Abstract
For the first time, bioavailability, pharmacokinetics, and biotransformation of selenium-enriched yeast (SeY) and sodium selenite (Na2SeO3) in rats were systemically compared by analyzing free selenomethionine (SeMet), total SeMet, and selenium (Se). After SeY and Na2SeO3 were orally administered to rats at a dose of 100 μg Se/kg, plasma free SeMet, total SeMet, and Se at various time points were determined by ultra-performance liquid chromatography-tandem mass spectrometry. Based on Se and total SeMet, the relative bioavailability values of SeY compared with Na2SeO3 were 144% and 272%, respectively. For the rats treated with SeY, 0.73–2.68% of total Se was biotransformed to free SeMet, 14.3–20.4% to SeMet-proteins and albumin-bound SeMet, and 75.9–82.3% to selenoproteins in plasma. SeY had higher bioavailability than Na2SeO3 based on Se and total SeMet levels. Plasma SeMet was the optimal biomarker of SeY status in vivo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
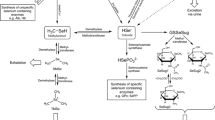
Selenium (Se) is incorporated into proteins in the form of selenocysteinyl and selenomethionyl residues to form the active center of selenoproteins and Se-containing proteins, and exhibits important biological functions for human health [1, 2]. Proteins containing Se in the form of selenocysteine (SeCys) are defined as selenoproteins, and proteins containing Se in the form of selenomethionine (SeMet) are called Se-containing proteins [1]. Plasma Se concentration is usually considered a biomarker of the short-term status and dietary intake of Se, and erythrocyte and tissue Se levels are representative of the long-term status [2]. Actually, total Se concentration does not reflect the functional activity of Se because the element is incorporated in selenoproteins and Se-containing proteins with different biological activities. Although plasma glutathione peroxidase (GPx) and selenoprotein P (SelP) have also been proposed as biomarkers of Se status, they reach saturation in a low maximum Se range of 100 (corresponding to 70 μg Se/day)–124 ng/mL (corresponding to 105 μg Se/day), respectively, which become invalid in high Se concentration [2]. Other selenoproteins have the similar drawbacks. It is known that at least 96–98% of total Se is protein-bound and occurs in the forms of SeCys and SeMet in human plasma [3]. The levels of SeCys and SeMet in plasma comprehensively reflect biological activity and status of Se. Free SeCys is not available due to its instability, while it is inaccurate, laborious, and costly for the indirect quantification of SeCys employing a proteolytic extract after derivatization with iodoacetamide [4, 5]. Therefore, the in vivo level of SeMet is the optimal biomarker of Se status and dietary intake, as well as of SeY quality [6].
There are two forms of Se, inorganic species including selenite and selenate, and organic species consisting of SeMet and SeCys residues, which are used as nutritional and therapeutic sources. Generally, SeMet-based supplements are significantly better than selenate- or selenite-based ones [7]. Se-enriched yeast (SeY) is the most popular matrix for Se supplement since SeMet and SeCys are the first and second abundant Se species, accounting for approximately 60–85% and 2–4% of total Se in SeY, respectively [8, 9]. Additionally, the content of inorganic Se (selenite) in SeY does not exceed 1% of total Se.
In our previous research [6], the quantification of free SeMet in plasma after the oral administration of SeY to rats was firstly established by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) in order to evaluate SeY. Up until now, SeMet from SeY for the biosynthesis of selenoproteins and Se-containing proteins has not been reported. To our knowledge, there are only three reports regarding the bioavailability of SeY, in which biological samples obtained at a single time point were falsely used for the bioavailability, resulting in wrong conclusions [10,11,12]. In the present investigation, bioavailability, pharmacokinetics, and biotransformation of SeY and sodium selenite in rats were firstly and systemically evaluated by using free SeMet, total SeMet, and Se in plasma.
