Abstract
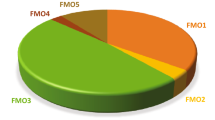
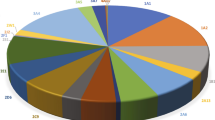
Data are presented on the formation of potentially toxic metabolites of drugs that are substrates of human drug metabolizing enzymes. The tabular data lists the formation of potentially toxic/reactive products. The data were obtained from in vitro experiments and showed that the oxidative reactions predominate (with 96% of the total potential toxication reactions). Reductive reactions (e.g., reduction of nitro to amino group and reductive dehalogenation) participate to the extent of 4%. Of the enzymes, cytochrome P450 (P450, CYP) enzymes catalyzed 72% of the reactions, myeloperoxidase (MPO) 7%, flavin-containing monooxygenase (FMO) 3%, aldehyde oxidase (AOX) 4%, sulfotransferase (SULT) 5%, and a group of minor participating enzymes to the extent of 9%. Within the P450 Superfamily, P450 Subfamily 3A (P450 3A4 and 3A5) participates to the extent of 27% and the Subfamily 2C (P450 2C9 and P450 2C19) to the extent of 16%, together catalyzing 43% of the reactions, followed by P450 Subfamily 1A (P450 1A1 and P450 1A2) with 15%. The P450 2D6 enzyme participated in an extent of 8%, P450 2E1 in 10%, and P450 2B6 in 6% of the reactions. All other enzymes participate to the extent of 14%. The data show that, of the human enzymes analyzed, P450 enzymes were dominant in catalyzing potential toxication reactions of drugs and their metabolites, with the major role assigned to the P450 Subfamily 3A and significant participation of the P450 Subfamilies 2C and 1A, plus the 2D6, 2E1 and 2B6 enzymes contributing. Selected examples of drugs that are activated or proposed to form toxic species are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A previous analysis of the published data on the participation of human oxidoreductase enzymes in the general metabolism of drugs showed that cytochrome P450 (P450, CYP) enzymes participate to the greatest extent, to the extent of 96% (Rendic and Guengerich 2015). The participation of other enzymes was: aldo–keto reductase (AKR) 1%, monoamine oxidase (MAO) 1%, flavin-containing monooxygenase (FMO) 2%, and the group of minor participating enzymes < 1%. Within the P450 Superfamily, the participation in the general metabolism of drugs (considering both drugs under development and marketed drugs) was: Subfamily 3A-33%, followed by the Subfamily 2C-24%, the Subfamily 1A-14%, and P450 2D6-13%. An estimate of the participation of P450 subfamilies in the number of reactions, taking into account just the metabolism of marketed drugs, was: Subfamily 3A-28%, Subfamily 2C-25%, Subfamily 1A-12%, and the 2D6 enzyme-13%. The contribution of other P450 enzymes ranged from 1 to 5% in both cases (data for the analysis collected up to 2015 (Rendic and Guengerich 2015). Thus, in addition to the P450 Subfamily 3A, a relatively large contribution to the general drug metabolism was shown for Subfamilies 2C and 1A and the P450 2D6 enzyme. The results of the analysis also showed that the main five P450 enzymes that catalyze the metabolism of marketed drugs are P450 3A4- 22%, 2D6 -14%, 2C19-9%, 2C9- 10%, and 1A2-8%, accounting for 63% of the marketed drug P450 metabolism reactions in humans (Rendic and Guengerich 2015). However, an analysis of recently approved drugs (2015–2020) showed that P450s are involved in 80% of the metabolism of these drugs and P450 3A4/3A5 in 52% (Bhutani et al. 2021). Another analysis of the reactions of drugs resulting in the formation of potentially toxic metabolites or intermediates (toxication reactions) as substrates of the P450 Families 1- 4 showed that 6% of the reactions involved reported toxication reactions (Rendic and Guengerich 2021). Of the 4039 reactions, 235 (6%) involve the formation of potentially toxic intermediates or metabolites. Clear domination of P450 3A4 in the catalysis of toxications compared with other P450s was also shown in this case, catalyzing 25% of the reactions (Rendic and Guengerich 2021).
The present report updates the data on the metabolism of drugs to potentially toxic/reactive products (toxication reactions) by human drug enzymes, including additional enzymes along with the well-studied P450 enzymes. The data on 40 enzymes (20 P450 and 20 other enzymes) that catalyze oxidative, reductive, hydrolysis, and/or conjugation reactions of drugs resulting in the formation of potentially toxic metabolites or intermediates are included in the analysis (Table 1). The results of the analysis are summarized in a total of 327 records organized in alphabetical order for an easier approach and are tabularly presented (Table 2 (drugs A-D), Table 3 (drugs E-J), and Table 4 (drugs J-Z). In the analysis included are also reactions and products that are not toxic but as substrates but in additional reactions products or intermediates formed exert toxic effects (e.g. products of hydroxylation reactions catalyzed by P450 enzymes and products of hydrolysis reactions).
Explanations of abbreviations of the enzyme names are presented in Table 1. Data presented in Table 2, 3, and 4 are used for calculations presented in Figs. 1 and 2. Minor enzymes were designated as those that participate with less than 10% of the data presented in Table 2, 3, and 4.
Results and discussion
The present report presents data on the activation of drugs to potentially toxic or reactive products catalyzed by human drug-metabolizing enzymes (Table 1). The data, including reactions resulting in the formation of reactive/toxic products formed on the activation of drugs and metabolites, are presented in Table 2, 3, and 4. The reactions presented are catalyzed by 40 human enzymes and include oxidative reactions (e.g., C-, N-, and S-oxidation, epoxide formation, dechloroethylation, O- and N-demethylation, and desulfuration). The data presented were obtained from in vitro experiments and showed that the oxidative reactions (e.g., C-, N-, and S-oxidation, epoxide formation, dechloroethylation, O- and N-demethylation, and desulfuration) predominate (with 96% of the total potential toxication reactions). Reductive reactions (e.g., reduction of nitro to amino group and reductive dehalogenation) participate to the extent of 4%. (Fig. 1).
The calculated participation of human metabolizing enzymes (Fig. 1, Table 1) in the toxication of drugs and their products shows the dominant role of P450 enzymes (72%), followed by MPO (7%), FMO (3%), AOX, (4%), SULT (5%), and a group of other enzymes (AADAC, ADH, CAT, CES, COX, CPR and POR, LPO, HB + H2O2, NR, NAT, MAO, XOR), which participate to the extent of 9%. In our previous analysis, the participation of human P450 enzymes in the general oxidative and reductive metabolism of drugs was calculated to be ~ 96% (Rendic and Guengerich 2015). The results of the present analysis, which takes into account only toxication reactions and includes additional enzymes (e.g., MPO, AOX, FMOs, and SULT), show a lower participation of P450 enzymes in the bioactivation of drugs to toxic products compared to the general metabolism of drugs.
The previous analysis of the bioactivation of drugs to toxic products within the Family 1–4 P450 enzymes showed that the P450 3A4 enzyme dominates by catalyzing ~ 25% of the reactions (Rendic and Guengerich 2021). Domination of the P450 Subfamily 3A and 2C enzymes was also shown in the present analysis, as the members of P450 Subfamily 3A (P450 3A4 and P450 3A5) participated in the bioactivation of drugs and products to an extent of 27% and the members of Subfamily 2C (P450 2C9, P450 2C19) to an extent of 16%. Other enzymes participated in the bioactivation reactions to the following extents P450 1A1 5%, P450 2D6 8%, P450 2E1 10%, and P450 2B6 6%. All other P450 enzymes (minor enzymes) (P450 1B1, 2C8, 2C18, 2C9.1, 2C9.2, 2C9.3, 2J2, 2A6, 2J2, and 4A11) participated to the extent of 14% (Fig. 2). For comparison, previous analyses of the participation of the human P450 families of enzymes in the general metabolism of drugs also showed the dominance of the P450 Subfamily 3A with 33%, that of the P450 Subfamily 2C with 24%, and that of the P450 2D6 and 1A2 enzymes at 13% and 14%, respectively (Rendic and Guengerich 2015). Thus, the results of the present analysis correspond to the data presented previously, but with a slightly lower participation of the P450 enzymes in the bioactivation of drugs to toxic products compared to the results of the participation of the enzymes in the general metabolism of the drugs. In both cases, however, the dominance of the Subfamily P450 3A and Subfamily 2C enzyme was shown.
The data show that some of the drugs were removed from the market because of toxic effects (e.g., amphetamine, danthron, ellipticine, enflurane, nomifensine, thalidomide, thioridazine, troglitazone, zomepirac, phenacetin, flunitrazepam, tolcapone). Some of these discontinued drugs returned to the market for applications under controlled conditions and for specific illnesses (e.g., thalidomide, amphetamine, ellipticine). The drugs are substrates in either oxidative or reductive metabolic reactions catalyzed by one or several enzymes, in which the formation of various reactive products is achieved by toxication reactions (Table 2, 3, and 4). Some reactive species formed in the course of drug metabolism may be deactivated in reactions with reduced glutathione (GSH).
Depending on the structural properties and enzymes involved, the drugs participate predominantly as substrates in oxidative reactions involving C, N, and S atoms. Reductive reactions are minor in the toxication of the drugs, e.g. reduction of nitro to amino groups (e.g., chloramphenicol, clonazepam, flunitrazepam, and flutamide). For example, toxication by reduction or oxidation of chloramphenicol and its metabolites is shown in Fig. 3. The reactions involve the reduction of the nitro to nitroso and N-hydroxy groups, oxidations with the formation of nitroxide radicals and reactive oxygen species, and consequent DNA damage. Additional reactions are the formation of a reactive acyl chloride from chloramphenicol as a P450 oxidation product, shown in rat liver microsomes. This reaction leads to the formation of additional reactive metabolite(s) that inhibit reaction(s) catalyzed by P450 enzymes, as reported in rats. This reaction might result in drug-drug interaction when chloramphenicol is coadministered with other drugs that are metabolized by the P450 enzymes, e.g. voriconazole (Fig. 3, Table 2 and references therein).
Drug bioactivation reactions usually comprise a set of reactions in which products may be toxic to cells. The structures representing some proposed or suggested activated products that might be formed during drug metabolism are shown in Fig. 4A, B, with examples of enzymes and drug substrates.
When a drug, in the course of its metabolism, forms reactive species, there might be a risk of toxic reactions. The reactions may be identified either in vitro or in vivo in the course of drug development (Fig. 4a, b). For instance, both reductive and oxidative dehalogenation reactions of halothane have led to the formation of reactive species (the formation of radicals) and toxic reactions (e.g., liver necrosis and cytotoxicity). The toxic reactions led to the withdrawal of the drug from use as an inhalation anesthetic drug in some countries (Rendic and Guengerich 2021). For enflurane, tolcapone, ellipticine derivatives, and troglitazone, reactive species are formed by oxidative reactions, including N-oxidation, C-hydroxylation, and dehydrogenation (Table 2, 3, and 4, and the references therein). The products of drugs formed by the reactions catalyzed by P450 enzymes might be activated either by additional oxidation or as conjugation. An example is the antiestrogen drug tamoxifen and its metabolite 3-hydroxytamoxifen (Droloxifen®), which is also utilized as a drug. 3-Hydroxytamoxifen is reported to be bioactivated by O-sulfation to form the therapeutically active form of the drug. However, catechols formed by C-3 and C4-hydroxylation, after oxidation to o-quinones, may covalently bind to cellular macromolecules. Hydroxylation at C-3 and consequent sulfate conjugation are considered detoxication reactions that result in the formation of therapeutically active drugs. However, α-hydroxylation and consequent sulfate conjugation are considered toxication reactions (Kim et al. 2005). Thus, bioactivation of hydroxylated products to toxic metabolites results from their oxidations to quinone(s), semiquinone(s), and epoxides at olefins and phenyl rings. Examples of the reactive species formed and the reactions of activation of tamoxifen into reactive (toxic) metabolites are presented in Figs. 4A, B, 5, and Table 4 and references therein.
The structurally related antiestrogenic drug toremifene can be metabolized as a substrate of P450 enzymes with the formation of an N-desmethyl product and an epoxide or by the formation of 4-hydroxy and toremifene α-hydroxy metabolites. Hydroxy metabolites of tormifene exert low toxic properties due to limited O-sulfation by hydroxysteroid sulfotransferases, resulting in limited formation of DNA adducts (Fig. 6, Table 4, and references therein).
