Abstract
Chromium (Cr) is required for carbohydrate, lipid, and protein metabolisms in humans and animals. Cr insufficiency is associated with diabetes and cardiovascular disease. Chromium-enriched yeast (CrY) is a widely used Cr dietary supplement, but its pharmacokinetics remains unavailable. CrY was orally administered to rats at a single dose of 1 mg Cr/kg, and plasma Cr concentration at different time points was measured by inductively coupled plasma mass spectrometry. Pharmacokinetics of CrY in rats was well fitted to a non-compartmental model. Plasma Cr concentration reached the maximum of 8.68 ± 2.87 ng/mL at 0.25 h, and gradually decreased to 4.05 ± 0.47 ng/mL at 24 h. CrY was rapidly absorbed into the blood and was slowly eliminated after the oral administration, which could lead to the accumulation of Cr in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trivalent chromium (Cr3+) is an important trace element for humans and animals in the regulation of carbohydrate, lipid, and protein metabolism (Zhang et al. 2021). Cr3+ is a biologically active form of chromodulin which plays an important role in the regulation of the cellular response to insulin. Cr insufficiency leads to impaired insulin function, inhibition of protein synthesis and energy production, disruption of carbohydrate metabolism, and to type 2 diabetes and heart disease (Vincent 2007). The Chinese adequate intake of Cr is 30 μg for adults aged 18 and over according to the Chinese Dietary References Intakes (Chinese Nutrition Society 2014). Due to poor absorption of inorganic Cr and dietary Cr (0.5–3%), more bioavailable organic Cr species have been developed, such as Cr picolinate, Cr nicotinate, and Cr-enriched yeast (CrY). CrY is obtained by fermentation in which added inorganic Cr3+ is bound to amino acids and peptides in the yeast cell. CrY exhibits low acute toxicity with an LD50 value of more than 5000 mg/kg, and low chronic toxicity with a NOAEL value of 2500 mg/kg/day in rats (Food EPoFaNSat 2012). No adverse effect was observed in non-diabetic and diabetic subjects supplemented with CrY at a dose range of 20–1000 μg Cr/day for 2 months to 7.8 years (Food EPoFaNSat 2012). CrY supplementation in type 2 diabetes mellitus patients for 12–14 weeks was reported to better control fasting blood glucose, serum cholesterol, triglyceride concentrations, and blood pressure (Racek et al. 2006, 2013). Unfortunately, no sufficient data was provided to confirm the bioavailability from CrY was better than that from inorganic Cr compounds because available bioavailability was based on the effects of CrY on insulin action (Authority EFS 2009).
To our knowledge, no pharmacokinetics of CrY is available although CrY is widely used in humans. Therefore, the purpose of the present study aims to investigate the pharmacokinetics of CrY in rats to disclose comprehensive and quantitative disposition of CrY for the first time.
Materials and methods
Materials
CrY with Cr content of 2280 mg/kg was supplied by the Angel Yeast Co. Ltd. (Yichang, China). Cr standard solution (1000 μg/mL) was purchased from Guobiao Testing and Certificate Co. Ltd. (Beijing, China). Analytical reagent–grade nitric acid was obtained from Merck Corp. (Darmstadt, Germany).
Determination of plasma Cr
A Thermo Scientific iCAP RQ ICP-MS equipped with a helium-filled collision cell was used for the determination of Cr at m/z of 52 in a single helium kinetic energy discrimination mode according to ICP-MS conditions of our previous method (Zhang et al. 2021). Germanium isotope (72Ge) with a concentration of 10 ng/mL was used as an internal standard to minimize non-spectral interference and instrumental drifts. Data acquisition and processing were performed with the Thermo Scientific Qtegra 2.8 software. Plasma Cr concentration was determined by ICP-MS following 1:20 dilution of 100 μL of plasma with 0.5% (v/v) HNO3 aqueous solution. Calibration curve was constructed in the concentration range of 0.1–10 ng/mL. The ICP-MS method was validated for its sensitivity, linearity, accuracy, and precision according to “Sect. 730 plasma spectrochemistry” of the USA Pharmacopeia 2021 edition. A calibration curve showed a good linearity with correlation coefficient of more than 0.997. The limit of detection (LOD) and lower limit of quantification (LLOQ) were 0.03 and 0.1 ng/mL, respectively. Accuracy and precision of the method 94.1–101.7% and 1.2–3.6%, respectively.