Materials and Methods
Materials
SeY with Se content of 2191 μg/g and SeMet content of 1701 μg Se/g was supplied by the Angel Yeast Co., Ltd. (Yichang, China). Sodium selenite (Na2SeO3, purity > 98%) was provided by the Vital Materials Co., Ltd. (Guangzhou, China). Protease XIV, lipase, L-SeMet (purity ≥ 98%), and methylselenocysteine (purity ≥ 95%) were from the Sigma-Aldrich Co. (St. Louis, MO, USA). HPLC-grade acetonitrile, formic acid, and water were obtained from the Fisher Scientific Inc. (Geel, Belgium). Standard solutions of Se (1 mg/mL) and yttrium (1 mg/mL) were available from the National Center for Standard Materials (Beijing, China). All other chemicals were of analytical reagent grade. The Se-deficient (Se < 0.02 mg/kg) diet with all other nutrients at the standard levels was provided by the Trophic Animal Feed High-tech Co. (Jiangsu, China) according to the AIN-93G formula.
Pharmacokinetic Study of SeY and Sodium Selenite
Animal experiments were adhered to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, eighth edition in 2011) and were approved by our Institutional Animal Care and Use Committee. In a temperature- and humidity-controlled facility with a 12-h light/dark cycle, 36 adult Sprague-Dawley rats weighing 305.7 ± 13.4 g were acclimatized for at least 7 days prior to the study and were fed with Se-deficient diets. During the whole experimental period, the animals were free access to demineralized water. The rats were equally divided into six groups (n = 6 for each group), and each group consisted of three males and three females. SeY and Na2SeO3 were dissolved in 1% (w/v) carboxymethylcellulose sodium aqueous solution and 0.9% (w/v) physiological saline, respectively. At a single dose of 100 μg Se/kg, SeY was intragastrically administered to three groups and Na2SeO3 was intragastrically given to the other three groups. At 0, 0.167, 0.5, 0.75, 1, 2, 4, 6, 8, 12, and 24 h following the administration of SeY or Na2SeO3, approximately 0.25 mL of blood was collected from each rat and centrifuged at 5000×g for 10 min to obtain plasma. At the end of each experiment, the rats were sacrificed using carbon dioxide. The plasma samples from each three groups receiving Na2SeO3 or SeY were used for the analysis of Se, free SeMet, or total SeMet.
Determination of Se, Free SeMet, and Total SeMet
Plasma Se levels were determined by a Thermo Finnigan Element II high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS) according to our previous method [13]. The Waters Acquity Xevo TQ UPLC-MS/MS was used for the determination of free SeMet in plasma according to our previous method [6]. For the analysis of total SeMet, 100 μL of enzyme solution (100 mg of protease XIV and 100 mg of lipase in 10 mL of tris buffer at pH 7.0) was added to 100 μL of plasma and incubated at 37 °C for 30 h. After centrifugation at 12,000g for 30 min, 50 μL of supernatant was vortex-mixed with 200 μL of acetonitrile. The solution was centrifuged at 12,000g for 30 min, filtered through a 0.22-μm membrane, and injected into the UPLC-MS/MS according to our previous method [6].
Pharmacokinetic Data Analysis
Non-compartmental analysis was employed to calculate the pharmacokinetic parameters: terminal half-life (t1/2, λz), volume of distribution (V), systemic clearance (Cl), area under the curve from 0 to the last sampling time t (AUC0–t), area under the curve from 0 to infinite time (AUC0–∞), and mean residence time from 0 to time t (MRT0–t).
The statistical analysis was performed using the one-way ANOVA with Scheffe post hoc test. Data are expressed as the mean ± SD. A value of p < 0.05 was considered statistically different.
Results and Discussion
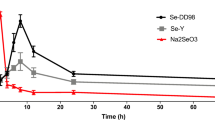
The concentration-time profiles of Se, free SeMet, and total SeMet in rat plasma after the oral administration of SeY or Na2SeO3 are shown in Fig. 1, and the pharmacokinetic parameters of Se, free SeMet, and total SeMet are presented in Tables 1 and 2.
