacted by myricetin, methyl eugenol (MEG), and cisplatin (CP). These three medications were found to promote cell death, cell cycle arrest, and caspase-3 activity, all of which suppressed the growth of cancer cells. Combining these chemicals also decreases mitochondrial membrane potential and increases the proportion of cells in the G0/G1 phase of the cell cycle (Yi et al. 2015). The prophylactic treatment of rats with myricetin for 21 days lowered the markers of inflammation, apoptosis, cardiac toxicity, and oxidative stress in the context of 5-fluorouracil’s reduced cardiotoxicity. It increased the antioxidative activity (Arafah et al. 2022). In a previous study, through an MTT assay, researchers detected the apoptosis-inducing mechanisms of myricetin, myricitrin, and quercitrin in the human prostate cancer cell line PC-3. At concentrations ranging from 37.5 to 300 mol/L, the combination of myricetin and myricitrin had a strong inhibitory effect on the proliferation of cancer cells (Xu 2013). Myricetin and temozolomide decreased the proliferation, migration, and invasion of U-87MG glioblastoma cells. However, combining myricetin and temozolomide did not demonstrate any beneficial effects. Myricetin inhibited the development of lamellipodia, focal adhesions, membrane ruffles, vasculogenic mimicry, and the phosphorylation of the ROCK2, paxillin, cortactin, PI3K/Akt, and JNK signalling pathways (Zhao et al. 2018).
Bioavailability of myricetin
Only after evaluating their efficacy, novelty, and minimum detrimental effects on organisms could the vast treasury of natural materials be utilised as pharmaceutical medications. Therefore, the solubility of biomolecules through diverse physiological processes is crucial for their efficacy. Myricetin is less hydrophilic yet significantly soluble in organic solvents such as acetone, dimethylformamide, tetrahydrofuran, and dimethylacetyl chloride (Chang et al. 2012). Various strategies, such as nanotechnology, are now being developed to enhance the bioavailability of myricetin (Xia et al. 2020). In one study, rats were given myricetin orally and intravenously in dose-dependent ways. It evaluated the bioavailability in the blood, and myricetin was found less orally due to inadequate absorption of the molecule (Dang et al. 2014). Solid lipid nanoparticles with about 30 µmol of myricetin were used against HT-29 cells, and colony formation, expression of Bax, Bcl2, and apoptosis-inducing factor were measured. In the HT-29 cells, the nano-loaded myricetin significantly boosted apoptosis, Bax, and AIF expression and decreased Bcl2 and MMP (Alidadi et al. 2022). The liposomal nanoformulation of myricetin not only increases the molecules’ bioavailability but also decreases the pro-oxidant properties. Accordingly, the zebrafish embryo showed an effect of nanocapsulated myricetin formulation, in which the chemical increased antioxidant activity against oxidative stressors (Agraharam et al. 2021). Myricetin microemulsion (MYR-ME) is a mixture of myricetin, Cremphor, Tween-80, Transcutol, WL1349, and distilled water that improves the molecule’s bioavailability by more than 1225 times compared to water, allowing for greater oral efficacy. The MYR-ME has potentially enhanced the antioxidative and antiproliferative activity against HepG2 human cancer cells and further increased 14.43 fold oral bioavailability of myricetin after oral administration of the emulsion to Sprague Dawley rats (Guo et al. 2016). The TPGS-modified liposome nanocarriers also have the potential to deliver myricetin via the oral route and increase the pharmaceutical efficacy of the molecules (Thant et al. 2021). Mesoporous silica nanoparticles treated with folic acid and filled with myricetin were utilised to treat non-small cell lung cancer. (NSCLC). Under in vitro environments, FA-conjugated nanocarriers improve the absorption of myricetin in lung cancer, thereby decreasing colony formation cell viability, dramatically enhancing apoptosis, and upregulating the expression of caspase-3 and PARP (Song et al. 2020). Myricetin-loaded NLCs and DXT induce apoptosis in MDA-MBA231 breast cancer cells by decreasing survivin, cyclin B1, and Mcl1 antiapoptotic genes and augmenting Bax and Bid proapoptotic proteins (Maroufi et al. 2020).
Challenges in using myricetin as a drug
Plant metabolites have a vast contribution to the progress of pharmaceutical cancer drug development. However, most bio-products always have challenges in using as safe drugs and optimise their optimisation. In the case of the bioactive molecule myricetin, it has shown a wide variety of anticancer activity against several cancer cell lines. However, it also poses a challenge for utilisation as a drug due to its pleiotropic nature and variability. Myricetin has multiple targets in signalling pathways. These targets can encumber tumour progression. They can also prevent metastasis and induce cell cycle inhibition. Additionally, myricetin can have other effects. The main challenge of the molecules is their poor bioavailability and solubility. This decreases their chemotherapeutic application. This is particularly true for large-scale utilisation in aqueous solutions. Furthermore, bio-molecule sensitivity against various abiotic factors, such as light and heat, can lead to their degradation and, consequently, the loss of their bioactivities (Albuquerque et al. 2021). Some studies have also limited the use of myricetin due to its toxic nature towards the biological cell. One of the reports observed that molecules above 450 μM lead to cellular damage in isolated guinea pig enterocytes (Semwal et al. 2016; Canada et al. 1989). Another limitation of the clinical use of myricetin is the lack of research on aspects of their route for administration, exact formulation, and doses for various types of cancer. It combines the administration of this drug with other bioactive compounds (Imran et al. 2021). The clinical trial research on myricetin molecules is minimal. However, some clinical survey suggests that the consumption of these molecules assist in the low incidence of prostate cancer, and regular consumption of myricetin with other flavonoids bio-molecule by menopausal females helps to reduce the risk of coronary heart disease.
