Abstract
To evaluate the effects of hypercholesterolemia on the relaxation function of the urinary bladder, we examined the physiological mechanisms involved in the isoproterenol-induced relaxation in isolated detrusor strips in vitro and voiding behavior in vivo in rats. Adult male Sprague–Dawley rats were fed standard (control, N = 16) or 4 % cholesterol diet (hypercholesterolemia, N = 17) for 4 weeks. Concentration–response curves for isoproterenol-induced relaxations in carbachol-precontracted detrusor muscle strips were recorded. The contributions of β2- and β3-adrenoceptors and ATP-dependent and Ca2+-dependent potassium channels to the relaxation response were investigated by using selective adrenergic agonists salbutamol and BRL 37344 and specific potassium channel inhibitors glibenclamide and charybdotoxin, respectively. Cystometrography was performed to assess bladder function. Hypercholesterolemic rats had higher serum cholesterol and low- and high-density lipoprotein levels than the controls with no sign of atherosclerosis. Isoproterenol-induced relaxation was significantly enhanced in the hypercholesterolemia group. Preincubation with the M2 receptor antagonist attenuated the relaxation response in both groups. The relaxation responses to isoproterenol and salbutamol were similar in both groups, while BRL 37344 appeared to produce a greater relaxant effect in the hypercholesterolemic rats. Also, the inhibitory effects of potassium channel inhibitors on relaxation responses were comparable among the groups. The cystometric findings revealed that threshold and basal pressure values were higher in the hypercholesterolemia group compared with controls. We showed that hypercholesterolemia leads to greater relaxation responses to isoproterenol, appears to impair the braking function of M2 cholinergic receptors on adrenoceptor-induced relaxations in the isolated detrusor muscle, and affects the voiding function in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The storage function of the urinary bladder requires relaxation of the detrusor smooth muscle (DSM) which is a sympathetic nervous system (SNS)-mediated function (Yamaguchi 2002; Frazier et al. 2008) involving activation of β-adrenoceptors (β-ARs) by neuronally released noradrenaline to accommodate increasing volumes of urine without major elevations in the intravesical pressure (Andersson and Arner 2004; Andersson 2009). Although all three β-AR subtypes are expressed in both rat and human DSM cells (Takeda et al. 2000; Andersson and Arner 2004; Michel and Vrydag 2006; Tyagi et al. 2009), DSM relaxation in humans is predominantly mediated by β3-ARs (Takeda et al. 1999; Michel and Vrydag 2006; Frazier et al. 2008) whereas rat DSM relaxation is mediated via both β2- and β3-ARs (Oshita et al. 1997; Yamazaki et al. 1998). Regardless of the subtypes expressed, activation of β-ARs results in increased intracellular cAMP levels, which in turn activate protein kinase A (PKA) to mediate DSM relaxation (Tanaka et al. 2005; Uchida et al. 2005). In addition, the involvement of different K+ channels in response to stimulation of β-ARs has also been shown to mediate relaxation in various smooth muscle tissues (Tanaka et al. 2005; Uchida et al. 2005; Ferro 2006). The K+ channels expressed in the urinary bladder include voltage-gated (Kv), small-conductance (SK), ATP-sensitive (KATP), and large-conductance Ca2+-activated K+ (BK) channels (Frazier et al. 2008). Although the last two provide the greatest contribution to the DSM tone, β-AR-stimulated bladder relaxation is mediated predominantly by BK channels (Frazier et al. 2005; Petkov and Nelson 2005; Frazier et al. 2008; Hristov et al. 2008). Thus, BK channels are considered to be the most important physiologically relevant K+ channels regulating DSM function (Hristov et al. 2008; Petkov 2012; Afeli and Petkov 2013; Parajuli and Petkov 2013). In addition to β-AR stimulation, nitric oxide (NO) has been claimed as a potential neurotransmitter involved in controlling the lower urinary tract (Monica et al. 2008; Cerruto et al. 2012).
Parasympathetic and sympathetic divisions of the autonomic nervous system work in different directions in the urinary bladder as elsewhere in the body. The contractile function of the DSM accounts for voiding and is controlled by the parasympathetic nervous system (PNS), whereas relaxation of the urinary bladder is regulated by the SNS activity and is responsible for urine storage (Yamaguchi 2002; Andersson and Arner 2004; Michel and Vrydag 2006; Hristov et al. 2008). However, the interaction between PNS and SNS should not be overlooked; for instance, muscarinic agonists have been reported to attenuate the relaxing effects of β-AR stimulation of the bladder in rats (Hegde et al. 1997; Michel and Sand 2009). Overactive bladder (OAB), the symptom complex including urgency with or without urge incontinence, frequency, and nocturia, is a subset of storage lower urinary tract symptoms (LUTS) often associated with detrusor overactivity (DO) (Huang et al. 2010; Yoshida et al. 2010b; Cerruto et al. 2012). Disturbances in the SNS and PNS dual control mechanism of urinary bladder function may underlie symptoms of OAB (Andersson 1988; Yoshida et al. 2010b; Hristov et al. 2013).
