Abstract
This is an overview of the metabolic activation of drugs, natural products, physiological compounds, and general chemicals by the catalytic activity of cytochrome P450 enzymes belonging to Families 1–4. The data were collected from > 5152 references. The total number of data entries of reactions catalyzed by P450s Families 1–4 was 7696 of which 1121 (~ 15%) were defined as bioactivation reactions of different degrees. The data were divided into groups of General Chemicals, Drugs, Natural Products, and Physiological Compounds, presented in tabular form. The metabolism and bioactivation of selected examples of each group are discussed. In most of the cases, the metabolites are directly toxic chemicals reacting with cell macromolecules, but in some cases the metabolites formed are not direct toxicants but participate as substrates in succeeding metabolic reactions (e.g., conjugation reactions), the products of which are final toxicants. We identified a high level of activation for three groups of compounds (General Chemicals, Drugs, and Natural Products) yielding activated metabolites and the generally low participation of Physiological Compounds in bioactivation reactions. In the group of General Chemicals, P450 enzymes 1A1, 1A2, and 1B1 dominate in the formation of activated metabolites. Drugs are mostly activated by the enzyme P450 3A4, and Natural Products by P450s 1A2, 2E1, and 3A4. Physiological Compounds showed no clearly dominant enzyme, but the highest numbers of activations are attributed to P450 1A, 1B1, and 3A enzymes. The results thus show, perhaps not surprisingly, that Physiological Compounds are infrequent substrates in bioactivation reactions catalyzed by P450 enzyme Families 1–4, with the exception of estrogens and arachidonic acid. The results thus provide information on the enzymes that activate specific groups of chemicals to toxic metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human cytochrome P450 (P450, CYP) enzymes catalyze a great number of metabolic reactions that have important effects on the biological activities (physiologic, therapeutic, and/or toxic) of xenobiotics such as drugs, natural products, general chemicals (e.g., environmental chemicals such as pesticides, pro-carcinogens), and physiological compounds. Their general role and significance for metabolism in humans has been discussed and reviewed previously. In addition, in previous publications efforts were made to estimate the participation of the activity of different groups of enzymes, e.g. oxidoreductase enzymes (FMO (microsomal flavin-containing monooxygenase), AKR (aldo–keto reductase), MAO (monoamine oxidase), and P450 enzymes), in the metabolism of natural products and physiological chemicals and general chemicals in humans. When the groups of chemicals were analyzed, the results showed the highest values for participation of P450 enzymes in the metabolism of drugs and general chemicals as substrates. For P450 enzymes the calculations also showed that, regarding drug metabolism, more than three-fourths of the human P450 reactions can be accounted for by a set of five P450s: 1A2, 2C9, 2C19, 2D6, and 3A4, with the largest fraction of the P450 reactions being catalyzed by P450 3A enzymes. Compared to other oxidoreductase enzymes and taking into consideration chemicals that are classified as carcinogens, our calculations showed that metabolic activations of the compounds to toxic metabolites are dominantly catalyzed by P450 enzymes (66% of bioactivations) and that, within this group, six P450s (1A1, 1A2, 1B1, 2A6, 2E1, and 3A4) accounted for 77% of the P450 activation reactions. In the present review we have updated and extended our calculations to general activation reactions forming potentially toxic metabolites as a consequence of metabolic activation of drugs, natural products, physiological compounds, and general chemicals (Rendic 2002; Rendic and Di Carlo 1997; Rendic and Guengerich 2012,2015). We recently reviewed the properties (mechanisms, induction, inhibition, toxic effects, and benefits) of human P450s belonging to the P450 Families 5–51 (i.e., 22 of the total 57 P450s) that are responsible for metabolism and biosynthesis of physiological compounds, including their substrate selectivity, information, and references (Rendic and Guengerich 2018). In the present paper, we update and discuss important aspects of many of the P450s belonging to Families 1–4, including the reactions and the roles in metabolic activation of xenobiotics (drugs, natural products, general chemicals) and physiological compounds.
Results and discussion
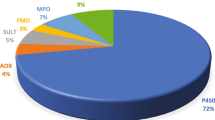
A synopsis of the data used for the analysis of the catalytic activity of P450 Families 1–4 is presented in Table 1. Data were collected from more than 5152 references. The total number of data entries for enzymatic reactions catalyzed by P450s belonging to 1–4 Families was 7686 of which 1114 (~ 15%) were defined as bioactivation reactions of different degrees. When considering the activation of all compounds the results show predominant participation of P450s 3A4, 1A2, and 1A1, followed by P450s 2E1 and 1B1. P450s 2C9, 2D6, 2A6, 2C19, and 2B6 also have significant participation in bioactivation reactions (Fig. 1).
Data analyzed were divided into four groups of compounds: General Chemicals, Drugs, Natural Products, and Physiological Compounds. Of the 2165 reactions for General Chemicals, 618 (29%) were classified as activations; for 4032 Drugs entries, 237 (6%) were classified as activations; for the 952 reactions under Natural Products, 186 (20%) were classified as activations; for the 530 Physiological Compounds, 75 reactions (14%) were classified as activations (Table 1).
General chemicals
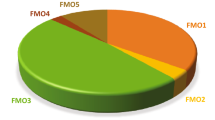
We reported previously that the metabolism of General Chemicals catalyzed by human enzymes is predominately catalyzed by P450 enzymes in humans (~ 92%) (Rendic and Guengerich 2015). Other enzymes, besides P450s, that participate in a greater extent include those in the AKR, FMO, and MAO families (Rendic and Guengerich 2015). P450 enzymes dominate in bioactivation of carcinogens (66%) over other xenobiotic-metabolizing enzymes (Rendic and Guengerich 2012). The present data show that among P450 enzymes, Family 1 enzymes (P450s 1A1, 1A2, B1) dominate in activations of General Chemicals, followed by P450s 2E1, 3A4, and 2A6 (Fig. 2).
The following examples illustrate the participation of P450 enzymes in the bioactivation of selected General Chemicals substrates.
