Abstract
A high salt diet leads to a decrease in vascular dilatation to agonists, but the vascular mechanisms involved in this process are not extensively studied. A group of male Wistar rats at the age of 3 months was transferred to a high-salt diet containing 8% NaCl (HS) for 3 months, while the second group received a normal-salt diet with a standard salt content (0.34%) (NS). At the end of the experiment, the rats were euthanized and the abdominal aorta and superior mesenteric artery (SMA) were extracted. The vascular segments were placed into a myograph, and the acetylcholine (ACh)-induced relaxation of the phenylephrine (PHE)-precontracted vascular segments was measured. A high-salt diet led to attenuate the relaxation of the SMA in a calcium-free solution. In response to ACh and sodium nitroprusside, a pronounced relaxation of the vascular segments was observed, while the ACh-induced vascular relaxation in HS rats showed a lower amplitude. Potassium channel blockers (TEA, TRAM-34, apamine) attenuated the ACh-induced relaxation of the SMA, but not the aorta. In the SMA of HS rats, a decrease in the relaxation under the effect of K+ channel blockers was more prominent. Inhibition of the production of endogenous hydrogen sulfide (H2S) also led to attenuate the ACh-induced relaxation of SMA segments. In SMA of HS rats, the degree of attenuation of the ACh-induced relaxation against the background of propargylglycine was larger than in NS rats. The data obtained in the study show that a long-term high-salt diet leads to a decrease in agonist-induced relaxation of the aortic segments and SMA due to a decrease in the production of NO by the endothelium. In the SMA of HS rats, a decrease in NO-mediated relaxation is partially compensated by the increasing role of EDHF in ACh-induced relaxation. The results of the study also show that one of the EDHFs in the rat SMA is H2S, the role of which in SMA relaxation increases in HS rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Epidemiological studies indicate the existence of a link between high salt consumption and human arterial hypertension [1]. Sodium overconsumption (more than 5 g per day, as defined by the World Health Organization) quite often leads to increase arterial pressure (AP) and to form stable arterial hypertension. The AP dependence on the dietary sodium chloride content was also demonstrated in some animals, for example, in Dahl-sensitive rats, although lacking in Wistar rats [2, 3].
Arterial hypertension induced by a high-salt diet is a powerful risk factor for a cerebral stroke, ischemic heart disease and cardiac insufficiency [4, 5]. At the same time, in vivo and in vitro studies have shown that excessive dietary salt can cause anatomical remodeling and functional disorders in small and large arteries, as well as inflammatory alterations in the microvascular endothelium, even in normotensive subjects, thus significantly increasing the risk of ischemic or hemorrhagic stroke and various ischemic heart diseases [5, 6]. It was established that in healthy young people a short-term (for 7 days) high-salt diet (regardless of the AP value, changes in the body composition, and fluid retention) worsens microvascular reactivity by affecting the endothelium-dependent vasodilatation [7]. It is also worth allowing for the influence of salt load on physical characteristics of vessels, specifically, their rigidity [8, 9], which is defined as a collagen/elastin balance in the vascular wall. It was shown that a high-salt load leads to increase collagen synthesis and reduce elastin content, inducing the development of fibrosis which increases vascular rigidity and thereby enhances endothelial dysfunction [10, 11].
The vascular endothelium is an important component of the vascular wall, which maintains its homeostasis, including vascular tone regulation. Under physical (shear stress) or chemical (neurotransmitters, hormones, cytokines) stimulation, endothelial cells secrete several factors a part of which has a vasoconstrictor effect while the other one promotes vascular dilatation [12]. It was established in numerous studies that vasodilators synthesized in the endothelium are nitrogen oxide (NO), vasodilating prostaglandins (PGs), specifically, prostacyclin PGI2, and endothelium-derived hyperpolarizing factor (EDHF) [13].
Multiple pathophysiological conditions, including cardiovascular, renal and metabolic, are underlain by an imbalance between the amounts of vasoconstrictors and vasodilators produced by the endothelium, with the deficiency of the latter being most common to occur [14]. Some authors demonstrated that a reduction in the level of NO bioavailability is a principal manifestation of endothelial dysfunction in small and large arteries of rats and mice fed a high-salt diet [15]. Besides, some evidence was obtained that a high dietary salt content is paralleled by an impairment of the endothelium-dependent arterial dilatation mediated by metabolites of arachidonic acid [16]. As for the role of endothelial hyperpolarization in the regulation of vascular tone in animals kept on a high-salt diet, it is necessary to point out that this mechanism is poorly studied and the data obtained are equivocal [17]. Félétou and Vanhoutte [18] believe that EDHF-mediated responses may play the role of the arterial vasodilator reserve under hypertension and some other pathologies, compensating, at least temporarily or partially, endothelial dysfunction when NO synthesis (bioavailability) is impaired.
Lastly, it is noteworthy that by now there are almost no studies addressing the role of H2S in the regulation of vascular tone in animals being on a high-salt diet. Meanwhile, it has been recently shown that H2S exerts plenty of beneficial effects on the vascular wall, which include a suppression of oxidative stress, inhibition of inflammation and enhancement of vasodilatation [19].