The prevalence of OAB concomitantly increases with the incidence of hypercholesterolemia (Devroey et al. 2011; Hall et al. 2011), especially with the increasing age (Yoshida et al. 2010b). Although the association between hyperlipidemic conditions and the risk of LUTS remains unclear, there is increasing evidence for an association between LUTS or detrusor dysfunction and hyperlipidemia. For example, hyperlipidemia associates with increased urinary frequency (Huang et al. 2010) and bladder overactivity in rats (Rahman et al. 2007) and contributes to the bladder dysfunction in rabbits (Yoshida et al. 2010a). Moreover, LUTS including storage, voiding, and postmicturition symptoms are associated with hypercholesterolemia in rats (Nomiya et al. 2012), rabbits (Azadzoi et al. 1999), and humans (Hall et al. 2011). We recently reported a relationship between hypercholesterolemia and DSM contractility (Balkanci et al. 2012). In the present study, we aimed to investigate the effect of diet-induced hypercholesterolemia in rats on the adrenergic relaxation response in the isolated detrusor strips in vitro and voiding behavior in vivo. To this end, we investigated the in vitro relaxation response of precontracted detrusor strips to (1) isoproterenol, a nonselective β-adrenergic receptor agonist, and (2) salbutamol and BRL 37344 and β2- and β3-AR selective agonists, respectively, in an effort to determine the contribution of the β-AR subtypes to the relaxation response. In addition, the role of potassium channel subtypes in the relaxation response to β-agonists was investigated using glibenclamide and charybdotoxin, ATP- and Ca2+-dependent K+ channel inhibitors, respectively. Finally, the effect of hypercholesterolemia on voiding function in vivo was assessed using cystometrography.
Materials and methods
Animals
All animals received humane care: Guiding Principles in the Care and Use of Laboratory Animals were strictly adhered to at all times together with the recommendations from the Declaration of Helsinki. The experimental protocol was approved by the Hacettepe University Institutional Ethics Committee for the Care and Use of Experimental Animals. Four- to 7-week-old, adult, male Sprague–Dawley rats, weighing 250–300 g, were assigned randomly to two groups: control (C) (N = 16) and hypercholesterolemia (HC) (N = 17). All rats were kept under environmentally controlled conditions at 21 ± 2 °C and 30–70 % relative humidity with 12-h dark–12-h light illumination sequence (the lights were on between 07.00 and 19.00 hours) with ad libitum access to tap water (drinking bottle) and food. The control group was fed standard pellet chow, and the hypercholesterolemia group was fed high-cholesterol diet (containing 4 % cholesterol and 0.2 % deoxycholate) for 4 weeks. Prior to the experiment day, animals were housed individually in metabolic cages where they had free access to water and food for 24 h to measure food and water consumption and urine output.
In vitro experiments
The animals were anesthetized with ether inhalation. The abdomen was opened through a midline incision, then the urinary bladder was excised and dissected in ice-cold, oxygenated Krebs–Henseleit solution (118.4 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25.0 mM NaHCO3, 2.5 mM CaCl2, and 12.2 mM glucose; pH 7.35–7.40). Having all the surrounding adipose and connective tissues cleared out, the bladder body was divided into four longitudinal strips (10 × 2 mm). The isolated strips were then transferred into the organ baths containing 15 ml of Krebs–Henseleit solution at 37 °C, gassed with a mixture of 95 % O2 and 5 % CO2. Isometric tension changes were recorded and analyzed using a force-displacement transducer (MAY FDT 05, Commat, Ankara, Turkey) and a computerized data acquisition/analysis system (BIOPAC MP30, Biopac Systems Inc., CA). The isolated detrusor strips were allowed to equilibrate for at least 60 min under a resting tension of 1 g until the spontaneous contractions became stable. During the stabilization period, the Krebs–Henseleit solution in the isolated organ baths was refreshed every 15 min.
Relaxation response of bladder strips
Initially, all strips were challenged with 0.1 mM acetylcholine, which was subsequently washed out with fresh physiological solution. This was repeated at 15-min intervals for 50 min until the resting tension was reached. The detrusor strips were first precontracted with the muscarinic agonist carbachol (CCh; 10 μM) and allowed to reach a stable tension for 15 min. Thereafter, cumulative isoproterenol concentration–response curves (1 nM–0.1 mM) were recorded. Only a single relaxation curve was obtained in each bladder strip to avoid desensitization. Similarly, cumulative isoproterenol concentration–response curves were obtained in the presence of methoctramine (M2 cholinergic receptor antagonist; 10 μM) and L-NG-nitroarginine methyl ester (L-NAME; NO synthase inhibitor; 0.1 mM). The methoctramine concentration used was within the effective range as previously reported (Stevens et al. 2007). Thereafter, the following experiments were conducted on the methoctramine preincubated strips to avoid reduction of isoproterenol-induced relaxations by M2 receptor-mediated cholinergic activity.
Effects of β-adrenoceptor agonists and K+ channel inhibitors
To test the effect of β-ARs and subtype variations, the cumulative concentration–response curves for the nonselective β-AR agonist isoproterenol (1 nM–0.1 mM), β2-adrenoceptor selective agonist salbutamol, and β3-adrenoceptor selective agonist BRL 37344 (1 nM–10 μM) were obtained.
Two different inhibitors were employed to evaluate the role of potassium channels in the isoproterenol-induced bladder relaxation: the ATP-sensitive K+ channel (KATP) inhibitor glibenclamide (10 μM) and the large conductance Ca2+-activated K+ channel (BK) inhibitor charybdotoxin (0.1 μM). Detrusor strips were preincubated with either charybdotoxin or glibenclamide for 15 min in the presence of methoctramine. Following equilibration, cumulative isoproterenol concentration–response curves (1 nM–0.1 mM) were obtained as described above.
