Abstract
Phenoxodiol is used for the treatment of malignancy. The substance is effective by triggering suicidal tumor cell death or apoptosis. At least in theory, phenoxodiol could similarly stimulate suicidal erythrocyte death or eryptosis. Eryptosis is characterized by cell shrinkage and breakdown of cell membrane asymmetry with phosphatidylserine translocation to the erythrocyte surface. Signaling of eryptosis includes increase of cytosolic Ca2+ activity ([Ca2+]i), formation of reactive oxygen species (ROS), and increase of ceramide abundance at the cell surface. The present study explored whether phenoxodiol induces eryptosis and whether it modifies Ca2+ entry, ROS, and ceramide. Using flow cytometry, phosphatidylserine exposure at the cell surface was quantified from annexin V binding, cell volume from forward scatter, [Ca2+]i from Fluo3 fluorescence, ROS from DCFDA-dependent fluorescence, and ceramide abundance utilizing specific antibodies. A 48-h exposure of human erythrocytes to phenoxodiol (100 μg/ml [416 μM]) significantly increased the percentage of annexin V binding cells, significantly decreased average forward scatter and Fluo3 fluorescence and significantly increased ceramide abundance, but did not significantly modify DCFDA fluorescence. The effect of phenoxodiol on annexin V binding tended to decrease following removal of extracellular Ca2+, an effect, however, not reaching statistical significance. In conclusion, phenoxodiol triggers eryptosis, an effect paralleled by increase of ceramide abundance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenoxodiol, an isoflavonoid (Fig. 1), is utilized in the treatment of malignancy (Aguero et al. 2005; Alvero et al. 2006; Choueiri et al. 2006b; Mor et al. 2006; Saif et al. 2009; Silasi et al. 2009; de Souza et al. 2010). The substance is at least in part effective by triggering suicidal death and sensitization against cytoxic treatment of tumor cells (Kamsteeg et al. 2003; Sapi et al. 2004; Straszewski-Chavez et al. 2004; Alvero et al. 2006, 2008; Choueiri et al. 2006a, b; Gamble et al. 2006; Mor et al. 2006, 2008; Yu et al. 2006; Herst et al. 2007, 2009; Kluger et al. 2007; Morre et al. 2007; Yagiz et al. 2007; De Luca et al. 2008, 2010; Aguero et al. 2010; Wu et al. 2011; Mahoney et al. 2012; Yao et al. 2012; Li et al. 2014; Isono et al. 2018; Miyamoto et al. 2018). Studies on structure-activity relationships (Silasi et al. 2009; Chen et al. 2015) revealed that the anticancer activity of phenoxodiol was superior to the parent molecule genistein (Silasi et al. 2009). Studies utilizing tumor xenograft mouse models demonstrated in vivo activity of phenoxodiol (Alvero et al. 2006, 2007; McPherson et al. 2009; Aguero et al. 2010; Yao et al. 2012; Li et al. 2014).
At least in theory, phenoxodiol could similarly trigger eryptosis, the suicidal death of erythrocytes characterized by breakdown of cell membrane asymmetry with phosphatidylserine translocation to the cell surface (Mischitelli et al. 2016a; Bissinger et al. 2019) and cell shrinkage (Lang et al. 2003). Signaling regulating eryptosis includes increase of cytosolic Ca2+ activity ([Ca2+]i) (Mischitelli et al. 2016b; Bissinger et al. 2019), ceramide appearance at the cell surface (Abed et al. 2012), and oxidative stress (Bissinger et al. 2019). Triggers of eryptosis include diverse cytotoxic drugs (Lang et al. 2013, 2017; Pretorius et al. 2016; Briglia et al. 2017; Bissinger et al. 2019).
The present study explored whether phenoxodiol is capable of stimulating eryptosis. Erythrocytes isolated from healthy volunteers were treated with phenoxodiol and phosphatidylserine surface abundance, cell volume, [Ca2+]i, ROS formation, and ceramide abundance were determined by flow cytometry.