The aim of this work was to assess the impact of a high-salt diet (8% NaCl for 3 months) on the endothelium-dependent dilatation of isolated vessels [abdominal aorta and superior mesenteric artery (SMA)] in Wistar rats. Specific goals were to determine the influence of a high-salt diet on the individual mechanisms of ACh-induced endothelium-dependent vasodilatation (mediated by NO, PGI2 and endothelial hyperpolarization), as well as those realized with the involvement of H2S.
MATERIALS AND METHODS
Experiments were carried out on adult male Wistar rats aged 3 months and weighing 230–260 g [Biocollection Center for Collective Use at Pavlov Institute of Physiology of the Russian Academy of Sciences (IPh RAS)]. Rats were kept under standard vivarium conditions, receiving food and water ad libitum. Twenty rats used in toto were randomly distributed into two groups differing in their dietary NaCl content. The group 1 rats received for 12 weeks a high-sodium diet containing 8% NaCl in a compound feed (high-salt or HS rats). The group 2 rats received a standard diet containing 0.34% NaCl (normal salt or NS rats). A high-salt diet was based on a standard granulated compound feed with granules sprinkled with a saturated NaCl solution and dried thereafter. In terms of protein, fat and carbohydrate contents, as well as nutritive values, the diets of both rat groups were identical. Experimental protocols complied with the principles of the Basel Declaration and were approved by the Ethics Committee at IPh RAS.
Rats were euthanized by exsanguination under Zoletil anesthesia (8 mg/kg). After opening the abdominal cavity, segments of the abdominal aorta and superior mesenteric artery (SMA), 7–8 mm wide, were excised. Fat and surrounding connective tissue were thoroughly removed, and the aorta and SMA were cut into ring-shaped segments with a length of 2 mm (19 aortic segments from HS and 22 from NS rats, 21 SMA segments from HS and 24 from NS rats). The prepared vascular segments were stored until use in a fridge in an ice-cold physiological solution saturated with a gas mixture (95% O2 and 5% CO2).
At the onset of the experiment, vascular segments were placed into a myograph chamber through which a physiological saline containing (mM) 120.4 NaCl, 5.9 KCl, 1.2 NaH2PO4, 15.5 NaHCO3, and 11.5 glucose and saturated with the same gas mixture was flowing continuously. The solution temperature was maintained at 37.0 ± 0.1°C, pH of the solution—at 7.4 ± 0.2. A vascular segment was placed in between two titanic wires, and using a micromanipulator, the latter were stretched until the development of a 20 mH passive tension, allowing wires to reach the resting tension for 30 min. Such a stretch was optimal to develop a reproducible maximum contraction in the solution containing 100 mM KCl (equimolar NaCl amount was replaced with KCl) [20]. A contraction elicited by a hyperpotassium solution was used as a reference to assess the contractile capacity of the aortic and SMA segments. The contraction force of vascular segments was measured using a force transducer FORT-10 (WPI, USA) operating in an isometric mode. From the transducer, information was fed into the Labmaster block (amplifier + analog-to-digital converter; IPh RAS) and then into a computer. Primary data processing was carried by using Labmaster and Microsoft Excel software programs.
After determining the reference contractile response in the solution containing 100 mM KCl, the vessels were bathed in a physiological solution for 30 min. The presence of intact endothelium in the vascular preparations was confirmed by observing the relaxation response of PHE-precontracted vascular segments to ACh [21]. In a part of preparations, the endothelium was removed mechanically. To assess the influence of a high-salt diet on resting tone and remodeling of the aortic and SMA walls, the contraction force of the vascular segments was measured after 20-min perfusion with a calcium-free saline containing 2 mM EGTA.
The subsequent experimental design was the following: after measuring aortic and SMA segments’ tension in hyperpotassium and calcium-free solutions, the vascular segments were washed with a physiological solution over 30 min. After that, the bath solution was successively added first with phenylephrine hydrochloride (PHE, 10–5 M) and then, upon contractile response entering the plateau phase, ACh chloride at various concentrations (to determine endothelium-dependent relaxation); finally, the relaxation value was assessed. To identify vasodilators produced by the endothelium, the following inhibitors and blockers were applied: an endothelial NO synthase (eNOS) inhibitor L-N5-(1-Iminoethyl)ornithine dihydrochloride (L-NIO, 10–5 М); a cyclooxygenase (COX) inhibitor diclofenac sodium salt (DF, 5 × 10–6 М); a nonspecific K+ channel blocker tetraethylammonium chloride (TEA, 10–3 М); an intermediate-conductance Ca2+-activated K+ channel blocker TRAM-34 (10–5 M); a small-conductance Ca2+-activated K+ channel blocker apamin (Apa, 10–6 M); a cystathionine-γ-lyase inhibitor DL-propargylglycine (PPG, 10–4 М). To bind residual Ca2+ in a calcium-free solution, ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA, 2 × 10–3 М) was added.
All chemical reagents were purchased from Sigma-Aldrich (USA). Inhibitors and blockers were added to physiological solution 20 min prior to PHE application. Immediately before experiments, the reagents were dissolved in distilled water at a concentration of 10 mM, and then the requisite amount of them was added to physiological solution. Diclofenac and TRAM-34 were pre-dissolved in methanol (20 mg/mL) and added to physiological solution in the requisite amounts immediately prior to the experiment.