Nimesulide is a nonsteroidal anti-inflammatory drug associated with hepatotoxicity. It was suggested that hepatotoxicity of nimesulide results from the oxidation of the product 4-amino-2-phenoxy-methanesulfonamide to a toxic diiminoquinone, catalyzed by P450 and MPO enzymes (Fig. 4A, Table 4, and references therein).
It has been reported that MPO, in the presence of H2O2 or HOCl, activates various drugs or their products. Examples include carbamazepine, diclofenac, indomethacin, fluperlapine, phenytoin, and thiabendazole metabolites. The products of MPO oxidation are either stable or reactive metabolites. The reactive products include free radicals, reactive oxygen species, quinones, and iminoquinone species (Tables 2, 3, and 4 and references therein, and Fig. 4a, b).
Carbamazepine is metabolized to toxic species by multiple reactions catalyzed by P450s and the enzyme MPO. P450-catalyzed reactions include the formation of a C10,11 epoxide (P450 3A4 as the major enzyme), hydroxylation at C2 and C3 (as major metabolites), and the formation of an iminoquinone and o-quinone. The formation of a toxic C10,11 epoxide is catalyzed by multiple P450 enzymes but is a minor reaction in the overall metabolism of carbamazepine. MPO, in the presence of H2O2, catalyzes the oxidation of the C2- and C3-hydroxy products of carbamazepine with the formation of free radicals and reactive oxygen species (Table 2 and references therein, and Figs. 4A, B and 7).
A major reaction of diclofenac metabolism is the C-4´ hydroxylation catalyzed by P450s, with P450 2C9 as the major enzyme. Other hydroxylated metabolites (C5-hydroxy, C4´,5-dihydroxy, and C3-hydroxy) account for < 10% of the products. Toxication of diclofenac, resulting in the formation of protein adducts, is linked to its oxidative metabolism and hydroxylated metabolites formed by P450 enzymes. The proposed toxic products are 1´,4´, and 2,5-quinoneimines, o-imine methide, and an arene oxide. MPO, in the presence of H2O2 or neutrophils, oxidizes diclofenac and 5-hydroxy diclofenac to a toxic quinoneimine (Table 2 and references therein, Figs. 4a and 8).
In the case of thalidomide, which was withdrawn from the market because of its teratogenicity, proposed toxic metabolites are formed by P450-catalyzed reactions (e.g., hydroxylations, epoxidation, oxidation to quinones, and formation of reactive oxygen species). The reactions are catalyzed by P450s (with P450 3A4 and 2C19 as the major enzymes), and NR enzymes (Figs. 4a, b, 9, Table 4 and references therein). The toxic species thus formed (epoxides and quinones) might be detoxicated by reaction with reduced GSH or may exert toxicity by reacting with cell macromolecules (e.g., proteins and DNA) (Chowdhury et al. 2010; Wani et al. 2017) (Fig. 9 and Table 4 and references therein).
< insert Fig. 9 here >
Referring to the data presented in Tables 2, 3, and 4, and Fig. 4A, B it has to be considered that when characterizing the candidate drug as potentially toxic or non-toxic there is a close relationship between the structure of a drug and the activity of enzymes present in the cells of the targeted tissue. The results presented in this report have been obtained in vitro, but the side/toxic effects have been clinically noted resulting in some cases the removal of the drug from the market (e.g. thalidomide and other drugs, Tables 2, 3, and 4). The final effect of the drug in vivo might be, influenced by polymorphism and/or inhibition/induction of the activity of the major enzyme whose activity is related to the toxic effect(s), which may significantly affect the formation of toxic products. For instance, a reaction that is characterized by a high Km value, or an enzyme characterized as a “minor enzyme„ may become dominant if the reaction/enzyme characterized by low Km values or when the “major “ enzyme is inhibited by drug overdose or other concomitantly administered drug/chemical/food, or if the active form of the enzyme is not present in the cell due to genetic polymorphism. Considering the structural characteristics of the candidate drug with the possibility to form potentially toxic metabolites/intermediates as obtained in vitro might lower the incidence of consequent more or less serious side effects to the patients in vivo and even affect the rate of drug application-related deaths (European Drug Report 2023).
Concluding remarks
In the present report, the in vitro data on the toxication of drugs were updated, and additional enzymes (e.g., SULT, AOX, MPO, and some minor participating enzymes) were included in the analysis. The present data show the continued predominant participation of P450 enzymes in the both metabolism and toxication of drugs, probably due to extensive research on this superfamily of enzymes during the last 30 years. P450 enzymes catalyze 78% of toxication reactions. The dominant enzymes catalyzing toxication reactions of drugs belong to the P450 Subfamily 3A, catalyzing 28% of the reactions. Significant participation of the P450 Subfamilies 2C and 1A and P450 2D6 enzyme was also noted at this time in the toxication of drugs and metabolites. The results of the present analysis confirm the previous finding that human P450 enzymes still predominate in the metabolic toxication of the drugs catalyzing the formation of reactive/toxic products, but more additional enzymes have been identified to catalyze the toxication of drugs. Research on the toxication of drugs should include other enzymes catalyzing oxidative/reductive reactions and performing candidate drug toxicity/risk management to prevent serious side effects, withdrawal of the candidate drug from the market, and even drug-related deaths.
Availability of data and materials
All data are available in the text and tables of the review.
References
Abbasi A, Joswig-Jones CA, Jones JP (2020) Site-directed mutagenesis at the MoCo site of the human aldehyde oxidase: interrogating the kinetic differences between human and cynomolgus monkey. Drug Metab Dispos 48:1364–1371. https://doi.org/10.1124/dmd.120.000187
Amano T, Fukami T, Ogiso T, Hirose D, Jones JP, Taniguchi T, Nakajima M (2018) Identification of enzymes responsible for dantrolene metabolism in the human liver: A clue to uncover the cause of liver injury. Biochem Pharmacol 151:69–78. https://doi.org/10.1016/j.bcp.2018.03.002
Apak TI, Duffel MW (2004) Interactions of the stereoisomers of a-hydroxytamoxifen with human hydroxysteroid sulfotransferase SULT2A1 and rat hydroxysteroid sulfotransferase STa. Drug Metab Dispos 32:1501–1508. https://doi.org/10.1124/dmd.104.000919
Avent KM, DeVoss JJ, Gillam EMJ (2006) Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites. Chem Res Toxicol 19:914–920. https://doi.org/10.1021/tx0600090
Baer BR, Wienkers LC, Rock DA (2007) Time-dependent inactivation of P450 3A4e by raloxifene; identification of Cys239 as the site of apoprotein alkylation. Chem Res Toxycol 20:954–964. https://doi.org/10.1021/tx7000337e
Bai J, Cederbaum AI (2004) Adenovirus-mediated overexpression of CYP2E1 increases sensitivity of HepG2 cells to acetaminophen induced cytotoxicity. Mol Cell Biochem 262:165–176. https://doi.org/10.1023/B:MCBI.0000038232.61760.9e
Berson A, Wolf C, Chachaty C, Fisch C, Fau D Eugene D, Loeper J, Gauthier JC, Beaune P, Pompon D (1993) Metabolic activation of the nitroaromatic antiandrogen flutamide by rat and human cytochromes P-450, including forms belonging to the 3A and 1A subfamilies. J Pharmacol Exp Ther 265:366–372. https://jpet.aspetjournals.org/content/265/1/366.long. Accessed Dec 2023
Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD (2007) CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharmaceut Sci 96:2224–2231. https://doi.org/10.1002/jps.20892
Bezerra LS, Santos-Veloso MAO, Bezerra Junior NDS, Fonseca LCD, Sales WLA (2018) Impacts of cytochrome P450 2D6 (CYP2D6) genetic polymorphism in tamoxifen therapy for breast cancer. Rev Bras Ginecol Obstet 40:794–799. https://doi.org/10.1055/s-0038-1676303
Boerma JS, Vermeulen NPE, Commandeur JNM (2014) One-electron oxidation of diclofenac by human cytochrome P450s as a potential bioactivation mechanism for the formation of 2′-(glutathion-S-yl)-deschloro-diclofenac. Chem-Biol Interact 207:32–40. https://doi.org/10.1016/j.cbi.2013.11.001
Bohnenstengel F, Hofmann U, Eichelbaum M, Kroemer HK (1996) Characterization of the cytochrome P450 involved in side-chain oxidation of cyclophosphamide in humans. Eur J Clin Pharmacol 51:297–301. https://doi.org/10.1007/s002280050201
Boocock DJ, Brown K, Gibbs AH, Sanchez E, Turteltaub KW, White IN (2002) Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis 23:1897–1901. https://doi.org/10.1093/carcin/23.11.1897
Borges S, Desta Z, Li L et al (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Therapeut 80:61–74. https://doi.org/10.1016/j.clpt.2006.03.013
Bort R, Macé K, Boobis A, Gómez-Lecón MJ, Pfeifer A, Castell J (1999) Hepatic metabolism of diclofenac: role of human CYP in the minor oxidative pathways. Biochem Pharmacol 58:787–796. https://doi.org/10.1016/S0006-2952(99)00167-7
Cardillo C. Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA (1997). Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension (Dallas, Tex. ), 30(1 Pt 1), 57–63. https://doi.org/10.1161/01.hyp.30.1.57
Cashman JR, Xiong YN, Xu L, Janowsky A (1999) N-Oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): Role in bioactivation and detoxication. J Pharmacol Exp Ther 288:1251–1260. https://jpet.aspetjournals.org/content/288/3/1251.long
Chang TK, Weber GF, Crespi CL, Waxman DJ (1993) Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53:5629–5637. https://aacrjournals.org/cancerres/article/53/23/5629/499502/
Chang TK, Yu L, Maurel P, Waxman DJ (1997) Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res 57:1946–1954. PMID: 9157990. https://aacrjournals.org/cancerres/article/57/10/1946/503144/
Charneira C, Godinho ALA, Oliveira MC, Pereira SA, Monteiro EC, Marques MM, Artunes AMM (2011) Reactive aldehyde metabolites from the anti-HIV drug abacavir: Amino acid adducts as possible factors in abacavir toxicity. Chem Res Toxicol 24:2129–2141. https://doi.org/10.1021/tx200337b
Chen J (1998) Enhanced levels of several mitochondrial mRNA transcripts and mitochondrial superoxide production during ethinyl estradiol-induced hepatocarcinogenesis and after estrogen treatment of HepG2 cells. Carcinogenesis 19:2187–2193. https://doi.org/10.1093/carcin/19.12.2187
Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, Trager WF, Nelson SD (1998) Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol 11:295–301. https://doi.org/10.1021/tx9701687
Chen G, Yin S, Maiti S, Shao X (2002a) 4-Hydroxytamoxifen sulfation metabolism. J Biochem Mol Toxicol 16:279–285. https://doi.org/10.1002/jbt.10048
