Abstract
Schisandrin A (Sch A) is a lignin extracted from the fruit of Schisandra chinensis, which has potential anti-inflammatory properties and is used for treating various inflammatory diseases. In this study, we aimed to evaluate the anti-inflammatory effects of Sch A and the underlying mechanisms in animal models of acute inflammation. First, the anti-inflammatory effects of Sch A were evaluated preliminarily in an animal model of xylene-induced ear edema. Sch A pretreatment significantly decreased the degree of edema and inhibited telangiectasia in the ear. Second, a mouse model of paw edema was used to investigate the anti-inflammatory effects and mechanisms of Sch A. Pretreatment with Sch A significantly inhibited carrageenan-induced paw edema in mice. Hematoxylin-eosin (HE) staining of paw tissues demonstrated that Sch A inhibited the infiltration of inflammatory cells in the mouse model of paw edema. Enzyme-linked immunosorbent assay (ELISA) results indicated that the levels of inflammatory factors decreased. The western blot and immunohistochemical assay results revealed that the toll-like receptor 4/nuclear factor kappa-B (TLR4/NF-κB) pathway could play a role in the anti-inflammatory functions of Sch A. The findings demonstrated that Sch A exerts anti-inflammatory effects and may provide possible strategies for the treatment of inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a basic pathophysiological process that enables the body to remove pathogens and repair damaged tissues following infection or injury (Tang et al. 2018). In physiological conditions, the inflammatory response allows the body to remove harmful stimuli and maintain homeostasis, thus promoting the repair and healing of damaged tissues (Shabbir et al. 2018). However, chronic inflammation caused by acute unresolved inflammation or persistent irritation can lead to the occurrence of many severe refractory diseases (Gilroy and De Maeyer 2015), such as bronchial asthma, Parkinson’s disease, and Alzheimer’s disease. Presently, there are two main classes of drugs for the treatment of inflammation: non-steroidal and steroidal anti-inflammatory drugs (Cui et al. 2019). However, both drug classes have multiple side effects; therefore, new anti-inflammatory drugs with better efficacy and fewer side effects need to be developed.
Schisandra chinensis is a traditional Chinese medicine, listed in Chinese Pharmacopeia, and is used to treat a wide variety of diseases (Panossian and Wikman 2008; Liang et al. 2014). Modern pharmacological studies show that Schisandra chinensis has anti-inflammatory actions (Song et al. 2019), is hepato-protective (Park et al. 2014), sedation and hypnosis, lowering of the blood sugar (Xu et al. 2015), anti-oxidant effects (Jang et al. 2014), enhanced immunity, and has anti-cancer effects. The main components of Schisandra chinensis are lignans, volatile oils, triterpenes, polysaccharides, and flavonoids, among which, lignans are the main characteristic active components. Schisandrin A (Sch A) is one of the main active constituents of lignans in Schisandra chinensis (Qiu et al. 2018). Previous studies have demonstrated that Sch A also possesses anti-inflammatory effects. In vitro, Sch A can inhibit lipopolysaccharide-induced inflammation in macrophages (Kwon et al. 2018). Additionally, Sch A has also been shown to suppress lipopolysaccharide-induced neuronal damage in microglial cells (Li et al. 2018). In vivo, Sch A can attenuate dextran sulfate sodium-induced ulcerative colitis (Zhang et al. 2016). In addition, Sch A can protect against cerebral ischemia-reperfusion injury (Zhou et al. 2019). However, the role of Sch A in ear edema is still unclear. The anti-inflammatory mechanism of Sch A in the paw edema has also not been investigated. This study aimed to determine the anti-inflammatory effect of Sch A in xylene-induced ear edema and the anti-inflammatory mechanism of Sch A in carrageenan-induced paw edema in mice. This study provides an important theoretical basis for the development and utilization of Sch A. Meanwhile, the study also provides new directions for researchers to produce novel and more effective anti-inflammatory drugs by modifying the structure of Sch A.