Statistical data processing was carried out using Microsoft Excel and StatSoft STATISTICA 6.1.478. Distribution normality of the data obtained was tested by computing a Shapiro–Whilk WW test. Data were presented as means ± standard error (M ± SE). Statistical significance of differences was determined using the Student’s t-test. Differences were considered significant at p < 0.05.
RESULTS
At the end of the experimental period, the weight of HS rats amounted to 370 ± 32 g (n = 10), while that of NS rats was 393 ± 21 g (n = 10). Systolic arterial pressure, as measured in the caudal artery by a tail cuff method was 130 ± 10 mmHg in HS rats and 129 ± 11 mmHg in NS rats. As follows from these data, a 3-month high-salt diet had no effect on the body weight and systolic arterial pressure.
Hyperpotassium solution (100 mM K+) led to a powerful tonic contraction of the vascular segments. The contraction force of these segments in HS rats was indistinguishable from that in NS rats. The application of calcium-free solution demonstrated that SMA segments of HS rats retained a larger residual tension compared to SMA segments of NS rats. The contraction force of aortic segments in HS rats in a calcium-free solution also was larger compared to NS rats, but this difference proved insignificant (Table 1). These data indicate an impairment of the vascular relaxation mechanisms in HS rats.
After assessing contractile responses of vascular segments, we turned to studying their capability of agonist-induced relaxation. At the first stage, we studied the endothelium-independent responses of aortic and SMA segments in HS and NS rats. After establishing a stable PHE-induced contraction, the bath solution was added with sodium nitroprusside (NP, 10–5 M) [20]. NP induced a pronounced relaxation of vascular segments. NP-induced aortic and SMA relaxation in NS rats was somewhat stronger compared to HS rats, however the difference were statistically insignificant (data not shown).
At the next stage, we studied endothelium-dependent relaxation of vascular segments. The bath solution was added with ACh in increasing concentrations (10–9–10–5 М). ACh induced dose-dependent relaxation of PHE-precontracted vessels in both rat groups. The averaged data on this experimental series are shown in Fig. 1. In these experiments, relaxation of aortic and SMA segments to ACh at a concentration of ≥ 10–7 in HS rats was weaker compared to NS rats. Denuded vessels did not react to the introduction of ACh to a bath solution at concentrations of 10–9–10–6 M, while at a concentration of 10–5 M ACh induced a small-amplitude contraction (data not shown). It is necessary to point out that relaxation of aortic and SMA segments in response to ACh application at a maximum concentration was significantly weaker compared to the NP-induced relaxation of these vessels.
Later on, we studied the mechanisms of endothelium-dependent dilatation of aortic and SMA segments in HS and NS rats. For this purpose, after PHE-precontracted relaxation of vascular segments, the bath solution was added with ACh, and the dilatation value was assessed. Then, after 30-min bathing of vascular segments with physiological solution, an eNOS inhibitor (L-NIO) and diclofenac were introduced separately or together. After 20 min, vascular segments were contracted by PHE, and ACh was added against this background. In the presence of inhibitors, relaxation of vascular segments was significantly weaker compared to the effect of ACh in a physiological solution. Data obtained in the series of experiments show that a major vasodilator in the studied vessels of NS rats is NO which in aortic segments provided up to 86% of ACh-induced vasorelaxation. A high-salt diet attenuated the ability of the endothelium to produce NO: in aortic segments of HS rats, NO accounted for less than 60% of the ACh-induced relaxation value. In SMA segments of NS rats, the proportion of no-NO-mediated relaxation was 29%, while in SMA of HS rats it increased up to 47%. The results of this series of experiments are shown in Fig. 2.
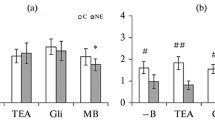
Considering the important role of endothelial hyperpolarization in dilatation of various arteries in different animals, as well as the involvement of Ca2+-activated K+ channels in this process [21], in the next series of experiments, we used various blockers of K+ channels: TEA as a nonspecific blocker of various K+ channels, TRAM-34 as a blocker of intermediate-conductance Ca2+-activated K+ channels, and apamin as a blocker of small-conductance Ca2+-activated K+ channels. The blockers were introduced into a physiological solution 20 min before precontraction and subsequent ACh-induced vasorelaxation. When studying aortic segments in HS and NS rats, it was established that all the K+ channel blockers we applied elicited a small ACh-induced decrement in the relaxation of aortic segments. In contrast to the aorta, in SMA segments of NS rats, TEA, TRAM-34 and apamin led to a pronounced decrement in ACh-induced relaxation. A maximum decrement in ACh-induced relaxation of SMA segments was observed after the application of a combination of the blockers TRAM-34 + apamin. In SMA segments of HS rats, a decrease in ACh-induced relaxation after the application of K+ channel blockers was more pronounced compared to the SMA responses in NS rats (Fig. 3).