One strip in each series was allocated as the time control. All experiments were conducted in the presence of 1 μM phentolamine, an α-adrenoceptor antagonist. At the end of the protocols, strips were weighed.
In vivo experiments
The C (N = 5) and HC (N = 7) rats anesthetized with urethane (1.5 g/kg, ip) were placed on an operating table in the supine position. Body temperatures of the animals were kept at 37.0 ± 0.1 °C using a rectal thermostatic probe-controlled incandescent lamp (100 W). Following a low abdominal midline incision, the urinary bladder was located and allowed to stabilize for 15 min. Then, a polyethylene catheter (no. 24) implanted and fixed into the dome of the urinary bladder was connected to both a volumetric pressure transducer (BIOPAC, BPT 300, Ankara, Turkey) and an infusion pump (Perfusor compact S, B. Braun) via a three-way stopcock. During the catheterization, the bladder was emptied if it was initially filled with urine. The tissues were covered with wet gauze to prevent desiccation. After instrumentation, all rats were allowed to stabilize for 30 min. Thereafter, infusion of normal saline was initiated at a rate of 6 ml/h at room temperature. This infusion rate was selected in light of the related literature (Rahman et al. 2007). All data were recorded and analyzed using a pressure transducer and a computerized data acquisition/analysis system (BIOPAC MP30, Biopac Systems Inc., CA). Following the stabilization period, measurements of micturition pressure (cmH2O), micturition intervals (s), threshold pressure (cmH2O), basal pressure (end micturition pressure, cmH2O), and micturition time (s) were obtained for a period of 30 min after the start of infusion. Moreover, bladder capacity (summation of the residual volume and mean urine (micturition) volume), micturition fraction (micturition volume × 100/bladder capacity, %), and compliance (bladder capacity/threshold pressure) were calculated. The urine was collected using a vial placed at the opening of the urethra. After each micturition, the vial was emptied and the collected urine volume was measured. At the end of the 30-min period, during the last voiding, infusion was stopped to terminate the cystometrographic evaluation. Residual volume was aspirated and measured. Then, the urinary bladder was excised and weighed.
Serum lipid profile
Following excision of the urinary bladder after both in in vivo and in vitro experiments, blood samples were collected by cardiac puncture to determine the serum lipid profile (total cholesterol, triglyceride, HDL, LDL, and VLDL) by diagnostic kits using a modular system autoanalyzer (Roche/Hitachi, IN) at the Clinical Biochemistry Laboratory of Hacettepe University Hospital.
Histopathological evaluation
After the experimental protocols were completed, the animals were sacrificed with exsanguination and the arch of aorta was excised and fixed in 10 % formaldehyde and processed by routine light microscopy tissue processing techniques. Sections were stained with hematoxylin–eosin and evaluated for atherosclerotic changes, as described previously (Cai et al. 2005).
Chemicals
All chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). Distilled water was used to dissolve all compounds except glibenclamide which was dissolved in dimethyl sulfoxide.
Calculations and statistical analysis
Data were analyzed by using a statistics software package for Windows (SPSS 15.0) (SPSS Inc., Chicago, IL). All values were reported as mean ± standard error of the mean (SEM). Contraction responses to CCh (10 μM) were normalized to the wet tissue weight (g/100 mg wet tissue weight). Relaxation responses were calculated as the percentages of the CCh-induced maximal contraction. E max (the highest relaxation response induced by the agonist) and pD2 (negative logarithm of the molar concentration of the agonist producing half of the maximal effect) values were calculated by using the SigmaPlot 10.0 for Windows (Systat Software, Inc., San Jose, CA) software to evaluate the efficacy and potency of the agonists, respectively. However, we have to mention that some of the E max values indicate the maximal response obtained by the highest concentration of the test agent applied, instead of the actual efficacy parameter. Therefore, some of the pD2 values may not reflect the actual potency. The number of animals and strips were indicated as N and n, respectively.
Normal distribution of the data was confirmed by the Kolmogorov–Smirnov test. The general metabolic profile, body and urinary bladder weights, serum cholesterol levels, and cystometric parameters were compared by Student’s t test for independent samples. Intra- and intergroup comparisons of cumulative concentration–response curves were performed by repeated measures analysis of variance followed by Tukey’s post hoc test for multiple comparisons. E max and pD2 values were compared by Student’s t test for independent samples. The differences were considered statistically significant when P < 0.05 (two-tailed).
Results
Animal characteristics and effects of high-cholesterol diet
High-cholesterol diet did not cause any significant difference in body weights, bladder weights, food and water consumption, and urine output of the rats (Table 1). However, total serum cholesterol, LDL, and HDL levels were higher in the HC group (P < 0.05), while triglyceride and VLDL levels were similar in both groups (Table 1). Histological evaluation of the aortic arch elicited no sign of atherosclerosis.
Relaxation response of bladder strips
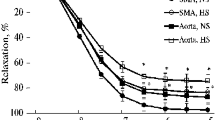
The amplitudes and frequencies of basal spontaneous contractions were similar in the HC (n = 17; 3.13 ± 0.24 g/100 mg wet tissue weight; 0.068 ± 0.005 Hz) and the NC groups (n = 16; 3.31 ± 0.26 g/100 mg wet tissue weight; 0.072 ± 0.004 Hz).The cholinergic receptor agonist carbachol (10 μM) induced a peak contraction of the DSM followed by a decline to a relatively stable plateau values over a period of approximately 15 min. The maximal contractile responses to CCh were 3.39 ± 0.51 and 2.92 ± 0.31 g/100 mg wet tissue weight for C and HC groups, respectively, immediately prior to addition of isoproterenol. Isoproterenol (1 nM–0.1 mM) administration resulted in concentration-dependent relaxations in the C and HC groups, being more prominent in the latter (P < 0.05) (Fig 1). Isoproterenol relaxed the bladder strips with a pD2 of 6.79 ± 0.18 and an E max of 35.64 ± 2.04 % in the C group and with a pD2 of 6.47 ± 0.17 and an E max of 46.55 ± 3.57 % in the HC group (Table 2).