Materials and methods
Animals
Male Kunming mice (body weight 20–25 g) were procured from the Jinan Pengyue Animal Center. All mice were kept in at a temperature of 20–24 °C, with standard 12-h light-dark cycles, and at a relative humidity of 54–59%. The mice were allowed to eat and drink ad libitum. The Ethics Committee of Jining Medical University approved the experimental protocols (Serial number: JNMC-2019-DW-002).
Chemicals
Sch A (purity > 98%) extracted and purified as previously described (Caichompoo et al. 2009) was purchased from Daosifu Biotechnology co., LTD. (Nanjing, China). Carrageenan was supplied by Bomei biotechnology (Hefei, China). All antibodies used in this research were provided by Bioworld Technology (MN, USA).
Xylene-induced ear edema
The mice were divided into four groups (n = 10): control, indomethacin (5 mg/kg), Sch A (25 mg/kg), and Sch A (50 mg/kg). The control group was provided with distilled water (10 ml/kg). The doses of Sch A were determined according to the reference (Zhou et al. 2019). All groups received treatment orally for seven consecutive days. One hour after the last administration, xylene (50 μL) was evenly applied to the front and back surface of the left ears, and the right ears were wiped with normal saline (as control). After 30 min, the mice were sacrificed and both ears were immediately excised. A section of each ear was sampled using an 8-mm perforator and then weighed. The degree of ear edema was calculated according to the following formula (Gong et al. 2019):
Carrageenan-induced paw edema
The mice were randomly divided into control, carrageenan, indomethacin (5 mg/kg), Sch A (25 mg/kg), and Sch A (50 mg/kg) groups, with 10 mice in each group. The control and carrageenan groups were treated with distilled water (10 ml/kg). All groups received treatment orally for seven consecutive days. One hour after the last administration, acute inflammation was induced in all groups except the control group using an intraplantar injection of 30 μL of 1% carrageenan in the left hind paw. The measurements were performed at 0, 1, 3, and 5 h after carrageenan injection using a PV-200 instrument (PV-200, TaiMeng Technology, Chengdu, China). Edema rate was calculated as previously described, using the following equation (Ma et al. 2013):
Histopathology analysis
The mice were sacrificed 5 h after the carrageenan was injected and the paw volumes were recorded. The paw tissues were then collected, fixed with 10% formaldehyde, and embedded in paraffin. Four micron slices were cut from the paraffin, and then stained with hematoxylin-eosin (HE). The histological changes of the paw were observed using an electron microscope.
ELISA detection
Paw tissues were homogenized and the homogenates were centrifuged as previously described (Wilches et al. 2019). The supernatants were collected to detect levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and myeloperoxidase (MPO) activity, according to the instructions of the ELISA kit (Kejing Biological Technology, Jiangsu, China).
Immunohistochemical analysis
The paw tissues of the mice in each group were embedded and sectioned following a standard protocol. The expression of NF-κB/p65 was detected using standard immunohistochemical staining, as previously described (El-Sheakh et al. 2015). The sections were incubated with the primary antibody (anti-NF-κB/p65), and horseradish peroxidase (HRP)-polymer was used as the secondary antibody. The sections were then colored with diaminobenzidine (DAB) and counterstained with methyl green.
Western blot analysis
Protein was extracted from the paw tissues using radio immunoprecipitation assay (RIPA) buffer (Solarbio, Beijing, China). The protein concentrations were determined using the bicinchoninic acid (BCA) method. The proteins were separated using sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked using 5% skim milk and incubated with primary antibodies for TLR4 (Bioworld Technology; BS3489; 1:750), p-p65 (Bioworld Technology; BS4137; 1:750), IκBα (Bioworld Technology; BS3601; 1:750), p-IκBα (Bioworld Technology; BS4105; 1:750), β-actin (Bioworld Technology; AP0060; 1:750), and secondary antibodies (Bioworld Technology; BS13278; 1:10000), according to the standard western blot procedure. Finally, the bands were visualized using enhanced chemiluminescence (ECL) (Beyotime, Shanghai, China). Band intensities were measured using the ImageJ software.
Statistical analysis
The values are presented as mean ± SD. The differences were analyzed using the one-way ANOVA test. All analyses were conducted using SPSS (version 20.0). P values < 0.05 were considered statistically significant.