In the recent years, studies of the mechanisms of arterial dilatation in different vascular regions put considerable emphasis on H2S, which is conventionally considered as a third gasotransmitter [22]. In the conclusive series of experiments, we studied the role of endogenous H2S in ACh-induced relaxation of aortic and SMA segments in HS and HN rats. To do this, we primarilly assessed the relaxation value of vascular segments in response to ACh added first to the physiological solution and then to the solution containing eNOS and cyclooxygenase inhibitors. Since a dominant enzyme synthesizing H2S in the cardiovascular system is cystathionine-γ-lyase (CSE) [23, 24], this solution was subsequently added with a CSE inhibitor propargylglycine (PPG). The introduction of PPG into the solution containing L-NIO and diclofenac led to a statistically significant decrease in ACh-induced relaxation. In SMA segments, the action of PPG was accompanied by a more pronounced decrease in vasorelaxation compared to that in the aorta, while in the SMA of HS rats the inhibitory effect of PPG was significantly larger compared to the SMA in NS rats (Fig. 4). These data allow suggesting that vasorelaxation induced by ACh in aortic and SMA segments is mediated not only by NO and PGI2 but also H2S.
ACh-induced relaxation of phenylephrine-precontracted aortic and SMA segments in rats fed a high-salt diet (HS) and control rat group (NS) in the presence of an endothelial NO-synthase inhibitor (L-NIO) and diclofenac (DF). Results are presented in % of ACh-induced relaxation values as mean ± SE. Differences between the same vessels in HS vs. NS rats are significant: #—p <0.01, *—p < 0.05.
ACh-induced relaxation of phenylephrine-precontracted aortic and SMA segemnts in rats fed a high-salt diet (HS) and control rats (NS) in the presence of K+ channel blockers: tetraethylammonium (TEA), TRAM-34, and apamine (Apa). Results are presented in % of the ACh-induced relaxation value as mean ± SE. Differences between SMA relaxation values in HS vs. NS rats are significant: #—p < 0.01, *—p < 0.05.
ACh-induced relaxation of phenylephrine-precontracted aortic and SMA segments of rats fed a high-salt diet (HS) and control rats (NS) in the presence of an eNOS inhibitor L-NIO, a cyclooxygenase inhibitor diclofenac (DF), and a cystathionine-γ-lyase inhibitor propargylglycine (PPG). Results are presented in % of ACh-induced relaxation values as mean ± SE. Differences between the same vessels in HS vs. NS rats are significant: #—p < 0.01, *—p < 0.05.
DISCUSSION
In the last 20 years, ample evidence has been obtained that a high-salt diet is closely associated with a higher risk of cardiovascular diseases independently of its effect on arterial blood pressure [25]. At the same time, it has become clear that in humans a high-salt diet leads to a functional deficiency of the vascular endothelium, which manifests itself in attenuating vascular reactions to various vasomotor irritants [26]. In experiments conducted on animals, it has also been established that a high-salt diet has a negative effect on vessels, typically disrupting the process of vasodilatation [27]. In these works, when studying the functional state of arteries in animals fed a high-salt diet, there were obtained significantly different results [28–30]. We suppose that this is due both to the difference in the experimental objects (artery’s type, location and size, animal species) and the diversity of the methods and protocols applied. The duration of a high-salt diet was also considerably variable.
In contrast to other works carried out on rats, in which the duration of a high-salt dies varied from 3 days to 4 weeks [28, 31], in our study, rats were kept on a high-salr diet for 12 weeks, which, in our opinion, must have led to pronounced changes in vascular tone regulation. Since in most of similar studies of arteries conducted both in vivo and in vitro there was revealed an impairment of the endothelium-dependent vasodilatation mechanisms, we also paid major attention to this problem.
Upon completion of a 12-week diet with a 8% NaCl content, animals’ body weight systolic arterial pressure were measured. A comparison of the data obtained in HA vs. NS rats demonstrated that a high-salt diet had no significant effect both on the body weight and arterial pressure.
When studying a maximum contraction force of aortic and SMA segments in HS and NS rats, there were found minimum differences, which could indirectly indicate the absence or weak remodeling of the vascular wall in HS rats. However, the effect of the calcium-free solution showed a decrease in the ability of the vascular wall to relax. Maximum differences between vascular reactions in HS vs. NS rats in the calcium-free solution was observed in studying SMA segments. The dilatatory effect of ACh on SMA segments in HS rats was also smaller compared to NS rats. Previously, agonist-induced remodeling and reduced reactivity were revealed in small arteries and arterioles of rats kept on a high-salt diet for 4 weeks [29]. In our study, the reduced response of SMA segments to the calcium-free solution may indicate remodeling of the large muscular arteries in rats kept for a long time on a high-salt diet.
It is commonly accepted that a major vasodilator produced by the endothelium is NO [32]. In our study, we first applied exogenous NO, i.e. assessed endothelium-independent relaxation of aortic and SMA segments. The introduction of nitroprusside (a source of exogenous NO) into the bath solution demonstrated the absence of significant differences between the analogous vessels in HS vs. NS rats. These results are of importance, because they indicate that a long-term high-salt diet does not lead to impair the NO-activated intracellular signaling mechanisms in vascular smooth muscle cells, i.e. smooth muscle cells retain a normal reactivity to the effect of NO.
