Abstract
Relapsed or refractory central nervous system (CNS) tumors still have poor prognosis, and, therefore, new treatment options are required. We retrospectively researched treatment results of patients with CNS tumors treated with nimotuzumab from 2010 to 2015. The study included nine patients with the diffuse intrinsic pontine glioma; eight with medulloblastoma; three each with anaplastic ependymoma, glioblastoma multiforme, and central nervous system primitive neuroectodermal tumor (CNS PNET); two patients with gliomatosis cerebri; and one patient each with other tumor types, including atypical teratoid rhabdoid tumor, thalamic astrocytoma, low-grade glial tumor, high-grade glial tumor, and cribriform neuroepithelial tumor. An objective response was observed in 10 of 33 patients with four patients showing a complete response, three a partial response, and three patients had stable disease. The 2-year overall survival (OS) and progression-free survival (PFS) rates were 35 ±9% and 19 ±8%, respectively. Due to the objective response in medulloblastoma, CNS PNET, and anaplastic ependymoma (MED group), survival rates of this group were analyzed. The 2-year OS and PFS for the MED group were 71 ±12% and 30 ±13%, respectively. The treatment was well tolerated. The treatment responses for medulloblastoma, CNS PNET, and anaplastic ependymoma have been promising. Likewise, some patients with relapsed or progressive CNS tumors may benefit through nimotuzumab-containing regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central nervous system (CNS) tumors are the most common solid tumors affecting children and adolescents (Smith et al. 2010). Treatment and possibility of cure depend on the type of tumor, its location within the brain, its resectability, possible spread, and the child’s age (Pollack and Jakacki 2011). Most children with CNS tumors need a combination of surgery, radiation therapy, and chemotherapy. The advancements in all three treatment areas in the last few decades have contributed to better outcomes (Pollack and Jakacki 2011).
The dismal prognosis for children with relapsed or refractory CNS tumors has been well documented, especially in patients who cannot undergo surgical treatment and have already received radiotherapy. The effective treatment of relapsed disease is controversial and challenging. Therefore, the researchers explore new treatment options for treating the disease (Fouladi et al. 2010).
Nimotuzumab is a humanized monoclonal antibody binding to the epidermal growth factor receptor (EGFR), highly overexpressed in the number of brain tumors (Egloff and Grandis 2008). High expression of EGFR protein in glioma is associated with tumor progression and enhanced tumorigenicity (Bredel et al. 1999; Khatua et al. 2012). The effect of nimotuzumab in gliomas is well understood, and its promising role as a therapeutic agent in patients with high-grade gliomas was reported (Lam et al. 2009). However, it has limited use in other brain tumors.
In this study, we retrospectively analyzed the efficacy and toxicity of nimotuzumab in patients with poor prognostic CNS tumors, especially with relapsed/refractory disease.
Materials and methods
This study was a retrospective analysis of a case series. We retrospectively reviewed a total of 33 patients diagnosed with CNS tumors treated with nimotuzumab-containing regimens either for the newly diagnosed patients or at progression in the Hacettepe University Oncology Institute from 2010 to 2015. Children and adolescents aged between 0 and 18 years with a measurable tumor before the start of nimotuzumab-containing regimen were eligible to be included in the study.
The patients’ medical records (gender, age, tumor entity, tumor location, the type of surgery), treatment history, and MRI scans were reviewed. The diagnosis of diffuse intrinsic pontine glioma (DIPG) and gliomatosis cerebri was clinically and radiologically made after the evaluation in the tumor board. The diagnosis of other tumor groups was made by an experienced pathologist. All MRIs were reviewed by the same neuroradiologist. MRI scans were obtained before the therapy, at 3-month intervals, and/or at the suspicion of progression on physical examination to detect therapy response. Tumor response criteria were determined by changes in size using width, transverse, and length measurements on MRI scans. Complete response (CR) was defined as the disappearance of the measurable disease, while a decrease in the sum of disease of ≥ 30% represented partial response (PR). An increase in the sum of disease ≥ 20% from the baseline represented progressive disease (PD). Stable disease (SD) was defined with the absence of CR, PR, or PD. Objective response (OR) was defined as all patients with either PR or CR (Therasse et al. 2000).