In the rats receiving Na2SeO3, Se levels showed a steep increase and plateaued at 2 h (Fig. 1a). No free SeMet was detected, and following enzymatic proteolysis, total SeMet slightly fluctuated in the range of 59.3–65.7 ng/mL (Fig. 1c). Total SeMet accounted for 4.74–12.79% of total Se post-dose and 12.85 ± 1.58% of total Se pre-dose, indicating that plasma SeMet was endogenous and completely protein-bound, and exogenous selenite was not transformed to SeMet. Exogenous selenite was readily taken up by erythrocytes within a few minutes, reduced to selenide by glutathione for the synthesis of selenoproteins, and then effluxed into the plasma, bound selectively to albumin and transferred to the liver, or excreted in urine or breath after being methylated [1, 14]. Selenite can be transformed to SeCys residues in plants and animals, and to SeMet residues only in plants [14].
IFor the rats treated with SeY, Se, free SeMet, and total SeMet were elevated to the maximum at 2 h and gradually decreased until 24 h (Fig. 1b, c). Compared with Na2SeO3, SeY exhibited significantly higher maximum plasma Se concentration because SeY was more easily absorbed and subsequently resulted in higher Se bioavailability [6]. After the administration of SeY, free SeMet and total SeMet accounted for 0.73–2.68% and 16.95–22.20% of total Se, respectively, which were in agreement with the reports stating that the non-protein-bound fraction of Se in plasma constituted 2–4% of total plasma Se after SeY supplementation [3], and total SeMet constituted 17.7–24.1% of total Se [4, 15]. Prior to the administration of SeY, free SeMet was not found and total SeMet accounted for 14.8 ± 1.8% of total Se, which were consistent with the findings of Na2SeO3 groups. The results indicated that free SeMet and increased protein-bound SeMet in plasma were attributable to SeY, in which 75.9–82.3% of total Se (deduction of total SeMet expressed in Se from total Se) contributed to the biosynthesis of selenoproteins. After the administration of SeY, protein-bound SeMet (the difference of total SeMet and free SeMet) varied in the range of 65.7–273 ng/mL, and accounted for 14.3–20.4% of total Se. It was previously reported that approximately 75% of total Se in human blood was present in plasma, in which Se was mainly present as SelP (68 ± 7%), GPx (25 ± 4%), and was associated to albumin (7 ± 4%) [16]. Compared with human plasma, more Se was utilized for the synthesis of SeMet proteins in rat plasma. When recognized as a Se species, exogenous SeMet from SeY can be transformed to SeCys via the trans-selenation pathway and lysed by β-lyase or directly by γ-lyase to selenide. In the meanwhile, SeMet can also be incorporated in its intact form into proteins by the Met codon without being distinguishing between SeMet and Met, or bound to albumin together with intact SeMet [1]. Following the enzymolysis, SeMet was released from Se-containing proteins and albumin.
In the present investigation, the authentic bioavailability of SeY has been reported for the first time. Based on total Se and total SeMet, the relative bioavailability values of SeY compared with Na2SeO3 were 144% and 272%, respectively. After the baseline correction, i.e., deduction of endogenous/pre-dose concentration, the relative bioavailability values were 257% and 3966%, respectively. The results showed that (1) SeMet was the optimal biomarker of SeY status in vivo, (2) SeY had higher bioavailability than Na2SeO3, and (3) Se bioavailability strongly depended on the chemical speciation of Se. The authentic bioavailability of SeY was affected by the digestion of SeY, the absorption of Se from the intestinal tract, the transport, and biotransformation of Se into biological active forms [17]. SeMet from SeY was almost completely absorbed through a Na+-dependent, carrier-mediated process [2]. Although total Se and total SeMet concentrations in SeY group were significantly higher than the corresponding baseline values, the relative bioavailability values were substantially influenced by the baseline correction, suggesting that higher Se dose could be administered if the dose might be well tolerated by rats. The decreasing acute and chronic toxicity sequences were selenite, SeMet, and SeY [8]; thus, more doses of SeY compared with Na2SeO3 could be administered to rats.
Conclusion
In conclusion, a majority of Se was biotransformed to selenoproteins following the oral administration of SeY to rats. SeY showed higher bioavailability than Na2SeO3. SeMet in plasma was the optimal biomarker of SeY status in vivo.