Polycyclic aromatic hydrocarbons (PAHs)
Examples (213 data entries) of the metabolic activation of a group of general chemicals (e.g., polycyclic aromatic hydrocarbons (PAHs), heterocyclic and aromatic amines, insecticides, organic solvents) are presented in Table 2. The majority of the data presented (75 data entries) involve PAHs and their metabolites. Of the 76 entries presented in Table 2, 24 are attributed as “high activity” or “high activation” and are catalyzed by P450 1A1, 1A2, 1B1, 2A13, and 2A6 enzymes. These data correlate well with experimental findings on the activation of PAHs by P450 enzymes (Shimada et al. 2013). The parent PAH compounds are not toxic per se but their products formed by hydroxylation and epoxidation reactions, catalyzed by P450 enzymes, are reactive and interact with cellular macromolecules. Consequently, the literature data on activation of PAHs are predominately focused on activation of the PAH metabolites (e.g., dihydrodiols possessing different stereochemical structures) to ultimate toxic dihydrodiol epoxides, as exemplified by the classic activation of benzo[a]pyrene (B[a]P) (Fig. 3).
The following examples are taken from Table 2 illustrate PAH compounds for which metabolic activation is attributed as “high activity reaction” and/or “high activation” (for references see Table 2):
P450 1A1: 5-Methylchrysene, trans-5-methylchrysene-1,2-diol, 7,12-dimethylbenz[a]anthracene (7,12-DMBA), trans-7,12-DMBA-3,4-diol, (±)-benzo[a]pyrene (B[a]P) -7,8-dihydrodiol, cis-(−)-B[a]P-7,8-dihydrodiol, trans-(+)-B[a]P-7,8-dihydrodiol, trans-(−)-B[a]P-7,8-dihydrodiol, dibenzo[a,l]pyrene (DB[a,l]P), trans-(−)-DB[a,l]P-(11R,12R)-diol
P4501A2: Dibenzo[a,l]pyrene (DB[a,l]P)
P4501B1: B[a]P, (+, −)-B[a]P-7,8-dihydrodiol, cis-(−)-B[a]P-7,8-dihydrodiol, trans-(+)-B[a]P-7,8-dihydrodiol, trans-5-Methylchrysene-1,2-diol, trans-7,12-DMBA-3,4-diol), dibenzo[a,l]pyrene (DB[a,l]P), trans-(−)-DB[a,l]P-(11R,12R)-diol
P450 2A13: trans-5-Methylchrysene-1,2-diol
P450 2A6: trans-5-Methylchrysene-1,2-diol
Heterocyclic and aromatic amines
Activation of heterocyclic, aromatic, and azoaromatic amines is represented by 58 cadsentries (Table 2) of which 15 are attributed as “high activity” and/or “high activation” catalyzed by P450 1A1, 1A2, 1B1, 2A13, 2A6, and 3A4. The reactions of activation or aromatic and heterocyclic amines are presented in Figs. 4 and 5 as illustrated by activation of 2-aminofluorene and MeIQx, respectively.
The following examples illustrate the metabolic activation of heterocyclic compounds by specific P450s (Table 2 and references therein):
P450 1A2: 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-6-methyldipyrido[1,2-a,3,2′-d]-imidazole (Glu-P-1)
P450 1A1: 2-Aminoanthracene (2-AA), 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1), 6-aminochrysene
P450 1A2: 2-Aminofluorene (2-AF)
P450 1B1: 2-AA
P450 2A6: 2-AA
P450 2A13: 2-AF
P450 3A4: 3-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1), 6-aminochrysene
Insecticides
Activation of organophosphate insecticides is represented by chlorpyrifos, diazinon, parathion, and azinphos-methyl (Table 2 and references therein). The compounds are metabolically activated to neurotoxic metabolites (i.e. oxon derivatives) by desulfuration reactions catalyzed by P450 enzymes . Chlorpyrifos (Fig. 6) and parathion (Fig. 7) are activated by P450 1A2, 2B6, 2D6, 2C8, 2C19, 3A4, and 3A5 enzymes, of which P450 2B6 is the most prominent at lower concentrations (20 µM) and having the highest kcat/Km value. In addition to the oxon derivative, chloropyrifos is also metabolized to the less toxic 3,5,6-trichloro-2-pyridinol by P450 3A4 (Jan et al. 2016; Crane et al. 2012a, 2012b; Croom et al. 2010; Mutch and Williams 2006).
Azinphos-methyl is activated primarily by P450 1A2 (at low concentrations), and 2B6 and 3A4 (at higher concentrations) (Table 2) (Buratti et al. 2002, 2003). The entries in Table 2 show that at lower concentrations organophosphates are activated predominately by P450 1A1, 1A2, 2B6, and 2C19 and at high concentrations by P450 3A4.