Results
Effect of Sch A on ear edema
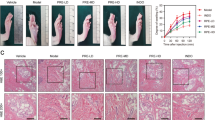
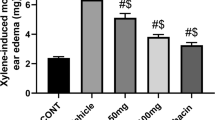
In the control group, the left ears showed a marked inflammatory response, including redness and edema, which was confirmed by calculating the degree of edema. Treatment with Sch A resulted in a dose-dependent reduction in xylene-induced ear edema in the mice (Fig. 1b). Meanwhile, there was an obvious reduction of redness on the left ears (Fig. 1a). The effect of Sch A (50 mg/kg) was comparable to the effect of indomethacin (5 mg/kg).
Carrageenan-induced mice paw edema
Carrageenan administration triggered a significant increase in the paw volume of the carrageenan group. Pretreatment with Sch A (25 and 50 mg/kg) markedly decreased paw edema at 1, 3, and 5 h after inflammation induction, indicating that Sch A (25 and 50 mg/kg) protected against paw edema induced by carrageenan (Fig. 2). Sch A (50 mg/kg) was comparable to indomethacin at the 5th hour after the inflammation was induced.
Effects of Sch A on carrageenan-induced paw edema: histopathological changes
The effects of Sch A on paw histopathological changes were evaluated by HE staining. In the carrageenan-induced paw edema animal model, the inflammatory cell was mainly neutrophils. Compared with the control group, the neutrophil infiltration was easily observed in the carrageen group (Fig. 1b). However, pretreatment with Sch A clearly diminished the carrageenan-induced histopathological changes (Fig. 3d and e).
Effects of Sch A on MPO activity
Compared with the control group, MPO activity obviously increased after carrageenan injection. In contrast, pretreatment with Sch A clearly inhibited MPO activity (Fig. 4).
Effects of Sch A on TNF-α and IL-1β levels
The levels of TNF-α and IL-1β remained low in the control group. However, levels of both TNF-α and IL-1β were significantly elevated in the carrageenan group. Pretreatment with Sch A significantly reduced the production of TNF-α and IL-1β in a dose-dependent manner (Fig. 5).
Effects of Sch A on TLR4 expression and NF-κB activation
The expression of TLR4 increased after carrageenan stimulation. Sch A treatment restored TLR4 expression to normal levels. Meanwhile, the injection of carrageenan resulted in increased levels of NF-κB and increased phosphorylation of IκBα and p65. Sch A treatment significantly attenuated these alterations (Figs. 6 and 7).
Representative NF-κB p65 immunohistochemistry in the paw tissue (magnification × 400). Paw sections from the control group (a) showed a small degree of immunostaining for NF-κB p65. On the other hand, induction with carrageenan significantly increased the immunohistochemical expression of NF-κB p65 in the carrageenan group (b). Pretreatment with indomethacin and Sch A markedly reduced positive nucleus staining for NF-κB p65 (c, d, e). The arrows indicate positive staining for NF-κB p65
Discussion
In this study, the anti-inflammatory bioactivity of Sch A was evaluated using xylene- and carrageenan-induced edema models, two classic animal models of acute inflammation.
In order to develop a preliminary understanding of the anti-inflammatory effects of Sch A, we used a mouse model of xylene-induced acute inflammatory ear edema. This model mainly causes the release of histamine, bradykinin, and other inflammatory mediators, resulting in local vasodilation, increased capillary permeability, inflammatory cell infiltration, and acute exudative inflammatory edema in the ear (Lee et al. 2019). In this study, pretreatment with Sch A markedly suppressed xylene-induced acute inflammation in the ear, as demonstrated by the decreasing degree of ear edema degree and reduced degree of ear redness, suggesting that Sch A possesses potent anti-inflammatory effects.