Pharmacokinetic study
Animal experiments were approved by the Institutional Animal Care and Use Committee of the Hubei Provincial Key Laboratory of Yeast Function. Six adult male Sprague–Dawley rats weighing 290.5 ± 6.3 g were acclimatized for at least 7 days in a 12-h light/dark cycle and constant temperature and humidity facility, and were allowed access to diet and water ad libitum. The rats were deprived of diet overnight for 12 h prior to dosing, although they were allowed free access to water during the whole experiment period. CrY was dissolved in 1% (w/v) carboxymethylcellulose sodium aqueous solution to obtain a concentration of 0.125 mg Cr/mL, and CrY was intragastrically administered to rats at a single dose of 1000 μg Cr/kg. At 0 (pre-dosing), 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, and 24 h after the administration, approximately 0.25 mL of blood was withdrawn from the jugular vein catheter of each rat and centrifuged at 5000 × g for 10 min to obtain plasma. All animals were sacrificed using carbon dioxide at the end of the experiment.
Pharmacokinetic data analysis
The oral pharmacokinetic parameters of CrY in rats were calculated using non-compartmental model of WinNonlin software, as follows: terminal half-life (t1/2, λz), volume of distribution (V), systemic clearance (Cl), area under the curve from 0 to the last sampling time t (AUC0-t), and mean residence time from 0 to time t (MRT0-t). Data are presented as the mean ± SD.
Results and discussion
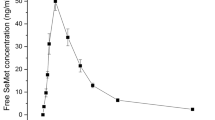
Pre-dosing plasma Cr concentrations fluctuated between LOD and LLOQ, which were much lower than other sampling time-points. Therefore, no baseline correction (subtraction of pre-dosing Cr concentration) was applied to other sampling time-point Cr concentrations due to negligible pre-dosing values as per Sect. 9011 guidance for bioavailability and bioequivalence of drug product in humans in the Chinese Pharmacopeia 2020. After the oral administration of CrY to rats, the concentration–time profile of Cr in plasma is shown in Fig. 1, and the pharmacokinetic parameters of Cr are presented in Table 1. Plasma Cr concentration reached the maximum of 8.68 ± 2.87 ng/mL at 0.25 h, rapidly declined to 4.38 ± 1.10 ng/mL at 4 h, and gradually decreased to 4.05 ± 0.47 ng/mL at 24 h, indicating that Cr from CrY was rapidly absorbed into the blood and was slowly eliminated after the oral administration.
Cr3+ in CrY was released following digestion in the gastrointestinal tract and was available for uptake into the body. The fluctuation trend of Cr concentrations observed for rat plasma in our experiment was consistent in magnitude with that in human plasma following a single dose ingestion of 10 mg Cr3+/L (Cr chloride) (Kerger et al. 1996). Similarly, blood Cr concentration increased from 1.8–2.2 ng/mL at baseline to 5.7–5.9 ng/mL in 68 adult diabetic and non-diabetic subjects following the oral administration of CrY at a dose of 1000 μg Cr/day for 6 months (Cheng et al. 2004). Peak time of CrY in the present research was much earlier than that of Cr picolinate and Cr malate in rats following oral gavage (Feng et al. 2018), indicating that CrY was rapidly absorbed. The absorption mechanism of Cr3+ in the intestine is still unclear, which involves passive transport and/or active transport (Food EPoFaNSat, 2012, 2010a, 2010b; O’Flaherty et al. 2001; Laschinsky et al. 2012; Febel et al. 2001). Following poor absorption of Cr3+ via the gastrointestinal tract, Cr3+ competitively binds to transferrin which Mn3+/2+ and Fe3+/2+ also compete for (Laschinsky et al. 2012), and is transported to the liver in the form of low molecular-weight Cr. Additionally, Cr3+ is also coordinated with low-molecular-weight ligands, such as nicotinic acid, amino acids, and glutathione (Pechancová et al. 2019), to be solubilized in vivo. Cr3+ does not penetrate red blood cells, suggesting that clearances into and out of red blood cells are not only rapid but also equal (Food EPoFANSat, 2010a, 2010b; O’Flaherty et al. 2001).
Absorbed extent of Cr3+ salts is dependent on their chemical species, and more amount of Cr3+ traverse cells and membranes when favorable ligand is complexed with Cr3+. For example, absorption fraction values of Cr3+ chloride and Cr3+ picolinate were 0.4% and 2.8%, respectively (O’Flaherty et al. 2001). In addition, absorption and disposition of Cr were influenced by its solubility and oxidation state (O’Flaherty 1993; Kottwitz et al. 2009). Approximately 1.8% of a soluble Cr3+ salt administered into the stomach and small intestine of nonfasted rats were found to be absorbed, indicating that Cr3+ exhibited low absorption fraction (O’Flaherty 1996). Interestingly, similar oral absorption extent of approximately 15% was observed for nonabsorbable Cr2O3 (Cr3+) and absorbable Na2CrO4 (Cr6+) regardless of chemical structure and solubility of Cr (Febel et al. 2001), in which absorption extent and absorption retention were erroneously applied by the authors.