At the initial stage of studying endothelium-dependent relaxation of aortic and SMA segments, there were plotted dose-effect curves for AСh application at concentrations of 10–9–10–5 М. It was shown that with ACh applied at concentrations ≥10–7 M, relaxation of aortic segments in NS rats was more than 75%, while that of SMA segments in NS rats—more than 85% of PHE-stimulated precontraction in these vessels. Differences in the reactions of vascular segments of HS vs. NS rats to the effect of ACh at concentrations ≥10–7 M were statistically significant. Analysis of the data obtained for the effect of nitroprusside and ACh on vascular segments of HS vs. NS rats demonstrated that long-term high-salt diet leads to impair the endothelial production of vasodilators in arteries of different types: both in collector (aorta) and distributive (SMA) arteries.
As is well known, under physiological conditions, endothelial cells in the vascular wall produce NO through activation of endothelial eNOS. Pathogenic factors (which a high-salt diet can be referred to) lead to express the genes encoding the synthesis of inducible NO synthase (iNOS) in a variety of cells, including smooth muscle cells in the vascular wall [33]. As we aimed to study the endothelium-dependent vascular tone regulation, we used L-NIO which is a selective eNOS inhibitor. The addition of L-NIO to a physiological solution led to significantly decrease the ACh-induced relaxation of vascular segments. A maximum decrease in relaxation was observed in aortic segments of NS rats, indicating that, under physiological conditions, a major endothelial vasodilator in these vessels is NO. Aortic segments in HS rats, in the presence of L-NIO, demonstrated a smaller reduction in the amplitude of ACh-induced relaxation. A comparison of these data indicates that the damaging effect of a high-salt diet on the aortic endothelium consists, specifically, in impairing the eNOS function.
In SMA segments of NS rats, L-NIO exerted a significantly smaller inhibitory effect compared to the aorta, indicative of a smaller role of NO as an endothelial vasodilator in these vessels. The considerable amplitude of ACh-induced relaxation against the background of the action of L-NIO also indicates that under physiological conditions the SMA endothelium, along with NO, produces other vasodilators too. In SMA segments of HS rats, the amplitude of ACh-induced relaxation in the presence of L-NIO decreased, thus confirming the damaging effect of a high-salt diet on eNOS in these vessels.
Since in some vessels an important role in vasodilatation is played by endothelium-produced prostaglandins [34], we studied the influence of a cyclooxygenase inhibitor diclofenac on ACh-induced relaxation of aortic and SMA segments. Diclofenac had virtually no effect on the relaxation of aortic and SMA segments in NS rats, indicating an insignificant role of prostanoids in the relaxation of these vessels under physiological conditions. A high-salt diet altered the ability of the endothelium to synthesize vasodilating prostanoids. In HS rats, in the presence of diclofenac, there was observed a significant decrease in ACh-induced relaxation of aortic and SMA segments, with the effect of diclofenac being more expressed in the SMA. These data demonstrate that a long-term high-salt diet results in cyclooxygenase activation in the vascular wall. Since diclofenac inhibits cyclooxygenases 1 and 2, it is logical to assume that a high-salt diet promotes the expression of genes responsible for the synthesis of cyclooxygenase 2 and the appearance of this enzyme in the vascular wall [35], thus contributing to the production of a significant amount of prostanoids involved in vasodilatation.
Because against the background of a co-application of NO synthase and cyclooxygenase inhibitors there was no complete inhibition of ACh-induced relaxation of SMA segments in NS and HS rats, our further study was focused on the possible role of the endothelium-derived hyperpolarization factor (EDHF) in vasorelaxation. The chemical nature of EDHF is still unidentified. It is thought that several substances may function as the EDHF: epoxyeicosatrienoic acid, H2O2, K+, H2S [36]. Despite the EDHF identity is unknown, the mechanism of its action is more or less clear: endothelial hyperpolarization develops mainly due to the opening of Ca2+-sensitive K+ channels, leading to K+ outflux from cells and the development of hyperpolarization followed by relaxation of smooth muscle cells [37]. In our study, we applied three K+ channel blockers: TEA, a nonselective blocker of various types of K+ channels; TRAM-34, a selective blocker of intermediate-conductance Ca2+-sensitive K+ channels; aramin, a selective blocker of small-conductance Ca2+-sensitive K+ channels. None of these blockers led to significant changes in the amplitude of ACh-induced relaxation of aortic segments in HS and NS rats, thus allowing the conclusion that the EDHF-mediated mechanism of relaxation in these vessels is lacking or is of extremely low efficiency.