Drugs
A present and historical view of the activation of drugs and their conversion to reactive metabolites as substrates of P450 enzymes has been reviewed recently by one of the authors of the present paper. It has been pointed out that P450 metabolic activity often prevents drug toxicity (for instance making drug elimination faster), but on the opposite side it can, in some cases, result in the conversion of drugs to reactive metabolites that cause toxicity (Guengerich 2020). The final properties of the products of drug-P450 enzyme reactions can also be significantly affected by factors such as (a) variations in the activity caused by genetic polymorphism and thus primarily on the level of single nucleotide variations (SNVs), or (b) by enzyme induction and/or inhibition of activity by environmental chemicals or by co-administered drug(s) (Guengerich and Rendic 2010; Guengerich 2020). Examples of drugs that are converted to toxic metabolites, due to the activity of P450 enzymes, are listed in Table 3. It must be emphasized that most drugs, used in recommended doses, are not or are only slightly toxic per se due to extensive testing in preclinical and clinical testing of drugs. However, as mentioned before, toxic metabolites might be formed under circumstances of enhanced dose, when applied with other drugs/chemicals that might redirect metabolism pathway to the formation of toxic metabolites, or when genetic polymorphism of the particular enzyme was not tested or observed in early drug testing. It is prudent to remember the words of Paracelsus, paraphrased, “the dose makes the poison (only the dose distinguishes a medicine from a poison)” (Borzelleca 2000). Selected examples of drugs taken from Table 3 are discussed, for which toxicity is related to metabolic conversion to toxic products and is known to occur during clinical use. In addition, therapeutic compounds are presented that are used as pro-drugs. Such pro-drugs are therapeutically inactive until activated by P450 enzymes but can became also cytotoxic in healthy cells/tissues when used in therapy (e.g., the anticancer drugs cyclophosphamide, ifosfamide, and AQ4N (banoxantrone; 1,4-bis{[2-(dimethylamino)ethyl]amino}-5,8-hydroxy-anthracene-9,10-dione bis-N-oxide)). Also included is the natural product drug ellipticine, which is used in cancer therapy and activated to a cytotoxic metabolite (Table 3). However, while the inherent toxicity of a drug might be lowered, the metabolites formed might be also less toxic and less therapeutically active. An example of such a drug is trabectedin (ecteinascidin 743), an anti-cancer drug of marine origin for which the side effects include myelosuppression, hepatotoxicity, and nausea and vomiting (Held-Warmkessel 2003). Trabectedin is metabolized by P450 3A4 (major enzyme) and in addition by P450s 2C9, 2C19, 2D6, and 2E1. Metabolic and inhibition studies revealed that the metabolites formed are less cytotoxic and less therapeutically active than the parent drug. Inhibitors of P450 enzymes significantly increased cytotoxicity of the drug in a human cell line model system (Reid et al. 2002; Brandon et al. 2005, 2006).
The numbers of activation reactions of drugs as substrates of human P450 enzymes are presented in Fig. 8, calculated from our records. Of the total of 4039 reactions, 235 (~ 6%) involve activation and formation of potentially toxic intermediates or metabolites. P450 3A4 clearly dominated in the formation of toxic metabolites compared with other P450s, catalyzing ~ 25% of the bioactivation reactions.
The following examples illustrate the participation of P450 enzymes in the bioactivation of selected drugs (Table 3).
AQ4N
AQ4N, an aliphatic amine di-N-oxide, is a potent topoisomerase II inhibitor and in clinical trials as a potential anticancer drug. It is inactive until enzymatically bioactivated to an active amine under the reductive conditions present in hypoxic tumor cells (Fig. 9) (Patterson 1993; Lalani et al. 2007; O'Rourke et al. 2008). Because the amine AQ4 is very toxic to normal cells, it is not inherently suitable for delivery as an anticancer drug. AQ4N is reported to be a substrate of several P450s (i.e. 1A1, 1A2, 1B1, 2B6, 2W1, 2S1, and 3A4) (Table 3 and references therein), but most efficiently by P450s 1A1 and 2B6 (Yakkundi et al. 2006)). Under reducing oxygen conditions (hypoxia) AQ4N is reduced to the cytotoxic AQ4-mono-N-oxide (AQ4M) and amine (AQ4) (Patterson et al. 1999). Of the enzymes involved in the metabolism of AQN4, P450 2W1 is highly expressed in some human colon and adrenal tumors and was suggested as tumor-specific enzyme. In addition, strong expression of P450 2S1 has been reported in tumors of epithelial origin and hypoxic tumors and the gene was found to be overexpressed under hypoxic conditions (Karlgren et al. 2005; Saarikoski et al. 2005; Rivera et al. 2007; Nishida et al. 2010; Xiao et al. 2011).
Cyclophosphamide and ifosfamide
Cyclophosphamide and ifosfamide are widely used anticancer agents that require metabolic activation by P450 enzymes (Figs. 10 and 11, respectively). While 4-hydroxylation yields DNA-alkylating and cytotoxic metabolites, N-dechloroethylation results in the generation of neuro- and nephrotoxic products. Cyclophosphamide and ifosfamide undergo extensive P450-catalyzed metabolism to yield both active (4-hydroxylated) and therapeutically inactive but neurotoxic N-dechloroethyl metabolites, and ovarian toxicity is a major concern with cyclophosphamide therapy. The human liver microsomal P450 metabolism of cyclophosphamide and ifosfamide 4-hydroxylation is well characterized (Table 3 and references therein). Of all P450 enzymes, P450 3A4 exhibited the highest N-dechloroethylation activity (bioactivation) toward both cyclophosphamide and ifosfamide, whereas P450 2B6 and P450 3A4 displayed high N-dechloroethylation activity toward ifosfamide but not cyclophosphamide. With cyclophosphamide as substrate, P450 3A4 was shown to catalyze ≥ 95% of liver microsomal N-dechloroethylation, whereas with ifosfamide as substrate, P450 3A4 catalyzed an average of ~ 70% of liver microsomal N-dechloroethylation (range 40–90%), with the balance of this activity catalyzed by P450 2B6 (range 10–70%, depending on the P450 2B6 content of the liver) (Huang and Waxman 2000). In the case of cyclophosphamide treatment, determination of selected P450 enzyme genotypes (such as frequencies of the variant alleles CYP2B6*5, CYP2C19*2, CYP2C9*2, and CYP3A5*3) has been proposed for predicting the risk of premature ovarian failure in lupus nephritis patients (Takada et al. 2004).
Acetaminophen
Acetaminophen (paracetamol, Tylenol®) is one of the most widely used drugs in the world. It is very safe when used at therapeutic doses. However, it is also involved in ~ ½ of the cases of drug-induced liver failure and well exemplifies the axiom of Paracelsus about the “the dose makes the poison” (Larson et al. 2005). When overdosed, the drug causes mitochondrial dysfunction and centrilobular necrosis in the liver in humans and experimental animals (Yoon et al. 2016). Normally most of the ingested drug is eliminated by glucuronidation and sulfation, catalyzed by UDP-glucuronosyltransferases (UGT1A1 and 1A6) and sulfotransferases (SULT1A1, 1A3/4, and 1E1), respectively, producing nontoxic conjugates (McGill and Jaeschke 2013) (Fig. 12).