Materials and methods
Erythrocytes, solutions, and chemicals
Erythrocytes were isolated from fresh Li-heparin-anticoagulated blood samples drawn from healthy volunteers in the blood bank of the University Clinic of Tübingen. The study is approved by the ethics committee of the University of Tübingen (184/2003 V). The blood was centrifuged at 120g for 20 min at 21 °C, platelets and leukocytes-containing supernatant discarded, and erythrocytes incubated at a hematocrit of 0.4% in Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1 MgSO4, 32 N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES; pH 7.4), 5 glucose, and 1 CaCl2, at 37 °C for 48 h. In nominally Ca2+-free solutions, 1 mM CaCl2 was replaced by 1 mM EGTA. Where indicated, erythrocytes were exposed for 48 h to phenoxodiol (Sigma, Schnelldorf, Germany) at concentrations ranging from 25 μg/ml (104 μM) to 500 μg/ml (2081 μM).
Annexin V binding and forward scatter
Erythrocytes were washed in Ringer solution containing 5 mM CaCl2 and then stained with annexin V FITC (1:200 dilution; ImmunoTools, Friesoythe, Germany) in this solution at 37 °C for 20 min protected against light. Annexin V abundance at the erythrocyte surface was quantified in a FACSCalibur (BD, Heidelberg, Germany) with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. Parallel forward scatter (FSC) was determined as measure of cell volume.
Intracellular Ca2+
After incubation and washing in Ringer solution, erythrocytes were loaded with Fluo-3/AM (Biotium, Hayward, USA) at 37 °C for 30 min in Ringer solution containing 5 mM CaCl2 and 5 μM Fluo-3/AM. Fluorescence intensity was measured with an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a FACSCalibur. The geomean of the Ca2+-dependent fluorescence was calculated.
Reactive oxygen species
Reactive oxygen species (ROS) was determined utilizing 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA). After incubation, washing in Ringer solution, and staining with 10 μM DCFDA (Sigma, Schnelldorf, Germany) in Ringer solution, erythrocytes were incubated at 37 °C for 30 min in the dark and washed two times in Ringer solution. ROS-dependent fluorescence intensity was measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a FACSCalibur (BD). The geomean of the DCFDA-dependent fluorescence was calculated.
Ceramide abundance
Ceramide abundance at the erythrocyte surface was quantified with a monoclonal antibody-based assay. Erythrocytes were stained for 1 h at 37 °C with 1 μg/ml anti-ceramide antibody (clone MID 15B4, Alexis, Grünberg, Germany) in PBS containing 0.1% bovine serum albumin (BSA) at a dilution of 1:10, washed twice with PBS-BSA, and stained for 30 min with polyclonal fluorescein isothiocyanate (FITC) conjugated goat anti-mouse IgG- and IgM-specific antibody (Pharmingen, Hamburg, Germany) diluted 1:50 in PBS-BSA. FITC abundance was quantified by flow cytometry at an excitation wavelength of 488 nm and an emission wavelength of 530 nm. The geomean of the ceramide-dependent fluorescence was calculated.
Statistics
Measured values are expressed as arithmetic means ± SD. Statistical analysis was made using ANOVA with Tukey’s test as post-test and t test as appropriate (n = number of different erythrocyte specimens studied).
Results
The effect of phenoxodiol on eryptosis was tested by determination of cell membrane scrambling and cell shrinkage.
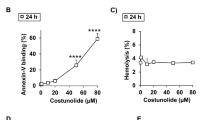
Erythrocytes were incubated for 48 h in Ringer solution without or with phenoxodiol (25–100 μg/ml [104–416 μM] or 100–500 μg/ml [416 μM–2080 μM]) and cell membrane scrambling quantified from phosphatidylserine abundance at the erythrocyte surface identified by determination of annexin V binding utilizing flow cytometry. As illustrated in Fig. 2, a 48-h exposure to phenoxodiol increased the percentage of phosphatidylserine exposing erythrocytes, an effect reaching statistical significance at 50 μg/ml (208 μM) phenoxodiol.