Chen Q, Ngui JS, Doss GA, Wang RW, Cai X, DiNinno FP, Lombardord TA, Hammond ML, Stearns RA, Evans DC, Baillie TA, Tang W (2002b) Cytochrome P450 3A4-mediated bioactivation of raloxifene: Irreversible enzyme inhibition and thiol adduct formation. Chem Res Toxicol 15:907–914. https://doi.org/10.1021/tx0200109
Chen C-S, Jounaidi Y, Waxman DJ (2005) Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expressed cytochromes P450. Drug Metab Dispos 33:1261–1267. https://doi.org/10.1124/dmd.105.004788
Chen Q, Doss GA, Tung EC, Braun MP, Didolkar V, Strauss JR, Wang RM, Stearns RA, Evans DC, Baillie TA, Tang W (2006a) Evidence for the bioactivation of zomepirac and tolmetin by an oxidative pathway: identification of glutathione adducts in vitro in human liver microsomes and in vivo in rats. Drug Metab Dispos 34:145–151. https://doi.org/10.1124/dmd.105.004341
Chen C, Meng L, Ma X, Krausz KW, Pommier Y, Idle JR, Gonzalez FJ (2006b) Urinary metabolite profiling reveals CYP1A2-mediated metabolism of NSC686288 (aminoflavone). Journal Pharmacol Exp Ther 318:1330–1342. https://doi.org/10.1124/jpet.106.105213
Cheung C, Yu A-M, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, Gonzalez FJ (2005) The CYP2E1-humanized transgenic mouse: role of CYP2E1 in acetaminophen hepatotoxicity. Drug Metab Dispos 33:449–457. https://doi.org/10.1124/dmd.104.002402
Cho H-J, Koh W-J, Ryu Y-J, Ki C-S, Nam M-H, Kim J-W, Lee S-Y (2007) Genetic polymorphisms of NAT2 and CYP2E1 associated with antituberculosis drug-induced hepatotoxicity in Korean patients with pulmonary tuberculosis. Tuberculosis 87:551–556. https://doi.org/10.1016/j.tube.2007.05.012
Choughule KV, Joswig-Jones CA, Jones JP (2015) Interspecies differences in the metabolism of methotrexate: an insight into the active site differences between human and rabbit aldehyde oxidase. Biochem Pharmacol 96:288–295. https://doi.org/10.1016/j.bcp.2015.05.010
Chowdhury G, Murayama N, Okada Y, Uno Y, Shimizu M, Shibata N, Guengerich FP, Yamazaki H (2010) Human liver microsomal cytochrome P450 3A enzymes involved in thalidomide 5-hydroxylation and formation of a glutathione conjugate. Chem Res Toxicol 23:1018–1024. https://doi.org/10.1021/tx900367p
Chowdhury G, Shibata N, Yamazaki H, Guengerich FP (2014) Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analogue of thalidomide. Chem Res Toxicol 27:147–156. https://doi.org/10.1021/tx4004215
Chugh R, Wagner T, Griffith KA, Taylor JM, Thomas DG, Worden FP, Leu KM, Zalupski MM, Baker LH (2007) Assessment of ifosfamide pharmacokinetics, toxicity, and relation to CYP3A4 activity as measured by the erythromycin breath test in patients with sarcoma. Cancer 109:2315–2322. https://doi.org/10.1002/cncr.22669
Clarke TA, Waskell LA (2003) The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos 31:53–59. https://doi.org/10.1124/dmd.31.1.53
Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang, Waxman DJ (1997) Human cytochrome P4502B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug Metab Dispos 25:985–993. https://dmd.aspetjournals.org/content/25/8/985.long
Coller JK, Krebsfaenger N, Klein K, Wolbold R, Nüssler A, Neuhaus P, Zanger UM, Eichelbaum M, Mürdter TE (2004) Large interindividual variability in the in vitro formation of tamoxifen metabolites related to the development of genotoxicity. Brit J Clin Pharmacol 57:105–111. https://doi.org/10.1046/j.1365-2125.2003.01970.x
Coulet M, Dacasto M, Eeckhoutte C, Larrieu G, Sfukaa J-F, Alvinerie M, Macé K, Pfeifer GP (1998) Identification of human and rabbit cytochromes P450 1A2 as major isoforms involved in thiabendazole 5-hydroxylation. Fund Clinical Pharmacol 12:225–235. https://doi.org/10.1111/j.1472-8206.1998.tb00946.x
Coulet M, Eeckhoutte C, Larrieu G, Sutra J-F, Alvinerie M, Macé K, Pfeifer A, Zucco F, Stammati AL, De Angelis I, Vignoli AL, Galtier P (2000) Evidence for cytochrome P4501A2-mediated protein covalent binding of thiabendazole and its passive intestinal transport: use of human and rabbit derived cells. Chem-Biol Interact 127:109–124. https://doi.org/10.1016/S0009-2797(00)00167-8
Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EMJ (2002) Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4′-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 30:869–874. https://doi.org/10.1124/dmd.30.8.869
Cribb AE, Spielberg SP, Griffin GP (1995) N4-Hydroxylation of sulfamethoxazole by cytochrome P450 of the cytochrome P4502C subfamily and reduction of sulfamethoxazole hydroxylamine in human and rat hepatic microsomes. Drug Metab Dispos 23:406–414. https://dmd.aspetjournals.org/content/23/3/406.long
Cuttle L, Munns AJ, Hogg NA, Scott JR, Hooper WD, Dickinson RG, Gillam EM (2000) Phenytoin metabolism by human cytochrome P450: involvement of P450 3A and 2C forms in secondary metabolism and drug-protein adduct formation. Drug Metab Dispos 28:945–950. https://dmd.aspetjournals.org/content/28/8/945.long
Damsten MC, De Vlieger JSB, Niessen WMA, Irth H, Vermeulen NPE, Commandeur JNM (2008) Trimethoprim: novel reactive intermediates and bioactivation pathways by cytochrome P450s. Chem Res Toxicol 21:2181–2187. https://doi.org/10.1021/tx8002593
Dehal SS, Kupfer D (1996) Evidence that the catechol 3,4-dihydroxytamoxifen is a proximate intermediate to the reactive species binding covalently to proteins. Cancer Res 56:1283–1290. https://aacrjournals.org/cancerres/article/56/6/1283/502932/Evidence-That-the-Catechol-3-4-Dihydroxytamoxifen
Dehal SS, Kupfer D (1997) CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res 57:3402–3406. https://aacrjournals.org/cancerres/article/57/16/3402/503545/CYP2D6-Catalyzes-Tamoxifen-4-Hydroxylation-in
Dehal SS, Kupfer D (1999) Cytochrome P-450 3A and 2D6 catalyze ortho hydroxylation of 4-hydroxytamoxifen and 3-hydroxytamoxifen (droloxifene) yielding tamoxifen catechol: involvement of catechols in covalent binding to hepatic proteins. Drug Metab Dispos 27:681–688. PMID: 10348797https://dmd.aspetjournals.org/content/27/6/681.long
Dekker SJ, Dohmen F, Vermeulen NPE, Commandeur JNM (2019) Characterization of kinetics of human cytochrome P450s involved in bioactivation of flucloxacillin: inhibition of CYP3A-catalysed hydroxylation by sulfaphenazole: bioactivation of flucloxacillin by human CYPs. Brit J Pharmacol 176:466–477. https://doi.org/10.1111/bph.14548
Den Braver MW, Den Braver-Sewradj SP, Vermeulen NPE, Commandeur JNM (2016) Characterization of cytochrome P450 isoforms involved in sequential two-step bioactivation of diclofenac to reactive p-benzoquinone imines. Toxicol Lett 253:46–54. https://doi.org/10.1016/j.toxlet.2016.04.022
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. Pharmacol Exp Ther 310:1062–1075. https://doi.org/10.1124/jpet.104.065607
Diamond S, Boer J, Maduskuie TP, Falahatpisheh N, Li Y, Yelesswaram S (2010) Species-specific metabolism of SGX523 by aldehyde oxidase and the toxicological implications. Drug Metab Dispos 38:1277–1285. https://doi.org/10.1124/dmd.110.032375
Dong H, Haining RL, Thummel KE, Rettie AE, Nelson SD (2000) Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab Dispos 28:1397–1400. https://dmd.aspetjournals.org/content/28/12/1397.long
Dragovic S, Boerma JS, Vermeulen NPE, Commandeur JNM (2013) Effect of human glutathione S-transferases on glutathione-dependent inactivation of cytochrome P450-dependent reactive intermediates of diclofenac. Chem Res Toxicol 26:1632–1641. https://doi.org/10.1021/tx400204d
Driscoll JP, Kornecki K, Wolkowski JP, Chupak L, Kalgutkar AS, O’Donnell JP (2007) Bioactivation of phencyclidine in rat and human liver microsomes and recombinant P450 2B enzymes: Evidence for the formation of a novel quinone methide intermediate. Chem Res Toxicol 20:1488–1497. https://doi.org/10.1021/tx700145k
Ekhart C, Doodeman VD, Rodenhuis S, Smits PHM, Beijnen JH, Huitema ADR (2009) Polymorphisms of drug-metabolizing enzymes (GST, CYP2B6 and CYP3A) affect the pharmacokinetics of thiotepa and tepa. Brit J Clin Pharmacol 67:50–60. https://doi.org/10.1111/j.1365-2125.2008.03321.x
European Drug Report 2023. https://www.emcdda.europa.eu/data/source-data/edr/2023/drug-induced-deaths_en#edr-2023-drd-table1
Falany JL, Pilloff DE, Leyh TS, Falany CN (2006) Sulfation of raloxifene and 4-hydroxytamoxifen by human cytosolic sulfotransferases. Drug metabolism and disposition: the biological fate of chemicals. Drug Metab Dispos 34:361–368. https://doi.org/10.1124/dmd.105.006551
Fan PW, Zhang F, Bolton JL (2000) 4-Hydroxylated metabolites of the antiestrogens tamoxifen and toremifene are metabolized to unusually stable quinone methides. Chem Res Toxicol 13:45–52. https://doi.org/10.1021/tx990144v
Fang J, Baker GB, Silverstone PH, Coutts RT (1997) Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol 17:227–233. https://doi.org/10.1023/A:1026317929335
Fang J, McKay G, Song J, Remillrd A, Li X, Midha K (2001) In vitro characterization of the metabolism of haloperidol using recombinant cytochrome P450 enzymes and human liver microsomes. Drug Metab Dispos 29:1638–1643. https://dmd.aspetjournals.org/content/29/12/1638.long
Fang JL, Loukotková L, Chitranshi P, Gamboa da Costa G, Beland FA (2018) Effects of human sulfotransferases on the cytotoxicity of 12-hydroxynevirapine. Biochem Pharmacol 155:455–467. https://doi.org/10.1016/j.bcp.2018.07.016
Fleming CM, Branch RA, Wilkinson GR, Guengerich FP (1992) Human liver microsomal N-hydroxylation of dapsone by cytochrome P-450 3A4. Mol Pharmacol 41:975–980. PMID: 1588928. https://molpharm.aspetjournals.org/content/41/5/975.long
Francois AA, Nishida CR, Ortiz de Montellano PR, Phillips IR, Shepard EA (2009) Human flavin-containing monooxygenase 2.1 catalyzes oxygenation of the antitubercular drugs thiacetazone and ethionamide. Drug Metab Dispos 37:178–186. https://doi.org/10.1124/dmd.108.024158
Frei E, Bieler CA, Arlt VM, Wiessler M, Stiborová M (2002) Covalent binding of the anticancer drug ellipticine to DNA in V79 cells transfected with human cytochrome P450 enzymes. Biochem Pharmacol 64:289–295. https://doi.org/10.1016/S0006-2952(02)01072-9
Fukami T, Iida A, Konishi K, Nakajima M (2016) Human arylacetamide deacetylase hydrolyzes ketoconazole to trigger hepatocellular toxicity. Biochem Pharmacol 116:153–161. https://doi.org/10.1016/j.bcp.2016.07.007
Ganesan S, Sahu R, Walker LA, Tekwani BL (2010) Cytochrome P450-dependent toxicity of dapsone in human erythrocytes. J Appl Toxicol 30:271–275. https://doi.org/10.1002/jat.1493
Gardner I, Leeder JS, Chin T, Zahid N, Uetrecht JP (1998) A comparison of the covalent binding of clozapine and olanzapine to human neutrophils in vitro and in vivo. Mol Pharmacol 53:999–1008. https://molpharm.aspetjournals.org/content/53/6/999.long
Gardner I, Popović M, Zahid N, Uetrecht JP (2005) A Comparison of the covalent binding of clozapine, procainamide, and vesnarinone to human neutrophils in vitro and rat tissues in vitro and in vivo. Chem Res Toxicol 18:1384–1394. https://doi.org/10.1021/tx050095o
