Abstract
The large number of plant-derived molecules have been explored in cancer cell lines and preclinical studies. As an outcome of these studies, decent number of isolated compounds expressed their candidature for being explored in clinical trials. The thrust behind development of plant-derived molecules is their low-cost and lesser side effects as compared to synthetic anticancer agents. Flavonoids are synthesized by plants as secondary metabolites and are a diverse chemical class possessing a wide spectrum of pharmacological activities such as anti-inflammatory, antiplatelet, antidiabetic, antimicrobial, cardioprotective, as well as anticancer activities. Various flavonoids especially genistein, myricetin, quercetin, luteolin, hesperidin, and epigallocatechin-3-gallate have been widely studied in cancer cell lines and animal models of tumorigenesis. In the recent past, the phase 1 and 2 clinical trials conducted with the aforementioned molecules have revealed promising results. Moreover, their supplementation reduced radiotherapy and chemotherapeutic agent-induced side effects in cancer patients. The present book chapter summarizes the subclasses of flavonoids with special reference to selected molecules from each class, which exhibited anticancer activity in cell lines and at present are being explored in various clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cancer is one of the leading causes of mortality, and every sixth death globally is due to cancer. About 8.9 million people died from different types of cancer in 2016. According to a recent report, the majority of deaths in cancer patients are predominantly due to cancer of the prostate, colorectum, breast, and lungs (Siegel et al. 2017). Even with the progress in the discovery of novel anticancer medications, cancer is still the leading cause of mortality worldwide (May 2014; Siegel et al. 2017). There is an increased incidence of cancer along with the unwanted side effects of chemotherapeutic agents. This has enforced the scientists to explore the future anticancer agents from natural sources. Notably, plant-derived anticancer agents have gained attention because of their low toxicity and better therapeutic efficacy (Pan et al. 2013). The plant-derived drugs have diverse mechanisms of action, but most of them cause apoptotic cell death by caspase or p53-dependent as well as p53-independent mechanisms. In addition, plant-derived drugs exhibit their anticancer activity through certain novel mechanisms such as autophagy, mitotic catastrophe, and senescence leading to cell death and necrosis-like programmed cell death (Gali-Muhtasib et al. 2015).
Plants synthesize a wide array of chemical compounds like flavonoids, alkaloids, glycosides, terpenoids, etc. Many of these compounds are produced by plant as secondary metabolites, which help the plants to respond against various environmental stimuli and stresses as well as genetically programmed developmental signals. It is estimated that more than 50% of modern pharmaceuticals have originated from the plant sources. In the recent times, there is an increasing contemplation in the scientific community about the importance of phytomedicines, phytochemistry, and pharmacological investigations of natural health products and diets for treating noncommunicable diseases, especially cancer, type 2 diabetes, obesity, cardiovascular, and neurodegenerative disorders. Partly, this is due to the realization that in folklore medicine, herbal remedies have been used effectively for treating different ailments. At present, there exists data from an overwhelming number of in vitro and in vivo studies showing beneficial effects of plant-based extracts and their bioactive ingredients. Many clinical studies with isolated ingredients from plants have revealed multiple health benefits in boosting immune function, anti-inflammation, antimicrobial, and antioxidant activities.
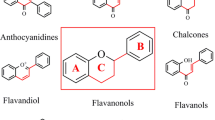
Flavonoids are polyphenolic substances, which are widely found in fruits, vegetables, and certain beverages. They are associated with various therapeutic activities and are present in various nutraceutical, pharmaceutical, medicinal, and cosmetic preparations. The basic structure of flavonoid contains flavan nucleus having 15 carbon atoms arranged in three rings (C6–C3–C6). The various classes of flavonoids exist as flavones (e.g., apigenin and kaenpteral), flavanones (e.g., hesperetin and fisetin), catechins (e.g., catechin and epigallocatechin gallate), and anthocyanins (e.g., cyanidin and delphinidin). The basic nucleus of flavonoid and its various subtypes is given in Fig. 9.1. In addition, the various food sources of various types of flavonoids are summarized in Table 9.1.
2 Flavonoids as Pharmacological Agents
Several types of flavonoids, flavanols, flavones, and flavanonols isolated from plants, vegetables, and fruits have shown multifarious biological activities, such as antioxidant, anti-inflammatory, antidiabetic, cardioprotective, as well as anticancer activity. Various pharmacological activities of flavonoids are depicted in Fig. 9.2.
3 Flavonoids as Anticancer Agents
3.1 Flavanols
3.1.1 Myricetin
Myricetin is a phenolic compound isolated from Myrica nagi Thunb. bark belonging to family Myricaceae (Lau-Cam and Chan 1973). It is found mostly in vegetables, berries, wines, and teas prepared from different plants mainly from families Primulaceae (Chua et al. 2011), Polygonaceae (El-Kader et al. 2013), Myricaceae (Jones et al. 2011), Pinaceae (Hergert 1956), and Anacardiaceae (Umadevi et al. 1988). It is 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone and occurs in free and bound form such as myricetin-3-O-(4″-acetyl)-α-L-arabinopyranoside, myricetin-3-O- β-D-xylopyranoside, myricetin-3-O-(3″-O-galloyl)-α-L-rhamnoside, etc. (De Leo et al. 2006; Kong et al. 2014). Myricetin exhibits its anticancer activity against several types of cancers. Myricetin affects Akt signaling in EGF-induced cell transformation by competing with ATP and thereby inhibits the expression of Akt. Thus, it is demonstrated that myricetin is an inhibitor of Akt which is overexpressed in the cancer cells. Moreover, it inhibits the cancer cell growth by inhibiting their entry into mitotic phase by targeting the kinase activity of cyclin B/CDK1 complexes. Studies suggest its antimitotic potential in treating liver cancer and its apoptotic cell death-promoting activity in various cell lines. In addition, myricetin targets the tumor metastasis and angiogenesis mechanisms by targeting several proteins including MMP-9, MMP-13, VGEF, and HIF-1 (Devi et al. 2015).
3.1.2 Quercetin
Quercetin (3,3,4,5,7-pentahydroxy flavanone) is a unique bioflavonoid found abundantly in fruits and vegetables such as grapes, tomatoes, Brassica vegetables, onions, and tea (Häkkinen et al. 1999; USDA 2011). Reports suggest that quercetin in combination with many naturally occurring compounds such as luteolin derivatives, resveratrol, 2-methoxyestradiol, ellagic acid, and synthetic drugs like cisplatin and doxorubicin resulted in synergistic anticancer activity (Akagi et al. 1995; Mertens-Talcott and Percival 2005; Nessa et al. 2011; Wang et al. 2012; Yang et al. 2015).