In SMA of NS rats, all three blockers led to reduce the amplitude of ACh-induced relaxation. A maximum effect was exerted by a combination of blockers TRAM-34 + apamin. The data obtained when studying vascular segments of HS rats proved to be of greatest interest. In the SMA of HS rats, the inhibitory effect of each of the applied K+ channel blocker was expressed far stronger compared to vessels of NS rats. A significant reduction in ACh-induced relaxation of SMA segments in HS rats was observed when applying each of the blockers, while the maximum effect was also obtained by a combination of the blockers TRAM-34 + apamin. The data obtained in this series of experiments allow the conclusion of the presence in the SMA endothelium of intermediate- and small-conductance Ca2+-sensitive K+ channels. In other words, the EDHF-mediated mechanism of vasodilatation functions quite effectively in these vessels. It is traditionally believed that the EDHF-mediated mechanism of vasodilatation works effectively in small arteries and arterioles only [38]. Moreover, there is evidence that its efficiency in these vessels is even more significant than the NO-mediated mechanism [39]. In several works, it was also demonstrated that intermediate- and small-conductance Ca2+-sensitive K+ channels are involved in agonist-induced vasorelaxation of the rat SMA [40, 41]. In our study, we obtained data not only proving the presence of intermediate- and small-conductance Ca2+-sensitive K+ channels in the rat SMA but also their considerably increasing role in relaxation processes in the SMA of rats fed a high-salt diet.
Over the last decade, when studying the vasodilatory mechanisms, much attention was focused on the third gasotransmitter, H2S [42]. We carried out a series of experiments to find out the possible role of H2S in ACh-induced relaxation of the rat aorta and SMA. It is well known that diverse H2S-producing enzymes have been identified in the wall of various arteries, including cystathionine-γ-lyase (CSE), cystathionine-β-synthase, and 3-mercaptopyruvate sulfurtransferase. Because CSE is present in the wall of most of the vessels studied [23, 24], in our experiments, we used propargylglycine (PPG) as an inhibitor of H2S synthesis, adding it to a solution already containing inhibitors of NO synthase and cyclooxygenase. The addition of PPG led to attenuate SMA relaxation in NS rats. In SMA segments of HS rats, a decrease in the amplitude of relaxation was significantly larger compared to the SMA of NS rats. PPG had a little influence on the relaxation of aortic segments in HS and NS rats. When comparing the data obtained with the results of application of the Ca2+-sensitive K+ channel blockers (the amplitude of relaxation weakening, its increasing in HS rats), we suppose that in the SMA of Wistar rats it is H2S that functions as an EDHF (or one of the EDHFs).
Our results demonstrate that long-term high-salt diet in rats leads to a restriction of the ability of the SMA to relax, as well as to changes in the reactions of arteries to vasodilators. A high-salt diet causes the attenuation of ACh-induced NO-mediate relaxation of the rat aorta and SMA, which is most pronounced in the aorta (Fig. 2). In the SMA, a diet with a high NaCl content results in an increasing efficiency of the EDHF-mediated mechanism of relaxation. Apparently, similar changes in endothelium-dependent vasodilatation in the rat SMA are typical of muscular arteries under a variety of vascular pathologies. It is well known that under pathological conditions leading to an impairment of the vascular function and accompanied by NO deficiency, the role of the EDHF-mediated mechanism of vasodilatation increases compensatorily [18, 43]. The results of this study also show that in the rat SMA the function of the EDHF is performed by H2S, the production of which increases when animals are kept on a long-term high-salt diet.
REFERENCES
Elijovich, F., Weinberger, M.H., Anderson, C.A., Appel, L.J., Bursztyn, M., Cook, N.R., Dart, R.A., Newton-Cheh, C.H., Sacks, F.M., and Laffer, C.L., Salt sensitivity of blood pressure: a scientific statement from the American Heart Association, Hypertension, 2016, vol. 68(3), pp. e7–e46. doi: 10.1161/HYP.0000000000000047
Swenson, S.J., Speth, R.C., and Porter, J.P., Effect of a perinatal high-salt diet on blood pressure control mechanisms in young Sprague-Dawley rats, Am. J .Physiol. Regul. Integr. Compar. Physiol., 2004, vol. 286(4), R764–R770. doi: 10.1152/ajpregu.00492.2003
Bagrov, A.Y., Agalakova, N.I., Kashkin, V.A., and Fedorova, O.V., Endogenous cardiotonic steroids and differential patterns of sodium pump inhibition in NaCl-loaded salt-sensitive and normotensive rats, Am. J. Hypertens., 2009, vol. 22, pp. 559–563.
Rust, P. and Ekmekcioglu, C., Impact of salt intake on the pathogenesis and treatment of hypertension, Adv. Exp. Med. Biol., 2017, vol. 956, pp. 61–84. doi: 10.1007/5584_2016_147
Marketou, M.E., Maragkoudakis, S., Anastasiou, I., Nakou, H., Plataki, M., Vardas, P.E., and Parthenakis, F.I., Salt-induced effects on microvascular function: A critical factor in hypertension mediated organ damage, J. Clin. Hypertens., 2019, vol. 21, pp. 749–757. doi: 10.1111/jch.13535
Strazzullo, P., D’Elia, L., Kandala, N.B., and Cappuccio, F.P., Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies, Br. Med. J., 2009, vol. 24, 339: b4567. doi: 10.1136/bmj.b4567
Barić, L., Drenjančević, I., Matić, A., Stupin, M., Kolar, L., Mihaljević, Z., Lenasi, H., Šerić, V., and Stupin, A., Seven-day salt loading impairs microvascular endothelium-dependent vasodilation without changes in blood pressure, body composition and fluid Status in healthy young humans, Kidney Blood Press Res., 2019, vol. 44(4), pp. 835–847. doi: 10.1159/000501747
Gates, P.E., Tanaka, H., Hiatt, W.R., and Seals, D.R., Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension, Hypertension, 2004, vol. 44, pp. 35–41.