Effect of phenoxodiol on phosphatidylserine exposure. a Histogram of annexin V binding of erythrocytes following exposure for 48 h to Ringer solution without (gray area) and with (black line) presence of 100 μg/ml (416 μM) phenoxodiol. b Arithmetic means ± SD (n = 20) of erythrocyte annexin V binding following incubation for 48 h to Ringer solution without (white bar) or with (black bars) phenoxodiol (25–100 μg/ml [104–416 μM]) or the solvent alone (DMSO, gray bar). c Arithmetic means ± SD (n = 15) of erythrocyte annexin V binding following incubation for 48 h to Ringer solution without (white bar) or with (black bars) phenoxodiol (100–500 μg/ml [416–2080 μM]) or the solvent alone (DMSO, gray bar). ***p < 0.001 indicates significant difference from the absence of phenoxodiol (ANOVA)
Erythrocyte shrinkage was quantified by measuring erythrocyte forward scatter utilizing flow cytometry. As a result, exposure to phenoxodiol (25–100 μg/ml [104–416 μM] or 100–500 μg/ml [416 μM–2080 μM]) decreased the average erythrocyte forward scatter, an effect reaching statistical significance at 50 μg/ml (208 μM) phenoxodiol (Fig. 3).
Effect of phenoxodiol on erythrocyte forward scatter. a Histograms of forward scatter of erythrocytes following exposure for 48 h to Ringer solution without (gray area) and with (black line) presence of 100 μg/ml (416 μM) phenoxodiol. b Arithmetic means ± SD (n = 27) of the erythrocyte forward scatter (FSC) following incubation for 48 h to Ringer solution without (white bar) or with (black bars) phenoxodiol (25–100 μg/ml [104–416 μM]) or the solvent alone (DMSO, gray bar). c Arithmetic means ± SD (n = 15) of the erythrocyte forward scatter (FSC) following incubation for 48 h to Ringer solution without (white bar) or with (black bars) phenoxodiol (100–500 μg/ml [416–2080 μM]) or the solvent alone (DMSO, gray bar). ***p < 0.001 indicates significant difference from the absence of phenoxodiol (ANOVA)
A next series of experiments addressed cytosolic Ca2+ activity ([Ca2+]i). Fluo3 fluorescence was employed in order to quantify [Ca2+]i. The erythrocytes were analyzed after a 48-h incubation in Ringer solution without or with phenoxodiol (25–100 μg/ml [104–416 μM]). As illustrated in Fig. 4, a 48-h exposure to phenoxodiol (25–100 μg/ml [104–416 μM]) decreased the Fluo3 fluorescence, an effect reaching statistical significance at each 25, 50, and 100 μg/ml (104, 208, 416 μM) phenoxodiol.
Effect of phenoxodiol on cytosolic Ca2+ activity. a Histogram of Fluo3 fluorescence of erythrocytes following exposure for 48 h to Ringer solution without (gray area) and with (black line) presence of 100 μg/ml (416 μM) phenoxodiol. b Arithmetic means ± SD (n = 27) of erythrocyte Fluo3 fluorescence following incubation for 48 h to Ringer solution without (white bar) or with (black bars) phenoxodiol (25–100 μg/ml [104–416 μM]) or the solvent alone (DMSO, gray bar). ***p < 0.001 indicates significant difference from the absence of phenoxodiol (ANOVA)
Further experiments explored whether phenoxodiol-induced cell membrane scrambling required entry of extracellular Ca2+. To this end, erythrocytes were incubated for 48 h in the absence or presence of 100 μg/ml (416 μM) phenoxodiol in the presence or nominal absence of extracellular Ca2+. As shown in Fig. 5, removal of extracellular Ca2+ did not significantly modify the effect of phenoxodiol on annexin V binding. Both in the presence and absence of extracellular Ca2+ did phenoxodiol significantly increase the percentage of annexin V binding erythrocytes. Accordingly, the phenoxodiol-induced cell membrane scrambling did not require entry of extracellular Ca2+.