Quercetin modulates the cell signaling by inhibiting the high-mobility group box protein 1 (HMGB1)-induced TNF-α and IL-1β expression, which further regulates activity of various pro-inflammatory cytokines (Degryse et al. 2001). Furthermore, quercetin considerably inhibits the degradation of IҡBα and nuclear translocation of NF-ҡB which is important for cytokine expression (Park et al. 2004; Kokkola et al. 2005). Quercetin has been demonstrated to prevent metastasis of breast cancer cells through suppression of matrix metalloproteinase-9 (MMP-9) in 12-O-tetradecanoyl phorbol-13-acetate (TPA)-treated MCF-7 cells (Lin et al. 2008). Oral administration of quercetin encapsulated with tamoxifen in PLGA nanoparticles significantly increased its bioavailability and attenuated breast cancer cell growth through induction of apoptosis (Jain et al. 2013).
3.1.3 Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene) is a naturally occurring polyphenol belonging to stilbenes. It is found mostly in peanuts, berries, grapes, and plant sources and also in red wine (Bielsalski 2007). In plants, resveratrol exists in two isomeric forms, i.e., trans-resveratrol and cis-resveratrol, and their glucosides, trans-piceid and cis-piceid. The anticancer potential of resveratrol was first published in 1997 (Jang et al. 1997).
Research reports suggest that resveratrol downregulates the Kras expression, prevents the formation and progression of colorectal tumors, and increases the expression of miR-96 (Saud et al. 2014). Furthermore, it also modulates the mitomycin C-mediated effects of colorectal cancer by inhibiting cell growth and upregulating p21 which blocks cell cycle at G0/G1 and G2/M phases (Ali and Braun 2014). It further regulates the metabolism of glucose and regulates GLUT1 in ovarian cancer cell lines. Resveratrol suppresses glucose uptake and inhibits plasma membrane GLUT1 localization linked with the inhibition of the activity of Akt in ovarian cancer cell lines (Gwak et al. 2015).
In a clinical trial study, Patel and his colleagues demonstrated that in colon cancer patients, resveratrol at dose levels of 0.5 and 1.0 g reduces tumor cell proliferation by 5% (Patel et al. 2010). In an another study, Brown et al. showed that resveratrol causes a decrease in circulating insulin-like growth factor (IGF)-I and IGF-binding proteins (IGFBP)-3 in healthy volunteers (Brown et al. 2010). This study demonstrated that resveratrol may affect the IGF axis probably by direct effect on IGF-I and IGFBP-3. These proteins may also serve as potential markers in chemopreventive efficacy in human clinical trials (Jogie-Brahim et al. 2009) (Fig. 9.3).
3.2 Flavones
3.2.1 Luteolin
Luteolin or 3′,4′,5,7-tetrahydroxyflavone is a flavonoid present in various fruits, vegetables, as well as medicinal herbs. Traditional Chinese medicine has used luteolin-rich herbs as anti-inflammatory and anticancer agent. These biological effects of luteolin are attributed to antioxidant or pro-oxidant activity (Lin et al. 2008). Luteolin has been noted to kill various types of cancer cells including leukemia, pancreatic tumor, hepatoma, and lung carcinoma (Huang et al. 1999; Lee et al. 2002, 2005; Cheng et al. 2005). Luteolin has been demonstrated to serve as anticancer agent by inhibiting cell proliferation, metastasis, angiogenesis, and induction of apoptosis. Luteolin promotes cytotoxicity in cancer cells by suppressing survival mechanisms such as PI3K/Akt pathway and stimulating the tumor suppressor p53 signaling (Han et al. 2002). Luteolin suppressed the cancer stem cell properties and their metastatic potential in prostate cancer cells (Tsai et al. 2016). Luteolin inhibits the human cytochrome P450 (CYP) 1 enzymes including CYP1A1, CYP1A2, and CYP1B1, which further suppress the activation of carcinogens (Kim et al. 2005). In vascular smooth muscle cells, luteolin inhibited the PDGF-mediated proliferation of endothelial cells and consequently inhibited the PDGF-induced activation of ERK, PI3K/Akt, and PLC-1 along with reduction of c-fos gene expression (Kim et al. 2005). Moreover, luteolin promotes JNK-mediated apoptosis by modulating bad or p53 pathways (Yu et al. 2004; Ju et al. 2007). Notably, JNK-mediated p53 activation governs the expression of Bax, which further regulates apoptosis (Yu et al. 2004). Luteolin has been demonstrated to induce endoplasmic reticulum stress and mitochondrial dysfunction which leads to apoptosis in gliomablastoma (Wang et al. 2017). Luteolin treatment induced G0/G1 phase arrest in SMMC-7721 hepatocarcinoma cell line. Luteolin promoted autophagy by increasing number of intracellular autophagosomes in cancer cells. Interestingly, chloroquine, an autophagy inhibitor, attenuated the anticancer effect of luteolin in hepatocarcinoma cell line (Cao et al. 2017). Another novel mechanism suggested for anticancer potential of luteolin is blockage of ribosomal S6 kinase (RSK). RSK is ERK regulated and is responsible for cell growth and its survival. Luteolin treatment blocked RSK-1 and demonstrated marked anticancer potential in MOLM-13 and Kasumi-1 leukemic cells (Deng et al. 2017). Luteolin inhibited the incidence rate of tumors and decreased tumor volume in 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinogenesis in rats (Samy et al. 2006). In another study, luteolin reduced DMBA-induced lung carcinogenesis in mice (Kasala et al. 2016). An early phase I clinical trial is under process to examine whether luteolin and nano-luteolin exert an inhibitory effect on tongue squamous cell carcinoma cell lines by inducing apoptosis and to assess if nano-luteolin has more efficient apoptotic activity than luteolin on tongue squamous cell carcinoma cell line (https://clinicaltrials.gov/ct2/show/NCT03288298).
3.2.2 Diosmetin
Diosmetin (3′,5,7-trihydroxy-4′-methoxyflavone) is the aglycone part of the flavonoid glycoside diosmin (3′, 5, 7-trihydroxy-4′-methoxyflavone-7-aminoglycoside) which occurs naturally in the genus Teucrium (Lamiaceae) and in Portuguese olive leaves (Meirinhos et al. 2005; Macedonia 2005; Spanakis et al. 2009). Intestinal microflora enzymes hydrolyze diosmin to its aglycone diosmetin before its absorption into the body (Kanaze et al. 2004). Pharmacologically, it has been established that diosmetin possesses different medicinal properties such as antimicrobial, antioxidant, anti-inflammatory, as well as anticancer activities (Chandler et al. 2010; Domínguez et al. 2011; Zhao et al. 2011). In a study, diosmetin is identified as a CYP1 substrate (Androutsopoulos et al. 2009a). CYP1A1 is one of the cytochrome P450 enzymes, which has been extensively examined for its capacity to activate compounds having carcinogenic potential. The exposure to environmental carcinogens is noted to increase the level of CYP1A1 expression through aryl hydrocarbon receptors. Diosmetin treatment inhibited cell proliferation of the human breast adenocarcinoma MCF-7 cells which were pre-induced with the potent CYP1 inducer 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Androutsopoulos et al. 2009a). Diosmetin inhibited the proliferation and progression of cell cycle in MDA-MB 468 cells by affecting CYP1 enzyme, whereas it had no aversive effect on normal breast cell lines MCF-10A. This shows its safety of use over other synthetic drugs. Diosmetin is also reported to induce G1 arrest in MDA-MB-468 cell lines. Interestingly, it is proclaimed that the diosmetin is metabolized to similar flavone luteolin in MDA-MB-468 breast cancer cell lines selectively through aromatic demethylation of the B ring by CYP1A1, CYP1B1, and the hepatic enzyme CYP1A2, which is not seen in MCF-7A cells (Androutsopoulos et al. 2009b).