All patients except one received radiotherapy previously. Nimotuzumab was used by the approval of the Ministry of Health for off-label use. Informed consent was obtained from the parents. All patients had a measurable disease before nimotuzumab therapy, which was administered as monotherapy or in combination with the other drugs. The treatment schedule was 150 mg/m2/dose intravenous administration based on dose in a study by Bartels et al., weekly in the first 12 weeks, then every alternate week until progression, or the end of 2 years (Bartels et al. 2014). If nimotuzumab was combined with vinorelbine, it was administered the same day with nimotuzumab at a dose of 20 mg/m2. When combined with temozolomide (TMZ) or cisplatin-etoposide, each 28-day cycle consisted of TMZ 180 mg/m2/day for 5 days or cisplatin 100 mg/m2 given on day 1 with etoposide 100 mg/m2 administered subsequently for 3 days. All patients had adequate hematologic, renal, and hepatic functions. Toxicity was graded utilizing the National Cancer Institute Common Toxicity Criteria (National Cancer Institute 2010). Toxicity was assessed after each course. EGFR expression analysis could not be performed as the kit was unavailable at the time of the study.
Calculations were made using the Statistical Package for Social Studies (SPSS, version 16). OS and PFS were estimated with the Kaplan-Meier method. PFS and OS were calculated from the date of the first nimotuzumab regimen infusion to the date of any radiological or clinical progression or death due to any cause and censored at the date of the latest follow-up for patients who were event-free and alive. For the comparison of different groups, the log-rank test was used (significance level p<0.05).
Results
A total of 33 patients comprising 10 females and 23 males received nimotuzumab at the Pediatric Oncology Department at the Hacettepe University from 2010 to 2015. At the time of the diagnosis, the median age was 7 (range, 0.1 to 17.5) years. Patients’ characteristics, according to tumor entity, are summarized in Table 1.
No further classification could be made in the pathological evaluation of three patients. The first one was low-grade glioma. The pathologist reported it as she could not do further evaluation between the pilomyxoid astrocytoma and the pilocytic astrocytoma. The second was the high-grade glioma (HGG). The tumor showed glial differentiation immunohistochemically with a high mitosis rate and Ki 67 index. Although it could not be specifically categorized, it was considered high grade glial tumor. The last one was thalamic astrocytoma, where the paraffin blocks from the outer center were evaluated by our pathologist. The findings were evaluated as the glial tumors in the high-grade astrocytic phenotype.
The primary tumor location was infratentorial in 20 patients (60.6%). A total of eight patients had seeding at the time of the diagnosis (six craniospinal, two spinal). There were 11 patients without tissue biopsy (nine with DIPG, two with gliomatosis cerebri). All participants except one (45 days infant with the cribriform neuroepithelial tumor) underwent radiotherapy previously.
Median follow-up time was 22 (range, seven to 99) months after the diagnosis. After initiation of treatment with nimotuzumab-containing regimens, patients were followed up for a median of 8 (range, 1 to 51) months.
Nimotuzumab was the first-line treatment in four patients (all with DIPG), second-line in 12 patients, third-line in 14 patients, and fourth-line treatment in three patients (87.8% relapsed/progressive disease). The duration of nimotuzumab therapy ranged from 21 days to 18 months (median 2.5 months). The mean number of nimotuzumab doses was 13.8 (median 10), from three to 42 doses.
After nimotuzumab therapy, objective response and stable disease were observed in 10 of the 33 patients (30.3%), and other patients progressed. The characteristic features of the patients with objective responses and stable disease are summarized in Table 2. Radiological features of medulloblastoma patients with complete response are summarized in Fig. 1. At the time of diagnosis, stable disease was observed in one of four patients who started nimotuzumab as the first-line treatment, and the others progressed (Table 2). One of the patients of central nervous system primitive neuroectodermal tumor (CNS PNET) in remission died 35 months after the diagnosis due to the secondary aplastic anemia associated with the previous medications (first-line cisplatin-etoposide, second-line vincristine-lomustine-procarbazine).
Resolution of the seeding metastases of patients with medulloblastoma on brain magnetic resonance imaging (MRI). a–d Postcontrast axial T1W image shows bilateral nodular contrast enhancement within the internal acoustic canal (a, arrow) and trigeminal nerves enhancement (b, arrow) in a 9-year old male patient. Six months later, follow-up MRI images demonstrate complete resolution of these lesions (c, d). e–h Postcontrast axial (e, g) and sagittal (f) T1W images show bilateral nodular contrast enhancement on the occipital lobes (e, f, arrows) and within right internal acoustic canal (g, arrow) in a 6-year old female patient. Three months later, follow-up MRI reveals complete resolution of the occipital lesions (h). i–l Axial diffusion-weighted image (DWI) demonstrates a lesion with restricted diffusion on the right lateral ventricle wall (i, arrows). This lesion is mild hyperintense on the axial FLAIR image (j), but does not show contrast enhancement (k). Two months later, follow-up DWI demonstrates resolution of this lesion (l, arrow).