The key mechanism in the hepatotoxicity is P450-catalyzed formation of the reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which depletes hepatic glutathione and accumulates to cause hepatocellular liver damage, including oxidative stress (Fig. 13). Approximately 5–9% of orally administered acetaminophen is metabolized by P450 2E1, 1A2, and 3A4 catalyzed oxidation reactions (Table 3 and references therein). At a toxic concentration the formation of the NAPQI-glutathione conjugate was highest with P450 3A4, having the lowest Km value of 130 μM (for comparison therapeutic concentrations of paracetamol are ~ 50 μM and toxic concentrations are ~ 1 mM) followed by P450 2E1 and P450 1A2 (Patten et al. 1993)). It has been proposed that human P450 3A4 is the major enzyme catalyzing acetaminophen oxidation to NAPQI (Laine et al. 2009). Other studies using inhibition studies with human recombinant enzymes indicated that P450 1A2 is the enzyme responsible for acetaminophen metabolic activation (Tan et al. 2008). In mice, the deletion of P450 2e1 or 1a2 blocks acetaminophen toxicity (Lee et al. 1996; Zaher et al. 1998).
Halothane
Halothane was previously the most widely used anesthetic agent and in 1963 was reported to cause liver necrosis in humans. It was shown that halothane liver necrosis was induced following pretreatment of rats with polychlorinated biphenyls, known inducers of P450 enzymes. The liver necrosis caused by halothane anesthesia could be prevented by administration of metyrapone, a rather non-selective inhibitor of P450 enzymes, prior to anesthesia. These findings indicated that halothane toxicity resulted from metabolic activation of halothane by P450 enzymes (Nastainczyk et al. 1982). In addition, halothane hepatotoxicity could be potentiated in rats by chronic administration of ethanol, an inducer of P450 2E1 (Takagi et al. 1983). Human P450s activate halothane by both reductive and oxidative metabolism (Table 3; Fig. 13), and oxidative dehalogenation by P450 2E1 is a major in vivo reaction with low Km values (and also results in lipid peroxidation). Limited participation of P450 2A6 activation has been indicated in vivo. Reductive activation of halothane is catalyzed by P450 2A6 and 3A4 enzymes (Table 3 and references therein). Halothane oxidation, the major metabolic pathway, leads to the production of the reactive electrophile trifluoroacetyl chloride, and subsequent acylation of liver proteins results in the formation of trifluoroacetylated protein neoantigens. Metabolic halothane reduction leads to the formation of the 2-chloro-1,1,1-trifluoroethyl radical by hemolytic cleavage of the C–Br bond (Fig. 13) (Kurth et al. 2014). Halothane has been replaced in most countries by other, less toxic, inhalation anesthetics due to its induced hepatitis, but there is still the possibility that it is in use in some developing countries.
17α-Ethynylestradiol
17α-Ethynylestradiol is in use as the estrogenic component of oral contraceptives (Bolt 1979). Similar to natural estrogens (see the Physiological Compounds section, vide infra) (Lacassagne 1932), 17α-ethynylestradiol is a weak carcinogen in rats, and carcinogenic activity has been associated with the formation of catechol metabolites (Fig. 14) (Zhu et al. 1993). C2-hydroxylation catalyzed by P450 enzymes (mainly P450 3A4) was found to be the main metabolic pathway of 17α-ethynylestradiol in individuals using oral contraceptives (Guengerich 1988)). This metabolite is excreted as 2-methoxyethynylestradiol after O-methylation (Back et al. 1984). Induction of P450 3A4 by rifampicin, barbiturates, or herbal remedies such as St. John’s wort can lead to increased clearance and unplanned pregnancy (Bolt et al. 1977; Guengerich 1988).
Natural products
Natural products, including herbal supplements, can have multiple effects on the activity of P450 enzymes, for instance inhibition or induction of activity and/or their expression (St. John’s wort, vide supra). By changing activity and/or expression of the enzymes and applied concomitantly with drugs, natural chemicals can provoke drug-phytochemical interactions. Such activity might result in altered therapeutic and or toxic properties of drugs (Guengerich and Rendic 2010; Rendic and Guengerich 2010). Examples of different classes of natural compounds that can be activated to toxic metabolites by cytochrome P450s (e.g., alkaloids, monoterpenes, mycotoxins, N-nitrosamines) are presented in Table 4. Natural products, as substrates of P450 enzymes, can be both activated to toxic and detoxicated to nontoxic products by P450 enzymes in different ways. For instance, aflatoxin B1 (AFB1) is activated to toxic and detoxicated to nontoxic metabolites by oxidative reactions, while aristolochic acid is activated by nitro reduction under (partially anaerobic conditions), and oxidative metabolism results in the formation of a nontoxic O-demethylated product. Estragole and safrole are examples in which metabolism by P450 enzymes to nontoxic metabolites can be followed by activation to toxic metabolites by conjugation to form a sulfate ester (Table 4 and references therein).
3-Methylindole (skatole) is formed in nature by microbial degradation of tryptophan and tyrosine (Carlson and Breeze 1984), but is also present in humans where it is formed by the decarboxylation of tryptophan in the large intestine. 3-Methylindole is a selective pulmonary toxicant and, in addition to intestinal formation and absorption, cigarette smoke is an additional source of 3-methylindole in smokers. 3-Methylindole may provoke pneumotoxicity and lung cancer by the activity of P450 1A1 and P450 2F1 (Weems et al. 2010). Toxicity of 3-methylindole depends on bioactivation by several reactions: epoxidation (3-methyloxindole formation, P450 1A1, 1A2, 1B1, 2E1, 2A6), C-hydroxylation (indole-3-carbinol formation, P450 1A1, 1A2, 1B1), and dehydrogenation (3-methyleneindolenine formation, P450 1A1, 1A2, 2A13, 2F1 (Table 4 and references therein).
The numbers of activation reactions catalyzed by human P450 enzymes reacting with natural products as substrates are presented in Fig. 15. Of the total of 952 reactions identified in our records, 152 (~ 16%) involve bioactivation and the formation of potentially toxic products. The major P450s involved in the activations are P450s 1A2 (~ 12%), P450s 2E1 and P450 3A4 (~ 11% each), followed by P450 1A1 and 2A6 (~ 10%).