Future perspective
Future research on myricetin for cancer treatment could concentrate on addressing its current limits and downsides and expanding its potential therapeutic applications. Some possible research areas are enhancing bioavailability, targeted delivery, clinical trials, combination therapy, and mechanisms of action. New formulations and administration methods may improve myricetin’s solubility, bioavailability, and efficacy. Researchers could investigate using targeted delivery systems for myricetin to increase specificity and decrease the likelihood of non-specific targeting of healthy cells (Afroze et al. 2020). Large-scale clinical trials are required to examine the safety and efficacy of myricetin in people, particularly in connection to specific forms of cancer. Researchers could investigate the potential benefits of combining myricetin with other cancer treatments, such as chemotherapy or radiation therapy, to improve their efficacy and lessen side effects (Albuquerque et al. 2021). Myricetin’s mechanisms of action on cancer cells, including its impact on specific signalling pathways and biological processes, require additional research.
Conclusion
Cancer is a multifaceted disease, and the genesis and progression of the disease involve simultaneous modulation of multiple biological pathways responsible for the growth, survival, and proliferation of cells. The treatment of cancer must target signalling cascades. Myricetin is a plant flavonoid. It is present in wine, tea, and medication. Myricetin affects cancer cell processes. Since it blocks several proteins and signalling pathways that promote cell proliferation and inhibit apoptosis, myricetin has been a promising cancer chemopreventive in numerous cancer models. It has remarkable antitumour efficacy since it also causes cell cycle arrest, prevents cell invasion and migration, and triggers autophagy and necroptosis. However, it has limited bioavailability, non-specific targeting, lack of clinical data, potential side effects, and interactions with other drugs. More research is needed to understand its safety and effectiveness and address its potential limitations and drawbacks. Future research on myricetin for cancer treatment could concentrate on bioavailability enhancement, targeted delivery, clinical trials, combination therapy, and action mechanisms. These investigations will aid in advancing knowledge of the compound’s potential and identifying methods for overcoming its current limits.
Data availability
All the generated data is summarised in the form of supporting references.
Abbreviations
- PAL:
-
Phenylalanine ammonia-lyase
- 4CL:
-
4-Coumaryl-CoA ligase
- CHS:
-
Chalcone synthase
- CHI:
-
Chalconeisomerase
- F3H:
-
Flavanone-3-hydroxylase
- F3′H:
-
Flavonoid-3′-hydroxylase
- FLS:
-
Flavonol synthase
References
Abadi AJ, Mirzaei S, Mahabady MK et al (2021) Curcumin and its derivatives in cancer therapy: potentiating antitumor activity of cisplatin and reducing side effects. Phytother Res 36:189–213. https://doi.org/10.1002/ptr.7305
Afroze N, Pramodh S, Hussain A et al (2020) A review on myricetin as a potential therapeutic candidate for cancer prevention. 3 Biotech 10:211. https://doi.org/10.1007/s13205-020-02207-3
Agraharam G, Girigoswami A, Girigoswami K (2021) Nanoencapsulated myricetin to improve antioxidant activity and bioavailability: a study on zebrafish embryos. Chemistry 4:1–17. https://doi.org/10.3390/chemistry4010001
Albuquerque BR, Heleno SA, Oliveira MBPP et al (2021) Phenolic compounds: current industrial applications, limitations and future challenges. Food Funct 12:14–29. https://doi.org/10.1039/d0fo02324h
Alidadi H, Ashtari A, Samimi A et al (2022) Myricetin loaded in solid lipid nanoparticles induces apoptosis in the HT-29 colorectal cancer cells via mitochondrial dysfunction. Mol Biol Rep 49:8537–8545. https://doi.org/10.1007/s11033-022-07683-9
Anwar S, Khan S, Anjum F et al (2022) Myricetin inhibits breast and lung cancer cells proliferation via inhibiting MARK4. J Cell Biochem 123:359–374. https://doi.org/10.1002/jcb.30176
Arafah A, Rehman MU, Ahmad A et al (2022) Myricetin (3,3′,4′,5,5′,7-hexahydroxyflavone) prevents 5-fluorouracil-induced cardiotoxicity. ACS Omega 7:4514–4524. https://doi.org/10.1021/acsomega.1c06475
Attwood PV, Muimo R (2017) The actions of NME1/NDPK-A and NME2/NDPK-B as protein kinases. Lab Invest 98:283–290. https://doi.org/10.1038/labinvest.2017.125