Ca2+ sensitivity of phenoxodiol-induced phosphatidylserine exposure. a, b Histograms of annexin V binding of erythrocytes following exposure for 48 h to Ringer solution without (gray areas) and with (black lines) phenoxodiol (100 μg/ml [416 μM]) in the presence (a) and absence (b) of extracellular Ca2+. c Means ± SD (n = 16) of annexin V binding of erythrocytes after a 48-h treatment with Ringer solution without (white bars) or with (black bars) phenoxodiol (100 μg/ml [416 μM]) in the presence (left bars, +Ca2+) and absence (right bars, −Ca2+) of Ca2+. ***p < 0.001 indicates significant difference from the absence of phenoxodiol (ANOVA)
ROS was determined utilizing DCFDA. As illustrated in Fig. 6, a 48-h exposure to phenoxodiol (100 μg/ml [416 μM]) did not significantly modify the DCFDA fluorescence of erythrocytes. Accordingly, phenoxodiol did not appreciably induce oxidative stress.
Effect of phenoxodiol on reactive oxygen species. a Histogram of DCFDA fluorescence in erythrocytes following exposure for 48 h to Ringer solution without (gray area) and with (black line) presence of 100 μg/ml (416 μM) phenoxodiol. b Arithmetic means ± SD (n = 22) of DCFDA fluorescence in erythrocytes following incubation for 48 h to Ringer solution without (white bar) or with (black bar) phenoxodiol (100 μg/ml [416 μM]). ***p < 0.001 indicates significant difference from the absence of phenoxodiol (ANOVA)
Ceramide abundance at the erythrocyte surface was determined utilizing specific antibodies. As shown in Fig. 7, a 48-h exposure to phenoxodiol (100 μg/ml [416 μM]) significantly increased the ceramide abundance at the erythrocyte surface.
Effect of phenoxodiol on ceramide abundance. a Histogram of ceramide abundance in erythrocytes following exposure for 48 h to Ringer solution without (gray area) and with (black line) presence of 100 μg/ml (416 μM) phenoxodiol. b Arithmetic means ± SD (n = 13) of ceramide abundance in erythrocytes following incubation for 48 h to Ringer solution without (white bar) or with (black bar) phenoxodiol (100 μg/ml [416 μM]). **p < 0.01 indicates significant difference from the absence of phenoxodiol (unpaired t test)
Discussion
The present study discloses a novel effect of phenoxodiol, i.e., the stimulation of eryptosis, the suicidal erythrocyte death, which is characterized by erythrocyte shrinkage and erythrocyte membrane scrambling with phosphatidylserine translocation to the erythrocyte surface (Bissinger et al. 2019). The phenoxodiol concentrations required for significant stimulation of eryptosis are in the range of concentrations reported in the plasma of patients under phenoxodiol treatment (Choueiri et al. 2006a). A linear correlation was observed between dosage and plasma concentrations reaching 60 μg/ml (250 μM) plasma concentration at a dosage of 27 mg/kg/24 h (Choueiri et al. 2006a).
The present study further sheds some light on the signaling involved in the stimulation of eryptosis by phenoxodiol. The effect of phenoxodiol on cell membrane scrambling was paralleled by an increase of ceramide abundance at the erythrocyte surface. Ceramide is a well-known stimulator of cell membrane scrambling. Ceramide is partially effective by sensitizing erythrocytes for the scrambling effect of Ca2+ (Bissinger et al. 2019). Phenoxodiol decreases cytosolic Ca2+ activity ([Ca2+]i) and the effect of phenoxodiol on cell membrane scrambling was not significantly modified by removal of extracellular Ca2+. The phenoxodiol-induced eryptosis apparently does not depend on Ca2+ entry. Along those lines, phenoxodiol triggered cell membrane scrambling even in the nominal absence of extracellular Ca2+. Phenoxodiol did not significantly modify the abundance of reactive oxygen species, another stimulator of eryptosis (Bissinger et al. 2019). The present observations, however, do not rule out the involvement of further signaling pathways. The present study does not define the direct molecular target of phenoxodiol. Ceramide is produced by acid sphingomyelinase, which is upregulated by platelet activating factor (PAF) (Lang et al. 2015). The cation channel is activated by prostaglandin E2, which is generated by cyclo-oxygenase (Lang et al. 2005). Possibly, phenoxodiol influences ceramide abundance and channel activity by directly or indirectly influencing PAF formation and cyclo-oxygenase activity. Several signaling pathways further have been shown to be sensitive to phenoxodiol (Table 1).