Concentration–response curves of isoproterenol in the carbachol (10 μM)-precontracted isolated detrusor strips in the control and hypercholesterolemic rats. Responses are the mean (±SEM) relaxation expressed as a percentage of the precontraction to CCh. Isoproterenol caused marked relaxations in hypercholesterolemia group compared to control group (*P < 0.05). ISO isoproterenol
The second series of experiments explored whether selective M2 receptor inhibition enhances the isoproterenol-induced relaxation response. Methoctramine (10 μM) preincubation significantly altered the isoproterenol concentration–response curves in the C and HC groups (Fig 2). As expected, methoctramine increased the maximum relaxation responses induced by isoproterenol in both groups (P < 0.05). Although the relaxation-enhancing effect of methoctramine appeared to be less pronounced in the HC group (Fig 2), the difference between the groups was not significant, and pD2 values were found to be similar (Table 2).
Effects of methoctramine (10 μM) and L-NAME (0.1 mM) incubation on isoproterenol-induced relaxations in rat-isolated detrusor strips in the control (upper panel) and hypercholesterolemia (lower panel) groups. Responses are the mean (±SEM) relaxation expressed as a percentage of the precontraction to CCh. Methoctramine but not L-NAME preincubation enhanced the isoproterenol-induced relaxations both in C and HC groups (*P < 0.05). ISO isoproterenol, Met methoctramine, L-NAME L-NG-nitroarginine methyl ester
In the third series of experiments, L-NAME (0.1 mM), a NO synthase inhibitor, failed to affect the efficacy and potency of isoproterenol in both groups (Table 2).
Relaxing effects of β-adrenoceptor agonists
Thereafter, all other experiments were conducted in the presence of methoctramine. Salbutamol (β2-adrenoceptor selective agonist) and BRL 37344 (β3-adrenoceptor selective agonist) also relaxed CCh precontracted bladder strips in vitro in a concentration-dependent manner (Fig 3). Concentration–response curves obtained in response to cumulative BRL 37344 and salbutamol (1 nM-10 μM) administrations were significantly different from the isoproterenol concentration–response curve in the C group, whereas in the HC group, only the salbutamol concentration–response curve was different from the isoproterenol concentration–response curve (P < 0.05).
Relaxing effects of isoproterenol, BRL 37344, and salbutamol in the presence of methoctramine (10 μM) in rat-isolated detrusor strips in the control (upper panel) and hypercholesterolemia (lower panel) groups. Responses are the mean (±SEM) relaxation expressed as a percentage of the precontraction to CCh. Isoproterenol elicited enhanced relaxation responses both in C and HC groups (*P < 0.05 vs BRL + Met, #P < 0.05 vs SAL + Met,). ISO isoproterenol, Met methoctramine, BRL BRL 37344, SAL salbutamol
E max values obtained with BRL 37344 and salbutamol were lower than that of isoproterenol in both the C and HC groups (P < 0.05) (Table 3). In addition, hypercholesterolemia seemed to cause greater relaxations in response to BRL 37344; however, this effect was not statistically significant (Fig 3).
pD2 values did not reveal any inter- nor intragroup differences statistically (Table 3).
Effects of K+ channel inhibitors
In the present study, we used two different inhibitors to investigate the role of two types of potassium channels in the isoproterenol-induced bladder relaxation: glibenclamide and charybdotoxin. Pretreatment of the detrusor strips with charybdotoxin, a large-conductance Ca2+-activated K+ channel inhibitor (0.1 μM), suppressed the relaxation induced by isoproterenol particularly in the C group (Fig 4).
Relaxing effects of isoproterenol in the presence of charybdotoxin (0.1 μM) or glibenclamide (10 μM) in rat-isolated detrusor strips in the control (upper panel) and hypercholesterolemia (lower panel) groups. Responses are the mean (±SEM) relaxation expressed as a percentage of the precontraction to CCh. Charybdotoxin inhibited the isoproterenol-induced relaxation responses in the C but not the HC group (*P = 0.05 vs ISO). ISO isoproterenol
Glibenclamide, an ATP-sensitive K+ channel inhibitor (10 μM), failed to attenuate the isoproterenol-induced relaxation responses.
None of the potassium channel blockers significantly altered the E max or pD2 of isoproterenol when the groups were compared according to the effect of diet (Table 4).
Cystometrography results
Table 5 displays the cystometric findings in the groups. Basal and threshold pressures were significantly higher in the HC group, whereas there was no significant difference in the micturition pressure between both groups. The shorter micturition interval, the higher functional bladder capacity, the increased residual volume, and the lower compliance values obtained in the HC group failed to reach statistical significance.
Discussion
The present study demonstrated that diet-induced hypercholesterolemia augmented the nonselective β-AR agonist-induced relaxation response of CCh-precontracted rat-isolated detrusor strips. Further investigation of the contribution of β2- and β3-AR and the role of the selected potassium channels on the relaxation response revealed no significant difference between groups. In the cystometric evaluations, hypercholesterolemic rats appeared to exhibit voiding patterns characteristic of OAB.