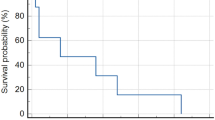
No significant adverse effects were reported. No allergic reactions or acneiform rashes were observed. We have not observed any effects of age in terms of efficacy and tolerability. The 2-year OS and PFS after the initiation of nimotuzumab treatment were 35 ±9% and 19 ±8%, respectively (Fig. 2a, b). Median OS and PFS were 25 ±6 and 3 ±0.5 months, respectively.
Due to the objective response in medulloblastoma, CNS PNET, and anaplastic ependymoma, survival rates of this group (MED group) were analyzed. OS and PFS for the 2 years after the initiation of nimotuzumab treatment for the MED group were 71 ±12% and 30 ±13%, respectively (Fig. 3a, b). In the HGG group (DIPG, glioblastoma multiforme, and anaplastic astrocytoma), median PFS and OS were 3 ±0.37 and 22 ±4.7 months, respectively. Although there was a trend estimated toward better OS and PFS in the MED group, the comparison between the MED group and HGG group did not show any significant difference in OS and PFS (p = 0.055) (Fig. 4).
a Kaplan-Meier curve for OS (overall survival) in the MED group (medulloblastoma, anaplastic ependymoma, primitive neuroectodermal tumor). b Kaplan-Meier curve for PFS (progression-free survival after nimotuzumab therapy) in the MED group (medulloblastoma, anaplastic ependymoma, primitive neuroectodermal tumor)
Discussion
The management of CNS tumors in children remains a challenge for pediatric oncologists and requires coordinated efforts of specialists (Khatua et al. 2012). Overall survival rates remain low despite the novel treatment approaches. The prognosis is poor with relapsed or refractory CNS tumors, especially for those who had received radiotherapy and/or without any chance of surgery. The poor prognosis of patients with relapsed or refractory brain tumors has led to research for new therapeutic strategies (Khatua et al. 2012; Lam et al. 2009).
The present report demonstrated the outcomes of nimotuzumab as monotherapy or in combination with chemotherapy in patients with diverse CNS tumors. Most of the patients were at the risk of poor outcomes as 87.8% had relapse/progressive disease, and only 30.3% underwent gross total resection. Evaluation of EGFR expression in the primary tumor specimens was not conducted as part of the study.
In this report, objective response and stable disease were observed in 30% of the patients, and the 2-year PFS after nimotuzumab therapy was 19 ±8%. The OS was 35 ±9% with a median OS of 25 months. In a study by Saurez et al., the study group was diagnosed with a progressive or recurrent brain tumor, nimotuzumab was administered as monotherapy or in combination, and the median OS was 19 months (Saurez et al. 2009). Our results were comparable with those results. Nimotuzumab proved to offer clinical benefits in patients with a progressive or recurrent brain tumor. While the patients were hospitalized during the other chemotherapy protocols, nimotuzumab could be administered in outpatient settings. Outpatient treatment provides an advantage for the patients.
Nimotuzumab, a recombinant humanized monoclonal immunoglobulin G1 antibody binding EGFR, blocks the binding of EGF and transforms growth factor-alpha to EGFR (Lam et al. 2009). It was designed to be used for adult cancers of epithelial origin (Van den Eynde et al. 2011). EGFR is involved in the development of high-grade astrocytic tumors (Garrido et al. 2011; Ramos et al. 2006) and is one of the main genetic factors affecting the prognosis of HGG. Amplification of EGFR in childhood is to a lesser degree than in adult HGG. HGG is an extremely aggressive lesion with poor outcomes of patients, despite advances in the treatment with a 5-year PFS of only 6–18% (Qaddoumi et al. 2009). Studies on HGG in children have demonstrated the efficacy of nimotuzumab. In a phase II trial by Bode et al., an objective response was achieved in 14 of 46 patients with HGG (Bode et al. 2006). The median survival time of responders and non-responders were 10 and 3.2 months, respectively. In another report by Sirachainan et al., the outcome of nimotuzumab-irinotecan therapy was demonstrated in newly diagnosed HGG (Sirachainan et al. 2017). The 5-year PFS and OS were 19.9% and 31.5%, respectively. Median survival time was 3.2 months in a phase II study by Bartels et al. (Bartels et al. 2014). In another study by Kebudi et al., the median survival time for progressive disease and the newly diagnosed patient group were 6 and 11 months, respectively (Kebudi et al. 2019).