The limitations of the present study include the lack of in vivo data and the uncertainty of the direct molecular target. Future studies may explore the influence of phenoxodiol on blood count and packed cell volume in vivo, and the influence of the drug on PAF (Lang et al. 2015) and/or cyclo-oxygenase activity (Lang et al. 2005). Moreover, future studies may address the possibility that phenoxodiol may favorably influence the clinical course of malaria by sensitizing erythrocytes for the eryptotic effect of Plasmodium infection and thus eryptosis and clearance of infected erythrocytes (Foller et al. 2009).
In conclusion, phenoxodiol triggers erythrocyte cell membrane scrambling, an effect paralleled by increase of ceramide abundance.
Change history
23 July 2019
The original version of this article contains several mistakes due to the missed corrections:
References
Abed M, Towhid ST, Mia S, Pakladok T, Alesutan I, Borst O, Gawaz M, Gulbins E, Lang F (2012) Sphingomyelinase-induced adhesion of eryptotic erythrocytes to endothelial cells. Am J Physiol Cell Physiol 303:C991–C999
Aguero MF, Facchinetti MM, Sheleg Z, Senderowicz AM (2005) Phenoxodiol, a novel isoflavone, induces G1 arrest by specific loss in cyclin-dependent kinase 2 activity by p53-independent induction of p21WAF1/CIP1. Cancer Res 65:3364–3373
Aguero MF, Venero M, Brown DM, Smulson ME, Espinoza LA (2010) Phenoxodiol inhibits growth of metastatic prostate cancer cells. Prostate 70:1211–1221
Alvero AB, O’Malley D, Brown D, Kelly G, Garg M, Chen W, Rutherford T, Mor G (2006) Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer 106:599–608
Alvero AB, Brown D, Montagna M, Matthews M, Mor G (2007) Phenoxodiol-topotecan co-administration exhibit significant anti-tumor activity without major adverse side effects. Cancer Biol Ther 6:612–617
Alvero AB, Kelly M, Rossi P, Leiser A, Brown D, Rutherford T, Mor G (2008) Anti-tumor activity of phenoxodiol: from bench to clinic. Future Oncol 4:475–482
Bissinger R, Bhuyan AAM, Qadri SM, Lang F (2019) Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J 286:826–854
Briglia M, Rossi MA, Faggio C (2017) Eryptosis: ally or enemy. Curr Med Chem 24:937–942
Chen Y, Cass SL, Kutty SK, Yee EM, Chan DS, Gardner CR, Vittorio O, Pasquier E, Black DS, Kumar N (2015) Synthesis, biological evaluation and structure-activity relationship studies of isoflavene based Mannich bases with potent anti-cancer activity. Bioorg Med Chem Lett 25:5377–5383
Choueiri TK, Mekhail T, Hutson TE, Ganapathi R, Kelly GE, Bukowski RM (2006a) Phase I trial of phenoxodiol delivered by continuous intravenous infusion in patients with solid cancer. Ann Oncol 17:860–865
Choueiri TK, Wesolowski R, Mekhail TM (2006b) Phenoxodiol: isoflavone analog with antineoplastic activity. Curr Oncol Rep 8:104–107
De Luca T, Bosneaga E, Morre DM, Morre DJ (2008) Downstream targets of altered sphingolipid metabolism in response to inhibition of ENOX2 by phenoxodiol. Biofactors 34:253–260
De Luca T, Morre DM, Morre DJ (2010) Reciprocal relationship between cytosolic NADH and ENOX2 inhibition triggers sphingolipid-induced apoptosis in HeLa cells. J Cell Biochem 110:1504–1511
de Souza PL, Russell PJ, Kearsley JH, Howes LG (2010) Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr Rev 68:542–555