The relatively low relaxation response to isoproterenol in this study could be explained by the fact that it is more potent at relaxing the KCl-precontracted strips or passive tension than that of the CCh-precontracted strips (Longhurst and Levendusky 1999; Frazier et al. 2005; Michel and Sand 2009; Witte et al. 2011). Interaction between the M2 cholinergic receptors and β-adrenergic receptor-induced relaxation may help in understanding this relative resistance (Michel and Sand 2009; Witte et al. 2011).
Since M2 receptors and the NO/cAMP pathway have been proposed as auxiliary mechanisms involved in the relaxation of CCh-precontracted DSM (with urothelium intact), their roles were also investigated in the present study. The concentration–response curves for isoproterenol were re-obtained in methoctramine-incubated strips, to determine the effect of muscarinic M2 receptor in isoproterenol-induced relaxations. M2 receptors are Gi protein-coupled seven-transmembrane receptors, interacting with smooth muscle relaxation through adenylyl cyclase inhibition (Ehlert et al. 2005). We found that methoctramine enhanced the isoproterenol-induced relaxation in both experimental groups. This finding is consistent with reports demonstrating an enhancement in the relaxant effect of isoproterenol against muscarinic agonist-induced contraction in the ileum and urinary bladder in M2 receptor-knockout mice (Matsui et al. 2003; Ehlert et al. 2005). Further, M2 receptors were shown to attenuate the relaxation responses induced by isoproterenol in rat detrusor strips (Witte et al. 2011). The difference between groups due to hypercholesterolemia-associated enhancement in the isoproterenol-induced relaxations disappeared with M2 receptor blockade. Therefore, we propose a less prominent inhibitory role for M2 muscarinic receptors on β-AR-mediated relaxation in the hypercholesterolemic rats.
The NO/cGMP pathway may possibly modulate several cellular systems found in the bladder wall. The accumulated data suggest a supportive role for NO in bladder relaxation during the filling phase along with other factors (Cerruto et al. 2012). However, we observed that nitric oxide synthase (NOS) inhibition did not alter the responses to isoproterenol in either groups in the present study, while chronic NO deficiency has been reported to result in overactive DSM (Monica et al. 2008). Similar with our findings, there are some previous studies reporting the lack of effect of NOS inhibitors on the CCh-induced contractions in mouse bladder (Ekman et al. 2009) and on the inhibition of maximal isoproterenol effects in rat bladder (Frazier et al. 2005).
We investigated in vitro relaxation response of CCh-precontracted detrusor strips to selective β2-AR agonist, salbutamol, and β3-AR agonist, BRL 37344, to determine the contribution of β2- and β3-adrenergic receptor subtypes to the adrenoceptor-mediated relaxation response. To the best of our knowledge, this is the first study investigating the relationship between hypercholesterolemia and contribution of β-agonists to the DSM relaxation response in rats. Alterations in bladder β-ARs in various pathological conditions such as hypertension and bladder outlet obstruction in rats (Michel and Barendrecht 2008) and decreased contribution of a β3-agonist to the relaxation response of the bladder with aging in human (Li et al. 2003) have been reported. Nevertheless, relaxation response was unchanged in diabetic rats compared to control animals (Longhurst et al. 2004). Moreover, it was reported that gender had small if any effects on relaxation response (Michel and Barendrecht 2008). Our findings indicate that diet-induced hypercholesterolemia does not change relative contributions of β-AR agonists to the relaxation response of detrusor strips. Although information regarding the effects of hypercholesterolemia and relaxation response of smooth muscle is limited, it was shown that hyperlipidemia significantly decreases endothelium-dependent and/or endothelium-independent relaxation in the vascular (Shishido et al. 2004), clitoral (Kim et al. 2002), and cavernous smooth muscles (Kim 2000). Further, the BRL 37344-induced relaxation was 30 and 38 % whereas salbutamol caused 23 and 26 % maximal relaxations in the C and HC groups, respectively. Compared to the C group, stimulation with BRL 37344 accounted for approximately two thirds of the maximal relaxation response in the HC group, implying a greater contribution of β3-ARs to the relaxation response in this group (60 vs. 30 % in C and 57 vs. 38 % in HC group). BRL 37344 was previously shown to inhibit CCh-induced tone in human DSM strips (Takeda et al. 1999). In addition, these β-AR agonists modulate other mechanisms involved in detrusor function, e.g., BRL 37344 was demonstrated to reduce both spontaneous phasic contractions and muscle tone (Hristov et al. 2008) and to inhibit EFS-induced contractions in human (Afeli et al. 2013) and rat (Afeli and Petkov 2013) DSM. Although the concentrations of BRL 37344 used in the present work were in accordance with the literature (Afeli and Petkov 2013; Afeli et al. 2013) proposing its β3-AR agonistic role, one should note that BRL 37344 has also been reported to be a partial agonist at rat β3-ARs and to have antagonistic properties at muscarinic receptors (Kubota et al. 2002; Vrydag and Michel 2007). In addition, another limitation of the current study is the lack of confirmation with selective antagonists to confirm the suggested increase in the β3/β2 ratio.