Awadelkareem AM, Al-Shammari E, Elkhalifa AEO et al (2022) Phytochemical and in silico ADME/Tox analysis of Eruca sativa extract with antioxidant, antibacterial and anticancer potential against Caco-2 and HCT-116 colorectal carcinoma cell lines. Molecules 27:1409. https://doi.org/10.3390/molecules27041409
Bai Y, Liu X, Chen Q et al (2021) Myricetin ameliorates ox-LDL-induced HUVECs apoptosis and inflammation via lncRNA GAS5 upregulating the expression of miR-29a-3p. Sci Rep 11:19637. https://doi.org/10.1038/s41598-021-98916-7
Barzegar A (2016) Antioxidant activity of polyphenolic myricetin in vitro cell- free and cell-based systems. Mol Biol Res Commun 5:87–95
Birbrair A, Zhang T, Wang Z-M et al (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307:C25–C38. https://doi.org/10.1152/ajpcell.00084.2014
Blonska M, Czuba ZP, Krol W (2003) Effect of flavone derivatives on interleukin-1beta (IL-1beta) mRNA expression and IL-1beta protein synthesis in stimulated RAW 264.7 macrophages. Scand J Immunol 57:162–166. https://doi.org/10.1046/j.1365-3083.2003.01213.x
Campos JJ, de Oliveira Azevedo A, de Souza Filho JD et al (2013) Bioguided isolation of myricetin-3-O-β-galactopyranoside with antinociceptive activity from the aerial part of Davilla elliptica St.-Hil. Journal of Ethnopharmacology 150:270–274. https://doi.org/10.1016/j.jep.2013.08.042
Canada AT, Watkins WD, Nguyen TD (1989) The toxicity of flavonoids to guinea pig enterocytes. Toxicol Appl Pharmacol 99:357–361. https://doi.org/10.1016/0041-008X(89)90018-5
Chang CJ, Tzeng T-F, Liou S-S et al (2012) Myricetin increases hepatic peroxisome proliferator-activated receptor α protein expression and decreases plasma lipids and adiposity in rats. Evidence-Based Complement Altern Med: eCAM 2012:787152. https://doi.org/10.1155/2012/787152
Chua LS, Latiff NA, Lee SY et al (2011) Flavonoids and phenolic acids from Labisia pumila (Kacip Fatimah). Food Chem 127:1186–1192. https://doi.org/10.1016/j.foodchem.2011.01.122
Ci Y, Zhang Y, Liu Y et al (2018) Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phytother Res 32:1373–1381. https://doi.org/10.1002/ptr.6071
Conley-LaComb M, Saliganan A, Kandagatla P et al (2013) PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol Cancer 12:85. https://doi.org/10.1186/1476-4598-12-85
Corell L, Armenta S, Esteve-Turrillas FA, de la Guardia M (2018) Flavonoid determination in onion, chili and leek by hard cap espresso extraction and liquid chromatography with diode array detection. Microchem J 140:74–79. https://doi.org/10.1016/j.microc.2018.04.014
Dang Y, Lin G, Xie Y et al (2014) Quantitative determination of myricetin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and its absolute bioavailability. Drug Res 64:516–522. https://doi.org/10.1055/s-0033-1363220
De Leo M, Braca A, Sanogo R et al (2006) Antiproliferative activity of Pteleopsis suberosa leaf extract and its flavonoid components in human prostate carcinoma cells. Planta Med 72:604–610. https://doi.org/10.1055/s-2006-931556
Dimas K, Demetzos C, Angelopoulou D et al (2000) Biological activity of myricetin and its derivatives against human leukemic cell lines in vitro. Pharmacol Res 42:475–478. https://doi.org/10.1006/phrs.2000.0716
Duthie SJ, Dobson VL (1999) Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur J Nutr 38:28–34. https://doi.org/10.1007/s003940050043
Duthie SJ, Collins AR, Duthie GG, Dobson VL (1997) Quercetin and myricetin protect against hydrogen peroxide-induced DNA damage (strand breaks and oxidised pyrimidines) in human lymphocytes. Mutat Res/genetic Toxicol Environ Mutagen 393:223–231. https://doi.org/10.1016/s1383-5718(97)00107-1
Elshamy AI, Ammar NM, Hassan HA et al (2020) Topical wound healing activity of myricetin isolated from Tecomaria capensis v. aurea. Molecules 25:4870. https://doi.org/10.3390/molecules25214870
Fan K-J, Yang B, Liu Y et al (2017) Inhibition of human lung cancer proliferation through targeting stromal fibroblasts by dihydromyricetin. Mol Med Rep 16:9758–9762. https://doi.org/10.3892/mmr.2017.7802
Feng J, Chen X, Wang Y et al (2015) Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell Biochem 408:163–170. https://doi.org/10.1007/s11010-015-2492-1
Fogelman E, Tanami S, Ginzberg I (2015) Anthocyanin synthesis in native and wound periderms of potato. Physiol Plant 153:616–626. https://doi.org/10.1111/ppl.12265
Gervasi T, Calderaro A, Barreca D et al (2022) Biotechnological applications and health-promoting properties of flavonols: an updated view. Int J Mol Sci 23:1710. https://doi.org/10.3390/ijms23031710