Foller M, Bobbala D, Koka S, Huber SM, Gulbins E, Lang F (2009) Suicide for survival--death of infected erythrocytes as a host mechanism to survive malaria. Cell Physiol Biochem 24:133–140
Gamble JR, Xia P, Hahn CN, Drew JJ, Drogemuller CJ, Brown D, Vadas MA (2006) Phenoxodiol, an experimental anticancer drug, shows potent antiangiogenic properties in addition to its antitumour effects. Int J Cancer 118:2412–2420
Herst PM, Petersen T, Jerram P, Baty J, Berridge MV (2007) The antiproliferative effects of phenoxodiol are associated with inhibition of plasma membrane electron transport in tumor cell lines and primary immune cells. Biochem Pharmacol 74:1587–1595
Herst PM, Davis JE, Neeson P, Berridge MV, Ritchie DS (2009) The anti-cancer drug, phenoxodiol, kills primary myeloid and lymphoid leukemic blasts and rapidly proliferating T cells. Haematologica 94:928–934
Isono M, Sato A, Asano T, Okubo K, Asano T (2018) Evaluation of therapeutic potential of phenoxodiol, a novel isoflavone analog, in renal cancer cells. Anticancer Res 38:5709–5716
Kamsteeg M, Rutherford T, Sapi E, Hanczaruk B, Shahabi S, Flick M, Brown D, Mor G (2003) Phenoxodiol--an isoflavone analog--induces apoptosis in chemoresistant ovarian cancer cells. Oncogene 22:2611–2620
Kluger HM, McCarthy MM, Alvero AB, Sznol M, Ariyan S, Camp RL, Rimm DL, Mor G (2007) The X-linked inhibitor of apoptosis protein (XIAP) is up-regulated in metastatic melanoma, and XIAP cleavage by Phenoxodiol is associated with carboplatin sensitization. J Transl Med 5:6
Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM (2003) Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol Cell Physiol 285:C1553–C1560
Lang PA, Kempe DS, Myssina S, Tanneur V, Birka C, Laufer S, Lang F, Wieder T, Huber SM (2005) PGE(2) in the regulation of programmed erythrocyte death. Cell Death Differ 12:415–428
Lang E, Modicano P, Arnold M, Bissinger R, Faggio C, Abed M, Lang F (2013) Effect of thioridazine on erythrocytes. Toxins (Basel) 5:1918–1931
Lang E, Bissinger R, Gulbins E, Lang F (2015) Ceramide in the regulation of eryptosis, the suicidal erythrocyte death. Apoptosis 20:758–767
Lang F, Bissinger R, Abed M, Artunc F (2017) Eryptosis - the neglected cause of anemia in end stage renal disease. Kidney Blood Press Res 42:749–760
Li Y, Huang X, Huang Z, Feng J (2014) Phenoxodiol enhances the antitumor activity of gemcitabine in gallbladder cancer through suppressing Akt/mTOR pathway. Cell Biochem Biophys 70:1337–1342
Mahoney S, Arfuso F, Rogers P, Hisheh S, Brown D, Millward M, Dharmarajan A (2012) Cytotoxic effects of the novel isoflavone, phenoxodiol, on prostate cancer cell lines. J Biosci 37:73–84
Mahoney S, Arfuso F, Millward M, Dharmarajan A (2014) The effects of phenoxodiol on the cell cycle of prostate cancer cell lines. Cancer Cell Int 14:110
McPherson RA, Galettis PT, de Souza PL (2009) Enhancement of the activity of phenoxodiol by cisplatin in prostate cancer cells. Br J Cancer 100:649–655
Mischitelli M, Jemaa M, Almasry M, Faggio C, Lang F (2016a) Ca2+ entry, oxidative stress, ceramide and suicidal erythrocyte death following diosgenin treatment. Cell Physiol Biochem 39:1626–1637
Mischitelli M, Jemaa M, Almasry M, Faggio C, Lang F (2016b) Triggering of erythrocyte cell membrane scrambling by emodin. Cell Physiol Biochem 40:91–103