Garton KJ, Yuen P, Meinwald J, Thummel KE, Kharasch ED (1995) Stereoselective metabolism of enflurane by human liver cytochrome P450 2E1. Drug Metab Dispos 23:1426–1430. PMID: 8689955. https://dmd.aspetjournals.org/content/23/12/1426.long
Gill H, Tingle M, Park B (1995) N-Hydroxylation of dapsone by multiple enzymes of cytochrome P450: Implications for inhibition of haemotoxicity. Brit J Clin Pharmacol 40:531–538. https://doi.org/10.1111/j.1365-2125.1995.tb05797.x
Glatt H (1997) Bioactivation of mutagens via sulfation. FASEB J 11:314–321. https://doi.org/10.1096/fasebj.11.5.9141497
Glatt H, Bartsch I, Christoph S, Coughtrie MWH, Falany CN, Hagen M, Landsiedel R, Pabel U, Phillips DH, Seidel A, Yamazoe Y (1998) Sulfotransferase-mediated activation of mutagens studied using heterologous expression systems. Chem-Biol Interact 109:195–219. https://doi.org/10.1016/s0009-2797(97)00133-6
Glatt H, Boeing H, Engelke CEH, Ma L, Kuhlow A, Pabel U, Pomplun T, Meinl W (2001) Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutat Res/fund Mol Mech Mutagen 482:27–40. https://doi.org/10.1016/S0027-5107(01)00207-X
Goda R, Nagai D, Akiyama Y, Nishikawa K, Ikemoto I, Aizawa Y, Nagata K, Yamazoe Y (2006) Detection of a new N-oxidized metabolite of flutamide, N-[4-nitro-3-(trifluoromethyl)phenyl]hydroxylamine, in human liver microsomes and urine of prostate cancer patients. Drug Metab Dispos 34:828–835. https://doi.org/10.1124/dmd.105.008623
Granvil CP, Madan A, Sharkawi M, Parkinson A, Wainer IW (1999) Role of CYP2B6 and CYP3A4 in the in vitro N-dechloroethylation of (R)- and (S)-ifosfamide in human liver microsomes. Drug Metab Dispos 27:533–541. https://dmd.aspetjournals.org/content/27/4/533.long
Grilo NM, Antunes AMM, Caixas U, Marinho AT, Charneira C, Oliveira MC, Monteriro EC, Marques MM, Perira SA (2013) Monitoring abacavir bioactivation in humans: Screening for an aldehyde metabolite. Toxicol Lett 219:59–64. https://doi.org/10.1016/j.toxlet.2013.02.021
Grilo NM, Charneira C, Pereira SA, Monteriro EC, Marques MM, Antunnes AMM (2014) Bioactivation to an aldehyde metabolite—possible role in the onset of toxicity induced by the anti-HIV drug abacavir. Toxicol Lett 224:416–423. https://doi.org/10.1016/j.toxlet.2013.10.036
Guengerich FP (1988) Oxidation of of 17α-ethinylestradiol by human liver cytochrome P-450. Mol Pharmacol 33: 500–508. https://molpharm.aspetjournals.org/content/33/5/500.long
Guengerich FP (1990a) Metabolism of 17α-ethinylestradiol in humans. Life Sci 47:1981–1988. https://doi.org/10.1016/0024-3205(90)90431-p
Guengerich FP (1990b) Inhibition of oral contraceptive steroid-metabolizing enzymes by steroids and drugs. Am J Obstet Gynecol 163:2159–2163. https://doi.org/10.1016/0002-9378(90)90557-n
Guengerich FP (1999) Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17. https://doi.org/10.1146/annurev.pharmtox.39.1.1
Guo X, Jia Y, Han L, Zhao Y, Li W, Zhang Z, Peng Y, Zheng J (2019a) Metabolic activation of tofacitinib mediated by myeloperoxidase in vitro. Chem Res Toxicol 32:2459–2465. https://doi.org/10.1021/acs.chemrestox.9b00280
Guo X, Li W, Li Q, Chen Y, Zhao G, Peng Y, Zheng J (2019b) Tofacitinib is a mechanism-based inactivator of cytochrome P450 3A4. Chem Res Toxicol 32:1791–1800. https://doi.org/10.1021/acs.chemrestox.9b00141
Hafner V, Albermann N, Haefeli WE, Ebinger F (2008) Inhibition of voriconazole metabolism by chloramphenicol in an adolescent with central nervous system aspergillosis. Antimicrob Agents Chemother 52:4172–4174. https://doi.org/10.1128/AAC.00805-08
Hagen N, Olsen AK, Andersen JV, Tjørnelund J, Hansen SH (2002) Characterization of mixtures of recombinant human cytochrome p450s as a screening model for metabolic stability in drug discovery. Xenobiotica 32:749–759. https://doi.org/10.1080/00498250210147124
Halpert J, Balfour C, Miller NE, Morgan ET, Dunbar D, Kaminsky LS (1985a) Isozyme selectivity of the inhibition of rat liver cytochromes P-450 by chloramphenicol in vivo. Mol Pharmacol 28:290–296. https://molpharm.aspetjournals.org/content/28/3/290.long
Halpert JR, Miller NE, Gorsky LD (1985b) On the mechanism of the inactivation of the major phenobarbital-inducible isozyme of rat liver cytochrome P-450 by chloramphenicol. J Biol Chem 260:8397–403. https://www.sciencedirect.com/science/article/pii/S0021925817394875?via%3Dihub
Hansten PD (2018) The underrated risks of tamoxifen drug interactions. Eur J Drug Metab Pharmacokinet 43:495–508. https://doi.org/10.1007/s13318-018-0475-9
Harleton E, Webster M, Bumpus NN, Kent UM, Rae JM, Hollenberg PF (2004) Metabolism of N,N ´, N ″-triethylenethiophosphoramide by CYP2B1 and CYP2B6 results in the inactivation of both isoforms by two distinct mechanisms. J Pharmacol Exp Ther 310:1011–1019. https://doi.org/10.1124/jpet.104.069112
Hashizume T, Yoshitomi S, Asahi S, Matsumura S, Chatani F, Oda H (2009) In vitro micronucleus test in HepG2 transformants expressing a series of human cytochrome P450 isoforms with chemicals requiring metabolic activation. Mut Res 677:1–7. https://doi.org/10.1016/j.mrgentox.2009.03.009
Hashizume T, Yoshitomi S, Asahi S, Uematsu R, Matsumura S, Chatani F, Oda H (2010) Advantages of human hepatocyte-derived transformants expressing a series of human cytochrome P450 isoforms for genotoxicity examination. Toxicol Sci 116:488–497. https://doi.org/10.1093/tox.sci/kfq154
Hazai E, Vereczkey L, Monostory K (2002) Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Commun 291:1089–1094. https://doi.org/10.1006/bbrc.2002.6541
He K, Woolf TF, Kindt EK, Fielfer Talaat RE (2001) Troglitazone quinone formation catalyzed by human and rat CYP3A: An atypical CYP oxidation reaction. Biochem Pharmacol 62:191–198. https://doi.org/10.1016/S0006-2952(01)00653-0
He K, Talaat RE, Pool WF, Reily MD, Reed JE, Bridges AJ, Woolf TF (2004) Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. Drug Metab Dispos 32:639–646. https://doi.org/10.1124/dmd.32.6.639
Henderson MC, Siddens LK, Morré JT, Krueger SK, Williams DE (2008) Metabolism of the anti-tuberculosis drug ethionamide by mouse and human FMO1, FMO2 and FMO3 and mouse and human lung microsomes. Toxicol Appl Pharmacol 233:420–427. https://doi.org/10.1016/j.taap.2008.09.017
Higuchi R, Fukami T, Nakajima M, Yokoi T (2013) Prilocaine- and lidocaine-induced methemoglobinemia is caused by human carboxylesterase-, CYP2E1-, and CYP3A4-mediated metabolic activation. Drug Metab Dispos 41:1220–1230. https://doi.org/10.1124/dmd.113.051714
Ho PC, Abbott FS, Zanger UM, Chang TKH (2003) Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogenom J 3:335–342. https://doi.org/10.1038/sj.tpj.6500210
Hollenberg PF, Kent UM, Bumpus NN (2008) Mechanism-based inactivation of human cytochromes P450s: experimental characterization, reactive intermediates, and clinical implications. Chem Res Toxicol 21:189–205. https://doi.org/10.1021/tx7002504, https://molpharm.aspetjournals.org/content/33/5/500.long
Hu Y, Dehal SS, Hynd G, Jones GB, Kupfer D (2003) CYP2D6-mediated catalysis of tamoxifen aromatic hydroxylation with an NIH shift: Similar hydroxylation mechanism in chicken, rat and human liver microsomes. Xenobiotica 33:141–151. https://doi.org/10.1080/0049825021000042733
Huang Z, Roy P, Waxman DJ (2000) Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol 59:961–972. https://doi.org/10.1016/S0006-2952(99)00410-4
Infante JR, Rugg T, Gordon M, Rooney I, Rosen L, Zeh K, Liu R, Burris HA, Ramanathan RK (2013) Unexpected renal toxicity associated with SGX523, a small molecule inhibitor of MET. Invest New Drugs 31:363–369. https://doi.org/10.1007/s10637-012-9823-9
Jacobson PA, Green K, Birnbaum A, Remmel RP (2002) Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of hiotepa to TEPA. Cancer Chemother Pharmacol 49:461–467. https://doi.org/10.1007/s00280-002-0453-3
Jamieson JD, Smith EB, Dalvie DK, Stevens GJ, Yanochko GM (2011) Myeloperoxidase-mediated bioactivation of 5-hydroxythiabendazole: a possible mechanism of thiabendazole toxicity. Toxicol in Vitro 25:1061–1066. https://doi.org/10.1016/j.tiv.2011.04.007
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Desta WRV, Z, Flockhart DA, Skaar TC, (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159. https://doi.org/10.1023/B:BREA.0000025406.31193.e8
Jordan CG, Rashidi MR, Laljee H, Clarke SE, Brown JE, Beedham C (1999) Aldehyde oxidase-catalyzed oxidation of methotrexate in the liver of guinea pig, rabbit and man. J Pharm Pharmacol 51:411–418. https://doi.org/10.1211/0022357991772619
Ju C, Uetrecht JP (1998) Oxidation of a metabolite of indomethacin (desmethyldeschlorobenzoylindomethacin) to reactive intermediates by activated neutrophils, hypochlorous acid, and the myeloperoxidase system. Drug Metab Dispos 26:676–680. https://dmd.aspetjournals.org/content/26/7/676.long
Kalgutkar AS, Taylor TJ, Venkatakrishnan K, Isin EM (2003) Assessment of the contributions of CYP3A4 and CYP3A5 in the metabolism of the antipsychotic agent haloperidol to its potentially neurotoxic pyridinium metabolite and effect of antidepressants on the bioactivation pathway. Drug Metab Dispos 31:243–249. https://doi.org/10.1124/dmd.31.3.243
Kalgutkar AS, Henne KR, Lame ME, Vaz ADN, Collin C, Soglia JR, Zhao SX, Hop CECA (2005a) Metabolic activation of the nontricyclic antidepressant trazodone to electrophilic quinone-imine and epoxide intermediates in human liver microsomes and recombinant P4503A4. Chem-Biol Interact 155:10–20. https://doi.org/10.1016/j.cbi.2005.03.036
Kalgutkar AS, Vaz ADN, Lame ME, Henne KR, Soglia J, Zhao SX, Abramov YA, Lombardo F, Collin C, Hendsch ZS, Hop CECA (2005b) Bioactivation of the nontricyclic antidepressant nefazodone to a reactive quinone-imine species in human liver microsomes and recombinant cytochrome P450 3A4. Drug Metab Dispos 33:243–253. https://doi.org/10.1124/dmd.104.001735
Kang P, Dalvie D, Smith E, Zhou S, Deese A (2007) Identification of a novel glutathione conjugate of flutamide in incubations with human liver microsomes. Drug Metab Dispos 35:1081–1088. https://doi.org/10.1124/dmd.107.014860
Kang P, Dalvie D, Smith E, Zhou S, Deese A, Nieman JA (2008) Bioactivation of flutamide metabolites by human liver microsomes. Drug Metab Dispos 36:1425–1437. https://doi.org/10.1124/dmd.108.020370
Kassahun K, Pearson PG, Tang W, McIntosh I, Leung K, Elmore C, Dean D, Wang R, Doss BTA (2001) Studies on the metabolism of troglitazone to reactive intermediates in vitro and in vivo. Evidence for novel biotransformation pathways involving quinone methide formation and thiazolidinedione ring scission. Chem Res Toxicol 14:62–70. https://doi.org/10.1021/tx000180q