3.2.3 Nobiletin
Nobiletin (5,6,7,8,3′, 4′-hexamethoxyflavone) is a major component of Citrus depressa and is noted to exhibit anticancer activity in various in vitro and in vivo studies. Literature reveals that nobiletin inhibits the proliferation of skin, breast, prostate, and colon carcinoma cell lines (Kandaswami et al. 1991). It inhibits the production of matrix metalloproteases which results in antiproliferative activity (Ishiwa et al. 2000). Nobiletin inhibits the invasion of human fibrosarcoma HT-1080 cells by suppressing the metalloproteinases and activating TIMP-1 production. Nobiletin inhibits the phosphorylation of mitogen-activated protein/extracellular signal-regulated kinase-1/2 (MEK-1/2). Notably, U0126, a MEK1/2 inhibitor, imitated the nobiletin’s action to reduce ability to decrease 12-O-tetradecanoyl phorbol-13-acetate (TPA)-stimulated production of proMMPs-1 and proMMPs-9 in human fibrosarcoma HT-1080 cells (Miyata et al. 2004). Moreover, in TPA-treated HT-1080 cells, nobiletin assisted the phosphorylation of c-Jun NH2-terminal kinase (JNK), which is an important downstream signal factor of the PI3K/Akt pathway. Among 40 different flavonoids, nobiletin showed the maximum antiproliferative activity in six human cancer cell lines (Murakami et al. 2000; Manthey and Guthrie 2002). It suppresses the prostaglandin E2 (PGE2) production and cyclooxygenase-2 expression in in vitro studies (Kohno et al. 2001). Studies demonstrated that administration of nobiletin-rich C. Reticulata peel extract for 1 year exhibits preventive effects on the progression of the cognitive impairment in donepezil-pre-administered Alzheimer disease patients without any side effects. Unfortunately, the research on nobiletin clinical application is quite limited, which might be due to the uncertainty of molecular targets. More clinical trials of nobiletin and its metabolites are still needed.
3.3 Flavanones
3.3.1 Hesperidin
Hesperidin (5,7,3′-trihydroxy-4′-methoxy-flavanone 7-rhamnoglucoside) belongs to the class of flavonoids called flavanones and is predominantly found in citrus fruits. Hesperidin has been noted to possess a diverse range of pharmacological activity attributing to its anti-inflammatory and antioxidant potential. In the last few years, hesperidin has gained attention of cancer biologists, and it has been screened extensively in vitro and in vivo for its antimutagenic and anticancer properties. In the endometrial cancer cells, hesperidin induced apoptosis by increasing Bax and decreasing Bcl2 and promoted cell death by downregulating estrogen receptor I (Cincin et al. 2018). Hesperidin has been noted to mitigate the migration and invasion of A549 cancer cells by inhibiting SDF-1/CXCR-4 cascade (Xia et al. 2018b). Hesperidin has been demonstrated to suppress azoxymethane-induced colon carcinogenesis in rats (Tanaka et al. 1997). Interestingly, hesperidin administration along with doxorubicin has been reported to increase laters’ anticancer activity along with reduction in its side effects in Ehrlich ascites carcinoma-bearing mice (Donia et al. 2018). Hesperidin treatment demonstrated anticancer activity by inducing endoplasmic reticulum stress and G0/G1 arrest in ovarian cancer cell line and A549 lung cancer cell line, respectively (Zhao et al. 2017; Xia et al. 2018a). Hesperidin attenuated diethylnitrosamine/carbon tetrachloride-induced hepatocarcinogenesis in rats through activation of PPAR-γ and Nrf-2/ARE/HO-1 signaling (Mahmoud et al. 2017). Notably, hesperidin demonstrated better cytotoxic activity against human hepatic cancer HepG2 cell line than other flavonoids such as neohesperidin, naringin, and naringenin. Moreover, hesperidin has been noted to induce apoptosis in HepG2 cells through mitochondrial as well as death receptor pathway (Banjerdpongchai et al. 2016). Hesperidin has been noted to upregulate tumor suppressor phosphatase and tensin homologue (PTEN) and reduce the expression of PI3K/Akt survival pathway in azoxymethane-induced colon carcinoma in mouse. Moreover, hesperidin-mediated restoration of glycogen beta-synthase-3 attenuated the proto-oncogenes such as c-jun, c-myc, and β-catenin, thereby resulting in anticancer activity in colon cells (Saiprasad et al. 2014) (Figs. 9.4 and 9.5).
3.4 Flavan-3-Ols
3.4.1 Epigallocatechin-3-Gallate
Green tea is extracted from the leaves of evergreen shrub Camellia sinensis and is almost consumed all over the world (Yang et al. 2009). Green tea mainly contains polyphenols and catechins such as epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate, epigallocatechin (EGC), and epicatechin (EC). Among all the above catechins, epigallocatechin-3-gallate (EGCG) possesses powerful anticancer activity due to its antioxidative potential (Katiyar and Mukhtar 1996). EGCG is the ester form of epigallocatechin and gallic acid. EGCG has also been reported to have beneficial effects in the treatment of neurodegenerative diseases (Hügel and Jackson 2012), cardiovascular diseases (Tipoe et al. 2007), cancer (Schramm 2013), diabetes (Thielecke and Boschmann 2009), and liver diseases (Xiao et al. 2014).
EGCG inhibits tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis by inhibiting 8-hydroxydeoxyguanosine formation (Xu et al. 1992). Furthermore, it inhibits dimethylarsinic acid and cisplatin-induced lung tumorigenesis in rodent models (Mimoto et al. 2000; An et al. 2008) and diethylnitrosamine-induced liver tumorigenesis by inhibiting insulin-like growth factor signaling in diabetic and obese C57BL/KsJ-db/db mice (Shimizu et al. 2011). EGCG inhibits angiogenesis and tumor growth in human pancreatic cancer and breast cancer by downregulating VEGF expression both in serum-deprived HT29 human colon cancer cells and in vivo (Jung et al. 2011; Shankar et al. 2013; Braicu et al. 2013). Moreover, EGCG inhibits invasion and metastasis in hypopharyngeal carcinoma cells by downregulating hepatocyte growth factor (HGF)-induced MMP-9 as well as activation of urokinase-type plasminogen activator (uPA) (Lim et al. 2008).
