Abstract
Purpose
Nimotuzumab is an IgG1 antibody that targets epidermal growth factor receptor (EGFR). Overexpression of EGFR is detected in some pediatric brain tumors including diffuse intrinsic pontine gliomas (DIPG)s.
Methods
Since May 2010, nimotuzumab, combined with carboplatin or vinorelbine or Temozolomide (TMZ), was administered during progressive disease (PD) after the use of the institutional protocol consisting of radiotherapy (RT) + TMZ and adjuvant TMZ. After May 2012, children with newly diagnosed disease received TMZ during RT, and nimotuzumab and TMZ after RT. Nimotuzumab was given as 150 mg/m2/dose once a week for 12 weeks, and then every other week with TMZ until PD. PD patients were switched to nimotuzumab + vinorelbine combination until death.
Results
Nimotuzumab was used in 24 children with DIPG (seven in the PD group, 17 in the newly diagnosed patient group). In the PD group, median survival time was 12 months (7–42 months); 1-year and 2-year overall survival (OS) rates were 42.9 ± 18% and 14.3 ± 13%, respectively. The median survival in this group, after the initiation of nimotuzumab was 6 months (3–8 months). In the newly diagnosed patient group, median survival time was 11 months (3–35 months) and median progression free survival was 4 months (1–21 months). The 1-year OS in this group was 35.3 ± 11% and 2 year OS was 11.8 ± 7%. Nimotuzumab ± chemotherapy was well tolerated with no major adverse effect.
Conclusion
Nimotuzumab-containing regimens are feasible and tolerable; it might be that some patients either with newly diagnosed DIPG or with progressive disease may benefit modestly from nimotuzumab-containing combinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite aggressive treatment, the prognosis of high-grade gliomas is poor. Particularly, diffuse intrinsic pontine glioma (DIPG) remains the grimmest and most frustrating brain tumor that has a median survival of only 9–12 months [1,2,3,4]. In the study of Hargrave et al. [2], a typical DIPG is defined by onset of symptoms ≤ 3 months before diagnosis, ≥ 2 signs of the neurologic triad (cranial nerve deficit, ataxia, or long tract signs) combined with specific magnetic resonance imaging (MRI) features (appearance of a poorly marginated tumor with mass effect occupying ≥ 50% of the axial diameter of the pons, hypointensity on T1 and hyperintensity on T2-images). Radiotherapy (RT) is the standard treatment for DIPGs, achieving a temporary neurologic improvement in nearly 70% of patients [1,2,3,4]. Different combinations of chemotherapy, RT, and radiosensitizers were attempted but failed to improve long-term survival [1,2,3,4,5]. Recent studies have shown amplification or overexpression of epidermal growth factor receptor (EGFR) in DIPGs [6,7,8]. Hence, EGFR inhibitors deserve studying as a target therapy in DIPGs.
Nimotuzumab is an IgG1 antibody that targets EGFR. Promising results of Phase I and II studies with nimotuzumab have been reported in high-grade gliomas including DIPG [9,10,11,12]. In our institution, nimotuzumab was administered initially during progressive disease (PD); then it was used as primary treatment after RT with TMZ. Our aim is to assess the outcome of DIPG patients who were treated with nimotuzumab-containing regimens.