The following examples illustrate the participation of P450 enzymes in the bioactivation of selected natural compounds (Table 4).
Aflatoxins
AFB1 is a potent hepatocarcinogen in animal models and also classified as a hepatocarcinogen in humans. AFB1 is metabolically activated by P450 enzymes to form cytotoxic and DNA-reactive intermediates (Fig. 16). AFB1 is activated to the toxic exo-8,9 epoxide most prominently by P450 Subfamily 3A enzymes in liver and P450 2A13 in the lung (Shimada and Guengerich 1989; Deng et al. 2018). In addition to its hepatotoxicity, AFB1 can be toxic in lungs (at least in animal models) due to the activity of P450 2A enzymes. P450 3A enzymes (3A4 and 3A5) oxidize AFB1 to the highly mutagenic exo-8,9-epoxide (Fig. 16), while P450 1A2 oxidizes it to a roughly equimolar mixture of toxic exo- plus the endo-epoxide, the latter of which is essentially non-mutagenic (Iyer et al. 1994). Both P450 3A4 and 1A2 enzymes also catalyze AFB1 detoxication reactions, i.e. 3α-hydroxylation in the case of P450 3A4 (aflatoxin Q1 formation) and 9a-hydroxylation in the case of P450 1A2 (aflatoxin M1 formation) (Rendic and Guengerich 2012)). This example illustrates that P450 enzymes can catalyze both activation and detoxication reactions acting on the same substrate. The toxic AFB1-exo-8,9-epoxide is detoxicated by glutathione (GSH) transferases by conjugation of GSH to the epoxide (Johnson et al. 1997; Deng et al. 2018; Yang et al. 2012). In addition to a being substrate of P450 enzymes, AFB1 is an inducer of P450 1A1, 1B1, and 3A4 in monocytes (Bahari et al. 2014), and the compound might enhance its own metabolism or metabolism of another substrate of the enzyme.
Artistocholic acid
Aristolochic acids constitute a group of compounds found naturally in many types of plants known as Aristolochiaceae, including Aristolochia and Asarum (wild ginger) grown worldwide. Aristolochic acid I and II are the predominant chemical toxins in the plants. Aristolochic acid compounds were shown to be the cause of a kidney disease called Chinese herb nephropathy, now renamed aristolochic acid nephropathy (Arlt et al. 2002; Schmeiser et al. 2009; Gökmen et al. 2013). Aristocholic acid is classified by the International Agency for Research on Cancer as a Group I carcinogen. This natural product has also been implicated in the development of another kidney disease, Balkan endemic nephropathy, and its associated urothelial malignancy. The disease is endemic in certain rural areas of Balkan countries located closed to the tributaries of the Danube river basin (Arlt et al. 2007; Grollman et al. 2007; Han et al. 2019). As already mentioned, aristolochic acid I is activated by reduction of the nitro group (under partially anaerobic conditions), and oxidative metabolism results in the formation of nontoxic O-demethylated metabolites. Nitro reduction of aristolochic acid I, considered as the major factor causing its toxicity, is required to exert its carcinogenic properties. The reaction catalyzed by P450s 1A1 and 1A2 results in the generation of N-hydroxyaristolactam I, which leads to the formation of a cyclic acyl nitrenium ion, the intermediate that either forms DNA adducts or rearranges to 7-hydroxyaristolactam I (Fig. 17). Aristolochic acid I oxidation to a nontoxic metabolite by O-demethylation of the methoxy group is catalyzed by the same enzymes, i.e. P450 1A1 and 1A2, with contribution from P450 2C9 and 3A4 (Table 4). The product of the reactions is 8-hydroxyaristolochic acid I, a detoxication product. The O-demethylated metabolite is excreted either in its free or conjugated form (Chan et al. 2006; Shibutani et al. 2010; Arlt et al. 2011; Stiborová et al. 2012a).
Estragole
Estragole is a common component of herbs and spices and is a natural constituent of basil oil. It is also a genotoxic hepatocarcinogen in rodents, and its potential toxic effect in humans is still under consideration. One of the major sources of human exposure to this phytochemical is Foeniculum vulgare Mill. (fennel) (Levorato et al. 2018). Toxicity is ascribed to its hydroxylation in position C1´, catalyzed by P450s 1A2, 2A6, 2C19, 2D6, and 2E1, of which P450s 1A2 and 2A6 are the major enzymes (Table 4 and references therein). Other enzymes can also contribute but at relatively high concentrations of estragole. The metabolite of P450 oxidation is not inherently toxic; however, C1´-hydroxylation of estragole is the first step in activation followed by sulfate conjugation by a sulfotransferase to produce genotoxic 3´-sulfoxyestragole (Fig. 18) (Monien et al. 2019).
Ethanol
Ethanol is widely consumed and is metabolically activated to toxic acetaldehyde (Fig. 19). The metabolism and activation of ethanol is primarily catalyzed by alcohol dehydrogenase and, to a lesser extent, catalase but under certain circumstances (e.g., high doses) P450 enzymes can also be involved. Many P450 enzymes in Families 1–4 oxidize ethanol to acetaldehyde at high concentrations, namely 1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 (Table 3), but P450 2E1 has the highest catalytic activity (i.e., specificity constant, kcat/Km).
The role of P450 2E1 in ethanol metabolism has been reviewed recently. P450 2E1, 3A4, and 1A2 were reported as P450s that are significantly involved in the oxidation of ethanol to acetaldehyde under conditions of high concentration (Km ~ 10 mM) and chronic use (Hamitouche et al. 2006; Guengerich and Avadhani 2018; Guengerich 2020).