Although Angel CrY did not contained Cr6+ detected by IC-ICP-MS (Zhang et al. 2021), European Food Safety Authority still specified Cr6+ ≤ 0.2% of total Cr in CrY considering various fermentation routes of CrY from some manufacturers (Food EPoFaNSat, 2012). Compared to soluble Cr3+ compounds, soluble Cr6+ compounds exhibited roughly tenfold greater fractional absorption in the gastrointestinal tract (O’Flaherty et al. 2001) because Cr6+ (CrO42−) structurally resembled sulfate and phosphate and was easily taken up by all cells and organs through sulfate transporters (Collins et al. 2010). Reducing agents in gastric juices, like ascorbate, glutathione, NADH, and sulfhydryls, reduced Cr6+ to Cr3+ in the gastrointestinal tract, which was a major pathway for detoxification in humans and rodents (Schlosser and Sasso 2014). Most of Cr6+ was rapidly reduced to Cr3+ by nonenzymatic mechanisms in the acidic environment of the stomach and was absorbed as Cr3+ in the small intestine (Zhang et al. 2021). In other organs, the reduction process was principally mediated by enzymes (O’Flaherty 1993).
Most Cr was cleared rapidly from the blood (Food EPoFANSat, 2010b), as demonstrated by the Cl value of 3.27 ± 1.30 L/h/kg in our research, and was prone to accumulate in the bone after oral or intraperitoneal administration to rats (O’Flaherty 1996). After absorption into the plasma, Cr was preferentially transferred to the bone surface and approximately 4% of the dose was incorporated into mineralizing new bone like vanadium (O’Flaherty 1993, 1996; Zhang et al. 2007). Most Cr3+ was slowly removed from tissues and excreted in the urine with small amounts eliminated in perspiration, bile, and feces (Food EPoFaNSat 2012, 2010a).
Conclusion
In summary, pharmacokinetics of CrY in rats following oral administration was firstly investigated and the results indicated that CrY was rapidly absorbed and slowly eliminated in vivo.
Data availability
All the data generated or analyzed during this study are contained within the article.
References
Authority EFS (2009) Inability to assess the safety of chromium-enriched yeast added for nutritional purposes as a source of chromium in food supplements and the bioavailability of chromium from this source, based on the supporting dossiers. EFSA J 7(6):1083. https://doi.org/10.2903/j.efsa.2009.1083
Cheng HH, Lai MH, Hou WC, Huang CL (2004) Antioxidant effects of chromium supplementation with type 2 diabetes mellitus and euglycemic subjects. J Agric Food Chem 52(5):1385–1389. https://doi.org/10.1021/jf035074j
Chinese Nutrition Society (2014) Chinese dietary reference intakes 2013. Science Press, China
Collins BJ, Stout MD, Levine KE, Kissling GE, Melnick RL, Fennell TR, Walden R, Abdo K, Pritchard JB, Fernando RA, Burka LT, Hooth MJ (2010) Exposure to hexavalent chromium resulted in significantly higher tissue chromium burden compared with trivalent chromium following similar oral doses to male F344/N rats and female B6C3F1 mice. Toxicol Sci 118(2):368–379. https://doi.org/10.1093/toxsci/kfq263
Febel H, Szegedi B, Huszar S (2001) Absorption of inorganic, trivalent and hexavalent chromium following oral and intrajejunal doses in rats. Acta Vet Hung 49(2):203–209. https://doi.org/10.1556/004.49.2001.2.10
Feng W, Li Q, Wang W, Zhao T, Feng Y, Li F, Mao G, Chen Y, Ding Y, Yang L, Wu X (2018) Pharmacokinetics and bioavailability of chromium malate and its influence on trace metals absorption after oral or intravenous administration. Indian J Pharmacol 50(2):75–83. https://doi.org/10.4103/ijp.IJP_505_17
Food EPoFANSat (2010a) Scientific opinion on the safety of trivalent chromium as a nutrient added for nutritional purposes to foodstuffs for particular nutritional uses and foods intended for the general population (including food supplements). EFSA Journal 8 (12):1882. https://doi.org/10.2903/j.efsa.2010.1882