The precise cellular mechanism of β-AR-mediated bladder relaxation has not been elucidated fully. The effects of β3-adrenoceptors are mainly mediated through cAMP/PKA pathway, but PKA-dependent and PKA-independent mechanisms may also take part in the DSM function, among which activation of K+, particularly BK channels, functionally interacts with β3-ARs-promoted DSM relaxation (Frazier et al. 2005; Petkov and Nelson 2005; Tanaka et al. 2005; Uchida et al. 2005; Ferro 2006; Hristov et al. 2008). Alterations in the expression of BK channels may cause urinary dysfunctions, such as overactive bladder and urinary incontinence (Meredith et al. 2004; Herrera et al. 2005). In our study, the relaxant effect of isoproterenol in precontracted detrusor strips was reduced by the BK channel inhibitor, charybdotoxin, in the C group, while it did not exert such an effect in the HC group. Although iberiotoxin could have been used for the same purpose, charybdotoxin was selected in the present study due to its similar, if not superior, reducing effect of isoproterenol relaxations than that of iberiotoxin in the precontracted bladder as previously reported (Frazier et al. 2005). In support of our findings, charybdotoxin was reported to potentiate the contraction of the urinary bladder in mice (Ekman et al. 2009) and to inhibit the relaxation induced by β-AR agonists in rat (Uchida et al. 2005) at concentrations of 0.3 and 0.1 μM, respectively. Recent studies reported decreased BK channel expression with aging (unpublished data by Petkov et al. as stated in (Petkov 2012)), BK channel dysfunction (Oger et al. 2011), and reduced BK channel activity in neurogenic DO (Hristov et al. 2013). Basal and nerve-mediated DSM contraction was enhanced, resulting in bladder overactivity and urinary incontinence in mice lacking functional BK channel subunits (Meredith et al. 2004). Our results derived from detrusor strips are in line with the reports stating that hypercholesterolemia may reduce BK channel activity (Dopico et al. 2012). Although the modulation of KATP channels by hypercholesterolemia was shown in other smooth muscles (Ren et al. 2001), we failed to show any effect of KATP channel blockade by glibenclamide on the relaxation response of bladder in both the C and HC groups.
In this study, experiments in rat-isolated DSM strips revealed a less significant braking effect for M2 receptors on isoproterenol-induced relaxation response in the hypercholesterolemic rats. Since we ended up with some clues for modulation of DSM relaxation function in in vitro experiments in the present study and contraction function in another recent study in diet-induced hypercholesterolemia, to observe the functional consequences of these in vitro results, in vivo cystometric experiments were performed. Previously, hyperlipidemia and/or hypercholesterolemia were associated with detrusor/bladder overactivity in cystometric studies (Azadzoi et al. 1999; Rahman et al. 2007; Son et al. 2007; Huang et al. 2010; Yoshida et al. 2010a). Our results indicating relatively more frequent voiding and decreased voided volume supports these findings of overactivity in the HC group in line with the increased residual volume, resulting in higher basal and threshold pressures. As stated by Nitti et al. (2013), administration of β3-AR agonist increases bladder capacity without changing the micturition pressure, residual volume, or voiding contraction during the storage phase of the voiding cycle (Nitti et al. 2013). In conflict with this, hypercholesterolemic rats exhibited increased capacity with higher basal and threshold pressures and residual volume, suggesting modification in in vivo response. Decreased micturition fraction in the HC group also indicates impairment in voiding. Since the detrusor contractility is intact, it is likely that the relaxation response of the bladder neck (which is completely different in innervation and receptor density) is involved. The response of the neck muscles was not included in our study, but it deserves to be investigated since reciprocal contraction and relaxations of DSM and bladder neck is crucial both for voiding and storage functions of the urinary bladder. The compliance was lower in the HC group in our study, similar with the results from a rat model of atherosclerosis-induced chronic bladder ischemia (Nomiya et al. 2012). As stated by Kohan, decreased bladder compliance reflects an increase in threshold pressure without a change in bladder capacity (Kohan et al. 2000) which is in accordance with our results. Nevertheless, regarding our in vivo findings, it should be noted that the only significant difference due to hypercholesterolemia obtained in the present study was in threshold pressure and basal pressure.
The decreased compliance in HC group in cystometrography may seem contradictory to our in vitro results. But keeping the volume–pressure curve in mind, one can easily figure it out that the higher basal pressure due to decreased micturition fraction makes the bladder work at the upper end of the curve, where compliance is naturally low. In our study, diet-induced hypercholesterolemia augmented relaxation responses in detrusor strips. However, it is not reflected to in vivo function possibly due to the higher residual volume and basal pressure.
In conclusion, the data of the current study confirm previous studies suggesting that M2 receptors modulate the action of β-agonists in the bladder and that this braking function of M2 receptors in the β-adrenergic relaxation of DSM is attenuated in hypercholesterolemic rats. The current results may also indicate that the ratio of β3/β2 receptors may change in hypercholesterolemic rats; however, this needs to be confirmed in additional studies. Additional studies are also required to determine the clinical implications of these results; however, they seem to suggest that a combination of antimuscarinics and β-agonists may be beneficial in the treatment of bladder disorders in patients with and without hypercholesterolemia.