After obtaining preliminary evidence demonstrating the anti-inflammatory effects of Sch A, we used a model of paw edema to further explore the anti-inflammatory effects of Sch A and its mechanisms. The carrageenan-induced paw edema animal model is used to evaluate the anti-inflammatory effects of drugs in a multitude of studies (Yonathan et al. 2006). Previous studies have shown that Sch A can suppress carrageenan-induced paw edema in comparison with Sch A and Schisandra B in the anti-inflammatory effect of the difference. Sch A produced an inhibition on carrageenan-induced paw edema in both long-term and acute treatment, while Schisandra B that was another important ingredient in Schisandra chinensis had the effect of inhibiting paw edema only during long-term treatment (Leong et al. 2016). The results obtained in our study indicate that Sch A markedly inhibited the carrageenan-induced edema and histopathological changes in the paw tissues. These results confirmed the anti-inflammatory properties of Sch A.
The adhesion and infiltration of white blood cells, especially neutrophils (PMN), are important features of acute inflammation (Sadeghi et al. 2013). MPO is the most reliable index used to evaluate the degree of infiltration of PMN aggregation. The aggregation and degree of infiltration of PMN can be quantified by determining MPO activity in local tissues (Loria et al. 2008). In this study, we found that MPO activity significantly increased in acute inflammatory mice paw edema tissues, suggesting that carrageenan-induced inflammation resulted in PMN aggregation and infiltration in inflammatory sites. Sch A markedly reduced MPO activity, indicating that Sch A can inhibit the aggregation and infiltration of neutrophils in inflammatory sites and play an anti-inflammatory role. These findings are consistent with the pathological results and the histological test results. IL-1β and TNF-α are important indicators of inflammation. Under physiological conditions, their concentrations are maintained at a low level. However, their levels significantly increase under inflammatory conditions (Hu et al. 2019). In this study, a considerable rise of IL-1β and TNF-α was detected in edema tissues. Pre-treatment with Sch A visibly inhibited the increase of IL-1β and TNF-α levels, indicating that Sch A may reduce the degree of inflammation by inhibiting the release of inflammatory cytokines.
NF-κB is a nuclear transcription factor that is closely related to inflammation in the body (Hayden and Ghosh 2011). In its resting state, NF-κB has no biological activity. However, when stimulated by cytokines, endotoxins, and other factors, NF-κB can initiate the transcription of target genes and promote the release of inflammatory factors (Liang et al. 2018). Previous studies have demonstrated that Sch A is able to modulate inflammation by inhibiting the NF-κB pathways. However, it is still uncertain whether the anti-inflammatory effect of Sch A is related to the upstream protein of NF-κB. TLRs are pattern-recognition receptors that play a pivotal role in the immune system (Yang et al. 2016). They can induce the release of various cytokines involved in the inflammatory response through activating the NF-κB signal transduction pathway. TLR4 is an important component of the TLRs/NF-κB signaling pathway that mediates inflammation. After stimulation of the tissues, TLR4 in the cell membrane recognizes the corresponding ligand and activates NF-κB, thereby inducing the continuous inflammatory response (Qi et al. 2019). In this study, the results demonstrated that the expression of TLR4 and downstream proteins, including p-IκBα and p-p65, were considerably upregulated after carrageenan induction. Simultaneously, there was visible immunostaining for NF-κB p65 in the carrageenan group. Pretreatment with Sch A noticeably reduced the expression of TLR4, p-IκBα, p-p65, and positive staining for NF-κB p65, indicating that Sch A may decrease inflammation by inhibiting the TLR4/NF-κB signaling pathway (Fig. 8). These results are similar to those of previous studies (Shalini et al. 2015; Kwon et al. 2018; Rai et al. 2018). Previous studies have also demonstrated that the mechanism of Sch A was related to inhibition of the mitogen-activated protein kinases (MAPK) and phosphatidylinositol-3 kinase (PI3K/Akt) signal pathways (Kwon et al. 2018). However, we did not explore the anti-inflammatory effect of Sch A on other inflammatory pathways. Therefore, future studies should be conducted to determine whether Sch A can regulate other inflammatory pathways.
In summary, the evidence obtained from this study demonstrated that Sch A protects against xylene- and carrageenan-induced edema. The underlying mechanism might be downregulation of the TLR4/NF-κB signaling pathway.