Safrole
Safrole is a natural compound categorized as an IARC Group 2B carcinogen. It is extracted from sassafras oil or certain other essential oils and also from betel quid. Safrole was reported to be a rodent hepatocarcinogen, and DNA adducts were identified in liver samples of patients having a history of betel quid chewing (Bolton et al. 1994; Chung et al. 2008). In addition, betel quid chewing is associated with oral and hypopharynx cancers (Shield et al. 2017; Chen et al. 2017). The metabolism of safrole was reported to be predominantly catalyzed by P450 1A2, with minor contributions by P450 2E1. It was suggested that the ortho-quinone metabolite may mediate safrole hepatotoxicity (Fig. 20; Table 4 and references therein). Safrole can also, as in the case of estragole, undergo bioactivation by sequential 1´-hydroxylation and sulfation, resulting in reactive intermediates capable of forming DNA adducts (Jeurissen et al. 2004). In addition, it has been reported that safrole is a mechanism-based inhibitor of P450 1A2 (Hu et al. 2019; Yang et al. 2018). It has been also reported that safrole induced P450 2A6 activity and tobacco specific 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolic activation, resulting in higher NNK-induced genotoxicity (Tsou et al. 2019).
N´-Nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)
The tobacco-specific nitrosamines NNN and NNK are potent carcinogens in animal models and are believed to be causative agents for esophageal cancer in smokers and those using chewing tobacco and snuff. Metabolic activation of NNN is required to exert carcinogenic potential (Fig. 21) and occurs through P450 catalyzed 2´- and 5´-hydroxylation, which generates unstable metabolites that decompose to 4-hydroxy-1-(3-pyridyl)-1-butanone ('keto alcohol') and 4-hydroxy-4-(3-pyridyl)butanal, respectively. The latter cyclizes to 5-(3-pyridyl)-2-hydroxytetrahydrofuran ('lactol'). P450s 2E1, 2A6, and 3A4 were identified as major catalysts for NNN 5´-hydroxylation in human liver microsomes (Yamazaki et al. 1992; Hecht 1998; Wong et al. 2005a; Patten et al. 1996, 1997; Carlson et al. 2016; Fan et al. 2019; Staretz et al. 1997; Fujita and Kamataki 2001a).
NNK, a potent tobacco-specific carcinogen, has been demonstrated to induce lung tumors in animals and is suspected to be a human carcinogen. P450s are the major enzymes responsible for the activation of NNK in lung and liver microsomes of rats and mice, as well as in the human liver. Human P450s 2A6 and 3A4 are involved in the activation of NNK (Smith et al. 1995; Staretz et al. 1997). In addition, it was demonstrated that P450s 1A2, 2A6, and 3A4 may be important for the activation of NNK to a DNA-methylating agent (‘keto aldehyde’) via the α-methylene hydroxylation pathway (Fig. 22). P450s 1A2, 2E1, and 2D6 are selective for α-methyl hydroxylation of NNK, leading to keto alcohol and a DNA-pyridyloxobutylating agent. P450 1A2 exhibits at least twice the specificity toward NNK bioactivation compared to P450 2E1 and catalyzed the formation of both, keto alcohol and 4-oxo-1-(3-pyridyl)-1-butanone (keto aldehyde) with the keto alcohol being the major product (Patten et al. 1996, 1997; Krishnan et al. 2009; Smith et al. 1996) (Table 4 and references therein).
Physiological compounds
Physiological substrates of P450 Family 1–4 enzymes include eicosanoids, estrogens (e.g., estradiol), fatty acids (e.g., arachidonic acid), cholesterol, fat-soluble vitamins (e.g., vitamins A, D3, E, and K), neurotransmitters (serotonin, tryptamine), leukotrienes, prostaglandins, fatty acids (e.g. arachidonic acid), bile acids (e.g. lithocholic, deoxycholic, cholic acid), corticosteroids, androgens (e.g., androstenedione, testosterone, dihydrotestosterone), and progesterone. In addition to being substrates of P450s Families 1–4, these compounds are predominately substrates of the enzymes belonging to Families 5–51 (with 22 enzymes) (Rendic and Di Carlo 1997; Rendic and Guengerich 2018). The data presented in Fig. 23 show the participation of Family 1–4 P450s in the activation of physiological compounds to some potentially toxic products. Of the total 530 metabolic reactions (data from our records), 75 (14%) involve bioactivation. The highest involvement is with P450s 1A1, 1A2, 1B1, 3A4, and 3A5 (~ 9% each), followed closely by P450 2C9 (8%). Physiological substrates in activation reactions include estrogenic hormones (17β-estradiol and estrone) and fatty acids (Table 5). In addition to being activated to toxic products, fatty acids (exemplified by arachidonic acid) can both down-regulate (Palacharla et al. 2017) or induce P450 activity by changing their expression (Finn et al. 2009). In some cases, the reaction products are not inherently reactive but may have deleterious signaling properties [e.g., 20-HETE, EETs (Sausville et al. 2018)].
Although a relatively low number of activations are ascribed to P450 enzymes interacting with physiological compounds, some of them are important because they can possibly cause either cancer (e.g., estrogenic hormones) or have an important impact on physiological processes related to high blood pressure (arachidonic acid).