References
Afeli SA, Petkov GV (2013) Functional BK channels facilitate the beta3-adrenoceptor agonist-mediated relaxation of nerve-evoked contractions in rat urinary bladder smooth muscle isolated strips. Eur J Pharmacol 711:50–56
Afeli SA, Rovner ES, Petkov GV (2013) BRL37344, a beta3-adrenergic receptor agonist, decreases nerve-evoked contractions in human detrusor smooth muscle isolated strips: role of BK channels. Urology 82(744):e741–747
Andersson KE (1988) Current concepts in the treatment of disorders of micturition. Drugs 35:477–494
Andersson KE (2009) Prospective pharmacologic therapies for the overactive bladder. Ther Adv Urol 1:71–83
Andersson KE, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986
Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB (1999) Overactivity and structural changes in the chronically ischemic bladder. J Urol 162:1768–1778
Balkanci ZD, Pehlivanoglu B, Bayrak S, Karabulut I, Karaismailoglu S, Erdem A (2012) The effect of hypercholesterolemia on carbachol-induced contractions of the detrusor smooth muscle in rats: increased role of L-type Ca2+ channels. Naunyn Schmiedeberg’s Arch Pharmacol 385:1141–1148
Cai GJ, Miao CY, Xie HH, Lu LH, Su DF (2005) Arterial baroreflex dysfunction promotes atherosclerosis in rats. Atherosclerosis 183:41–47
Cerruto MA, Asimakopoulos AD, Artibani W, Del Popolo G, La Martina M, Carone R, Finazzi-Agro E (2012) Insight into new potential targets for the treatment of overactive bladder and detrusor overactivity. Urol Int 89:1–8
Devroey D, Senesael E, Moerenhout T, Van De Vijver E, Vandevoorde J (2011) Follow-up of a cardiovascular prevention campaign. Cent Eur J Public Health 19:190–196
Dopico AM, Bukiya AN, Singh AK (2012) Large conductance, calcium- and voltage-gated potassium (BK) channels: regulation by cholesterol. Pharmacol Ther 135:133–150
Ehlert FJ, Griffin MT, Abe DM, Vo TH, Taketo MM, Manabe T, Matsui M (2005) The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol Exp Ther 313:368–378
Ekman M, Andersson KE, Arner A (2009) Signal transduction pathways of muscarinic receptor mediated activation in the newborn and adult mouse urinary bladder. BJU Int 103:90–97
Ferro A (2006) Beta-adrenoceptors and potassium channels. Naunyn Schmiedeberg’s Arch Pharmacol 373:183–185
Frazier EP, Mathy MJ, Peters SL, Michel MC (2005) Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol? J Pharmacol Exp Ther 313:260–267
Frazier EP, Peters SL, Braverman AS, Ruggieri MR Sr, Michel MC (2008) Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedeberg’s Arch Pharmacol 377:449–462
Hall SA, Chiu GR, Link CL, Steers WD, Kupelian V, McKinlay JB (2011) Are statin medications associated with lower urinary tract symptoms in men and women? Results from the Boston Area Community Health (BACH) Survey. Ann Epidemiol 21:149–155
Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM (1997) Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol 120:1409–1418
Herrera GM, Etherton B, Nausch B, Nelson MT (2005) Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289:R402–R409
Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV (2008) Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295:C1344–1353
Hristov KL, Afeli SA, Parajuli SP, Cheng Q, Rovner ES, Petkov GV (2013) Neurogenic detrusor overactivity is associated with decreased expression and function of the large conductance voltage- and Ca(2+)-activated K(+) channels. PLoS ONE 8:e68052
Huang YC, Shindel AW, Ning H, Lin G, Harraz AM, Wang G, Garcia M, Lue TF, Lin CS (2010) Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol 183:1232–1240
Kim SC (2000) Hyperlipidemia and erectile dysfunction. Asia J Androl 2:161–166
Kim HW, Kim SC, Seo KK, Lee MY (2002) Effects of estrogen on the relaxation response of rabbit clitoral cavernous smooth muscles. Urol Res 30:26–30
Kohan AD, Danziger M, Vaughan ED Jr, Felsen D (2000) Effect of aging on bladder function and the response to outlet obstruction in female rats. Urol Res 28:33–37
Kubota Y, Nakahara T, Yunoki M, Mitani A, Maruko T, Sakamoto K, Ishii K (2002) Inhibitory mechanism of BRL37344 on muscarinic receptor-mediated contractions of the rat urinary bladder smooth muscle. Naunyn Schmiedeberg’s Arch Pharmacol 366:198–203
Li G, Li K, Li Z, Wang P (2003) Age-dependent changes in beta-adrenoceptor function in human detrusor and possible mechanisms. Chin Med J 116:1511–1514
Longhurst PA, Levendusky M (1999) Pharmacological characterization of beta-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br J Pharmacol 127:1744–1750
Longhurst PA, Levendusky MC, Bezuijen MW (2004) Diabetes mellitus increases the rate of development of decompensation in rats with outlet obstruction. J Urol 171:933–937
Matsui M, Griffin MT, Shehnaz D, Taketo MM, Ehlert FJ (2003) Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M2 receptor knockout mice. J Pharmacol Exp Ther 305:106–113
Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW (2004) Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279:36746–36752
Michel MC, Barendrecht MM (2008) Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol Ther 117:297–312
Michel MC, Sand C (2009) Effect of pre-contraction on beta-adrenoceptor-mediated relaxation of rat urinary bladder. World J Urol 27:711–715
Michel MC, Vrydag W (2006) Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol 147(Suppl 2):S88–119