References
Caichompoo W, Zhang QY, Hou TT, Gao HJ, Qin LP, Zhou XJ (2009) Optimization of extraction and purification of active fractions from Schisandra chinensis (Turcz.) and its osteoblastic proliferation stimulating activity. Phytother Res 23:289–292
Cui LK, Han YM, Ma YK (2019) Calycosin ameliorates inflammatory paw edema in mice via inhibiting NF-kappa B activation. Int J Pharmacol 15:744–751
El-Sheakh AR, Ghoneim HA, Suddek GM, el SM A (2015) Antioxidant and anti-inflammatory effects of flavocoxid in high-cholesterol-fed rabbits. Naunyn Schmiedeberg's Arch Pharmacol 388:1333–1344
Gilroy D, De Maeyer R (2015) New insights into the resolution of inflammation. Semin Immunol 27:161–168
Gong LL, Yang S, Liu H, Zhang W, Ren LL, Han FF, Lv YL, Wan ZR, Liu LH (2019) Anti-nociceptive and anti-inflammatory potentials of Akebia saponin D. Eur J Pharmacol 845:85–90
Hayden MS, Ghosh S (2011) NF-kappaB in immunobiology. Cell Res 21:223–244
Hu YF, Wang L, Xiang L, Wu JS, Huang WG, Xu CS, Meng XL, Wang P (2019) Pharmacokinetic-pharmacodynamic modeling for coptisine challenge of inflammation in LPS-stimulated rats Scientific Reports 9:1450
Jang HI, Do GM, Lee HM, Ok HM, Shin JH, Kwon O (2014) Schisandra Chinensis Baillon regulates the gene expression of phase II antioxidant/detoxifying enzymes in hepatic damage induced rats. Nutr Res Pract 8:272–277
Kwon DH, Cha HJ, Choi EO, Leem SH, Kim GY, Moon SK, Chang YC, Yun SJ, Hwang HJ, Kim BW, Kim WJ, Choi YH (2018) Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-kappaB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int J Mol Med 41:264–274
Lee YY, Saba E, Irfan M, Kim M, Yi-Le Chan J, Jeon BS, Cho SK, Rhee MH (2019) The anti-inflammatory and anti-nociceptive effects of Korean black ginseng. Phytomedicine 54:169–181
Leong PK, Wong HS, Chen JH, Chan WM, Leung HY, Ko KM (2016) Differential action between Schisandrin A and Schisandrin B in eliciting an anti-inflammatory action: the depletion of reduced glutathione and the induction of an antioxidant response. PLoS One 11:e0155879
Li S, Xie R, Jiang C, Liu M (2018) Schizandrin A alleviates LPS-induced injury in human keratinocyte cell Hacat through a MicroRNA-127-dependent regulation. Cell Physiol Biochem 49:2229–2239
Liang CQ, Luo RH, Yan JM, Li Y, Li XN, Shi YM, Shang SZ, Gao ZH, Yang LM, Zheng YT, Xiao WL, Zhang HB, Sun HD (2014) Structure and bioactivity of triterpenoids from the stems of Schisandra sphenanthera. Arch Pharm Res 37:168–174
Liang Y, Shen T, Ming Q, Han G, Zhang Y, Liang J, Zhu D (2018) Alpinetin ameliorates inflammatory response in LPS-induced endometritis in mice. Int Immunopharmacol 62:309–312
Loria V, Dato I, Graziani F, Biasucci LM (2008) Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediat Inflamm 2008:135625
Ma Y, Li Y, Li X, Wu Y (2013) Anti-inflammatory effects of 4-methylcyclopentadecanone on edema models in mice. Int J Mol Sci 14:23980–23992
Panossian A, Wikman G (2008) Pharmacology of Schisandra chinensis bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol 118:183–212
Park HJ, Lee SJ, Song Y, Jang SH, Ko YG, Kang SN, Chung BY, Kim HD, Kim GS, Cho JH (2014) Schisandra chinensis prevents alcohol-induced fatty liver disease in rats. J Med Food 17:103–110
Qi C, Shao Y, Liu X, Wang D, Li X (2019) The cardioprotective effects of icariin on the isoprenaline-induced takotsubo-like rat model: involvement of reactive oxygen species and the TLR4/NF-kappaB signaling pathway. Int Immunopharmacol 74:105733