Kawashiro T, Yamashita K, Zhao XJ, Koyama E, Tani M, Chjiba K, Ishizaki T (1998) A study on the metabolism of etoposide and possible interactions with antitumor or supporting agents by human liver microsomes. J Pharmacol Exp Ther 286:1294–1300. https://jpet.aspetjournals.org/content/286/3/1294.long
Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A (2010) Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 38:92–99. https://doi.org/10.1124/dmd.109.029132
Kharasch ED, Thummel KE (1993) Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology 79:795–807. https://doi.org/10.1097/00000542-199310000-00023
Kharasch ED, Thummel KE, Mautz D, Bosse S (1994) Clinical enflurane metabolism by cytochrome P450 2E1. Clin Pharmacol Ther 55:434–440. https://doi.org/10.1038/clpt.1994.53
Kharasch ED, Hankins DC, Thummel KE (1995) Human kidney methoxyflurane and sevoflurane metabolism. Anesthesiology 82:689–699. https://doi.org/10.1097/00000542-199503000-00011
Kharasch ED, Hankins DC, Fenstamaker K, Cox K (2000) Human halothane metabolism, lipid peroxidation, and cytochromes P450 2A6 and P450 3A4. Eur J Clin Pharmacol 55:853–859. https://doi.org/10.1007/s002280050707
Kiang TKL, Ho PC, Anari MR, Tong V, Abbott FS, Chang TKH (2006) Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol Sci 94:261–271. https://doi.org/10.1093/toxsci/kfl096
Kim SY, Suzuki N, Santosh Laxmi YR, Reiger R, Shibutani S (2003) α-Hydroxylation of tamoxifen and toremifene by human and rat cytochrome P450 3A subfamily enzymes. Chem Res Toxicol 16:1138–1144. https://doi.org/10.1021/tx0300131
Kim SY, Laxmi YR, Suzuki N, Ogura K, Watabe T, Duffel MW, Shibutani S (2005) Formation of tamoxifen-DNA adducts via O-sulfonation, not O-acetylation, of α-hydroxytamoxifen in rat and human livers. Drug Metab Dispos 33:1673–1678. https://doi.org/10.1124/dmd.105.005330
Kinobe RT, Parkinson OT, Mitchell DJ, Gillam EMJ (2005) P450 2C18 catalyzes the metabolic bioactivation of phenytoin. Chem Res Toxicol 18:1868–1875. https://doi.org/10.1021/tx050181o
Kishino Y, Hasegawa T, Kato A, Nishiya Y, Rozhnal V, Watanabe K, Takasaki W, Yamoto T, Mori K (2019) Effect of inter-individual variability in human liver cytochrome P450 isozymes on cyclophosphamide-induced micronucleus formation. Mut Res/genet Toxicol Environ Mutagen 838:37–45. https://doi.org/10.1016/j.mrgentox.2018.11.016. (PMID: 30678826)
Kobayashi Y, Fukami T, Higuchi R, Nakajima M, Yokoi T (2012a) Metabolic activation by human arylacetamide deacetylase, CYP2E1, and CYP1A2 causes phenacetin-induced methemoglobinemia. Biochem Pharmacol 84:1196–1206. https://doi.org/10.1016/j.bcp.2012.08.015
Kobayashi Y, Fukami T, Shimizu M, Nakajima M, Yokoi T (2012b) Contributions of arylacetamide deacetylase and carboxylesterase 2 to flutamide hydrolysis in human liver. Drug Metab Dispos 40:1080–1084. https://doi.org/10.1124/dmd.112.044537
Komatsu T, Yamazaki H, Asahi S, Gillam EM, Guengerich FP, Nakajima M, Yokoi T (2000) Formation of a dihydroxy metabolite of phenytoin in human liver microsomes/cytosol: roles of cytochromes P450 2C9, 2C19, and 3A4. Drug Metab Dispos 28:1361–1368. PMID: 11038165. https://dmd.aspetjournals.org/content/28/11/1361.long
Konishi K, Fukami T, Gotoh S, Nakajima M (2017) Identification of enzymes responsible for nitrazepam metabolism and toxicity in human. Biochem Pharmacol 140:150–160. https://doi.org/10.1016/j.bcp.2017.06.114
Korzekwa KR, Krishnamachary N, Shou M, Ogai A, Parise RA, Rettie AE, Gonzalez FJ, Tracy TS (1998) Evaluation of atypical cytochrome P450 kinetics with two-substrate models: evidence that multiple substrates can simultaneously bind to cytochrome P450 active sites. Biochemistry 37:4137–4147. https://doi.org/10.1021/bi9715627
Kotrbová V, Mrázová B, Moserová M, Martínek V, Hodek P, Hudecek J, Frei E, Stiborová M (2011) Cytochrome b5 shifts oxidation of the anticancer drug ellipticine by cytochromes P450 1A1 and 1A2 from its detoxication to activation, thereby modulating its pharmacological efficacy. Biochem Pharmacol 82:669–680. https://doi.org/10.1016/j.bcp.2011.06.003
Kranendonk M, Alves M, Antunes P, Rueff J (2014) Human sulfotransferase 1A1-dependent mutagenicity of 12-hydroxy-nevirapine: the missing link? Chem Res Toxicol 27:1967–1971. https://doi.org/10.1021/tx5003113
Kudo S, Ishizaki T (1999) Pharmacokinetics of haloperidol: an update. Clin Pharmacokin 37:435–456. https://doi.org/10.2165/00003088-199937060-00001
Kumar S, Samuel K, Subramanian R, Braun MP, Stearns RA, Chiu SH, Evans DC, Baillie TA (2002) Extrapolation of diclofenac clearance from in vitro microsomal metabolism data: Role of acyl glucuronidation and sequential oxidative metabolism of the acyl glucuronide. J Pharmacol Exp Ther 303:969–978. https://doi.org/10.1124/jpet.102.038992
Kurth MJ, Yokoi T, Gershwin ME (2014) Halothane-induced hepatitis: paradigm or paradox for drug-induced liver injury. Hepatology 60:1473–1475. https://doi.org/10.1002/hep.27253
Lacroix C, Phan Hoang T, Nouveau J, Guyonnaud C, Laine G, Duwoos H, Lafont O (1989) Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur J Clin Pharmacol 36:395–400. https://doi.org/10.1007/BF00558302
Lai WG, Gardner I, Zahid N, Uetrecht JP (2000) Bioactivation and covalent binding of hydroxyfluperlapine in human neutrophils: implications for fluperlapine-induced agranulocytosis. Drug Metab Dispos 28:255–263. PMID: 10681368. https://dmd.aspetjournals.org/content/28/3/255.long
Laine JE, Auriola S, Pasanen M, Juvonen RO (2009) Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 39:11–21. https://doi.org/10.1080/00498250802512830
Lakehal F, Dansette PM, Becquemont L, Lasnier E, Delelo R, Balladur P, Poupon R, Beaune PH, Housset, (2001) Indirect cytotoxicity of flucloxacillin toward human biliary epithelium via metabolite formation in hepatocytes. Chem Res Toxicol 14:694–701. https://doi.org/10.1021/tx0002435
Lauer B, Tuschl G, Kling M, Mueller SO (2009) Species-specific toxicity of diclofenac and troglitazone in primary human and rat hepatocytes. Chem-Biol Interact 179:17–24. https://doi.org/10.1016/j.cbi.2008.10.031
Lecoeur S, Bonierbale E, Challine D, Gautier JC, Dansette VP, PM, Catinot R. Ballet F, Mansuy D, Beaune PH, (1994) Specificity of in vitro covalent binding of tienilic acid metabolites to human liver microsomes in relationship to the type of hepatotoxicity: comparison with to directly hepatotoxic drugs. Chem Res Toxicol 7:434–442. https://doi.org/10.1021/tx00039a023
Leemann T, Transon C, Dayer P (1993) Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4′-hydroxylation in human liver. Life Sci 52:29–34. https://doi.org/10.1016/0024-3205(93)90285-B
Lewis BC, Korprasertthaworn P, Miners JO (2016) Impaired dacarbazine activation and 7-ethoxyresorufin deethylation in vitro by polymorphic variants of CYP1A1 and CYP1A2: implications for cancer therapy. Pharmacogenet Genom 26:453–461. https://doi.org/10.1097/FPC.0000000000000236
Li F, Chordia MD, Huang T, Macdonald TL (2009) In vitro nimesulide studies toward understanding idiosyncratic hepatotoxicity: Diiminoquinone formation and conjugation. Chem Res Toxicol 22:72–80. https://doi.org/10.1021/tx800152r
Liu ZC, Uetrecht JP (2000) Metabolism of ticlopidine by activated neutrophils: implications for ticlopidine-induced agranulocytosis. Drug Metab Dispos 28:726–730 (PMID: 10859143)
Lolkema MP, Bohtes HH, Arkenau HT, Lampo A, Barale E, de Jonge MJA, van Doorn, de Bonno JS, Eskens FALM, (2015) The c-Met tyrosine kinase inhibitor JNJ-38877605 causes renal toxicity through species-specific insoluble metabolite formation. Clin Cancer Res 21:2297–2304. https://doi.org/10.1058/1078-0432.CCR-14-3258
López-Garcia MP, Dansette PM, Valadon P, Amar C, Beaune PH, Guengerich FP, Mansuy D (1993) Human-liver cytochromes P-450 expressed in yeast as tools for reactive-metabolite formation studies. Oxidative activation of tienilic acid by cytochromes P-450 2C9 and 2C10. Eur J Biochem 213:223–232. https://doi.org/10.1111/j.1432-1033.1993.tb17752.x
López-Garcia MP, Dansette PM, Mansuy D (1994) Thiophene derivatives as new mechanism-based inhibitors of cytochromes P-450: inactivation of yeast-expressed human liver cytochrome P-450 2C9 by tienilic acid. Biochemistry 33:166–175. https://doi.org/10.1021/bi00167a022LuH,WangJJ,Chan
Lu Y, Cederbaum AI (2006) Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci 89:515–523. https://doi.org/10.1093/toxsci/kfj031
Lu Y, Cederbaum A (2007) The mode of cisplatin-induced cell death in CYP2E1-overexpressing HepG2 cells: modulation by ERK, ROS, glutathione, and thioredoxin. Free Rad Biol Med 43:1061–1075. https://doi.org/10.1016/j.freeradbiomed.2007.06.021
Lu W, Uetrecht JP (2008) Peroxidase-mediated bioactivation of hydroxylated metabolites of carbamazepine and phenytoin. Drug Metab Dispos 36Pearce:1624–1636. https://doi.org/10.1124/dmd.107.019554
Lu H, Wang JJ, Chan KK, Philip PA (2006) Stereoselectivity in metabolism of ifosfamide by CYP3A4 and CYP2B6. Xenobiotica 36:367–385. https://doi.org/10.1080/00498250600598486
Lu H-F, Lai T-Y, Hsia T-C, Tang Y-J, Yang J-S, Chiang J-H, Lu C-C, Liu C-M, Wang H-L, Chung J-G (2010) Danthron induces DNA damage and inhibits DNA repair gene expressions in GBM 8401 human brain glioblastoma multiforms cells. Neurochem Res 35:1105–1110. https://doi.org/10.1007/s11064-010-0161-z
Maggs JL, Williams D, Pirmohamed M, Park BK (1995) The metabolic formation of reactive intermediates from clozapine, a drug associated with agranulocytosis in man. J Pharmacol Exp Ther 275:1463–1475. https://jpet.aspetjournals.org/content/275/3/1463.long
Mancy A, Antignac M, Minoletti C, Dijols S, Mouries V, Ha Duong N-T, Battioni P, Dansette PM, Mansuy D (1999) Diclofenac and its derivatives as tools for studying human cytochromes P450 active sites: particular efficiency and regioselectivity of P450 2Cs. Biochemistry 38:14264–14270. https://doi.org/10.1021/bi991195u
Manyike P, Kharasch E, Kalhorn T, Slattery J (2000) Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther 67:275–282. https://doi.org/10.1067/mcp.2000.104736
Martinkova E, Dontenwill M, Frei E, Stiborová M (2009) Cytotoxicity of and DNA adduct formation by ellipticine in human U87MG glioblastoma cancer cells. Neuro Endocrinol Lett 30 Suppl 1:60–66. https://www.nel.edu/userfiles/articlesnew/NEL300709A09.pdf
Maseneni S, Donzelli M, Taegtmeyer AB, Brecht K, Krähenbühl S (2012) Toxicity of clopidogrel and ticlopidine on human myeloid progenitor cells: Importance of metabolites. Toxicology 299:139–145. https://doi.org/10.1016/j.tox.2012.05.017
Maseneni S, Donzelli M, Brecht K, Krähenbühl S (2013) Toxicity of thienopyridines on human neutrophil granulocytes and lymphocytes. Toxicology 308:11–19. https://doi.org/10.1016/j.tox.2013.03.002
Masmirembwa CM, Otter C, Berg M, Jönsson M, Leidvik B, Jonsson E, Johansson, Bäckman, Edlund A, Andersson TB (1999) Heterologous expression and kinetic characterization of human cytochromes P-450: Validation of a pharmaceutical tool for drug metabolism research. Drug Metab Dispos 27:1117–1122. PMID: 10497136. https://dmd.aspetjournals.org/content/27/10/1117.long