Monica FZ, Bricola AA, Bau FR, Freitas LL, Teixeira SA, Muscara MN, Abdalla FM, Porto CS, De Nucci G, Zanesco A, Antunes E (2008) Long-term nitric oxide deficiency causes muscarinic supersensitivity and reduces beta(3)-adrenoceptor-mediated relaxation, causing rat detrusor overactivity. Br J Pharmacol 153:1659–1668
Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S (2013) Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 189:1388–1395
Nomiya M, Yamaguchi O, Andersson KE, Sagawa K, Aikawa K, Shishido K, Yanagida T, Kushida N, Yazaki J, Takahashi N (2012) The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn 31:195–200
Oger S, Behr-Roussel D, Gorny D, Bernabe J, Comperat E, Chartier-Kastler E, Denys P, Giuliano F (2011) Effects of potassium channel modulators on myogenic spontaneous phasic contractile activity in human detrusor from neurogenic patients. BJU Int 108:604–611
Oshita M, Hiraoka Y, Watanabe Y (1997) Characterization of beta-adrenoceptors in urinary bladder: comparison between rat and rabbit. Br J Pharmacol 122:1720–1724
Parajuli SP, Petkov GV (2013) Activation of muscarinic M3 receptors inhibits large-conductance voltage- and Ca2+-activated K+ channels in rat urinary bladder smooth muscle cells. Am J Physiol Cell Physiol 305:C207–214
Petkov GV (2012) Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9:30–40
Petkov GV, Nelson MT (2005) Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288:C1255–1263
Rahman NU, Phonsombat S, Bochinski D, Carrion RE, Nunes L, Lue TF (2007) An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int 100:658–663
Ren YJ, Xu XH, Zhong CB, Feng N, Wang XL (2001) Hypercholesterolemia alters vascular functions and gene expression of potassium channels in rat aortic smooth muscle cells. Acta Pharmacol Sin 22:274–278
Shishido T, Tasaki K, Takeishi Y, Takasaki S, Miyamoto T, Itoh M, Takahashi H, Kubota I, Ito T, Katano Y, Wakabayashi I, Tomoike H (2004) Chronic hypertriglyceridemia in young watanabe heritable hyperlipidemic rabbits impairs endothelial and medial smooth muscle function. Life Sci 74:1487–1501
Son H, Lee SL, Park WH, Park K, Park S, Kang MS, Kim DY, Kim SW, Paick JS (2007) New unstable bladder model in hypercholesterolemia rats. Urology 69:186–190
Stevens LA, Chapple CR, Chess-Williams R (2007) Human idiopathic and neurogenic overactive bladders and the role of M2 muscarinic receptors in contraction. Eur Urol 52:531–538
Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T, Hatano A, Takahashi K, Nomura S (1999) Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther 288:1367–1373
Takeda H, Yamazaki Y, Akahane M, Igawa Y, Ajisawa Y, Nishizawa O (2000) Role of the beta(3)-adrenoceptor in urine storage in the rat: comparison between the selective beta(3)-adrenoceptor agonist, CL316, 243, and various smooth muscle relaxants. J Pharmacol Exp Ther 293:939–945
Tanaka Y, Horinouchi T, Koike K (2005) New insights into beta-adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin Exp Pharmacol Physiol 32:503–514
Tyagi P, Thomas CA, Yoshimura N, Chancellor MB (2009) Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol : Off J Braz Soc Urol 35:76–83
Uchida H, Shishido K, Nomiya M, Yamaguchi O (2005) Involvement of cyclic AMP-dependent and -independent mechanisms in the relaxation of rat detrusor muscle via beta-adrenoceptors. Eur J Pharmacol 518:195–202
Vrydag W, Michel MC (2007) Tools to study beta3-adrenoceptors. Naunyn Schmiedeberg’s Arch Pharmacol 374:385–398
Witte LP, de Haas N, Mammen M, Stangeland EL, Steinfeld T, Aiyar J, Michel MC (2011) Muscarinic receptor subtypes and signalling involved in the attenuation of isoprenaline-induced rat urinary bladder relaxation. Naunyn Schmiedeberg’s Arch Pharmacol 384:555–563
Yamaguchi O (2002) Beta3-adrenoceptors in human detrusor muscle. Urology 59:25–29
Yamazaki Y, Takeda H, Akahane M, Igawa Y, Nishizawa O, Ajisawa Y (1998) Species differences in the distribution of beta-adrenoceptor subtypes in bladder smooth muscle. Br J Pharmacol 124:593–599
Yoshida M, Masunaga K, Nagata T, Satoji Y, Shiomi M (2010a) The effects of chronic hyperlipidemia on bladder function in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurourol Urodyn 29:1350–1354
Yoshida M, Masunaga K, Nagata T, Yono M, Homma Y (2010b) The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: pathophysiology and pharmacotherapy of overactive bladder. J Pharmacol Sci 112:128–134
Acknowledgments
This study was supported by Hacettepe University Research Foundation (HUBAB; 01-G-019 and 07-D09-101-03) and The Scientific and Technological Research Council of Turkey (TUBITAK: 106-S-244).
The authors wish to thank Gülsen Öner, PhD, Professor of Physiology, for her critical and initiative contribution to this study; Rıfkı Finci, MD, Professor of Pathology, for the histopathological evaluation of the aortic arch; and S. Remzi Erdem, MD, PhD, Professor of Pharmacology, for the critical reading of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayrak, S., Balkanci, Z.D., Pehlivanoğlu, B. et al. Does hypercholesterolemia affect the relaxation of the detrusor smooth muscle in rats? In vitro and in vivo studies. Naunyn-Schmiedeberg's Arch Pharmacol 388, 761–771 (2015). https://doi.org/10.1007/s00210-014-1060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1060-7