The consumption of green tea exerts beneficial effects even after a single dose. The levels of prostaglandin E2 (stimulates colorectal carcinogenesis) in tissue were reduced in normal subjects after 4 h of green tea consumption (August et al. 1999). The derivatives of green tea have shown effectiveness against various malignancies such as cervical, hepatic, and prostate, without toxicity in patients with premalignant conditions (Ahn et al. 2003; Bettuzzi et al. 2006; Luo et al. 2006). However, in a phase 2 clinical trial of China, there is no marked effect observed in biomarkers of esophageal squamous carcinogenesis from decaffeinated green tea (Wang et al. 2002).
In a single-arm trial testing, the effectiveness of EGCG against radiation-induced dermatitis in the patients of breast cancer demonstrated promising results. Topical application of EGCG reduces the pain in 85.7%, itching in 87.8%, tenderness in 79.6%, and burning feel in 89.8% patients, who underwent radiotherapy (Zhu et al. 2016). In a phase II clinical trial, EGCG treatment ameliorated the acute radiation-induced esophagitis (ARIE) in patients with stage III lung cancer. ARIE is one of the dose-dependent toxicities complicated by thoracic radiotherapy (Zhao et al. 2015). The supplementation of green extract having high concentration of EGCG for 12 months showed no observed change in the mammographic density in all postmenopausal women, but a marked reduction in percent mammographic density (PMD) was observed in 50–55-year-old women suggesting the effectiveness of green tea supplementation in preventing breast cancer (Samavat et al. 2017). The use of EGCG in bladder cancer in patients has demonstrated prosperous result in phase II clinical trial (Gee et al. 2017) (Fig. 9.6).
3.4.2 Pomegranate-Derived Polyphenols
Punica granatum is a small tree of family Punicaceae, commonly known as pomegranate. The fruits of the plant are used in many cultures. Of note, the name Punica has been derived from the Roman name of city Carthage, where best pomegranates have been known to grow. This tree is native to Persia but is now cultivated in America, Mediterranean area, and Asia. A class of tannins known as punicalagins unique to pomegranates has been demonstrated to possess excellent free radical scavenging properties (Gil et al. 2000; Noda et al. 2002).
Scientific reports suggest the potential role of pomegranate in the prevention as well as treating various types of cancer such as skin cancer, lung cancer, breast cancer, and prostate cancer because of its antioxidant nutrients. It slows down the propagation of cancer cells and accelerates their death. It also diminishes the blood supply to tumors and makes them smaller by starvation (Adhami et al. 2009).
Polyphenols obtained from the fermented juice and pericarp of pomegranate inhibit the proliferation and invasion of cells by inhibiting the secretory phospholipase (Lansky et al. 2005; Seeram et al. 2007; Espín et al. 2007). Standardized pomegranate extract having ellagitannins and ellagic acid suppresses the expression of androgen receptor through the inhibition of androgen-synthesizing enzymes. Moreover, pomegranate juice or extract inhibits the CYP enzyme, induces apoptosis and inhibits tumor growth, and decreases the serum PSA levels (Malik et al. 2005; Espín et al. 2007; Rettig et al. 2008; Paller et al. 2013). In skin cancer, it protects the fibroblasts from cell death and facilitates the skin repair (Aslam et al. 2006; Pacheco-Palencia et al. 2008; Hayouni et al. 2011). It also inhibits the skin edema, hyperplasia, and leukocytic infiltration induced by UV-B (Afaq et al. 2010; Khan et al. 2011).
In a human study, drinking of 8 oz. pomegranate juice per day increased the amount of time it took for their prostate-specific antigen (PSA) to double in patients who had surgery or radiation therapy for treating prostate cancer. Notably, the patients doubling PSA levels a short period of time have more risk of getting prostate cancer. Daily consumption of pomegranate juice increased the time of PSA levels to double from about 15 months to 54 months (Hajleh and Al-Dujali 2016).
3.5 Isoflavones
3.5.1 Genistein
Genistein (4′,5,7-trihydroxyisoflavone) was originally isolated from Genista tinctoria Linn. (Dyer’s broom) in 1899. It is predominant isoflavone of soy products (Perkin and Newbury 1899). Genistein structurally resembles estrogen, and therefore isoflavones have also been known as phytoestrogens. Genistein can thus bind to estrogen receptors due to structural similarity (Kuiper et al. 1997). It is documented that genistein can inhibit the growth of various cell lines such as prostate, leukemia, lymphoma, breast, lung, head, and neck cancer cells both in vitro and in vivo (Taylor et al. 2009). Various studies have reported the role of genistein as anticancer in every step of tumor progression. Genistein has been noted to attenuate the growth of cancer cells by inhibiting PTK-mediated signaling pathways (Akiyama et al. 1987; Sakla et al. 2007). It also exerts its inhibitory effect on all steps of cancer progress through apoptosis and cell cycle arrest, regulating the AKT/IKK/NF-ĸb, androgen mediated and other signaling pathways in the development of carcinogenesis. Studies showed that genistein modulates the expression of genes that regulates cell cycle and growth and thereby inhibits progression of cancer (Pavese et al. 2010).
Genistein is an isoflavone, which means its B ring is attached to the heterocyclic ring at the C3 position instead of C2 (Jacob, Hagai and Soliman 2011). It is a prominently found in soy products, (Herman et al. 1995; Barnes 1995). It inhibits cancer cell growth and induces apoptosis by modulating the expression of genes related to apoptotic pathways and inhibits Akt activation and NF-ĸB in cancer cells (Li et al. 1999; Davis et al. 1999). Genistein inhibits the invasive potential of human prostate cancer cell lines which suggest that it could inhibit the metastatic growth of prostate cancer (Santibanez et al. 1997). The in vitro studies using microarray shown that the genistein regulates the expression of genes involved in angiogenesis, cell cycle, cell growth, cell signal transduction, metastasis, and tumor cell invasion (Li and Sarkar 2002). In targeting the breast cancer, genistein possesses higher affinity toward ERβ subunit of estrogen receptor (ER) comparable to other isoflavones. This is attributed to the presence of a phenolic hydroxyl group, which is required for the formation of an intramolecular hydrogen bonding. The low concentrations of genistein (EC50 4 nM) overexpress gene expression and reduce proliferation more efficiently when ERβ is present. At higher doses, it stimulates the proliferation of MCF7/ERα cells which is counted as bad effects. At the end of 30-day clinical trial on adults, early-stage breast cancer patients (mainly HER2-negative and ER-positive), those with soy supplementation and high plasma genistein, had overexpression of tyrosine kinase receptor FGFR2 and other genes regulating proliferation pathways and cell cycle (Shike et al. 2014).