Geybels MS, Verhage BAJ, Arts ICW et al (2013) Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol 177:1388–1398. https://doi.org/10.1093/aje/kws419
Goyal R, Bala R, Sindhu RK et al (2022) Bioactive based nanocarriers for the treatment of viral infections and SARS-CoV-2. Nanomaterials 12:1530. https://doi.org/10.3390/nano12091530
Guo RX, Fu X, Chen J et al (2016) Preparation and characterisation of microemulsions of myricetin for improving its antiproliferative and antioxidative activities and oral bioavailability. J Agric Food Chem 64:6286–6294. https://doi.org/10.1021/acs.jafc.6b02184
Gupta G, Siddiqui MA, Khan MM et al (2020) Current pharmacological trends on myricetin. Drug Res 70:448–454. https://doi.org/10.1055/a-1224-3625
Ha TK, Jung I, Kim ME et al (2017) Anticancer activity of myricetin against human papillary thyroid cancer cells involves mitochondrial dysfunction–mediated apoptosis. Biomed Pharmacother 91:378–384. https://doi.org/10.1016/j.biopha.2017.04.100
Hagenacker T, Hillebrand I, Wissmann A et al (2010) Anti-allodynic effect of the flavonoid myricetin in a rat model of neuropathic pain: involvement of p38 and protein kinase C mediated modulation of Ca2+ channels. Eur J Pain 14:992–998. https://doi.org/10.1016/j.ejpain.2010.04.005
Han S-H, Lee J-H, Woo J-S et al (2022a) Myricetin induces apoptosis through the MAPK pathway and regulates JNK-mediated autophagy in SK-BR-3 cells. Int J Mol Med 49:54. https://doi.org/10.3892/ijmm.2022.5110
Han S-H, Lee J-H, Woo J-S et al (2022b) Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon 8:e09309. https://doi.org/10.1016/j.heliyon.2022.e09309
Hassan SM, Khalaf MM, Sadek SA, Abo-Youssef AM (2017) Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice. Pharm Biol 55:766–774. https://doi.org/10.1080/13880209.2016.1275704
Hou W, Hu S, Su Z et al (2018) Myricetin attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Future Med Chem 10:2253–2264. https://doi.org/10.4155/fmc-2018-0172
Huang H, Chen AY, Ye X et al (2015) Myricetin inhibits proliferation of cisplatin-resistant cancer cells through a p53-dependent apoptotic pathway. Int J Oncol 47:1494–1502. https://doi.org/10.3892/ijo.2015.3133
Imran M, Saeed F, Hussain G et al (2021) Myricetin: a comprehensive review on its biological potentials. Food Sci Nutr 9:5854–5868. https://doi.org/10.1002/fsn3.2513
Jahan N, Khalil-Ur-Rahman AS, Asi M (2013) Phenolic acid and flavonol contents of gemmo-modified and native extracts of some indigenous medicinal plants. Pak J Bot 45:1515–1519
Jain A, Madu CO, Lu Y (2021) Phytochemicals in chemoprevention: a cost-effective complementary approach. J Cancer 12:3686–3700. https://doi.org/10.7150/jca.57776
Jan F, Jan B, Akbar Dar M, et al (2022) A review on traditional uses, phytochemistry, and pharmacological activities of Verbascum thapsus. Edible Plants Health Dis 483–500. https://doi.org/10.1007/978-981-16-4959-2_16
Javed Z, Khan K, Herrera-Bravo J et al (2022) Myricetin: targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int 22:239. https://doi.org/10.1186/s12935-022-02663-2
Jayakumar JK, Nirmala P, Kumar BAP, Kumar AP (2014) Evaluation of protective effect of myricetin, a bioflavonoid in dimethyl benzanthracene-induced breast cancer in female Wistar rats. South Asian J Cancer 03:107–111. https://doi.org/10.4103/2278-330x.130443
Ji A, Hu L, Ma D et al (2022) Myricetin induces apoptosis and protective autophagy through endoplasmic reticulum stress in hepatocellular carcinoma. Evidence-Based Complement Altern Med: Ecam 2022:3115312. https://doi.org/10.1155/2022/3115312
Jiao D, Zhang XD (2016) Myricetin suppresses p21-activated kinase 1 in human breast cancer MCF-7 cells through downstream signaling of the β-catenin pathway. Oncol Rep 36:342–348. https://doi.org/10.3892/or.2016.4777
Jose J, Dhanya AT, Haridas KR et al (2016) Structural characterisation of a novel derivative of myricetin from Mimosa pudica as an anti-proliferative agent for the treatment of cancer. Biomed Pharmacother = Biomed Pharmacother 84:1067–1077. https://doi.org/10.1016/j.biopha.2016.10.020
Jung SK, Lee KW, Byun S et al (2008) Myricetin suppresses UVB-induced skin cancer by targeting fyn. Can Res 68:6021–6029. https://doi.org/10.1158/0008-5472.can-08-0899
Jung SK, Lee KW, Byun S et al (2010) Myricetin inhibits UVB-induced angiogenesis by regulating PI-3 kinase in vivo. Carcinogenesis 31:911–917. https://doi.org/10.1093/carcin/bgp221
Jung SK, Lee KW, Bode AM et al (2012) Abstract 167: Myricetin suppresses UVB-induced photoaging, skin cancer, and angiogenesis by targeting multiple kinases. Can Res 72:167–167. https://doi.org/10.1158/1538-7445.am2012-167