Methods
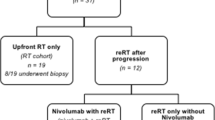
We retrospectively reviewed a total of 24 DIPG patients treated with nimotuzumab-containing regimens either for the newly diagnosed patients or at progression in two centers, Istanbul University Oncology Institute and Bezmialem Vakif University during January 2010–January 2017. Eligibility criteria of the patients included ≤ 3 months of clinical features of cranial nerve deficits, motor nerve disability, or cerebellar dysfunction. All patients had MRI with evidence of a large expensile lesion with irregular margins occupying ≥ 50% of the axial diameter of the pons, hypointensity on T1, and hyperintensity on T2 images. Radiological criteria also included encasement of the basilar artery, extension into the mesencephalon and/or medulla oblongata and/or cerebellar peduncle as well as contrast enhancement with or without necrosis. The diagnosis of DIPG was clinically made after evaluation by a pediatric oncologist, radiation oncologist, neurosurgeon, and radiologist in the weekly multidisciplinary tumor board. All patients were discussed and MRIs were reviewed by the same neurooncologists. Cranial MRI was obtained from all patients at diagnosis and was repeated every 3 months during treatment. All patients received RT as the standard treatment. Since 2004, temozolomide (TMZ) was used concomitantly during RT, followed by TMZ after RT as adjuvant chemotherapy for 12 cycles. From May 2010, nimotuzumab, combined with carboplatin or vinorelbine or TMZ, were administered during progressive disease (PD). After May 2012, children with newly diagnosed DIPG, received TMZ during RT, and nimotuzumab and TMZ after RT. Since no standard agents have demonstrated clinically significant superior activity and since in our previous report of DIPG patients who received RT and TMZ had a significantly higher overall survival than patients who only received RT [5], we decided to continue to use TMZ as a backbone both during and after RT. Following the promising reports with nimotuzumab [9,10,11], we planned to add nimotuzumab to the treatment. Nimotuzumab was used by the approval of the Ministry of Health as off-label use. Nimotuzumab was given as 150 mg/m2/dose once a week for 12 weeks and then every other week with TMZ 200 mg/m2/day for 5 days every 28 days until PD. In PD patients under TMZ + nimotuzumab treatment, TMZ was stopped and either vinorelbine 25 mg/m2/day or carboplatin 350 mg/m2/day was combined with nimotuzumab every 2 weeks until death or for up to 2 years. The treatment schedule for patients with newly diagnosed DIPG and at progression is shown in Fig. 1.
Informed consent was obtained from the parents. All patients had adequate hematologic, renal, and hepatic functions (hemoglobin level > 10 g/dl, total leukocyte count > 4000/mm3 with absolute neutrophil count > 1500/mm3 and platelet count > 100,000/mm3; liver enzyme level < 2.5 upper limit of normal; and serum creatinine level < 1.5 upper limit of normal). Toxicity was graded using the National Cancer Institute Common Toxicity Criteria [13]. Toxicity was evaluated after each course of nimotuzumab-containing chemotherapy.
Results
Among 24 (16 male, 8 female) patients, there were 7 patients with a median age of 6 years (4–11 years) in the PD group; 17 patients with a median age of 7 years (3–14 years) in the newly diagnosed patient group. In seven patients, with PD (Table 1), two had significant clinical improvement for 8 months (Table 1, patients 1 and 2); however, both died at 12 months since the initial diagnosis. One of these patients attended primary school with a good quality of life for 6 months (Table 1, patient 1). The other five patients with PD deteriorated under nimotuzumab treatment at a median of 3 months (1–4 months) and all died at a median of 17 months (7–42 months) since the initial diagnosis (Table 1, patients 3–7). Considering all patients with PD, the median survival from initial diagnosis was 12 months (7–42 months); 1 year OS was 42.9 ± 18% and 2 year OS was 14.3 ± 13% (Table 2). The median survival in this PD group, after the initiation of nimotuzumab was 6 months (3–8 months).
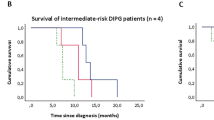
In 17 newly diagnosed patients who received nimotuzumab containing treatment (Table 3), 11 patients had either clinical improvement or remained stable at a median of 6 (3–18) months; however, all died at a median of 11 months (3–35) since diagnosis. One of these patients with clinical improvement for 18 months studied in high school with a good quality of life living in a dormitory (Table 3, patient 1). The tumor of this patient was biopsied when he had PD and revealed anaplastic astrocytoma. The other patient biopsied in this group revealed glioblastome multiforme (Table 3, patient 6). The number 2 patient having clinical improvement for 10 months attended primary school with no major complications (Table 3, patient 2). Another patient in this group had PD at 6 months of initial diagnosis; remained stable under nimotuzumab + vinorelbine combination for 24 months and lived for 35 months since the initial diagnosis (Table 3, patient 5). This patient also successfully attended the kindergarden more than a year. The remaining six patients in this group, having no response, deteriorated during RT and all died at a median of 4 months (3–8 months) (Table 3, patients 12–17).