In our study, the median survival time after nimotuzumab therapy was lower than that stated in the literature. This can be explained by using nimotuzumab as the second option in most of our patients. Besides, we hypothesized that our HGG group did not have EGFR overexpression.
The use of nimotuzumab in brain tumors other than HGG has been limited. A study by Cabanas et al. reported nine anaplastic ependymomas and six children with other tumors, including CNS PNET, neuroblastoma, medulloblastoma, and thalamic tumors (Cabanas et al. 2014). Although efficacy assessment was not the aim of that study, the median survival time for ependymomas was 52.2 months, and mean survival time for other tumors was 26.1 months. In another study (Saurez et al. 2009), the study group was diagnosed with a progressive or recurrent brain tumor, and two of five patients with ependymoblastoma were alive (one with a complete response and another with stable disease). Similarly, in our study, an objective response was observed in medulloblastoma, CNS PNET, and anaplastic ependymoma. Besides, 2-year PFS after the initiation of nimotuzumab treatment for this group was 30 ±13%. Although no statistically significant difference was found between the MED and HGG groups (p= 0.055), the results of the MED group were better than the HGG group. Although the results of the MED group were promising, a small number of patients limited our study.
The amplification and overexpression of the EGFR family were reported in ependymomas (Gilbertson et al. 2002) and medulloblastomas (Gilbertson et al. 1997; Gajjar et al. 2004), making them rational therapeutic targets. In a report by Jakacki et al., EGFR and ERBB receptor expression in children with refractory solid tumors was studied (Jakacki et al. 2008). The highest levels of EGFR expression were observed in medulloblastoma and ependymoma samples. The data suggested a possible role for EGFR as a therapeutic target in pediatric medulloblastoma and ependymoma (Jakacki et al. 2008). Although we did not analyze EGFR expression in our samples, we hypothesized that nimotuzumab responders with medulloblastoma, CNS PNET, and anaplastic ependymoma could have EGFR expression.
In our study, no significant adverse effects were reported. This minimal toxicity was consistent with more recent studies (Qaddoumi et al. 2009; Crombet et al. 2003). As compared to the other EGFR-targeting agents, nimotuzumab has shown promising clinical results with fewer adverse reactions (Crombet et al. 2003).
This study had some limitations such as retrospective nature, the limited number of patients, heterogeneity of the tumors, diversity of chemotherapy protocols, and lack of tissue biopsy in one-third of the patients. Besides, none of the biopsy specimens was analyzed for EGFR overexpression.
In conclusion, relapsed or refractory CNS tumors still have an extremely poor prognosis. Our treatment response of medulloblastoma, CNS PNET, and anaplastic ependymoma was promising. Nimotuzumab therapy may contribute to increase the survival of medulloblastoma, CNS PNET, and anaplastic ependymoma. Likewise, in patients with relapsed or refractory CNS tumors, nimotuzumab may be a treatment option. The efficacy of nimotuzumab remains to be proven in prospective clinical trials.