Kalff J, Robinson R (1925) XXVIII.—a synthesis of myricetin and of a galangin monomethyl ether occurring in galanga root. J Chem Soc Trans 127:181–184. https://doi.org/10.1039/CT9252700181
Kang BY, Kim SH, Cho D, Kim TS (2005) Inhibition of interleukin-12 production in mouse macrophagesvia decreased nuclear factor-κB DNA binding activity by myricetin, a naturally occurring flavonoid. Arch Pharmacal Res 28:274–279. https://doi.org/10.1007/bf02977791
Kang NJ, Jung SK, Lee KW, Lee HJ (2011) Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann N Y Acad Sci 1229:124–132. https://doi.org/10.1111/j.1749-6632.2011.06122.x
Kang HR, Moon JY, Ediriweera MK et al (2020) Dietary flavonoid myricetin inhibits invasion and migration of radioresistant lung cancer cells (A549-IR) by suppressing MMP-2 and MMP-9 expressions through inhibition of the FAK-ERK signaling pathway. Food Sci Nutr 8:2059–2067. https://doi.org/10.1002/fsn3.1495
Kenouche S, Sandoval-Yañez C, Martínez-Araya JI (2022) The antioxidant capacity of myricetin. A molecular electrostatic potential analysis based on DFT calculations. Chem Phys Lett 801:139708. https://doi.org/10.1016/j.cplett.2022.139708
Khan MI, Bouyahya A, Hachlafi NEL et al (2022) Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: a review on recent investigations. Environ Sci Pollut Res Int 29:24411–24444. https://doi.org/10.1007/s11356-021-17795-7
Khatoon E, Banik K, Harsha C et al (2020) Phytochemicals in cancer cell chemosensitisation: current knowledge and future perspectives. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.06.014
Kim GD (2017) Myricetin inhibits angiogenesis by inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in endothelial cells. J Cancer Prev 22:219–227. https://doi.org/10.15430/jcp.2017.22.4.219
Kim ME, Ha TK, Yoon JH, Lee JS (2014) Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res 34:701–706
Knickle A, Fernando W, Greenshields AL et al (2018) Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem Toxicol 118:154–167. https://doi.org/10.1016/j.fct.2018.05.005
Kong N-N, Fang S-T, Wang J-H et al (2014) Two new flavonoid glycosides from the halophyte Limonium franchetii. J Asian Nat Prod Res 16:370–375. https://doi.org/10.1080/10286020.2014.884081
Lee YS, Choi EM (2010) Myricetin inhibits IL-1β-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int Immunopharmacol 10:812–814. https://doi.org/10.1016/j.intimp.2010.04.010
Lee HS, Ha AW, Kim WK (2012) Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nurs Res Pract 6:294–300. https://doi.org/10.4162/nrp.2012.6.4.294
Lee SE, Son GW, Park HR et al (2015) Integrative analysis of miRNA and mRNA profiles in response to myricetin in human endothelial cells. BioChip J 9:239–246. https://doi.org/10.1007/s13206-015-9309-5
Li J, Qu W, Cheng Y et al (2014) The inhibitory effect of intravesical fisetin against bladder cancer by induction of p53 and down-regulation of NF-kappa B pathways in a rat bladder carcinogenesis model. Basic Clin Pharmacol Toxicol 115:321–329. https://doi.org/10.1111/bcpt.12229
Li M, Chen J, Yu X et al (2019) Myricetin suppresses the propagation of hepatocellular carcinoma via down-regulating expression of YAP. Cells 8:358. https://doi.org/10.3390/cells8040358
Li Y, Zhang J, Zhou H, Du Z (2022b) Anticancer effects of natural phytochemicals in anaplastic thyroid cancer (Review). Oncol Rep 48:156. https://doi.org/10.3892/or.2022.8368
Li M, Zha G, Chen R, et al (2021) Anticancer effects of myricetin derivatives in non‐small cell lung cancer in vitro and in vivo. Pharmacology Research & Perspectives 10. https://doi.org/10.1002/prp2.905
Li Q, Tan Q, Ma Y, et al (2022a) Myricetin suppresses ovarian cancer in vitro by activating the p38/Sapla signaling pathway and suppressing intracellular oxidative stress. Front Oncol 12. https://doi.org/10.3389/fonc.2022.903394
Lin GB (2012) Research advances of myricetin. J Int Pharm Res 39:483–487
Liu T, Peng F, Cao X et al (2021) Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega 6:30826–30833. https://doi.org/10.1021/acsomega.1c05256
Ma L, Cao X, Wang H et al (2019) Discovery of myricetin as a potent inhibitor of human flap endonuclease 1, which potentially can be used as sensitizing agent against HT-29 human colon cancer cells. J Agric Food Chem 67:1656–1665. https://doi.org/10.1021/acs.jafc.8b05447