Masubuchi Y, Nakano T, Ose A, Horie T (2001) Differential selectivity in carbamazepine-induced inactivation of cytochrome P450 enzymes in rat and human liver. Arch Toxicol 75:538–543. https://doi.org/10.1007/s002040100270
May DG, Porter J, Wilkinson GR, Branch RA (1994) Frequency distribution of dapsone N-hydroxylase, a putative probe for P4503A4 activity, in a white population. Clin Pharmacol Ther 55:492–500. https://doi.org/10.1038/clpt.1994.62
McCune JS, Risler LJ, Phillips BR, Thummel KE, Blough SDD (2005) Contribution of CYP3A5 to hepatic and renal ifosfamide N-dechloroethylation. Drug Metab Dispos 33:1074–1081. https://doi.org/10.1124/dmd.104.002279
McLean L, Soto U, Agama K, Francis J, Jimenez R, Pommier Y, Sowers L, Brantley E (2008) Aminoflavone induces oxidative DNA damage and reactive oxidative species-mediated apoptosis in breast cancer cells. Int J Cancer 122:1665–1674. https://doi.org/10.1002/ijc.23244
Melet A, Assrir N, Jean P, Lopez-Garcia MP, Marques-Soares C, Jaouen M, Dansette PM, Sari M-A, Mansuy D (2003) Substrate selectivity of human cytochrome P450 2C9: Importance of residues 476, 365, and 114 in recognition of diclofenac and sulfaphenazole and in mechanism-based inactivation by tienilic acid. Arch Biochem Biophys 409:80–91. https://doi.org/10.1016/S0003-9861(02)00548-9
Meng LH, Shankavaram U, Chen C, Agama K, Fu HQ, Gonzalez FJ, Weinstein J, Pommier Y (2006) Activation of aminoflavone (NSC 686288) by a sulfotransferase is required for the antiproliferative effect of the drug and for induction of histone gamma-H2AX. Cancer Res 66:9656–9664. https://doi.org/10.1158/0008-5472.CAN-06-0796
Minoda Y, Kharasch ED (2001) Halothane-dependent lipid peroxidation in human liver microsomes is catalyzed by cytochrome P4502A6 (CYP2A6). Anesthesiology 95:509–514. https://doi.org/10.1097/00000542-200108000-00037
Mitra AK, Thummel KE, Kalhorn TF, Kharasch ED, Unadkat JD, Slattery JT (1995) Metabolism of dapsone to its hydroxylamine by CYP2E1 in vitro and in vivo. Clin Pharmacol Ther 58:556–566. https://doi.org/10.1016/0009-9236(95)90176-0
Miyamoto G, Zahid N, Uetrecht JP (1997) Oxidation of diclofenac to reactive intermediates by neutrophils, myeloperoxidase, and hypochlorous acid. Chem Res Toxicol 10:414–419. https://doi.org/10.1021/tx960190k
Moore CD, Reilly CA, Yost GS (2010a) CYP3A4-mediated oxygenation versus dehydrogenation of raloxifene. Biochemistry 49:4466–4475. https://doi.org/10.1021/bi902213r
Moore CD, Shahrokh K, Sontum SF, Cheatham TE, Yost GS (2010b) Improved cytochrome P450 3A4 molecular models accurately predict the Phe215 requirement for raloxifene dehydrogenation selectivity. Biochemistry 49:9011–9019. https://doi.org/10.1021/bi101139q
Murray M, Butler AM, Stupans I (1994) Competitive inhibition of human liver microsomal P450 3A-dependent steroid 6-b-hydroxylation activity by cyclophosphamide in vitro. Pharmacol Exp Ther 270:645–649
Nakajima M, Komagata S, Fujiki Y, Ebi H, Itoh K, Mukai H, Yokoi T, Minami H (2007) Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmaco Genom 17:431–445. https://doi.org/10.1097/FPC.0b013e328045c4fb
Nakamura H, Torimoto N, Ishii I, Ariyoshi N, Nakasa H, Ohmori S, Kitada M (2003) CYP3A4 and CYP3A7-mediated carbamazepine 10,11-epoxidation are activated by differential endogenous steroids. Drug Metab Dispos 31:432–438. https://doi.org/10.1124/dmd.31.4.432
Neuman MG, Shear NH, Jacobson-Brown PM, Katz GG, Neilson HK, Malkiewicz IM, Cameron RG, Abbott F (2001) CYP2E1-mediated modulation of valproic acid-induced hepatocytotoxicity. Clin Biochem 34:211–218. https://doi.org/10.1016/S0009-9120(01)00217-X
Ngui JS, Tang W, Stearns RA, Shou M, Miller RR, Zhang Y, Lin JH, Baillie TA (2000) Cytochrome P450 3A4-mediated interaction of diclofenac and quinidine. Drug Metab Dispos 28:1043–1050. PMID: 10950847. https://dmd.aspetjournals.org/content/28/9/1043.long
Niwa T, Kageyama A, Kishimoto K, Yabusaki Y, Ishibashi F, Katagiri M (2002) Amino acid residues affecting the activities of human cytochrome P450 2C9 and 2C19. Drug Metab Dispos 30:931–936. https://doi.org/10.1124/dmd.30.8.931
Notley LM, De Wolf CJF, Wunsch RM, Lancaster RG, Gillam EMJ (2002) Bioactivation of tamoxifen by recombinant human cytochrome P450 enzymes. Chem Res Toxicol 15:614–622. https://doi.org/10.1021/tx0100439
Notley LM, Crewe KH, Taylor PJ, Lennard MS, Gillam EMJ (2005) Characterization of the human cytochrome P450 forms involved in metabolism of tamoxifen to its α-hydroxy and α,4-dihydroxy derivatives. Chem Res Toxicol 18:1611–1618. https://doi.org/10.1021/tx050140s
Obach RS, Dalvie DK (2006) Metabolism of nomifensine to a dihydroisoquinolinium ion metabolite by human myeloperoxidase, hemoglobin, monoamine oxidase A, and cytochrome P450 enzymes. Drug Metab Dispos 34:1310–1316. https://doi.org/10.1124/dmd.106.010173
Ogiso T, Fukami T, Mishiro K, Konishi K, Jones JP, Nakajima M (2018) Substrate selectivity of human aldehyde oxidase 1 in reduction of nitroaromatic drugs. Arch Biochem Biophys 659:85–92. https://doi.org/10.1016/j.abb.2018.10.017
Ohkuma Y, Hiraku Y, Kawanishi S (2001) Sequence-specific DNA damage induced by carcinogenic danthron and anthraquinone in the presence of Cu(II), cytochrome P450 reductase and NADPH. Free Rad Res 34:595–604. https://doi.org/10.1080/10715760100300491
Ohnishi S, Murata M, Ida N, Oikawa S, Kawanishi S (2015) Oxidative DNA damage induced by metabolites of chloramphenicol, an antibiotic drug. Free Rad Res 49:1165–1172. https://doi.org/10.3109/10715762.2015.1050963
Pabani UK, Khan Z, Ali L, Shah SK, Khan JA (2023) Allopurinol-induced uncommon dermatological emergency of toxic epidermal necrolysis (TEN). Cureus 15:e44812. https://doi.org/10.7759/cureus.44812
Pan LP, Wijnant P, De Vriendt C, Rosseel MT, Belpaire, (1997) Characterization of the cytochrome P450 isoenzymes involved in the in vitro N-dealkylation of haloperidol. Brit J Clin Pharmacol 44:557–564. https://doi.org/10.1046/j.1365-2125.1997.t01-1-00629.x
Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS (1993) Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 6:511–518. https://doi.org/10.1021/tx00034a019
Pearce RE, Uetrecht JP, Leeder JS (2005) Pathways of carbamazepine bioactivation in vitro: II. The role of human cytochrome P450 enzymes in the formation of 2-hydroxyiminostilbene. Drug Metab Dispos 33:1819–1826. https://doi.org/10.1124/dmd.105.004861
Pearce RE, Lu W, Wang Y, Uetrecht JP, Correia AM, Leeder JS (2008) Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine. Drug Metab Dispos 36:1637–1649. https://doi.org/10.1124/dmd.107.019562
Pelkonen O, Myllynen P, Taavitsainen P, Boobis AR, Watts Lake BG (2001) Carbamazepine: a “blind” assessment of CYP-associated metabolism and interactions in human liver-derived in vitro systems. Xenobiotica 31:321–343. https://doi.org/10.1080/00498250110055479
Perwitasari DA, Atthobari J, Wilffert B (2015) Pharmacogenetics of isoniazid-induced hepatotoxicity. Drug Metab Rev 47:222–228. https://doi.org/10.3109/03602532.2014.984070
Philip PA, Ali-Sadat, Doehmer J, Kocarek T, Akhtar A, Lu H, Chan KK (1999) Use of V79 cells with stably transfected cytochrome P450 cDNAs in studying the metabolism and effects of cytotoxic drugs. Cancer Chemother Pharmacol 43:59–67. https://doi.org/10.1007/s002800050863
Qian L, Ortiz de Montellano PR (2006) Oxidative activation of thiacetazone by the Mycobacterium tuberculosis flavin monooxygenase EtaA and human FMO1 and FMO3. Chem Res Toxicol 19:443–449. https://doi.org/10.1021/tx050328b
Quintanilha JCF, De Sousa VM, Visacri MB, Amaral LS, Santos RMM, Zambrano T, Salazar LA, Moriel P (2017) Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol 80:223–233. https://doi.org/10.1007/s00280-017-3358-x
Rahman MA, Kodidela S, Sinha N, Haque S, Shukla PK, Rao R, Kumar S (2019) Plasma exosomes exacerbate alcohol- and acetaminophen-induced toxicity via CYP2E1 pathway. Sci Rep 9:6571. https://doi.org/10.1038/s41598-019-43064-2
Raucy JL, Lasker JM, Lieber CS, Black M (1989) Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch Biochem Biophys 271:270–283. https://doi.org/10.1016/0003-9861(89)90278-6
Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM (1999) Metabolic activation of dacarbazine by human cytochromes P450: the role of CYP1A1, CYP1A2, and CYP2E1. Clin Cancer Res 5:2192–2197. https://aacrjournals.org/clincancerres/article/5/8/2192/287759/Metabolic-Activation-of-Dacarbazine-by-Human
Relling MV, Nemec J, Schuetz EG, Schuetz JD, Gonzalez FJ, Korzekwa KR (1994) O-Demethylation of epipodophyllotoxins is catalyzed by human cytochrome P450 3A4. Mol Pharmacol 45:352–358. https://molpharm.aspetjournals.org/content/45/2/352.long
Ren S, Yang JS, Kalhorn TF, Slattery JT (1997) Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res 57:4229–4235. PMID 9331082. https://aacrjournals.org/cancerres/article/57/19/4229/657383/Oxidation-of-Cyclophosphamide-to-4
Rendic S, Guengerich FP (2015) Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol 28:38–42. https://doi.org/10.1021/tx500444e
Rendic SP, Guengerich FP (2021) Human Family 1–4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update. Arch Toxicol 95:395–472. https://doi.org/10.1007/s00204-020-02971-4
Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL (2001) Human CYP1B1 and anticancer agent metabolism: Mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther 296:537–541. https://jpet.aspetjournals.org/content/296/2/537.long
Rodriguez RJ, Miranda CL (2000) Isoform specificity of N-deacetyl ketoconazole by human and rabbit flavin-containing monooxygenases. Drug Metab Dispos 28:1083–1086. PMID: 10950853. https://dmd.aspetjournals.org/content/28/9/1083.long
Roe AL, Snawder JE, Benson RW, Roberts DW, Casciano DA (1993) HepG2 cells: An in vitro model for P450-dependent metabolism of acetaminophen. Biochem Biophys Res Commun 190:15–19. https://doi.org/10.1006/bbrc.1993.1003
Roy P, Tretyakov O, Wright J, Waxman DJ (1999a) Stereoselective metabolism of ifosfamide by human P-450s 3A4 and 2B6. Favorable metabolic properties of R-enantiomer. Drug Metab Dispos 27:1309–1318. https://dmd.aspetjournals.org/content/27/11/1309.long
Roy P, Yu LJ, Crespi CL, Waxman DJ (1999b) Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos 27:655–666. PMID: 10348794. https://dmd.aspetjournals.org/content/27/6/655.long
Sadeque AJ, Fisher MB, Korzekwa KR, Gonzalez JF, Rettie AE (1997) Human CYP2C9 and CYP2A6 mediate formation of the hepatotoxin 4-ene-valproic acid. J Pharmacol Exp Ther 283:698–703. https://jpet.aspetjournals.org/content/283/2/698.long
Salem Z, Murray T, Yunis AA (1981) The nitroreduction of chloramphenicol by human liver tissue. J Lab Clin Med 97:881–886