Bagrov, A.Y. and Lakatta, E.G., The dietary sodium-blood pressure plot “stiffens”, Hypertension, 2004, vol. 44, pp. 22–24.
Zieman, S.J., Melenovsky, V., and Kass, D.A., Mechanisms, pathophysiology, and therapy of arterial stiffness, Arteriosclerosis, Thrombosis and Vasc. Biol., 2005, vol. 25, pp. 932–943.
Johnson, C.P., Baugh, R., Wilson, C.F., and Burns, J., Age related changes in the tunica media of the vertebral artery: implications for the assessment of vessels injured by trauma, J. Clin. Pathol., 2001, vol. 54, pp. 139–145.
Vanhoutte, P.M., Shimokawa, H., Feletou, M., and Tang, E.H., Endothelial dysfunction and vascular disease—a 30th anniversary update, Acta Physiol., 2017, vol. 219, pp. 22–96.
Zhao, Y., Vanhoutte, P.M., and Leung, S.W., Vascular nitric oxide: beyond eNOS, J. Pharmacol. Sci., 2015, vol. 129, pp. 83–94.
Ravarotto, V., Simioni, F., Pagnin, E., Davis, P.A., and Calo, L.A., Oxidative stress–chronic kidney disease–cardiovascular disease: a vicious circle, Life Sci., 2018, vol. 210, pp. 125–131. doi: 10.1016/j.lfs.2018.08.067
Nurkiewicz, T.R. and Boegehold, M.A., High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase, Am. J. Physiol. Regul. Integr. Compar. Physiol., 2007, vol. 292(4), R1550–R1556. doi: 10.1152/ajpregu.00703.2006
Zhu, J., Huang, T., and Lombard, J.H., Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries, J. Vasc. Res., 2007, vol. 44(5), pp. 382–390. doi: 10.1159/000102955
Lenda, D.M., Sauls, B.A., and Boegehold, M.A., Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt, Am. J. Physiol., 2000, vol. 279, pp. H7–H14.
Feletou, M. and Vanhoutte, P.M., EDHF: an update, Clin. Sci. (London), 2009, vol. 117, pp. 139–155. doi: 10.1042/CS20090096
Kanagy, N.L., Szabo, C., and Papa-petropoulos, A., Vascular biology of hydrogen sulfide, Am. J. Physiol. Cell. Physiol., 2017, vol. 312(5), pp. C537–C549. doi: 10.1152/ajpcell.00329.2016
Nyborg, N.C., Baandrup, U., Mikkelsen, E.O., and Mulvany, M.J., Active, passive and myogenic characteristics of isolated rat intramural coronary resistance arteries, Pflügers Arch., 1987, vol. 410, pp. 664–670.
Garland, C.J. and Dora, K.A., EDH: endothelium-dependent hyperpolarization and microvascular signaling, Acta Physiol. (Oxford), 2017, vol. 219(1), pp. 152–161. doi: 10.1111/apha.12649
Wagner, F., Asfar, P., Calzia, E., Radermacher, P., and Szabó, C., Bench-to-bedside review: Hydrogen sulfide—the third gaseous transmitter: applications for critical care, Crit. Care., 2009, vol. 13(3), p. 213. doi: 10.1186/cc7700
Polhemus, D.J. and Lefer, D.J., Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease, Circ. Res., 2014, vol. 114, pp. 730–737.
Kolluru, G.K., Bir, S.C., Yuan, S., Shen, X., Pardue, S., Wang, R., and Kevil, C.G., Cystathionine γ-lyase regulates arteriogenesis through NO-dependent monocyte recruitment, Cardiovasc. Res., 2015, 107(4): 590-600. 2015. Doi: 10.1093/cvr/cvv198. Epub 2015 Jul 20
Tuomilehto, J., Jousilahti, P., Rastenyte, D., Moltchanov, V., Tanskanen, A., Pietinen, P., and Nissinen, A., Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study, Lancet, 2001, vol. 357, pp. 848–851.