Mao M, Huang M (2018) Myricetin attenuates lung inflammation and provides protection against lipopolysaccharide-induced acute lung injury by inhibition of NF-κB pathway in rats. Trop J Pharm Res 16:2585. https://doi.org/10.4314/tjpr.v16i11.3
Maroufi NF, Vahedian V, Mazrakhondi SAM et al (2020) Sensitisation of MDA-MBA231 breast cancer cell to docetaxel by myricetin loaded into biocompatible lipid nanoparticles via sub-G1 cell cycle arrest mechanism. Naunyn Schmiedebergs Arch Pharmacol 393:1–11. https://doi.org/10.1007/s00210-019-01692-5
Marrero AD, Quesada AR, Martínez-Poveda B, Medina MÁ (2022) Antiangiogenic phytochemicals constituent of diet as promising candidates for chemoprevention of cancer. Antioxidants 11:302. https://doi.org/10.3390/antiox11020302
Martens S, Preuß A, Matern U (2010) Multifunctional flavonoid dioxygenases: flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 71:1040–1049. https://doi.org/10.1016/j.phytochem.2010.04.016
Mendes V, Vilaça R, de Freitas V et al (2015) Effect of myricetin, pyrogallol, and phloroglucinol on yeast resistance to oxidative stress. Oxidative Med Cell Longev 2015:782504. https://doi.org/10.1155/2015/782504
Micek A, Godos J, Del Rio D, et al (2021) Dietary flavonoids and cardiovascular disease: a comprehensive dose–response meta‐analysis. Mol Nutr Food Res 2001019. https://doi.org/10.1002/mnfr.202001019
Mondal S, Jana J, Sengupta P et al (2016) Myricetin arrests human telomeric G-quadruplex structure: a new mechanistic approach as an anticancer agent. Mol BioSyst 12:2506–2518. https://doi.org/10.1039/c6mb00218h
Oh JH, Karadeniz F, Lee JI et al (2020) Anticatabolic and anti-inflammatory effects of myricetin 3-O-β-d-galactopyranoside in UVA-irradiated dermal cells via repression of MAPK/AP-1 and activation of TGFβ/Smad. Molecules 25:1331. https://doi.org/10.3390/molecules25061331
Ono K, Nakane H, Fukushima M et al (1990) Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular DNA and RNA polymerases. Eur J Biochem 190:469–476. https://doi.org/10.1111/j.1432-1033.1990.tb15597.x
Park H, Park S, Bazer FW et al (2018) Myricetin treatment induces apoptosis in canine osteosarcoma cells by inducing DNA fragmentation, disrupting redox homeostasis, and mediating loss of mitochondrial membrane potential. J Cell Physiol 233:7457–7466. https://doi.org/10.1002/jcp.26598
Parvez MK, Al-Dosari MS, Arbab AH et al (2020) Bioassay-guided isolation of anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharm J 28:550–559. https://doi.org/10.1016/j.jsps.2020.03.006
Phillips PA, Sangwan V, Borja-Cacho D et al (2011) Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett 308:181–188. https://doi.org/10.1016/j.canlet.2011.05.002
Qi S, Feng Z, Li Q et al (2017) Myricitrin modulates NADPH oxidase-dependent ROS production to inhibit endotoxin-mediated inflammation by blocking the JAK/STAT1 and NOX2/p47phox pathways. Oxid Med Cell Longev 2017:9738745. https://doi.org/10.1155/2017/9738745
Qu X, Li Q, Song Y et al (2020) Potential of myricetin to restore the immune balance in dextran sulfate sodium-induced acute murine ulcerative colitis. J Pharm Pharmacol 72:92–100. https://doi.org/10.1111/jphp.13197
Rajabi S, Maresca M, Yumashev AV et al (2021) The most competent plant-derived natural products for targeting apoptosis in cancer therapy. Biomolecules 11:534. https://doi.org/10.3390/biom11040534
Rajendran P, Maheshwari U, Muthukrishnan A et al (2021) Myricetin: versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS-reliant mitochondria-facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol Cell Biochem 476:57–68. https://doi.org/10.1007/s11010-020-03885-6
Rao KV, Seshadri TR (1948) Nuclear oxidation in flavones and related compounds. Proc Indian Acad Sci - Section A 28. https://doi.org/10.1007/bf03171085
Rinaldo D, Silva MA, Rodrigues CM et al (2006) Preparative separation of flavonoids from the medicinal plant Davilla elliptica St. Hill. by high-speed counter-current chromatography. Quim Nova 29:947–949. https://doi.org/10.1590/S0100-40422006000500011
Sato M, Murakami K, Uno M et al (2013) Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J Biol Chem 288:23212–23224. https://doi.org/10.1074/jbc.m113.464222
Semwal D, Semwal R, Combrinck S, Viljoen A (2016) Myricetin: a dietary molecule with diverse biological activities. Nutrients 8:90. https://doi.org/10.3390/nu8020090
Senggunprai L, Tuponchai P, Kukongviriyapan V et al (2018) Myricetin ameliorates cytokine-induced migration and invasion of cholangiocarcinoma cells via suppression of STAT3 pathway. J Cancer Res Ther. https://doi.org/10.4103/jcrt.jcrt_287_17