17β-Estradiol and estrone
Estrogenic hormones (e.g., 17β-estradiol and estrone) can induce tumors in various organs of experimental animals (Lacassagne 1932). In humans, elevated circulating estrogen levels increase the risk of breast and uterine cancer. Estrogens can act as hormone stimulating cell proliferators and also as procarcinogens, inducing genetic damage (Yager 2000; Liehr 2000). 17β-Estradiol and estrone are eliminated from the body by metabolic conversion to inactive metabolites that are excreted in the urine and/or feces following oxidations and conjugation reactions. The first step in the metabolism of estrogens is hydroxylation catalyzed by P450 enzymes (Fishman et al. 1970; Zhu and Lee 2005). A large number of hydroxylated metabolites are formed and catalyzed by P450 Family 1–4 (Table 5); however, we focus here on reactions leading to the formation of activated and toxic metabolites. Activations of 17β-estradiol and estrone by hydroxylation at positions C2 and C4 have been suggested to be major reactions involved in mammary carcinogenesis and other cancers (Cavalieri and Rogan 2006; Cavalieri et al. 2006). The data (Table 5 and references therein) also show that formation of the major metabolite of 17β-estradiol, 2-hydroxyestradiol, is mainly catalyzed by P450s 1A2 and 3A4, and by P450 1A1 in extrahepatic tissues. P450 1B1, which is highly expressed in estrogen target tissues including mammary, ovary, and uterus, selectively catalyzes the 4-hydroxylation of 17β-estradiol (Guengerich et al. 2003; Chun and Kim 2016; Wen et al. 2007) Formation of catechols of estrone and estradiol is considered as a part of the carcinogenic process, in that these compounds can readily be further oxidized to reactive quinones, semiquinones, and reactive oxygen species are formed (Bolton and Thatcher 2008). 4-Hydroxyestradiol can generate free radicals from redox cycling, with the formation of corresponding semiquinone and quinone forms causing cellular damage. Local formation of 4-hydroxyestradiol in breast and endometrial cancers has been reported (Tsuchiya et al. 2005; Hayes et al. 1996; Spink et al. 1997; Liehr 2000; Shimada et al. 1999; Bolton 2002; Bolton and Thatcher 2008; Fussell et al. 2011). Estradiol-3,4-quinone is more reactive with DNA than estradiol-2,3-quinone, and the relative reactivities of estradiol-3,4-quinone and estradiol-2,3-quinone to form depurinating adducts have been correlated with the carcinogenicity, mutagenicity, and cell-transforming activity of their precursors, the catechol estrogens 4-hydroxyestradiol and 2-hydroxyestradiol (Zahid et al. 2006).
Numerous P450s have been detected in breast tumor or adjacent tissue, including P450s 1A1, 1B1, 2A5, 2B6, 2C9, 2D6, 2E1, 2J2, 2S1, 2U1, 3A4, 3A5, 3A43, 4A11, 4V2, 4X1, 4Z1, 26A1, and of course 19A1 (Hellmold et al. 1998; Huang et al. 1996; Iscan et al. 2001; Schmidt et al. 2004). Of these, three enzymes are involved to a major extent in estradiol hydroxylation (i.e. P450s 1A1, 1B1, and 3A4) (Fig. 24). P450 2C9 is also involved in the conversion of both estradiol and estrone, with low activity in forming C4- and C16-hydroxylated products (Table 5). P450 enzymes involved in estrogen metabolism are expressed in both tumor and non-tumor breast tissue; however, higher levels of P450 1B1 and 3A4 were found more often in non-tumor tissue than in tumor tissue. It has been suggested that local activation of estrogen to potentially reactive metabolites by the P450s in breast tissue may play a role in initiating and promoting the carcinogenic process (Modugno et al. 2003).
In breast tumor cells, P450 1A1 and 1B1 mRNA levels and rates of both estradiol 2- and 4-hydroxylation were elevated following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Spink et al. 1998). In addition, the inhibitory effects of ketoconazole, cyclosporin A, and cimetidine (inhibitors of P450 enzymes) toward P450 3A4-catalyzed estradiol 2-hydroxylation were reported, and the IC50 values were 7 nM, 64 nM, and 290 µM, respectively (Satoh et al. 2000). It was also reported that non-ortho-substituted polychlorinated biphenyl congeners can, depending on the structure, induce or inhibit P450 1B1 and 1A1 activity and consequently that they might affect the formation of 2- and 4- hydroxylated metabolites of estradiol and the potential for mammary tumorigenesis (Spink et al. 2002a; Pang et al. 1999). Resveratrol was reported to strongly inhibit the TCDD-induced aryl hydrocarbon receptor DNA binding activity, the expression of P450 1A1 and 1B1, and P450 1A1 and 1B1 catalytic activities in MCF-10A breast cancer cells. Resveratrol also reduced the formation of 2- and 4-hydroxyestradiol from 17β-estradiol by recombinant human P450s 1A1 and 1B1, respectively. Furthermore, resveratrol significantly attenuated intracellular reactive oxygen species formation and oxidative DNA damage, and the cytotoxicity induced by the catechol estrogens (Chen et al. 2004). In addition to chemicals that induce or inhibit the activity of P450 enzymes, genetic variation of the enzymes (e.g., P450 1B1) can also affect the metabolic activation and carcinogenesis of 17β-estradiol and estrone, although the effects have not been shown to be large (Shimada et al. 1999; Watanabe et al. 2000). Changes in the expression levels of estrogen-metabolizing P450s not only alter the activity of substrates but may also have physiological effects in liver and target tissues (Chun and Kim 2016).
Arachidonic acid
Arachidonic acid metabolites are key mediators involved in the pathogenesis of numerous cardiovascular, pulmonary, inflammatory, and thromboembolic diseases. Thromboxane A2 is produced by the action of thromboxane synthase (P450 5A1) on the prostaglandin endoperoxide H2 (PGH2), a product of the enzymatic transformation of arachidonic acid by the cyclooxygenases (Rendic and Guengerich 2018). Arachidonic acid is metabolized in a number of tissues (liver, kidney, lung, brain, and the vasculature) by P450 enzymes that form hydroxyeicosatetraenoic acids (HETEs) or epoxides (epoxyeicosatrienoic acids, EETs) (Fig. 25). The reactions occur in different organs (brain, kidney, lung, vasculature, liver). EETs and HETEs have different biological properties, based on sites of production, and can be stored in tissue lipids and released in response to hormonal stimuli.
20-HETE has both pro- and anti-hypertensive actions that result from modulation of vascular and kidney function. 20-HETE is a potent vasoconstrictor, and upregulation of the production of this compound can contribute to the elevation of endothelial dysfunction and the increase in peripheral vascular resistance associated with some forms of hypertension. In kidney, 20-HETE exerts anti-hypertensive action by inhibiting sodium reabsorption by the kidney in both the proximal tubule and thick ascending limb of Henle (Williams et al. 2010; Garcia et al. 2017; Zhang et al. 2018; Roman 2002). Formation of 20-HETE is catalyzed by human P450s 4A11, 4F2, and F3B and the epoxygenation of arachidonic acid to EETs is catalyzed by P450s 2C8, 2C9, 2C19, and 2J2 and (to a much lesser extent) by P450 2W1 (Table 5 and references therein). The arachidonic acid products 20-HETE and EETs compose a group of compounds that participate in the regulation of liver metabolic activity and hemodynamics, may be involved in abnormalities related to liver diseases (e.g., cirrhosis), and play a key role in the pathophysiology of portal hypertension and renal failure (Sacerdoti et al. 2003). Arachidonic acid, as a model for metabolic activation of polyunsaturated fatty acids, produced a concentration- and time-dependent toxicity to Hep G2-MV2E1-9 cells, which express P450 2E1, proposed to be related to reactive oxygen intermediates and lipid peroxidation (Chen et al. 1997).