Takase, H., Sugiura, T., Kimura, G., Ohte, N., and Dohi, Y., Dietary sodium consumption predicts future blood pressure and incident hypertension in the Japanese normotensive general population, J. Am. Heart Assoc., 2015, vol. 29, 4(8), e001959. doi: 10.1161/JAHA.115.001959
Boegehold, M.A., The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension, J. Vasc. Res., 2013, vol. 50, pp. 458–467. doi: 10.1159/000355270
Cavka, A., Jukic, I., Ali, M., Goslawski, M., Bian, J.T., Wang, E., Drenjancevic, I., and Phillips, S.A., Short-term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure, J. Hypertens., 2016, vol. 34, pp. 676–684. doi: 10.1097/HJH.0000000000000852
Cordaillat, M., Fort, A., Virsolvy, A., Elghozi, J.L., Richard, S., and Jover, B., Nitric oxide pathway counteracts enhanced contraction to membrane depolarization in aortic rings of rats on high-sodium diet, Am. J. Physiol. Regul. Integr. Compar. Physiol., 2007, vol. 292(4), R1557–R1562. doi: 10.1152/ajpregu.00624.2006
Allen, L.A., Schmidt, J.R., Thompson, C.T., Carlson, B.E., Beard, D.A., and Lombard, J.H., High salt diet impairs cerebral blood flow regulation via salt-induced angiotensin II suppression, Microcirculation, 2019, vol. 26(3), e12518. doi: 10.1111/micc.12518
Liu, Y., Rusch, N.J., and Lombard, J.H., Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet, Hypertension, 1999, vol. 33(2), pp. 686–688. doi: 10.1161/01.hyp.33.2.686
Vanhoutte, P.M., Shimokawa, H., Feletou, M., and Tang, E.H., Endothelial dysfunction and vascular disease—a 30th anniversary update, Acta Physiol. (Oxford), 2017, vol. 219(1), pp. 22–96. doi: 10.1111/apha.12646
Persichini, T., Cantoni, O., Suzuki, H., and Colasanti, M., Cross-talk between constitutive and inducible NO synthase: an update, Antioxid. Redox Signal., 2006, vol. 8(5–6), pp. 949–954. doi: 10.1089/ars.2006.8.949
Cavka, A., Cosic, A., Jukic, I., Jelakovic, B., Lombard, J.H., Phillips, S.A., Seric, V., Mihaljevic, I., and Drenjancevic, I., The role of cyclo-oxygenase-1 in high-salt diet-induced microvascular dysfunction in humans, J. Physiol., 2015, vol. 593(24), pp. 5313–5324. doi: 10.1113/JP271631. Epub 2015 Dec 7
Kido, M., Ando, K., Onozato, M.L., Tojo, A., Yoshikawa, M., Ogita, T., and Fujita T., Protective effect of dietary potassium against vascular injury in salt-sensitive hypertension, Hypertension, 2008, vol. 51(2), pp. 225–231. doi: 10.1161/HYPERTENSIONAHA.107.098251
Félétou, M. and Vanhoutte, P.M., EDHF: an update, Clin. Sci. (London), 2009, vol. 117(4), pp. 139–155. doi: 10.1042/CS20090096
Félétou, M., Endothelium-dependent hyperpolarization and endothelial dysfunction, J. Cardiovasc. Pharmacol., 2016, vol. 67(5), pp. 73–87. doi: 10.1097/FJC.0000000000000346
Garland, C.J. and Dora, K.A., EDH: endothelium-dependent hyperpolarization and microvascular signaling, Acta Physiol. (Oxford), 2017, vol. 219(1), pp. 152–161. doi: 10.1111/apha.12649
Coleman, H.A., Tare, M., and Parkington, H.C., Nonlinear effects of potassium channel blockers on endothelium-dependent hyperpolarization, Acta Physiol. (Oxford), 2017, vol. 219(1), pp. 324–334. doi: 10.1111/apha.12805
Ando, M., Matsumoto, T., Kobayashi, S., Iguchi, M., Taguchi, K., and Kobayashi, T., Differential participation of calcium-activated potassium channel in endothelium-dependent hyperpolarization-type relaxation in superior mesenteric arteries of spontaneously hypertensive rats, Can. J. Physiol. Pharmacol., 2018, vol. 96(8), pp. 839–844. doi: 10.1139/cjpp-2017-0557
Stankevicius, E., Lopez-Valverde, V., Rivera, L., Hughes, A.D., Mulvany, M.J., and Simonsen, U.F., Combination of Ca2+ -activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery, Br. J. Pharmacol., 2006, vol. 149(5), pp. 560–572. doi: 10.1038/sj.bjp.0706886
Cirino, G., Vellecco, V., and Bucci, M., Nitric oxide and hydrogen sulfide: the gasotransmitter paradigm of the vascular system, Br. J. Pharmacol., 2017, vol. 174(22), pp. 4021–4031. doi: 10.1111/bph.13815
Bellien, J., Thuillez, C., and Joannides, R., Contribution of endothelium-derived hyperpolarizing factors to the regulation of vascular tone in humans, Fundam. Clin. Pharmacol., 2008, vol. 22(4), pp. 363–377. doi: 10.1111/j.1472-8206.2008.00610.x
Funding
This work was supported by the Program “Fundamental scientific research for the long-term development and provision of social and state competitiveness” (47_110_LD&PC, section 64.1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All applicable international, national and institutional principles of handling and using experimental animals for scientific purposes were observed.
This study did not involve human subjects as research objects.
СONFLICT OF INTEREST
Authors of this study have no conflict of interest.
Additional information
Translated by A. Polyanovsky
The original online version of this article was revised: the issue date is not January 2020, but January 2021
Rights and permissions
About this article
Cite this article
Lobov, G.I., Ivanova, G.T. Regulation of Arterial Tone in Rats Fed a Long-Term High-Salt Diet. J Evol Biochem Phys 57, 145–155 (2021). https://doi.org/10.1134/S0022093021010142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021010142