Sharma R, Rana A, Kumar D, Kumar S (2021) Exploration of bioactive constituents from abandoned parts of the tea plant. Sustain Agric Rev 56:143–179. https://doi.org/10.1007/978-3-030-84405-9_6
Sharma P, Khan MA, Najmi AK, et al (2022) Myricetin-induced apoptosis in triple-negative breast cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Med Oncol 39. https://doi.org/10.1007/s12032-022-01856-z
Shih Y-W, Wu P-F, Lee Y-C et al (2009) Myricetin suppresses invasion and migration of human lung adenocarcinoma A549 cells: possible mediation by blocking the ERK signaling pathway. J Agric Food Chem 57:3490–3499. https://doi.org/10.1021/jf900124r
Shuaib M, Kushwaha PP, Prajapati KS, et al (2021) Effect of dietary phytochemicals in obesity and cancer. Obes Cancer:163–184. https://doi.org/10.1007/978-981-16-1846-8_9
Siddiqui AJ, Jahan S, Singh R et al (2022) Plants in anticancer drug discovery: from molecular mechanism to chemoprevention. Biomed Res Int 2022:5425485. https://doi.org/10.1155/2022/5425485
Soleimani M, Sajedi N (2020) Myricetin apoptotic effects on T47D breast cancer cells is a P53-independent approach. Asian Pac J Cancer Prev 21:3697–3704. https://doi.org/10.31557/apjcp.2020.21.12.3697
Song Y, Zhou B, Du X et al (2020) Folic acid (F.A.)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed Pharmacother 125:109561. https://doi.org/10.1016/j.biopha.2019.109561
Sultana B, Anwar F (2008) Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem 108:879–884. https://doi.org/10.1016/j.foodchem.2007.11.053
Sun F, Zheng XY, Ye J et al (2012) Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr Cancer 64:599–606. https://doi.org/10.1080/01635581.2012.665564
Sun W, Tao Y, Yu D et al (2018) Myricetin exerts potent anticancer effects on human skin tumor cells. Trop J Pharm Res 17:1067. https://doi.org/10.4314/tjpr.v17i6.13
Sun W-L, Li X-Y, Dou H-Y et al (2021) Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Reports 36:109641. https://doi.org/10.1016/j.celrep.2021.109641
Taheri Y, Suleria HAR, Martins N, et al (2020) Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complement Med Ther 20. https://doi.org/10.1186/s12906-020-03033-z
Tan C-Y, Chang CL (2018) NDPKA is not just a metastasis suppressor - be aware of its metastasis-promoting role in neuroblastoma. Lab Inv: J Tech Methods Pathol 98:219–227. https://doi.org/10.1038/labinvest.2017.105
Tan G, Uson-Lopez RA, Rahman MdM et al (2018) Myricetin enhances on apoptosis induced by serum deprivation in PC12 cells mediated by mitochondrial signaling pathway. Environ Toxicol Pharmacol 57:175–180. https://doi.org/10.1016/j.etap.2017.12.016
Thant Y, Wang Q, Wei C et al (2021) TPGS conjugated pro-liposomal nano-drug delivery system potentiate the antioxidant and hepatoprotective activity of myricetin. J Drug Del Sci Technol 66:102808. https://doi.org/10.1016/j.jddst.2021.102808
Tranchimand S, Tron T, Gaudin C, Iacazio G (2006) First chemical synthesis of three natural depsides involved in flavonol catabolism and related to quercetinase catalysis. Synth Commun 36:587–597. https://doi.org/10.1080/00397910500406534
Tsai S-H (1999) Suppression of TNFa-mediated NFkB activity by myricetin and other flavonoids through downregulating the activity of IKK in ECV304 cells. J Cell Biochem 74:606–615. https://doi.org/10.1002/(SICI)1097-4644(19990915)74:4%3c606::AID-JCB10%3e3.0.CO;2-W
Wang S-J, Tong Y, Lu S et al (2010a) Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Med 76:1492–1496. https://doi.org/10.1055/s-0030-1249780
Wang ZH, Ah Kang K, Zhang R et al (2010b) Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ Toxicol Pharmacol 29:12–18. https://doi.org/10.1016/j.etap.2009.08.007
Wang L, Feng J, Chen X et al (2014) Myricetin enhance chemosensitivity of 5-fluorouracil on esophageal carcinoma in vitro and in vivo. Cancer Cell Int 14:71. https://doi.org/10.1186/s12935-014-0071-2
Wang F, Song Z-Y, Qu X-J et al (2018) M10, a novel derivative of myricetin, prevents ulcerative colitis and colorectal tumor through attenuating robust endoplasmic reticulum stress. Carcinogenesis 39:889–899. https://doi.org/10.1093/carcin/bgy057
Wang M, Ren S, Bi Z et al (2022) Myricetin reverses epithelial-endothelial transition and inhibits vasculogenic mimicry and angiogenesis of hepatocellular carcinoma by directly targeting PAR1. Phytother Res: PTR 36:1807–1821. https://doi.org/10.1002/ptr.7427
Xia W, Zheng B, Li T et al (2020) Fabrication, characterisation and evaluation of myricetin adsorption onto starch nanoparticles. Carbohydr Polym 250:116848. https://doi.org/10.1016/j.carbpol.2020.116848