Sarich T, Kalhorn T, Magee S, al-Sayegh F, Adams S, Slattery J, Goldstein J, Nelson S, Wright J (1997) The effect of omeprazole pretreatment on acetaminophen metabolism in rapid and slow metabolizers of S-mephenytoin. Clin Pharmacol Ther 62:21–28. https://doi.org/10.1016/S0009-9236%2897%2990148-Xb
Shebley M, Jushchyshyn MI, Hollenberg PF (2006) Selective pathways for the metabolism of phencyclidine by cytochrome P450 2B enzymes: identification of electrophilic metabolites, glutathione, and N-acetyl cysteine adducts. Drug Metab Dispos 34:375–383. https://doi.org/10.1124/dmd.105.007047
Shen S, Marchick MR, Davis MR, Doss GA, Pohl LR (1999) Metabolic activation of diclofenac by human cytochrome P450 3A4: role of 5-hydroxydiclofenac. Chem Res Toxicol 12:214–222. https://doi.org/10.1021/tx9802365
Shen C, Meng Q, Zhang G, Hu W (2008) Rifampicin exacerbates isoniazid-induced toxicity in human but not in rat hepatocytes in tissue-like cultures. J Pharmacol 153:784–791. https://doi.org/10.1038/sj.bjp.0707611
Shet MS, McPhaul M, Fisher CW, Stallings NR, Estabrook RW (1997) Metabolism of the antiandrogenic drug (flutamide) by human CYP1A2. Drug Metab Dispos 25:1298–1303 (PMID: 9351907)
Shibutani S, Shaew PM, Suzuki N, Dasaradhi L, Duffel MW, Terashima I (1998b) Sulfation of α-hydroxytamoxifen catalyzed by human hydroxysteroid sulfotransferase results in tamoxifen-DNA adducts. Carcinogenesis 19:2007–2011. https://doi.org/10.1093/carcin/19.11.2007
Shibutani S, Dasaradhi L, Terashima I, Banoglu E, Duffel MW (1998a) α-Hydroxytamoxifen is a substrate of hydroxysteroid (alcohol) sulfotransferase, resulting in tamoxifen DNA adducts. Cancer Res 58:647–653. https://aacrjournals.org/cancerres/article/58/4/647/504765/Hydroxytamoxifen-Is-a-Substrate-of-Hydroxysteroid
Shibutani S, Ravindernath A, Terashima I, Suzuki N, Laxmi YR, Kanno Y, Suzuki M, Apak TI, Sheng JJ, Duffel MW (2001) Mechanism of lower genotoxicity of toremifene compared with tamoxifen. Cancer Res 61:3925–3931. https://aacrjournals.org/cancerres/article/61/10/3925/507395/Mechanism-of-Lower-Genotoxicity-of-Toremifene
Shih T-Y, Pai C-Y, Yang P, Chang W-L, Wang N-C, Hu OY-P (2013) A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob Agents Chemother 57:1685–1690. https://doi.org/10.1128/AAC.01866-12
Shin J-G, Kane K, Flockhart DA (2001) Potent inhibition of CYP2D6 by haloperidol metabolites: stereoselective inhibition by reduced haloperidol: inhibition of CYP2D6 by haloperidol metabolites. Brit J Clin Pharmacol 51:45–52. https://doi.org/10.1046/j.1365-2125.2001.01313.x
Sinclair J, Jeffery E, Wrighton S, Kostrubsky V, Szakacs WS, Sinclair P (1998) Alcohol-mediated increases in acetaminophen hepatotoxicity: role of CYP2E and CYP3A. Biochem Pharmacol 55:1557–1565. https://doi.org/10.1016/s0006-2952(97)00656-4. (PMID: 9633991)
Singla N, Gupta D, Birbian N, Singh J (2014) Association of NAT2, GST and CYP2E1 polymorphisms and anti-tuberculosis drug-induced hepatotoxicity. Tuberculosis 94:293–298. https://doi.org/10.1016/j.tube.2014.02.003
Smith KS, Smith PL, Heady TN, Trugman JM, Harman WD, Macdonald (2003) In vitro metabolism of tolcapone to reactive intermediates: relevance to tolcapone liver toxicity. Chem Res Toxicol 16:123–128. https://doi.org/10.1021/tx025569n
Spracklin DK, Kharasch ED (1998) Human halothane reduction in vitro by cytochrome P450 2A6 and 3A4: identification of low and high KM isoforms. Drug Metab Dispos 26:605–607. https://dmd.aspetjournals.org/content/26/6/605.long
Spracklin DK, Thummel KE, Kharasch ED (1996) Human reductive halothane metabolism in vitro is catalyzed by cytochrome P450 2A6 and 3A4. Drug Metab Dispos 24:976–983. https://dmd.aspetjournals.org/content/24/9/976.long
Spracklin DK, Hankins DC, Fisher JM, Thummel KE, Kharasch ED (1997) Cytochrome P450 2E1 is the principal catalyst of human oxidative halothane metabolism in vitro. J Pharmacol Exp Ther 281:400–411. https://jpet.aspetjournals.org/content/281/1/400.long
Sridar C, D’Agostino J, Hollenberg PF (2012) Bioactivation of the cancer chemopreventive agent tamoxifen to quinone methides by cytochrome P4502B6 and identification of the modified residue on the apoprotein. Drug Metab Dispos 40:2280–2288. https://doi.org/10.1124/dmd.112.047266
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764. https://doi.org/10.1093/jnci/djg108
Stiborová M, Bieler CA, Wiessler M, Frei E (2001) The anticancer agent ellipticine on activation by cytochrome P450 forms covalent DNA adducts. Biochem Pharmacol 62:1675–1684. https://doi.org/10.1016/S0006-2952(01)00806-1
Stiborová M, Bořek-Dohalská L, Hodek P, Mráz J, Frei E (2002) New selective inhibitors of cytochromes P450 2B and their application to antimutagenesis of tamoxifen. Arch Biochem Biophys 403:41–49. https://doi.org/10.1016/S0003-9861(02)00259-X
Stiborová M, Sejbal J, Bořek-Dohalská L, Aimová D, Poljaková J, Fosterová K, Rupertová M, Wiesner J, Hudecek J, Wiessler M, Frei E (2004) The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res 64:8374–8380. https://doi.org/10.1158/0008-5472.CAN-04-2202
Stiborová M, Rupertova M, Schmeiser HH, Frei E (2006b) Molecular mechanisms of antineoplastic action of an anticancer drug ellipticine. Biomed Papers (med Fac Univ Palacky Olomouc Czech Repub) 150:13–23. https://doi.org/10.5507/bp.2006.002
Stiborová M, Borek-Dohalská L, Aimová D, Kotrbová V, Kukacková K, Janouchová K, Rupertová M, Ryslavá, Hudecek J, Frei E (2006b) Oxidation pattern of the anticancer drug ellipticine by hepatic microsomes - similarity between human and rat systems. Gen Physiol Biophys 25:245–261. PMID: 17197724. http://www.gpb.sav.sk/2006_03_245.pdf
Stiborová M, Poljaková J, Martínková E, Borek-Dohalská L, Eckschlager T, Kizek R, Frei E (2011a) Ellipticine cytotoxicity to cancer cell lines—a comparative study. Interdiscipl Toxicol 4:98–105. https://doi.org/10.2478/v10102-011-0017-7
Stiborová M, Rupertová M, Frei E (2011b) Cytochrome P450- and peroxidase-mediated oxidation of anticancer alkaloid ellipticine dictates its anti-tumor efficiency. Biochim Biophys Acta-Proteins Proteom 1814:175–185. https://doi.org/10.1016/j.bbapap.2010.05.016. (PMID: 20576524)
Stiborová M, Indra R, Moserová M, Cerná V, Rupertová M, Martínek V, Eckschlager T, Kizek R, Frei E (2012a) Cytochrome b5 increases cytochrome P450 3A4-mediated activation of anticancer drug ellipticine to 13-hydroxyellipticine whose covalent binding to DNA is elevated by sulfotransferases and N,O-acetyltransferases. Chem Res Toxicol 25:1075–1085. https://doi.org/10.1021/tx3000335
Stiborová M, Poljaková J, Martínková E, Ulrichová J, Simánek V, Dvorák Z, Frei, (2012b) Ellipticine oxidation and DNA adduct formation in human hepatocytes is catalyzed by human cytochromes P450 and enhanced by cytochrome b5. Toxicology 302:233–241. https://doi.org/10.1016/j.tox.2012.08.004
Stiborová M, Černá V, Moserová M, Moserová M, Mrizová I, Arlt VM, Frei E (2014) The anticancer drug ellipticine activated with cytochrome P450 mediates DNA damage determining its pharmacological efficiencies: studies with rats, hepatic cytochrome P450 reductase null (HRN™) mice and pure enzymes. Int J Mol Sci 16:284–306. https://doi.org/10.3390/ijms16010284
Styles JA, Davies A, Lim CK, De Matteis F, Stanley LA, White INH, Yuan Z-X, Smith LL (1994) Genotoxicity of tamoxifen epoxide and toremifene in human lymphoblastoid cells containing human cytochrome P450s. Carcinogenesis 15:5–9. https://doi.org/10.1093/carcin/15.1.5
Szökő É, Tábi T, Borbás T, Dalmadi B, Tihanyi K, Magyar K (2004) Assessment of the N-oxidation of deprenyl, methamphetamine, and amphetamine enantiomers by chiral capillary electrophoresis: an in vitro metabolism study. Electrophoresis 25:2866–2875. https://doi.org/10.1002/elps.200406023
Tan WK, Tan ARY, Sivanandam P, Goh EJH, Yap ZP, Sabrulla NF, Austin-Muttitt K, Mullings JGL, Lau AJ (2020) In vitro inhibition of human aldehyde oxidase activity by clinically relevant concentrations of gefitinib and erlotinib: comparison with select metabolites, molecular docking analysis, and impact on hepatic metabolism of zaleplon and methotrexate. J Pharmacol Exp Ther 374:295–307. https://doi.org/10.1124/jpet.120.265249
Tang W (2003) The metabolism of diclofenac—enzymology and toxicology perspectives. Curr Drug Metab 4:319–329. https://doi.org/10.2174/1389200033489398
Tang W, Stearns RA, Wang RW, Chiu S-HL, Baillie TA (1999) Roles of human hepatic cytochrome P450s 2C9 and 3A4 in the metabolic activation of diclofenac. Chem Res Toxicol 12:192–199. https://doi.org/10.1021/tx9802217
Tateishi Y, Shibazaki C, Takahashi K, Nakamura S, Kazuki Y, Mashino T, Ohe T (2022) Synthesis and evaluation of tofacitinib analogs designed to mitigate metabolic activation. Drug Metab Pharmacokinet 43:100439. https://doi.org/10.1016/j.dmpk.2021.100439
Tettey JN, Maggs JL, Rapeport WG, Pirmohamed M, Park BK (2001) Enzyme-induction dependent bioactivation of troglitazone and troglitazone quinone in vivo. Chem Res Toxicol 14:965–974. https://doi.org/10.1021/tx0001981
Thummel K, Slattery JT, Ro H, Chien JY, Nelson SD, Lown KE, Watkins PB (2000) Ethanol and production of the hepatotoxic metabolite of acetaminophen in healthy adults. Clin Pharmacol Therapeut 67:591–599. https://doi.org/10.1067/mcp.2000.106574
Tingle MD, Jewell H, Maggs JL, O’Neill PM, Park BK (1995) The bioactivation of amodiaquine by human polymorphonuclear leucocytes in vitro: chemical mechanisms and the effects of fluorine substitution. Biochem Pharmacol 50:1113–1119. https://doi.org/10.1016/0006-2952(95)00236-S
Uetrecht JP (1992) Metabolism of clozapine by neutrophils: possible implications for clozapine-induced agranulocytosis. Drug Saf 7:51–56. https://doi.org/10.2165/00002018-199200071-00011
Uetrecht JP (1995) Myeloperoxidase as a generator of drug free radicals. Biochem Soc Sympos 61:163–170. https://doi.org/10.1042/bss0610163
Uetrecht J, Zahid N, Tehim A, Fu JM, Rakhit S (1997) Structural features associated with reactive metabolite formation in clozapine analogues. Chem-Biol Interact 104:117–129. https://doi.org/10.1016/S0009-2797(97)00017-3
Usuki E, Pearce R, Parkinson A, Castagnoli N (1996) Studies on the conversion of haloperidol and its tetrahydropyridine dehydration product to potentially neurotoxic pyridinium metabolites by human liver microsomes. Chem Res Toxicol 9:800–806. https://doi.org/10.1021/tx960001y
Usuki E, Van der Schyf CJ, Castagnoli N (1998) Metabolism of haloperidol and its tetrahydropyridine dehydration product HPTP. Drug Metab Rev 30:809–826. https://doi.org/10.3109/03602539808996331
Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B, Nicod L, Desmeules J, Hochstrasser D (2006) CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol 62:423–429. https://doi.org/10.1007/s00228-006-0111-5