Data Availability
Yes
Code availability
N/A
References
Bartels U, Wolff J, Gore L, Dunkel I, Gilheeney S, Allen J, Goldman S, Yalon M, Packer RJ, Korones DN, Smith A, Cohen K, Kuttesch J, Strother D, Baruchel S, Gammon J, Kowalski M, Bouffet E (2014) Phase 2 study of safety and efficacy of nimotuzumab in pediatric patients with progressive diffuse intrinsic pontine glioma. Neuro-Oncol 16:1554–1559
Bode U, Buchen S, Warmuth-Metz M, Pietsch T, Bach F, Fleischhack G. (2006) Final report of a phase II trial of nimotuzumab in the treatment of refractory and relapsed high-grade gliomas in children and adolescents. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007
Bredel M, Pollack IF, Hamilton RL, James CD (1999) Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res 5:1786–1792
Cabanas R, Saurez G, Alert J, Reyes A, Valdes J, Gonzalez MC, Pedrayes JL, Valle L, Infante M, Avila M, Herrera R, Hechavarria E, Rios M, Fernández A, Lorenzo Luaces P, Crombet Ramos T (2014) Prolonged use of Nimotuzumab in children with central nervous system tumors: safety and feasibility. Cancer Biother Radiopharm 29(4):173–178
Crombet T, Torres L, Neninger E, Catalá M, Solano ME, Perera A, Torres O, Iznaga N, Torres F, Pérez R, Lage A (2003) Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother 26(2):139–148
Egloff AM, Grandis JR (2008) Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol 35:286–297
Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, Gajjar A, Kun LE, Boyett JM, Gilbertson RJ (2010) Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol 28:4221–4227
Gajjar A, Hernan R, Kocak M (2004) Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol 22:984–993
Garrido G, Tikhomirov IA, Rabasa A, Yang E, Gracia E, Iznaga N, Fernández LE, Crombet T, Kerbel RS, Pérez R (2011) Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther 11:373–382
Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J (1997) Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res 57:3272–3280
Gilbertson RJ, Bentley L, Hernan R, Junttila TT, Frank AJ, Haapasalo H, Connelly M, Wetmore C, Curran T, Elenius K, Ellison DW (2002) ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res 8:3054–3064
Jakacki RI, Hamilton M, Gilbertson RJ, Blaney SM, Tersak J, Krailo MD, Ingle AM, Voss SD, Dancey JE, Adamson PC (2008) Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children’s Oncology Group Phase I Consortium Study. J Clin Oncol 26(30):4921–4927
Kebudi R, Cakir FB, Bay SB, Gorgun O, Altınok P, Iribas A, Agaoglu FY, Darendeliler E (2019) Nimotuzumab-containing regimen for pediatric diffuse intrinsic pontine gliomas: a retrospective multicenter study and review of the literature. Childs Nerv Syst 35(1):83–89
Khatua S, Sadighi ZS, Pearlman ML, Bochare S, Vats TS (2012) Brain tumors in children—current therapies and newer directions. Indian J Pediatr 79:922–927
Lam C, Bouffet E, Bartels U (2009) Nimotuzumab in pediatric glioma. Future Oncol 5(9):1349–1361
National Cancer Institute. Common terminology criteria for adverse events v4.03 (CTCAE). June 14, 2010. Available at evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06 14_QuickReference_5x7.pdf
Pollack IF, Jakacki RI (2011) Childhood brain tumors: epidemiology, current management, and future directions. Nat Rev Neurol 7:495–506
Qaddoumi I, Sultan I, Gajjar A (2009) Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the surveillance, epidemiology, and end results database. Cancer 115:5761–5770
Ramos TC, Figueredo J, Catala M et al (2006) Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther 5:375–379
Saurez G, Cabanas R, Zaldívar M, Garnier T, Iglesias B, Piedra P, Castillo MR, Longchong M, Iznaga N, Lage A (2009) Clinical experience with nimotuzumab in Cuban pediatric patients with brain tumors, 2005 to 2007. Medicc Rev 11(3):27–33
Sirachainan N, Boongird A, Swangsilpa T, Klaisuban W, Lusawat A, Hongeng S (2017) Reported outcomes of children with newly diagnosed high-grade gliomas treated with nimotuzumab and irinotecan. Childs Nerv Syst 33(6):893–897
Smith MA, Seibel NL, Altekruse SF, Ries LAG, Melbert DL, O'Leary M, Smith FO, Reaman GH (2010) Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28:2625–2634
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Van den Eynde M, Baurain JF, Mazzeo F, Machiels JP (2011) Epidermal growth factor receptor targeted therapies for solid tumours. Acta Clin Belg 66:10–17
Author information
Authors and Affiliations
Contributions
HSS, AV, TB, and İB wrote the article. RG made radiologic support. BA, BY, NK, TK, and CA collected the article. HSS and AV made statistics. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
N/A (retrospective study)
Consent to participate
Yes
Consent for publication
Yes
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 12 kb)
Rights and permissions
About this article
Cite this article
Susam-Sen, H., Varan, A., Bajin, İ. et al. Nimotuzumab therapy in the treatment of pediatric central nervous system tumors: single-center experience. Naunyn-Schmiedeberg's Arch Pharmacol 394, 1769–1777 (2021). https://doi.org/10.1007/s00210-021-02109-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02109-y