Genistein inhibited the HER2 expression, phosphorylation, and promoter activity through ER-independent manner (Sakla et al. 2007). MDA-MB-231 cell lines treated with varying concentrations (5–10–20 μM) of genistein demonstrated induction of apoptosis and G2/M cell cycle arrest in a dose as well as time-dependent manner. This effect is due to genistein inhibition of NFĸB through NOTCH-1 signaling, which affects Bcl-2 and Bcl-xl expression as a consequence of NFĸB inhibition (Pan et al. 2012). In a phase 2 chemoprevention trial on bladder cancer, the daily oral dose of genistein (300 mg/day and 600 mg/day) for 14–21 days before the surgery targets p-EGFR (endothelial growth factor receptor). The difference between p-EGFR staining of placebo arm and genistein arm is significantly different in 300 mg/day group but not in 600 mg/day (Messing et al. 2012). Genistein displayed a possible bimodal effect on bladder cancer tissue EGFR phosphorylation. Phase 2 studies of genistein were conducted in patients with prostate cancer. In this study, before undergoing radical prostatectomy for localized prostate cancer, patients were randomized to treatment with 2 mg genistein per kg of body weight versus no treatment (Xu et al. 2009). Normal prostate epithelial cells were excised selectively from prostate tissue by laser capture microdissection after the treatment, and these cells represent an “at-risk” target-type cells which are decent target for compound which arrests the conversion to an invasive phenotype. The qRT-PCR used to measure levels of MMT-2 transcript demonstrated that genistein reduced the MMT-2 gene expression to 24% of the level observed in control subjects. This study establishes the possibility of inhibiting prometastatic processes through a targeted therapeutic intervention in human subjects (Xu et al. 2009). In another phase 2 trial, patients having progressive prostate cancer when treated with soy milk for 12 months 3 times a day reduced the rise in PSA antigen as compared to its increase in patients before entering the study. Moreover, in a third phase 2 trial of genistein, men with prostate cancer were administered soy extract for 6 months, and it was concluded that the therapy was well tolerated with less than 10% patients experiencing mild diarrhea, and in 17% of patients, there was reduction of PSA levels (deVere White et al. 2004; Pendleton et al. 2008). Administration of genistein has been noted to influence various genes responsible for cell proliferation in randomized double-blind clinical trial of patients with localized prostate cancer (Bilir et al. 2017). Moreover, treatment with AXP107-11 (the crystalline form of genistein) in phase I trial of pancreatic cancer patients in combination with gemcitabine demonstrated a favorable pharmacokinetic profile along with its increased bioavailability without any toxicity (Lohr et al. 2016) (Fig. 9.7).
3.6 Anthocyanins
3.6.1 Cyanidin
Anthocyanins are widely distributed in human diets and are used for food color, suggesting that we ingest the considerable amount of anthocyanins from plant-based daily diets. In six different tumor cell lines (K562, PC3, HT-29, M-14, MCF-7, and DU145), it effectively halted the growth of cancer cells at lower GI50 concentrations than quercetin (Murphy et al. 2003). Cyanidin significantly attenuated zymosan-mediated inflammation in rodents. It suppressed the peritoneal exudates, tumor necrosis factor-α (TNF-α) interleukin-1 β (IL-1β) and IL-6, and cytokine-induced neutrophil chemoattractant-1 protein (CINC-1) levels (Tsuda et al. 2002). The zymosan elevated serum-α 2-macroglobulin, and decrease in serum albumin and transferrin level was corrected by cyanidin in vivo. Ingestion of cyanidin-3-glucoside (C3G) in ApcMin mice reduced the intestinal adenomas in a dose-dependent manner (Cooke et al. 2006). Total C3G concentration in mice was 43 ng/g and 8.1 μg/g tissue, respectively, in the intestinal mucosa and 7.2 and 12.3 μg/ml in the urine (Cooke et al. 2006). In a 13C-tracer clinical trial, total eight participants consumed 500 mg isotopically labeled C3G (6,8,10,3′,5’-13C5-C3G). The maximal elimination rate of C3G is seen after 6–24 h in feces while minimal in blood after 30 min. Although several studies have been done with cyanidin, yet very few have been conducted for anticancer activities (Fig. 9.8).
The data of various flavonoids which are clinically tested in various types of cancer patients are discussed in Table 9.2.
4 Conclusion
So far, tremendous information has been gathered by various studies exploring the role of flavonoids as potential anticancer agents in laboratories. The prosperous findings of cell lines and preclinical studies compelled the clinicians to further take up the flavonoids in clinical trials. In human trials, the flavonoids have demonstrated prosperous results. In addition, their supplementation reduced chemotherapy- and radiotherapy-induced complications in cancer patients. However, most of these trials are single centric and enrolled relatively small number of patients. The validity of flavonoids as potential anticancer agents is yet to be proven in multicentric trials involving large number of patients. In conclusion, the outcome of clinical studies is promising and presents flavonoids as potential anticancer agents.
References
Adhami VM, Khan N, Mukhtar H (2009) Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr Cancer 61:811–815
Afaq F, Khan N, Syed DN, Mukhtar H (2010) Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem Photobiol 86:1318–1326
Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, Bae SM, Lee IP (2003) Protective effects of green tea extracts (polyphenol E and EGCG) on human cervical lesions. Eur J Cancer Prev 12:383–390
Akagi K, Hirose M, Hoshiya T, Mizoguchi Y, Ito N, Shirai T (1995) Modulating effects of ellagic acid, vanillin and quercetin in a rat medium term multi-organ carcinogenesis model. Cancer Lett 94:113–121
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe SI, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262:5592–5595
Ali I, Braun DP (2014) Resveratrol enhances mitomycin C-mediated suppression of human colorectal cancer cell proliferation by upregulation of p21WAF1/CIP1. Anticancer Res 34:5439–5446
An Y, Li Z, Wang S, Wang Z (2008) Inhibition of (−) epigallocatechin gallate on dimethyl arsenic acid promoting lung tumorigenesis through the induction of oxidative stress in mice. J Hyg Res 37:748–750
Androutsopoulos VP, Mahale S, Arroo RR, Potter G (2009a) Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep 21:1525–1528
Androutsopoulos V, Wilsher N, Arroo RR, Potter GA (2009b) Bioactivation of the phytoestrogen diosmetin by CYP1 cytochromes P450. Cancer Lett 274:54–60
Aslam MN, Lansky EP, Varani J (2006) Pomegranate as a cosmeceutical source: pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J Ethnopharmacol 103:311–318
August DA, Landau J, Caputo D, Hong J, Lee MJ, Yang CS (1999) Ingestion of green tea rapidly decreases prostaglandin E2 levels in rectal mucosa in humans. Cancer Epidemiol Biomarkers Prev 8:709–713
Banjerdpongchai R, Wudtiwai B, Khaw-On P, Rachakhom W, Duangnil N, Kongtawelert P (2016) Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumour Biol 37:227–237