Xianghui R, Hongju Z, Cheng Z et al (2018) Bioactivities of myricetin derivatives containing piperazine acidamide moiety†. Chem J Chinese Universities 39:1197. https://doi.org/10.7503/cjcu20170740
Xie J, Zheng Y (2017) Myricetin protects keratinocyte damage induced by UV through IκB/NFκb signaling pathway. J Cosmet Dermatol 16:444–449. https://doi.org/10.1111/jocd.12399
Xie Y, Wang Y, Xiang W et al (2020) Molecular mechanisms of the action of myricetin in cancer. Mini Rev Med Chem 20:123–133. https://doi.org/10.2174/1389557519666191018112756
Xu R (2013) Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro. Food Chem 138:48–53. https://doi.org/10.1016/j.foodchem.2012.09.102
Yan T, Tao Y, Wang X et al (2021) Preparation, characterisation and evaluation of the antioxidant capacity and antitumor activity of myricetin microparticles formated by supercritical antisolvent technology. J Supercrit Fluids 175:105290. https://doi.org/10.1016/j.supflu.2021.105290
Yang W, Su J, Li M et al (2021) Myricetin induces autophagy and cell cycle arrest of HCC by inhibiting MARCH1-regulated Stat3 and p38 MAPK signaling pathways. Front Pharmacol 12:709526. https://doi.org/10.3389/fphar.2021.709526
Ye C, Zhang C, Huang H et al (2018) The natural compound myricetin effectively represses the malignant progression of prostate cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4 interaction. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem Pharmacol 48:1230–1244. https://doi.org/10.1159/000492009
Yeshi K, Crayn D, Ritmejerytė E, Wangchuk P (2022) Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 27:313. https://doi.org/10.3390/molecules27010313
Yi J-L, Shi S, Shen Y-L et al (2015) Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int J Clin Exp Pathol 8:1116–1127
Zhang C, Zhang G, Liao Y, Gong D (2017) Myricetin inhibits the generation of superoxide anion by reduced form of xanthine oxidase. Food Chem 221:1569–1577. https://doi.org/10.1016/j.foodchem.2016.10.136
Zhang J, Aray B, Zhang Y et al (2023) Synergistic effect of cucurbitacin E and myricetin on anti-non-small cell lung cancer: Molecular mechanism and therapeutic potential. Phytomedicine 111:154619. https://doi.org/10.1016/j.phymed.2022.154619
Zhao J, Hong T, Dong M et al (2012) Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis. Mol Med Rep 7:565–570. https://doi.org/10.3892/mmr.2012.1225
Zhao H-F, Wang G, Wu C-P et al (2018) A multi-targeted natural flavonoid myricetin suppresses lamellipodia and focal adhesions formation and impedes glioblastoma cell invasiveness and abnormal motility. CNS Neurol Disord Drug Targets- CNS Neurolo Disord 17:557–567. https://doi.org/10.2174/1871527317666180611090006
Zhong X, Wang X, Chen L, et al (2017) Synthesis and biological activity of myricetin derivatives containing 1,3,4-thiadiazole scaffold. Chem Central J 11. https://doi.org/10.1186/s13065-017-0336-7
Zhou Z, Mao W, Li Y et al (2019) Myricetin inhibits breast tumor growth and angiogenesis by regulating VEGF/VEGFR2 and p38MAPK signaling pathways. Anat Rec 302:2186–2192. https://doi.org/10.1002/ar.24222
Zhou H, Xu L, Shi Y et al (2023) A novel myricetin derivative with anticancer properties induces cell cycle arrest and apoptosis in A549 cells. Biol Pharm Bull 46:42–51. https://doi.org/10.1248/bpb.b22-00483
Zhou P, Zhao X-N, Ma Y-Y, et al (2022) Virtual screening analysis of natural flavonoids as trimethylamine (TMA)-lyase inhibitors for coronary heart disease. J Food Biochem e14376. https://doi.org/10.1111/jfbc.14376
Zhu M-L, Zhang P-M, Jiang M et al (2020) Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement Med Ther 20:209. https://doi.org/10.1186/s12906-020-02965-w
Author information
Authors and Affiliations
Contributions
Suneel Kumar: Data collection and drafting of the article Nitin Swamy: Data collection and drafting of the article Hardeep Singh Tuli: Final approval of the version to be published Seema Rani: Data analysis and interpretation Abhijeet Garg: Illustrations and diagrams Deepa Mishra: Diagrams Hadi Sajid Abdulabbas: Critical revision of the article Sardul Singh Sandhu: The conception of the work. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Swamy, N., Tuli, H.S. et al. Myricetin: a potential plant-derived anticancer bioactive compound—an updated overview. Naunyn-Schmiedeberg's Arch Pharmacol 396, 2179–2196 (2023). https://doi.org/10.1007/s00210-023-02479-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02479-5