In the whole newly diagnosed patient group of 17 patients, the median survival from initial diagnosis was 11 months (3–35) months and median progression free survival was 4 months (1–21 months). The 1-year and 2-year OS were 35.3 ± 11% and 11.8 ± 7%, respectively (Table 2).
Nimotuzumab + TMZ / nimotuzumab + carboplatin / or nimotuzumab + vinorelbine was well tolerated with no major adverse effect. We did not observe major adverse effect such as allergic reaction or changes in vital signs during any courses of nimotuzumab combinations. After 8 cycles of TMZ + nimotuzumab, the dose of TMZ was decreased by 25% in two patients (Table 3, patients 1 and 2), and the interval of TMZ was increased to 42 days in the last 4 cycles in one patient due to thrombocytopenia (Table 3, patient 1). There was mild thrombocytopenia in 7 patients under nimotuzumab + TMZ, probably due to TMZ toxicity with no transfusion requirement. No patients required erythrocyte tansfusions. There was no hospitalization due to febrile neutropenia or due to any other drug toxicity. Sixteen patients (3 patients in the PD group and 13 patients in the newly diagnosed patient group) had nutritional support by nasogastric tubing when the gagging reflex elapsed and three patients in the newly diagnosed patient group were fed through percutaneous endoscopic gastrostomy.
Discussion
Gilbertson et al. [6] determined EGFR/ERBB1 amplification and overexpression in 28 samples (18 surgical biopsies and 10 postmortem specimens) of DIPGs and they observed a significant increase in ERBB1 expression in higher tumor grades. Ballester et al. [8] reported EGFR overexpression in 16 of 24 (66%) postmortem DIPG samples and suggested that this could be a potential therapeutic target in a large percentage of cases. However, on the other hand, larger series have shown that EGFR mutation/amplification is a rare event in pediatric DIPG/HGG [14].
The first pediatric results with EGFR antibodies, come from phase II and phase III studies, carried out in Germany in patients with high-grade gliomas including DIPG [9, 10]. The phase II multicentre trial was designed to explore the feasibility and efficacy of nimotuzumab in 47 children and adolescents with resistant and relapsed high-grade gliomas [9]. Eleven patients showed stable disease and partial remission and median OS was extended for responders (10 months) compared to nonresponders (3.2 months) (Table 4). Another Phase II study reported from North America used a quite similar nimotuzumab regimen in 44 progressive DIPG patients and found that time to progression following nimotuzumab for the responding patients (n = 4) ranged from 119 days to 335 days; median survival time was 3.2 months [15] (Table 4). In our study, likewise Bode’s report, in seven patients with progressive disease, there were two patients with clinical improvement for 8 months (240 days). In this PD group, median OS was 12 months (7–42 months) which is much higher than the results reported by Bartels et al. [15] and is promising. In the MD Anderson data, where nimotuzumab was administered to 13 of 31 DIPG patients at progression, the median progression-free survival was reported as 2 months in the whole group; this group also emphasized repeat radiation as the most effective treatment at relapse in terms of PFS [16] (Table 4).
The phase III trial enrolled 42 children with newly diagnosed DIPG from Germany, Italy, and Russia during 2006 to 2007 [10]. Four had partial remission (9.8%) and 27 had (65.8%) stable disease. The median PFS was 5.9 months and the median OS was 9.7 months with a significantly longer survival in radiological responders than in non-responders (Table 4). In 2011, the same group reported the results of 37 newly diagnosed patients where the median PFS was 7 months and OS was 11 months [11] (Table 4). When we evaluate our newly diagnosed patient group, median PFS was 4 months and median OS was 11 months. Our results in newly diagnosed patients receiving nimotuzumab are similar to those reported by Bode et al. [10] and Massimino et al. [11] (Table 2). Three long survivors in this group lived for 22, 27, and 35 months (Table 3, patients 1, 5, and 7); two of them could attend school and one even lived in the school dormitory for 18 months (Table 3, patient 1). The median OS since diagnosis in patients with progressive disease in our study is similar to that of the results of the newly diagnosed patient group (12 months vs 11 months, respectively). In our previous report of DIPG patients before the nimotuzumab era [5], the median survival time among all patients (n = 50) was 13 months (1–160 months). In this previous report [5], patients who received RT and TMZ had a significantly higher overall survival than patients who only received RT (at 1 year 55% versus 42%; at 2 years 37% vs 0%; p = 0.018).