Barnes S (1995) Effect of genistein on in vitro and in vivo models of cancer. J Nutr 125:777–783
Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A (2006) Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res 66:1234–1240
Bielsalski HK (2007) Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care 10:724–728
Bilir B, Sharma NV, Lee J, Hammarstrom B, Svindland A, Kucuk O, Moreno CS (2017) Effects of genistein supplementation on genome-wide DNA methylation and gene expression in patients with localized prostate cancer. Int J Oncol 51:223–234
Braicu C, Gherman CD, Irimie A, Berindan-Neagoe I (2013) Epigallocatechin-3-gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J Nanosci Nanotechnol 13:632–637
Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE (2010) Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 70:9003–9011
Cao Z, Zhang H, Cai X, Fang W, Chai D, Wen Y, Chen H, Chu F, Zhang Y (2017) Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma. Cell Physiol Biochem 43:1803–1812
Chandler D, Woldu A, Rahmadi A, Shanmugam K, Steiner N, Wright E, Benavente-Garcia O, Schulz O, Castillo J, Münch G (2010) Effects of plant-derived polyphenols on TNF-α and nitric oxide production induced by advanced glycation endproducts. Mol Nutr Food Res 54:141–150
Cheng AC, Huang TC, Lai CS, Pan MH (2005) Induction of apoptosis by luteolin through cleavage of Bcl-2 family in human leukemia HL-60 cells. Eur J Pharmacol 509:1–10
Chua LS, Latiff NA, Lee SY, Lee CT, Sarmidi MR, Aziz RA (2011) Flavonoids and phenolic acid from Labisia pumila (Kacip Fatimah). Food Chem 127:1186–1192
Cincin ZB, Kiran B, Baran Y, Cakmakoglu B (2018) Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed Pharmacother 103:336–345
Cooke D, Schwarz M, Boocock D, Winterhalter P, Steward WP, Gescher AJ, Marczylo TH (2006) Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the ApcMin mouse model of intestinal carcinogenesis-relationship with tissue anthocyanin levels. Int J Cancer 119:2213–2220
Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD (2013) Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a 13C-tracer study. Am J Clin Nutr 97:995–1003
Davis JN, Kucuk O, Sarkar FH (1999) Genistein inhibits NF-kB activation in prostate cancer cells. Nutr Cancer 35:167–174
De Leo M, Braca A, Sanogo R, Cardile V, deTommasi N, Russo A (2006) Antiproliferative activity of Pteleopsis suberosa leaf extract and its flavonoid components in human prostate carcinoma cells. Planta Med 72:604–610
Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME (2001) The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol 152:1197–1206
Deng L, Jiang L, Lin X, Tseng KF, Lu Z, Wang X (2017) Luteolin, a novel p90 ribosomal S6 kinase inhibitor, suppresses proliferation and migration in leukemia cells. Oncol Lett 13:1370–1378
deVere White RW, Hackman RM, Soares SE, Beckett LA, Li Y, Sun B (2004) Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology 63:259–263
Devi KP, Rajavel T, Habtemariam S, Nabavi SF, Nabavi SM (2015) Molecular mechanisms underlying anticancer effects of myricetin. Life Sci 42:19–25
Domínguez M, Avila JG, Nieto A, Céspedes CL (2011) Anti-inflammatory activity of Penstemon gentianoides and Penstemon campanulatus. Pharm Biol 49:118–124
Donia TIK, Gerges MN, Mohamed TM (2018) Amelioration effect of Egyptian sweet orange hesperidin on Ehrlich ascites carcinoma (EAC) bearing mice. Chem Biol Interact 285:76–84
El-Kader AM, El-Readi MZ, Ahmed AS, Nafady AM, Wink M, Ibraheim ZZ (2013) Polyphenols from aerial parts of Polygonum bellardii and their biological activities. Pharm Biol 51:1026–1034
Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, Tomás-Barberán FA (2007) Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem 55:10476–10485
Flaig TW, Glodé M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, Su LJ, Li Y, Harrison G, Agarwal R, Crawford ED (2010) A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate 70:848–855
Gali-Muhtasib H, Hmadi RA, Kareh M, Tohme R, Darwiche ND (2015) Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis 20:1531–1562
Gann PH, Deaton RJ, Rueter EE, Van Breemen RB, Nonn L, Macias V, Han M, Ananthanarayanan V (2015) A phase II randomized trial of lycopene-rich tomato extract among men with high-grade prostatic intraepithelial neoplasia. Nutr Cancer 67:1104–1112
Garcia FA, Cornelison T, Nuño T, Greenspan DL, Byron JW, Hsu CH, Alberts DS, Chow HH (2014) Results of a phase II randomized, double-blind, placebo-controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia. Gynecol Oncol 132:377–382
Gee JR, Saltzstein DR, Kim K, Kolesar J, Huang W, Havighurst TC, House MG (2017) A phase II randomized, double-blind, presurgical trial of Polyphenon E in bladder cancer patients to evaluate pharmacodynamics and bladder tissue biomarkers. Cancer Prev Res 10:298–307
Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA (2000) Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 48:4581–4589
Gwak H, Haegeman G, Tsang BK, Song YS (2015) Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol Carcinog 54:1529–1540
Hajleh MA, Al-Dujaili ASE (2016) Anti-cancer activity of pomegranate and its biophenols; general review. EC Nutr 6:28–52
Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR (1999) Content of the Flavonols quercetin, myricetin, and 17. Kaempferol in 25 edible berries. J Agric Food Chem 47:2274–2279
Han DH, Denison MS, Tachibana H, Yamada K (2002) Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci Biotechnol Biochem 66:1479–1487
Hayouni EA, Miled K, Boubaker S, Bellasfar Z, Abedrabba M, Iwaski H, Oku H, Matsui T, Limam F, Hamdi M (2011) Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine 18:976–984
Hergert HL (1956) The flavonoids of lodgepole pine bark. J Org Chem 21:534–537
Herman C, Adlercreutz T, Goldin BR, Gorbach SL, Höckerstedt KA, Watanabe S, Hämäläinen EK, Markkanen MH, Mäkelä TH, Wähälä KT, Hase TA (1995) Soybean phytoestrogen intake and cancer risk. J Nutr 125:757–770
Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, Kandaswami C, Middleton E, Lee MT (1999) Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol 128:999–1010
Hügel HM, Jackson N (2012) Redox chemistry of green tea polyphenols: therapeutic benefits in neurodegenerative diseases. Mini Rev Med Chem 2:380–387
Ishiwa J, Sato T, Mimaki Y, Sashida Y, Yano M, Ito A (2000) A citrus flavonoid, nobiletin, suppresses production and gene expression of matrix metalloproteinase 9/gelatinase B in rabbit synovial fibroblasts. J Rheumatol 27:20–25
Jacob V, Hagai T, Soliman K (2011) Structure-activity relationships of flavonoids. Curr Org Chem 15:2641–2657
Jain AK, Thanki K, Jain S (2013) Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol Pharm 10:3459–3474
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC (1997) Cancer chemoprotective activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Jogie-Brahim S, Feldman D, Oh Y (2009) Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev 30:417–437
Jones JR, Lebar MD, Jinwal UK, Abisambra JF, Koren J, Blair L, O’Leary JC, Davey Z, Trotter J, Johnson AG (2011) The diarylheptanoid (+)-aR, 11S-myricanol and two flavones from bayberry (Myrica cerifera) destablize the microtubule associated protein tau. J Nat Prod 74:38–44