Vyas PM, Roychowdhury S, Koukouritaki SB, Hines RN, Krueger SK, Williams DE, Nauseef WM, Svensson CK (2006) Enzyme-mediated protein haptenation of dapsone and sulfamethoxazole in human keratinocytes: II. Expression and role of flavin-containing monooxygenases and peroxidases. J Pharmacol Exp Ther 319:497–505. https://doi.org/10.1124/jpet.106.105874
Walker D, Flinois J-P, Monkman SC, Beloc C, Boddy AV, Cholerton S, Daly AK, Lind MJ, Pearson ADJ, Beaune PH, Idle JR (1994) Identification of the major human hepatic cytochrome P450 involved in activation and N-dechloroethylation of ifosfamide. Biochem Pharmacol 47:1157–1163. https://doi.org/10.1016/0006-2952(94)90387-5
Wang C, Wang P, Yang L-P, Pan J, Yang X, Ma Y-Y (2017) Association of CYP2C9, CYP2A6, ACSM2A, and CPT1A gene polymorphisms with adverse effects of valproic acid in Chinese patients with epilepsy. Epilepsy Res 132:64–69. https://doi.org/10.1016/j.eplepsyres.2017.02.015
Wani TH, Chakrabarty A, Shibata N, Yamazaki H, Guengerich FP, Chowdhury G (2017) The dihydroxy metabolite of the teratogen thalidomide causes oxidative DNA damage. Chem Res Toxicol 30:1622–1628. https://doi.org/10.1021/acs.chemrestox.7b00127
Watanabe A, Fukami T, Nakajima M, Takamiya M, Aoki Y, Yokoi T (2009) Human arylacetamide deacetylase is a principal enzyme in flutamide hydrolysis. Drug Metab Dispos 37:1513–1520. https://doi.org/10.1124/dmd.109.026567
Watanabe A, Fukami T, Takahashi S, Koayashi Y, Nakagawa N, Nakajima M, Yokoi T (2010) Arylacetamide deacetylase is a determinant enzyme for the difference in hydrolase activities of phenacetin and acetaminophen. Drug Metab Dispos 38:1532–1537. https://doi.org/10.1124/dmd.110.033720
Watanabe M, Watanabe N, Maruyama S, Kawashiro T (2015) Comparative metabolic study between two selective estrogen receptor modulators, toremifene and tamoxifen, in human liver microsomes. Drug Metab Pharmacokinet 30:325–333. https://doi.org/10.1016/j.dmpk.2015.05.004. (PMID: 26423799)
Wen B, Zhou M (2009) Metabolic activation of the phenothiazine antipsychotics chlorpromazine and thioridazine to electrophilic iminoquinone species in human liver microsomes and recombinant P450s. Chem-Biol Interact 181:220–226. https://doi.org/10.1016/j.cbi.2009.05.014
Wen B, Ma L, Rodrigues AD, Zhu M (2008a) Detection of novel reactive metabolites of trazodone: evidence for CYP2D6-mediated bioactivation of m-chlorophenylpiperazine. Drug Metab Dispos 36:841–850. https://doi.org/10.1124/dmd.107.019471
Wen B, Ma L, Zhu M (2008b) Bioactivation of the tricyclic antidepressant amitriptyline and its metabolite nortriptyline to arene oxide intermediates in human liver microsomes and recombinant P450s. Chem-Biol Interact 173:59–67. https://doi.org/10.1016/j.cbi.2008.02.001
Wen B, Chen Y, Fitch WL (2009) Metabolic activation of nevirapine in human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos 37:1557–1562. https://doi.org/10.1124/dmd.108.024851
White IN (2003) Tamoxifen: Is it safe? Comparison of activation and detoxication mechanisms in rodents and in humans. Curr Drug Metab 4:223–239. https://doi.org/10.2174/1389200033489451
White IN, De Matteis F (2001) The role of CYP forms in the metabolism and metabolic activation of HCFCs and other halocarbons. Toxicol Lett 124:121–128. https://doi.org/10.1016/s0378-4274(00)00288-5
White IN, De Matteis F, Gibbs AH, Lim CK, Wolf DR, Henderson C, Smith LL (1995) Species differences in the covalent binding of [14C]tamoxifen to liver microsomes and the forms of cytochrome P450 involved. Biochem Pharmacol 49:1035–1042. https://doi.org/10.1016/0006-2952(95)98498-X
Whitehouse LW, Lodge BA, Thomas BAW, BH, (1987) Metabolic disposition of pyrazinamide in the rat: identification of a novel in vivo metabolite common to both rat and human. Biopharm Drug Dispos 8:307–318. https://doi.org/10.1002/bdd.2510080402
Winter HR, Wang Y, Unadkat JD (2000) CYP2C8/9 mediate dapsone N-hydroxylation at clinical concentrations of dapsone. Drug Metab Dispos 28:865–868. PMID: 10901692. https://dmd.aspetjournals.org/content/28/8/865.long
Wolkenstein P, Tan C, Lecoeur S, Wechsler J, Garcia-Martin N, Charue D, Bagot M, Beaune P (1998) Covalent binding of carbamazepine reactive metabolites to P450 isoforms present in the skin. Chem-Biol Interact 113:39–50. https://doi.org/10.1016/S0009-2797(98)00021-0
Wu D, Cederbaum A (2004) Glutathione depletion in CYP2E1-expressing liver cells induces toxicity due to the activation of p38 mitogen-activated protein kinase and reduction of nuclear factor-κB DNA binding activity. Mol Pharmacol 66:749–760. https://doi.org/10.1124/mol.104.002048
Wu J, Dong H, Cai Z, Yu Y (1997) Stable expression of human cytochrome CYP2B6 an CYP1A1 in Chinese hamster CHL cells: their use in micronucleus assays. Chinese Med Sci (=Chung-kuo i hsueh tsa chih) 12:148–155
Yamamoto T, Moriwaki Y, Takahashi S, Hada T, Higashino K (1987) In vitro conversion of pyrazinamide into 5-hydroxypyrazinamide and that of pyrazinoic acid into 5-hydroxypyrazinoic acid by xanthine oxidase from human liver. Biochem Pharmacol 36:3317–3318. https://doi.org/10.1016/0006-2952(87)90654-X
Yamazaki H, Inuoe K, Chiba K, Ozawa N, Kawai T, Suzuki Y, Goldstein JA, Guengerich FP, Shimada T (1998) Comparative studies on the catalytic roles of cytochrome p450 2C9 and its Cys and Leu-variants in the oxidation of warfarin, flurbiprofen, and diclofenac by human liver microsomes. Biochem Pharmacol 56:243–251. https://doi.org/10.1016/s0006-2952(98)00133-6
Yamazaki H, Shibata A, Suzuki M, Nakjima M, Shimada N, Guengerich FP, Yokoi T (1999) Oxidation of troglitazone to a quinone-type metabolite catalyzed by cytochrome P-450 2C8 and P-450 3A4 in human liver microsomes. Drug Metab Dispos 27:1260–1266. https://dmd.aspetjournals.org/content/27/11/1260.long
Yang M, Chordia MD, Li F, Huang T, Linden J, Macdonald TL (2010) Neutrophil- and myeloperoxidase-mediated metabolism of reduced nimesulide: evidence for bioactivation. Chem Res Toxicol 23:1691–1700. https://doi.org/10.1021/tx1001496
Yoshitomi S, Ikemoto K, Takahashi J, Miki H, Namba M, Asahi S (2001) Establishment of the transformants expressing human cytochrome P450 subtypes in HepG2, and their applications on drug metabolism and toxicology. Toxicol in Vitro 15:245–256. https://doi.org/10.1016/S0887-2333(01)00011-X
Yukinaga H, Takami T, Shioyama S-H, Tozuka Z, Masumoto H, Okazaki O, Sudo K-I (2007) Identification of cytochrome P450 3A4 modification site with reactive metabolite using linear ion trap-fourier transform mass spectrometry. Chem Res Toxicol 20:1373–1378. https://doi.org/10.1021/tx700165q
Yunis AA (1984) Differential in-vitro toxicity of chloramphenicol, nitroso-chloramphenicol, and thiamphenicol. Sexually Transm Dis 11(Suppl 4):340–342. https://doi.org/10.1097/00007435-198410001-00005
Yunis AA, Lim LO, Arimura GK (1986) DNA damage induced by chloramphenicol and nitroso-chloramphenicol: protection by N-acetylcysteine. Respiration 50(Suppl 1):50–55. https://doi.org/10.1159/000195088
Yunis AA, Arimura GK, Isildar M (1987) DNA damage induced by chloramphenicol and its nitroso derivative: damage in intact cells. Am J Hematol 24:77–84. https://doi.org/10.1002/ajh.2830240110
Zahno A, Bouitbir J, Maseneni S, Lindinger PW, Brecht K, Krähenbühl S (2013) Hepatocellular toxicity of clopidogrel: mechanisms and risk factors. Free Radic Biol Med 65:208–216. https://doi.org/10.1016/j.freeradbiomed.2013.06.007
Zand R, Nelson SD, Slattery JT, Thummel KE, Kalhorn TF, Adams SP, Wright JM (1993) Inhibition and induction of cytochrome P4502E1-catalyzed oxidation by isoniazid in humans. Clin Pharmacol Therap 54:142–149. https://doi.org/10.1038/clpt.1993.125
Zhai Y, Wang L, Yang F, Feng G, Feng S, Cui T, An L, He X (2016) The mechanism and risk factors of clopidogrel-induced liver injury. Drug Chem Toxicol 39:367–374. https://doi.org/10.3109/01480545.2015.1122606
Zhang Z, Fu J, Yao B, Zhang X, Zhao P, Zhou Z (2011) In vitro genotoxicity of danthron and its potential mechanism. Mut Res/genetic Toxicol Environ Mutagenesis 722:39–43. https://doi.org/10.1016/j.mrgentox.2011.02.006. (PMID: 21354327)
Zhao SX, Dalvie DK, Kelly JM, Soglia JR, Frederick KS, Smith EB, Obach RS, Kalgutkar AS (2007) NADPH-dependent covalent binding of [3H]paroxetine to human liver microsomes and S-9 fractions: identification of an electrophilic quinone metabolite of paroxetine. Chem Res Toxicol 20:1649–1657. https://doi.org/10.1021/tx700132x
Zhao M, Zhang T, Li G, Qiu F, Sun Y, Zhao L (2017) Associations of CYP2C9 and CYP2A6 polymorphisms with the concentrations of valproate and its hepatotoxin metabolites and valproate-induced hepatotoxicity. Basic Clin Pharmacol Toxicol 121:138–143. https://doi.org/10.1111/bcpt.12776
Zhao XJ, Kawashiro T, Ishizaki T (1998) Mutual inhibition between quinine and etoposide by human liver microsomes. Evidence for cytochrome P4503A4 involvement in their major metabolic pathways. Drug Metab Dispos 26:188–191. (PMID: 9456308). https://dmd.aspetjournals.org/content/26/2/188.long
Zheng N, Pang S, Oe T, Felix CA, Wehrli S, Blair IA (2006) Characterization of an etoposide-glutathione conjugate derived from metabolic activation by human cytochrome P450. Curr Drug Metab 7:897–911. https://doi.org/10.2174/138920006779010638
Zhou L, Erickson RR, Hardwick JP, Park SS, Wrighton SA, Holtzman JL (1997) Catalysis of the cysteine conjugation and protein binding of acetaminophen by microsomes from a human lymphoblast line transfected with the cDNAs of various forms of human cytochrome P450. J Pharmacol Exp Ther 281:785–790. (PMID: 9152386). https://jpet.aspetjournals.org/content/281/2/785.long
Zhu BT, Roy D, Liehr JG (1993) The carcinogenic activity of ethinyl estrogens is determined by both their hormonal characteristics and their conversion to catechol metabolites. Endocrinology 132:577–583. https://doi.org/10.1210/endo.132.2.8381068
Zhuo X, Zheng N, Felix CA, Blair IA (2004) Kinetics and regulation of cytochrome P450-mediated etoposide metabolism. Drug Metab Dispos 32:993–1000. https://dmd.aspetjournals.org/content/32/9/993.long
Zientek M, Jiang Y, Youdim K, Obach RS (2010) In vitro-in vivo correlation for intrinsic clearance for drugs metabolized by human aldehyde oxidase. Drug Metab Dispos 38:1322–1327. https://doi.org/10.1124/dmd.110.033555
Acknowledgements
The authors thank Jan Dragašević for assistance in the preparation of the manuscript.
Funding
One of the authors (F. P. G.) was supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) grant number R01 GM118122 [to F. P. G.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rendic, S.P., Guengerich, F.P. Formation of potentially toxic metabolites of drugs in reactions catalyzed by human drug-metabolizing enzymes. Arch Toxicol 98, 1581–1628 (2024). https://doi.org/10.1007/s00204-024-03710-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-024-03710-9