Massimino et al. [12] published another study in which they used nimotuzumab together with vinorelbine in newly diagnosed 25 patients. After a median follow-up of 14 months, 12/20 were alive, their PFS/OS at 12 months were 32%/81%, respectively and the OS at 2-yrs was reported as 28%. In the report of Hoffman et al. [17], where they compared clinical, radiologic, and histomolecular characteristics between short-term survivors and long-term survivors of DIPG, neoadjuvant, or adjuvant systemic therapy correlated with long-term survival in both univariable and multivariable analyses. In this large 1130 patient study, multivariable logistic regression demonstrated higher odds of long-term survival with use of EGFR inhibitors (e.g., gefitinib, erlotinib, nimotuzumab, rindopepimut, cetuximab) at diagnosis [17]. In the study of Massimino et al. [12], they also re-irradiated 11 of 16 patients at progression. Their median PFS and OS were 8.5 and 15 months, respectively (Table 4). Repeat radiation may have contributed to the improved results in this cohort, as has been reported by Janssens et al. and Lassaletta A et al. [18, 19]. There is no known effective therapy for recurrent disease. With increasing evidence of the safety of re-irradiation in other pediatric brain tumors, there is growing interest in considering re-irradiation for progressive DIPG. Lassaletta et al. [19] observed clinical improvement in most of the patients, confirming the benefit of this approach in a palliative setting. Steroids were completely avoided in six patients and were discontinued in four patients by the end of the re-irradiation. However, evaluation of quality of life is lacking in these reports. Data of quality of life may become critical for parents in making decision to proceed to re-irradiation vs palliative care. None of the patients in the present study were re-irradiated. One patient (Table 1, patient 3) who developed a new lesion just outside the initial radiation field received radiation to the new site. Re-irradiation in selected patients had begun recently in our centers.
There are limitations in this study because of its retrospective nature. Only two of the 24 patients could be biopsied. One was an anaplastic astrocytoma and survived for 27 months with a good quality of life. The other was a glioblastome multiforme who lived for 11 months. None of the specimens could be analyzed for H3 mutations. Biopsy of a DIPG with typical imaging findings is justified when the patient is part of an ethically approved clinical study [20]. We may speculate that if we could do biopsies in all samples and correlate the responders to nimotuzumab-containing regimens; this could be a guide for future studies using nimotuzumab. However, currently, there is no prognostic implication associated with treatment outcome according to EGFR expression in DIPGs [8].
In conclusion, DIPG has still very poor prognosis. Although, the addition of nimotuzumab to chemotherapy did not change the dismal prognosis, it might be that, some patients either with newly diagnosed DIPG or with progressive disease may benefit modestly from anti-EGFR-containing combinations.