Ju W, Wang X, Shi H, Chen W, Belinsky SA, Lin Y (2007) A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-κB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol 71:1381–1388
Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM (2011) EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer 84:844–850
Kanaze FI, Bounartzi MI, Niopas I (2004) A validated HPLC determination of the flavone aglycone diosmetin in human plasma. Biomed Chromatogr 18:800–804
Kandaswami C, Perkins E, Soloniuk DS, Drzewiecki G, Middleton E Jr (1991) Antiproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro. Cancer Lett 56:147–152
Kasala ER, Bodduluru LN, Barua CC, Gogoi R (2016) Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed Pharmacother 82:568–577
Katiyar S, Mukhtar H (1996) Tea in chemoprevention of cancer. Int J Oncol 8:221–238
Khan N, Syed DN, Pal HC, Mukhtar H, Afaq F (2011) Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-kappaB and MAPK signaling pathways in mouse skin (dagger). Photochem Photobiol 88:1126–1134
Kim HJ, Lee SB, Park SK, Kim HM, Park YI, Dong MS (2005) Effects of hydroxyl group numbers on the B-ring of 5, 7-dihydroxyflavones on the differential inhibition of human CYP 1A and CYP1B1 enzymes. Arch Pharm Res 28:1114–1121
Kohno H, Yoshitani SI, Tsukio Y, Murakami A, Koshimizu K, Yano M, Tokuda H, Nishino H, Ohigashi H, Tanaka T (2001) Dietary administration of citrus nobiletin inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Life Sci 69:901–913
Kokkola R, Andersson A, Mullins G, Östberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE (2005) RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 61:1–9
Kong NN, Fang ST, Wang JH, Wang ZH, Xia CH (2014) Two new flavonoid glycosides from the halophyte Limonium franchetti. J Asian Nat Prod Res 16:370–375
Kuiper G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863–870
Lansky EP, Jiang W, Mo H, Bravo L, Froom P, Yu W, Harris NM, Neeman I, Campbell MJ (2005) Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Investig New Drugs 23:11–20
Lau-Cam CA, Chan HH (1973) Flavonoids from Comptonia peregrina. Phytochemistry 12:1829
Lee HJ, Wang CJ, Kuo HC, Chou FP, Jean LF, Tseng TH (2005) Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicol Appl Pharmacol 203:124–131
Lee LT, Huang YT, Hwang JJ, Lee PP, Ke FC, Nair MP, Kanadaswam C, Lee MT (2002) Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res 22:1615–1627
Li Y, Sarkar FH (2002) Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr 132:3623–3631
Li Y, Upadhyay S, Bhuiyan M, Sarkar FH (1999) Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene 18:3166
Lim YC, Park HY, Hwang HS, Kang SU, Pyun JH, Lee MH, Choi EC, Kim CH (2008) Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett 271:140–152
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH, Wang LM, Chen YC (2008) Quercetin inhibition of tumor invasion via suppressing PKCdelta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 29:1807–1815
Lohr JM, Karimi M, Omazic B, Kartalis N, Verbeke CS, Berkenstam A, Frodin JE (2016) A phase I dose escalation trial of AXP107-11, a novel multi-component crystalline form of genistein, in combination with gemcitabine in chemotherapy-naive patients with unresectable pancreatic cancer. Pancreatology 16:640–645
Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, Zhang L (2006) Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis 27:262–268
Macedonia R (2005) In vitro antioxidant activity of some Teucrium species (Lamiaceae). Acta Pharma 55:207–214
Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR (2017) Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact 277:146–158
Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H (2005) Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A 102:14813–14818
Manthey JA, Guthrie N (2002) Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem 50:5837–5843
May M (2014) Statistics: attacking an epidemic. Nature 509:S50–S51
Meirinhos J, Silva BM, ValentÃo P, Seabra RM, Pereira JA, Dias A, Andrade PB, Ferreres F (2005) Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat Prod Res 19:189–195
Mertens-Talcott SU, Percival SS (2005) Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 218:141–151
Messing E, Gee JR, Saltzstein DR, Kim K, diSant'Agnese A, Kolesar J, Harris L, Faerber A, Havighurst T, Young JM, Efros M (2012) A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer Prev Res 5:621–630
Mimoto J, Kiura K, Matsuo K, Yoshino T, Takata I, Ueoka H, Kataoka M, Harada M (2000) (−)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis 21:915–919
Miyata Y, Sato T, Yano M, Ito A (2004) Activation of protein kinase C βII/ε-c-Jun NH2-terminal kinase pathway and inhibition of mitogen-activated protein/extracellular signal-regulated kinase 1/2 phosphorylation in antitumor invasive activity induced by the polymethoxy flavonoid, nobiletin. Mol Cancer Ther 3:839–847
Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, Kuwahara S, Takahashi Y, Ogawa K, Yano M, Tokuda H (2000) Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res 60:5059–5066
Murphy BT, MacKinnon SL, Yan X, Hammond GB, Vaisberg AJ, Neto CC (2003) Identification of triterpene hydroxycinnamates with in vitro antitumor activity from whole cranberry fruit (Vaccinium macrocarpon). J Agric Food Chem 51:3541–3545
Nessa MU, Beale P, Chan C, Yu JQ, Huq F (2011) Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res 31:3789–3797
Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF (2009) Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res 1:25–37
Nguyen MM, Ahmann FR, Nagle RB, Hsu CH, Tangrea JA, Parnes HL, Sokoloff MH, Gretzer MB, Chow HH (2011) Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prev Res 5:290–298
Noda Y, Kaneyuki T, Mori A, Packer L (2002) Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem 50:166–171
Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU (2008) Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultravioletirradiated human skin fibroblasts. J Agric Food Chem 56:8434–8441
Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, Stockton BR, Hertzman BL, Efros MD, Roper RP, Liker HR (2013) A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis 16:50–55
Pan H, Zhou W, He W, Liu X, Ding Q, Ling L, Zha X, Wang S (2012) Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int J Mol Med 30:337–343
Pan S, Zhou S, Gao S, Yu Z, Zhang S, Tang M, Sun J, Ma D, Han Y, Fong W, Ko K (2013) New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med 2013:627375
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377
Patel KR, Brown VA, Jones DJ, Brotton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner JE, Steward WP, Gescher AJ, Brown K (2010) Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res 70:7392–7399
Pavese JM, Farmer RL, Bergan RC (2010) Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev 29:465–482
Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, Rosser CJ (2008) Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 8:132
Perkin AG, Newbury FG (1899) Lxxix.– the colouring matters contained in dyer's broom (Genista tinctoria) and heather (Calluna vulgaris). J Chem Soc Trans 75:830–839