Miyamoto M, Takano M, Aoyama T, Soyama H, Ishibashi H, Kato K, Iwahashi H, Takasaki K, Kuwahara M, Matuura H, Sakamoto T, Yoshikawa T, Furuya K (2018) Phenoxodiol increases cisplatin sensitivity in ovarian clear cancer cells through XIAP down-regulation and autophagy inhibition. Anticancer Res 38:301–306
Mor G, Fu HH, Alvero AB (2006) Phenoxodiol, a novel approach for the treatment of ovarian cancer. Curr Opin Investig Drugs 7:542–548
Mor G, Montagna MK, Alvero AB (2008) Modulation of apoptosis to reverse chemoresistance. Methods Mol Biol 414:1–12
Morre DJ, Chueh PJ, Yagiz K, Balicki A, Kim C, Morre DM (2007) ECTO-NOX target for the anticancer isoflavene phenoxodiol. Oncol Res 16:299–312
Pretorius E, du Plooy JN, Bester J (2016) A comprehensive review on eryptosis. Cell Physiol Biochem 39:1977–2000
Saif MW, Tytler E, Lansigan F, Brown DM, Husband AJ (2009) Flavonoids, phenoxodiol, and a novel agent, triphendiol, for the treatment of pancreaticobiliary cancers. Expert Opin Investig Drugs 18:469–479
Sapi E, Alvero AB, Chen W, O’Malley D, Hao XY, Dwipoyono B, Garg M, Kamsteeg M, Rutherford T, Mor G (2004) Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol Res 14:567–578
Silasi DA, Alvero AB, Rutherford TJ, Brown D, Mor G (2009) Phenoxodiol: pharmacology and clinical experience in cancer monotherapy and in combination with chemotherapeutic drugs. Expert Opin Pharmacother 10:1059–1067
Straszewski-Chavez SL, Abrahams VM, Funai EF, Mor G (2004) X-Linked inhibitor of apoptosis (XIAP) confers human trophoblast cell resistance to Fas-mediated apoptosis. Mol Hum Reprod 10:33–41
Wu LY, De Luca T, Watanabe T, Morre DM, Morre DJ (2011) Metabolite modulation of HeLa cell response to ENOX2 inhibitors EGCG and phenoxodiol. Biochim Biophys Acta 1810:784–789
Yagiz K, Wu LY, Kuntz CP, James Morre D, Morre DM (2007) Mouse embryonic fibroblast cells from transgenic mice overexpressing tNOX exhibit an altered growth and drug response phenotype. J Cell Biochem 101:295–306
Yao C, Wu S, Li D, Ding H, Wang Z, Yang Y, Yan S, Gu Z (2012) Co-administration phenoxodiol with doxorubicin synergistically inhibit the activity of sphingosine kinase-1 (SphK1), a potential oncogene of osteosarcoma, to suppress osteosarcoma cell growth both in vivo and in vitro. Mol Oncol 6:392–404
Yu F, Watts RN, Zhang XD, Borrow JM, Hersey P (2006) Involvement of BH3-only proapoptotic proteins in mitochondrial-dependent phenoxodiol-induced apoptosis of human melanoma cells. Anti-Cancer Drugs 17:1151–1161
Acknowledgments
The authors acknowledge the meticulous preparation of the manuscript by Lejla Subasic.
Funding
The study was supported by the Deutsche Forschungsgemeinschaft, DFG grant “Gi proteins and platelets” (NU 53/13-1) and Deutscher Akademischer Austaauschdienst (DAAD).
Author information
Authors and Affiliations
Contributions
BN, CF, and FL conceived and designed research. MF and AMB conducted experiments and analyzed data. FL wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fink, M., Bhuyan, A.A.M., Nürnberg, B. et al. Triggering of eryptosis, the suicidal erythrocyte death, by phenoxodiol. Naunyn-Schmiedeberg's Arch Pharmacol 392, 1311–1318 (2019). https://doi.org/10.1007/s00210-019-01681-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01681-8