References
Freeman CR, Farmer JP (1998) Pediatric brain stem gliomas: a review. Int J Radiat Phys 40:265–271
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248
Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ (2012) Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev 38:27–35
Kebudi R, Cakir FB (2013) Management of diffuse intrinsic pontine gliomas in children: recent developments. Pediatr Drugs 15:351–362
Kebudi R, Cakir FB, Yaman Agaoglu F et al (2013) Pediatric diffuse intrinsic pontine glioma patients from a single center. Childs Nerv Syst 29:583–588
Gilbertson RJ, Hill DA, Hernan R (2003) ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem gliomas. Clin Cancer Res 9:3620–3624
Zarghooni M, Bartels U, Lee E, Buczkowicz P, Morrison A, Huang A, Bouffet E, Hawkins C (2010) Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADPribose) polymerase as potential therapeutic targets. J Clin Oncol 28:1337–1344
Ballester LY, Wang Z, Shandilya S, Miettinen M, Burger PC, Eberhart CG, Rodriguez FJ, Raabe E, Nazarian J, Warren K, Quezado MM (2013) Morphologic characteristics and immunohistochemical profile of diffuse intrinsic pontine gliomas. Am J Surg Pathol 37:1357–1364
Bode U, Buchen S, Warmuth-Metz M, Pietsch T, Bach F, Fleischhack G (2007) Final report of a phase II trial of nimotuzumab in the treatment of refractory and relapsed high-grade gliomas in children and adolescents [abstract]. J Clin Oncol 25 Suppl. 2006
Bode U, Windelberg M, Massimino M et al (2008) Phase III trial of nimotuzumab for the treatment of newly diagnosed diffuse intrinsic pontine gliomas in children and adolescents [abstract]. J Clin Oncol 26.Suppl.2008
Massimino M, Bode U, Biassoni V, Fleischhack G (2011) Nimotuzumab for pediatric diffuse intrinsic pontine gliomas. Expert Opin Biol Ther 11:247–256
Massimino M, Biassoni V, Miceli R et al (2014) Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neuro-Oncol 118:305–312
National Cancer Institute. Common terminology criteria for adverse events v4.03 (CTCAE). June 14, 2010. Available at evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
Mackay A, Burford A, Carvalho D et al (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32(4):520–537.e5
Bartels U, Wolff J, Gore L, Dunkel I, Gilheeney S, Allen J, Goldman S, Yalon M, Packer RJ, Korones DN, Smith A, Cohen K, Kuttesch J, Strother D, Baruchel S, Gammon J, Kowalski M, Bouffet E (2014) Phase 2 study of safety and efficacy of nimotuzumab in pediatric patients with progressive diffuse intrinsicpontine glioma. Neuro-Oncology 16:1554–1559
Wolff JE, Rytting ME, Vats TS, Zage PE, Ater JL, Woo S, Kuttesch J, Ketonen L, Mahajan A (2012) Treatment of recurrent diffuse intrinsic pontine glioma: the MD Anderson Cancer Center experience. J Neuro-Oncol 106:391–397
Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N et al (2018) Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG registries. J Clin Oncol 36(19):1963–1972
Janssens GO, Gandola L, Bolle S, Mandeville H, Ramos-Albiac M, van Beek K, Benghiat H, Hoeben B, Morales la Madrid A, Kortmann RD, Hargrave D, Menten J, Pecori E, Biassoni V, von Bueren AO, van Vuurden DG, Massimino M, Sturm D, Peters M, Kramm CM (2017) Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 73:38–47
Lassaletta A, Strother D (2018) Reirradiation in patients with diffuse intrinsic pontine gliomas: the Canadian experience. Pediatr Blood Cancer 65(6):e26988
Walker DA, Liu J, Kieran M, Jabado N, Picton S, Packer R, St. Rose C, (on behalf of the CPN Paris 2011 Conference Consensus Group), van Meeteren AS, Carvalho A, van Damme A, Depreitere B, Gustavsson B, Due Tonnessen BJ, Bertozzi-Salamon AI, Brentrup A, Raybaud C, Jones C, Dufour C, Dorfer C, Sainte-Rose C, Malluci C, Hargrave D, Walker D, van Vuurden D, de Carli E, Bouffet E, van Calenbergh F, Frappaz D, Frassanito P, Goodrich J, Baechli H, Grill J, Ternier J, Cappelen J, Caird J, Pereira J, Riffaud L, Baroncini M, Walker M, Kieran M, Ozek M, Jabado N, Nysom K, Varlet P, Goodden J, Bertolini P, Perilongo G, Mercier P, Grundy R, Kortmann RD, Packer R, Pfister S, Constantini S, Sgouros S, Holm S, Czech T, Merchant T, Stokland T, Ridola V, Vandertop P (2013) A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro-Oncology 15(4):462–468
Acknowledgements
We thank Prof Dr. Serra Sencer and Prof Dr. Kubilay Aydın, from the Istanbul University, Istanbul Medical Faculty, Department of Radiology, for the radiologic evaluation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kebudi, R., Cakir, F.B., Bay, S.B. et al. Nimotuzumab-containing regimen for pediatric diffuse intrinsic pontine gliomas: a retrospective multicenter study and review of the literature. Childs Nerv Syst 35, 83–89 (2019). https://doi.org/10.1007/s00381-018-4001-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-4001-9