Pop EA, Fischer LM, Coan AD, Gitzinger M, Nakamura J, Zeisel SH (2008) Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis and estrogenic outcomes in healthy, postmenopausal women – a phase I clinical trial. Menopause 15:684–692
Rettig MB, Heber D, An J, Seeram NP, Rao JY, Liu H, Klatte T, Belldegrun A, Moro A, Henning SM, Mo D (2008) Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappa B dependent mechanism. Mol Cancer Ther 7:2662–2671
Saiprasad G, Chitra P, Manikandan R, Sudhandiran G (2014) Hesperidin induces apoptosis and triggers autophagic markers through inhibition of Aurora – a mediated phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and glycogen synthase kinase-3 beta signalling cascades in experimental colon carcinogenesis. Eur J Cancer 50:2489–2507
Sakla MS, Shenouda NS, Ansell PJ, MacDonald RS, Lubahn DB (2007) Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine 32:69–78
Samavat H, Ursin G, Emory TH, Lee E, Wang R, Torkelson CJ, Mimi CY (2017) A randomized controlled trial of green tea extract supplementation and mammographic density in postmenopausal women at increased risk of breast cancer. Cancer Prev Res 10:710–718
Samy RP, Gopalakrishnakone P, Ignacimuthu S (2006) Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem Biol Interact 164:1–14
Santibanez JF, Navarro A, Martinez J (1997) Genistein inhibits proliferation and in vitro invasive potential of human prostatic cancer cell lines. Anticancer Res 17:1199–1204
Saud SM, Li W, Morris NL, Matter MS, Colburn NH, Kim YS, Young MR (2014) Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis 35:2778–2786
Schramm L (2013) Going green: the role of the green tea component EGCG in chemoprevention. J Carcinog Mutagen 4:142–156
Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, Sartippour M, Harris DM, Rettig M, Suchard MA, Pantuck AJ (2007) Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem 55:7732–7737
Shankar S, Marsh L, Srivastava RK (2013) EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol Cell Biochem 372:83–94
Shike M, Doane AS, Russo L, Cabal R, Reis-Filho JS, Gerald W, Cody H, Khanin R, Bromberg J, Norton L (2014) The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. J Natl Cancer Inst 106. https://doi.org/10.1093/jnci/dju189
Shimizu M, Sakai H, Shirakami Y, Yasuda Y, Kubota M, Terakura D, Baba A, Ohno T, Hara Y, Tanaka T, Moriwaki H (2011) Preventive effects of (−)-epigallocatechin gallate on diethyl nitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Prev Res 4:396–403
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics. CA Cancer J Clin 67:7–30
Spanakis M, Kasmas S, Niopas I (2009) Simultaneous determination of the flavonoid aglycones diosmetin and hesperetin in human plasma and urine by a validated GC/MS method: in vivo metabolic reduction of diosmetin to hesperetin. Biomed Chromatogr 23:124–131
Tanaka T, Makita H, Kawabata K, Mori H, Kakumoto M, Satoh K, Hara A, Sumida T, Tanaka T, Ogawa H (1997) Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 18:957–965
Taylor CK, Levy RM, Elliott JC, Burnett BP (2009) The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev 67:398–415
Thielecke F, Boschmann M (2009) The potential role of green tea catechins in the prevention of the metabolic syndrome – a review. Phytochemistry 70:11–24
Tipoe GL, Leung TM, Hung MW, Fung ML (2007) Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Haematol Disord Drug Targets 7:135–144
Tsai PH, Cheng CH, Lin CY, Huang YT, Lee LT, Kandaswami CC, Lin YC, Lee KP, Hung CC, Hwang JJ, Ke FC, Chang GD, Lee MT (2016) Dietary flavonoids luteolin and quercetin suppressed cancer stem cell properties and metastatic potential of isolated prostate cancer cells. Anticancer Res 36:6367–6380
Tsuda T, Horio F, Osawa T (2002) Cyanidin 3-O-β-D-glucoside suppresses nitric oxide production during a zymosan treatment in rats. J Nutr Sci Vitaminol 48:305–310
Umadevi I, Daniel M, Sabnis SD (1988) Chemotaxonomic studies on some members of Ancardiaceae. Proc Plant Sci 98:205–208
USDA Database for the Flavonoid – Content of Selected Foods (2011) http://www.16.nal.usda.gov/fnic/foodcomp/Data/Flav/av.pdf
Wang LD, Zhou Q, Feng CW, Liu B, Qi YJ, Zhang YR, Gao SS, Fan ZM, Zhou Y, Yang CS, Wei JP (2002) Intervention and follow-up on human esophageal precancerous lesions in Henan, northern China, a high incidence area for esophageal cancer. Gan to kagaku ryoho. Cancer Chemother 29:159–172
Wang G, Zhang J, Liu L, Sharma S, Dong Q (2012) Quercetin potentiates doxorubicin mediated antitumor effects against liver cancer through p53/Bcl-xl. PLoS One 7:e51764
Wang Q, Wang H, Jia Y, Pan H, Ding H (2017) Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother Pharmacol 79:1031–1041
Xia R, Sheng X, Xu X, Yu C, Lu H (2018a) Hesperidin induces apoptosis and G0/G1 arrest in human non-small cell lung cancer A549 cells. Int J Mol Med 41:464–472
Xia R, Xu G, Huang Y, Sheng X, Xu X, Lu H (2018b) Hesperidin suppresses the migration and invasion of non-small cell lung cancer cells by inhibiting the SDF-1/CXCR-4 pathway. Life Sci 201:111–120
Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TYH, Fung ML, Tipoe GL (2014) Epigallocatechingallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD,PI3K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr 53:187–199
Xu Y, Ho CT, Amin SG, Han C, Chung FL (1992) Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res 52:3875–3879
Xu L, Ding Y, Catalona WJ, Yang XJ, Anderson WF, Jovanovic B, Wellman K, Killmer J, Huang X, Scheidt KA, Montgomery RB (2009) Mek4 function, genistein treatment, and invasion of human prostate cancer cells. J Natl Cancer Inst 101:1141–1155
Yang CS, Wang X, Lu G, Picinich SC (2009) Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer 9:429–439
Yang F, Song L, Wang H, Wang J, Xu Z, Xing N (2015) Combination of quercetin and 2-methoxyestradiol enhances inhibition of human prostate cancer LNCaP and PC-3 cells xenograft tumor growth. PLoS One 10:e0128277
Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A (2004) JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell 13:329–340
Zhao R, Chen Z, Jia G, Li J, Cai Y, Shao X (2011) Protective effects of diosmetin extracted from Galium verum L. on the thymus of U14-bearing mice. Can J Physiol Pharmacol 89:665–673
Zhao H, Xie P, Li X, Zhu W, Sun X, Sun X, Chen X, Xing L, Yu J (2015) A prospective phase II trial of EGCG in treatment of acute radiation-induced esophagitis for stage III lung cancer. Radiother Oncol 114:351–356
Zhao J, Li Y, Gao J, De Y (2017) Hesperidin inhibits ovarian cancer cell viability through endoplasmic reticulum stress signaling pathways. Oncol Lett 14:5569–5574
Zhu W, Jia L, Chen G, Zhao H, Sun X, Meng X, Zheng M (2016) Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget 7:48607
Conflict of Interest
The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, B., Singh, H., Singh, D., Singh, A.P., Buttar, H.S., Arora, S. (2019). Flavonoids as Potential Anticancer Agents in Clinics: Where Have We Reached So Far?. In: Singh Tuli, H. (eds) Current Aspects of Flavonoids: Their Role in Cancer Treatment . Springer, Singapore. https://doi.org/10.1007/978-981-13-5874-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-5874-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5873-9
Online ISBN: 978-981-13-5874-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)