Food EPoFANSat (2010b) Scientific opinion on the safety of chromium picolinate as a source of chromium added for nutritional purposes to foodstuff for particular nutritional uses and to foods intended for the general population. EFSA Journal 8 (12):1883. https://doi.org/10.2903/j.efsa.2010.1883
Food EPoFaNSat (2012) Scientific opinion on ChromoPrecise® cellular bound chromium yeast added for nutritional purposes as a source of chromium in food supplements and the bioavailability of chromium from this source. EFSA Journal 10 (11):2951. https://doi.org/10.2903/j.efsa.2012.2951
Kerger BD, Paustenbach DJ, Corbett GE, Finley BL (1996) Absorption and elimination of trivalent and hexavalent chromium in humans following ingestion of a bolus dose in drinking water. Toxicol Appl Pharmacol 141(1):145–158. https://doi.org/10.1006/taap.1996.0271
Kottwitz K, Laschinsky N, Fischer R, Nielsen P (2009) Absorption, excretion and retention of 51Cr from labelled Cr-(III)-picolinate in rats. Biometals 22(2):289–295. https://doi.org/10.1007/s10534-008-9165-4
Laschinsky N, Kottwitz K, Freund B, Dresow B, Fischer R, Nielsen P (2012) Bioavailability of chromium (III)-supplements in rats and humans. Biometals 25(5):1051–1060. https://doi.org/10.1007/s10534-012-9571-5
O’Flaherty EJ (1993) A pharmacokinetic model for chromium. Toxicol Lett 68(1–2):145–158. https://doi.org/10.1016/0378-4274(93)90127-j
O’Flaherty EJ (1996) A physiologically based model of chromium kinetics in the rat. Toxicol Appl Pharmacol 138(1):54–64. https://doi.org/10.1006/taap.1996.0097
O’Flaherty EJ, Kerger BD, Hays SM, Paustenbach DJ (2001) A physiologically based model for the ingestion of chromium (III) and chromium (VI) by humans. Toxicol Sci 60(2):196–213. https://doi.org/10.1093/toxsci/60.2.196
Pechancová R, Pluháček T, Milde D (2019) Recent advances in chromium speciation in biological samples. Spectrochim Acta, Part B 152:109–122. https://doi.org/10.1016/j.sab.2018.12.008
Racek J, Trefil L, Rajdl D, Mudrová V, Hunter D, Senft V (2006) Influence of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and markers of oxidative stress in subjects with type 2 diabetes mellitus. Biol Trace Elem Res 109(3):215–230. https://doi.org/10.1385/bter:109:3:215
Racek J, Sindberg CD, Moesgaard S, Mainz J, Fabry J, Müller L, Rácová K (2013) Effect of chromium-enriched yeast on fasting plasma glucose, glycated haemoglobin and serum lipid levels in patients with type 2 diabetes mellitus treated with insulin. Biol Trace Elem Res 155(1):1–4. https://doi.org/10.1007/s12011-013-9758-9
Schlosser PM, Sasso AF (2014) A revised model of ex-vivo reduction of hexavalent chromium in human and rodent gastric juices. Toxicol Appl Pharmacol 280(2):352–361. https://doi.org/10.1016/j.taap.2014.08.010
Vincent JB (2007) The nutritional biochemistry of chromium (III) (2007). Elsevier, Netherlands
Zhang SQ, Chen GH, Lu WL, Zhang Q (2007) Effects on the bones of vanadyl acetylacetonate by oral administration: a comparison study in diabetic rats. J Bone Miner Metab 25(5):293–301. https://doi.org/10.1007/s00774-007-0759-7
Zhang SQ, Cheng SH, Shen S, Luo BY, Zhang Y (2021) Speciation analysis of chromium in chromium-enriched yeast by ion chromatography-inductively coupled plasma mass spectrometry. Biol Trace Elem Res 199(1):338–343. https://doi.org/10.1007/s12011-020-02149-0
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, and performance. The first draft of the manuscript was written by Shuang-Qing Zhang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval
All procedures were performed according to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, eighth edition in 2011) and were approved by the Institutional Animal Care and Use Committee of the Hubei Provincial Key Laboratory of Yeast Function.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, SQ., Qin, XW. & Zhang, Y. Pharmacokinetics of chromium-enriched yeast in rats following oral administration. Naunyn-Schmiedeberg's Arch Pharmacol 396, 167–170 (2023). https://doi.org/10.1007/s00210-022-02334-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02334-z