References
Suzuki KT (2005) Metabolomics of selenium: Se metabolites based on speciation studies. J Health Sci 51(2):107–114
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54. https://doi.org/10.1039/c3mt00185g
Combs GF Jr, Clark LC, Turnbull BW (2001) An analysis of cancer prevention by selenium. Biofactors 14(1-4):153–159
Encinar JR, Schaumloffel D, Ogra Y, Lobinski R (2004) Determination of selenomethionine and selenocysteine in human serum using speciated isotope dilution-capillary HPLC-inductively coupled plasma collision cell mass spectrometry. Anal Chem 76(22):6635–6642. https://doi.org/10.1021/ac049280h
Bier K, Vacchina V, Szpunar J, Bertinc G, Lobinnski R (2008) Simultaneous derivatization of selenocysteine and selenomethionine in animal blood prior to their specific determination by 2D size-exclusion ion-pairing reversed-phase HPLC-ICP MS. J Anal At Spectrom 23:508–513
Zhang SQ, Zhang HB, Zhang Y (2018) Quantification of selenomethionine in plasma using UPLC-MS/MS after the oral administration of selenium-enriched yeast to rats. Food Chem 241:1–6. https://doi.org/10.1016/j.foodchem.2017.08.068
Hoefig CS, Renko K, Kohrle J, Birringer M, Schomburg L (2011) Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J Nutr Biochem 22(10):945–955. https://doi.org/10.1016/j.jnutbio.2010.08.006
Rayman MP, Infante HG, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Br J Nutr 100(2):238–253. https://doi.org/10.1017/S0007114508922522
European Food Safety Authority (2008) Selenium-enriched yeast as source for selenium added for nutritional purposes in foods for particular nutritional uses and foods (including food supplements) for the general population - scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. EFSA J 6(7):766. https://doi.org/10.2903/j.efsa.2008.766
Yoshida M, Fukunaga K, Tsuchita H, Yasumoto K (1999) An evaluation of the bioavailability of selenium in high-selenium yeast. J Nutr Sci Vitaminol (Tokyo) 45(1):119–128
Takahashi K, Suzuki N, Ogra Y (2017) Bioavailability comparison of nine bioselenocompounds in vitro and in vivo. Int J Mol Sci 18(3):506. https://doi.org/10.3390/ijms18030506
Bogye G, Alfthan G, Machay T (1998) Bioavailability of enteral yeast-selenium in preterm infants. Biol Trace Elem Res 65(2):143–151
Liu X, Piao J, Huang Z, Zhang SQ, Li W, Tian Y, Yang X (2014) Determination of 16 selected trace elements in children plasma from china economical developed rural areas using high resolution magnetic sector inductively coupled mass spectrometry. J Anal Methods Chem 2014:975820. https://doi.org/10.1155/2014/975820
Suzuki KT, Ogra Y (2002) Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se. Food Addit Contam 19(10):974–983. https://doi.org/10.1080/02652030210153578
Nyman DW, Suzanne Stratton M, Kopplin MJ, Dalkin BL, Nagle RB, Jay Gandolfi A (2004) Selenium and selenomethionine levels in prostate cancer patients. Cancer Detect Prev 28(1):8–16. https://doi.org/10.1016/j.cdp.2003.11.002
Dumont E, Vanhaecke F, Cornelis R (2006) Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem 385(7):1304–1323. https://doi.org/10.1007/s00216-006-0529-8
Reyes LH, Encinar JR, Marchante-Gayon JM, Alonso JI, Sanz-Medel A (2006) Selenium bioaccessibility assessment in selenized yeast after “in vitro” gastrointestinal digestion using two-dimensional chromatography and mass spectrometry. J Chromatogr A 1110(1-2):108–116. https://doi.org/10.1016/j.chroma.2006.01.088
Funding
The work was partly supported by the Hubei Provincial Natural Science Foundation of China (2018CFB612).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Animal experiments were adhered to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, eighth edition in 2011) and were approved by our Institutional Animal Care and Use Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, SQ., Shen, S. & Zhang, Y. Comparison of Bioavailability, Pharmacokinetics, and Biotransformation of Selenium-Enriched Yeast and Sodium Selenite in Rats Using Plasma Selenium and Selenomethionine. Biol Trace Elem Res 196, 512–516 (2020). https://doi.org/10.1007/s12011-019-01935-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01935-9