Qiu F, Liu H, Duan H, Chen P, Lu SJ, Yang GZ, Lei XX (2018) Isolation, Structural elucidation of three new triterpenoids from the stems and leaves of Schisandra chinensis (Turcz) Baill. Molecules 23:1624
Rai U, Rawal A, Singh S (2018) Evaluation of the anti-inflammatory effect of an anti-platelet agent crinumin on carrageenan-induced paw oedema and granuloma tissue formation in rats. Inflammopharmacology 26:769–778
Sadeghi H, Hajhashemi V, Minaiyan M, Movahedian A, Talebi A (2013) Further studies on anti-inflammatory activity of maprotiline in carrageenan-induced paw edema in rat. Int Immunopharmacol 15:505–510
Shabbir A, Batool SA, Basheer MI, Shahzad M, Sultana K, Tareen RB, Iqbal J, Saeed-ul-Hassan (2018) Ziziphora clinopodioides ameliorated rheumatoid arthritis and inflammatory paw edema in different models of acute and chronic inflammation. Biomed Pharmacother 97:1710–1721
Shalini V, Jayalekshmi A, Helen A (2015) Mechanism of anti-inflammatory effect of tricin, a flavonoid isolated from Njavara rice bran in LPS induced hPBMCs and carrageenan induced rats. Mol Immunol 66:229–239
Song FJ, Zeng KW, Chen JF, Li Y, Song XM, Tu PF, Wang XM (2019) Extract of Fructus Schisandrae chinensis inhibits neuroinflammation mediator production from microglia via NF-kappa B and MAPK pathways. Chin J Integr Med 25:131–138
Tang L, Luo JR, Li DT, Ge R, Ma YL, Xu F, Liang TG, Ban SR, Li QS (2018) Anti-inflammatory effects of 4-o-methyl-benzenesulfonyl benzoxazolone (MBB) in vivo and in vitro as a novel NSAIDs lead compound. Pharmacol Rep 70:558–564
Wilches I, Jimenez-Castillo P, Cuzco N, Clos MV, Jimenez-Altayo F, Penaherrera E, Jerves-Andrade L, Tobar V, Vander Heyden Y, Leon-Tamariz F, Vila E (2019) Anti-inflammatory and sedative activities of Peperomia galioides: in vivo studies in mice. Nat Prod Res 29:1–5
Xu W, Zhou Q, Yin JJ, Yao Y, Zhang JL (2015) Anti-diabetic effects of polysaccharides from Talinum triangulare in streptozotocin (STZ)-induced type 2 diabetic male mice. Int J Biol Macromol 72:575–579
Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W, Deng C, Fan C, Di S, Sun Y, Yi W (2016) The emerging role of toll-like receptor 4 in myocardial inflammation. Cell Death Dis 7:e2234
Yonathan M, Asres K, Assefa A, Bucar F (2006) In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol 108:462–470
Zhang WF, Yang Y, Su X, Xu DY, Yan YL, Gao Q, Duan MH (2016) Deoxyschizandrin suppresses dss-induced ulcerative colitis in mice. Saudi J Gastroenterol 22:448–455
Zhou F, Wang M, Ju J, Wang Y, Liu Z, Zhao X, Yan Y, Yan S, Luo X, Fang Y (2019) Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res 11:199–209
Funding
This research was funded by the Shandong provincial college students innovation and entrepreneurship training program (S201910443039).
Author information
Authors and Affiliations
Contributions
LC and LX designed the study. WZ, ZY, and CX performed the experiments. XS and ZC analyzed the data. LC wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Ethics Committee of Jining Medical University approved the experimental protocols (Jining, China).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, L., Zhu, W., Yang, Z. et al. Evidence of anti-inflammatory activity of Schizandrin A in animal models of acute inflammation. Naunyn-Schmiedeberg's Arch Pharmacol 393, 2221–2229 (2020). https://doi.org/10.1007/s00210-020-01837-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01837-x