Concluding remarks
The data on activation of xenobiotics and endobiotics catalyzed by P450 enzymes in Families 1–4 are divided into groups of General Chemicals, Drugs, Natural Products, and Physiological Compounds. The metabolites formed are direct toxicants reacting with cell macromolecules in many cases. However, in selected cases the metabolites are not direct toxicants but participate as substrates in additional metabolic reactions (e.g., conjugation reactions) and the resulting products are final toxicants (e.g., estragole). In other cases, the product elicits physiological responses through indirect biological activities (e.g., 20-HETE, EETs). We have emphasized the observed higher number of activations of three groups of compounds (General Chemicals, Drugs, and Natural Products) yielding activated metabolites and the lower fraction of Physiological Compounds involved as substrates in activation reactions catalyzed by P450 enzymes belonging to Families 1–4, exemplified by estrogen hormones and arachidonic acid. In the group of General Chemicals, P450s 1A1, 1A2, and 1B1 are dominant in the formation of activated metabolites, followed by P450s 3A4 and 2E1 (Fig. 2); in the group Drugs (Fig. 9) P450 3A4 dominates in the formation of activated metabolites. In the group of Natural Products, P450s 1A2, 3A4, and 2E1 dominate in the formation of activated metabolites, followed by P450s 1A1 and 2A6 (Fig. 16); in the group of Physiological Compounds there was no clearly dominant P450 but the highest number of activations is attributed to P450s 1A, 1B1 and 3A (Fig. 23). The results show that Physiological Compounds are substrates infrequently in bioactivation reactions catalyzed by P450 enzymes belonging to Families 1–4, with the exception of estrogens and arachidonic acid.
The results presented to give information on the enzymes that dominate in the bioactivation of specific group of chemicals and might be used as a guide on which enzymes to direct research when testing their bioactivation to toxic metabolites.
Data availability
All the data are available in the text and tables of the review.
References
Aklillu E, Oscarson M, Hidestrand M, Leidvik B, Otter C, Ingelman-Sundberg M (2002) Functional analysis of six different polymorphic CYP1B1 enzyme variants found in an Ethiopian population. Mol Pharmacol 61(3):586–594. https://doi.org/10.1124/mol.61.3.586
Al-Subeihi AA, Alhusainy W, Kiwamoto R et al (2015) Evaluation of the interindividual human variation in bioactivation of methyleugenol using physiologically based kinetic modeling and Monte Carlo simulations. Toxicol Appl Pharmacol 283(2):117–126. https://doi.org/10.1016/j.taap.2014.12.009
Alvarez-Diez TM, Zheng J (2004) Mechanism-based inactivation of cytochrome P450 3A4 by 4-ipomeanol. Chem Res Toxicol 17(2):150–157. https://doi.org/10.1021/tx034143l
Amato G, Grasso E, Longo V, Gervasi PG (2001) Oxidation of N, N-dimethylformamide and N, N-diethylformamide by human liver microsomes and human recombinant P450s. Toxicol Lett 124(1–3):11–19. https://doi.org/10.1016/s0378-4274(01)00324-1
Aoyama T, Gonzalez FJ, Gelboin HV (1989) Human cDNA-expressed cytochrome P450 IA2: mutagen activation and substrate specificity. Mol Carcinog 2(4):192–198. https://doi.org/10.1002/mc.2940020405
Arlt VM, Stiborova M, Schmeiser HH (2002) Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis 17(4):265–277. https://doi.org/10.1093/mutage/17.4.265
Arlt VM, Stiborová M, vom Brocke J et al (2007) Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis 28(11):2253–2261. https://doi.org/10.1093/carcin/bgm082
Arlt VM, Levová K, Bárta F et al (2011) Role of P450 1A1 and P450 1A2 in bioactivation versus detoxication of the renal carcinogen aristolochic acid I: studies in Cyp1a1-/-, Cyp1a2-/-, and Cyp1a1/1a2-/- mice. Chem Res Toxicol 24(10):1710–1719. https://doi.org/10.1021/tx200259y
Aryal P, Terashita T, Guengerich FP, Shimada T, Oda Y (2000) Use of genetically engineered Salmonella typhimurium OY1002/1A2 strain coexpressing human cytochrome P450 1A2 and NADPH-cytochrome P450 reductase and bacterial O-acetyltransferase in SOS/umu assay. Environ Mol Mutagen 36(2):121–126. https://doi.org/10.1002/1098-2280(2000)36:2%3c121::aid-em6%3e3.0.co;2-p
Asai H, Imaoka S, Kuroki T, Monna T, Funae Y (1996) Microsomal ethanol oxidizing system activity by human hepatic cytochrome P450s. J Pharmacol Exp Ther 277(2):1004–1009
Avent KM, DeVoss JJ, Gillam EMJ (2006) Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites. Chem Res Toxicol 19(7):914–920. https://doi.org/10.1021/tx0600090
Back DJ, Maggs JL, Purba HS, Newby S, Park BK (1984) 2-Hydroxylation of ethinyloestradiol in relation to the oxidation of sparteine and antipyrine. Br J Clin Pharmacol 18(4):603–607. https://doi.org/10.1111/j.1365-2125.1984.tb02511.x
Badawi AF, Cavalieri EL, Rogan EG (2001) Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16a-hydroxylation of 17b-estradiol. Metabolism 50(9):1001–1003. https://doi.org/10.1053/meta.2001.25592
Baer BR, Rettie AE, Henne KR (2005) Bioactivation of 4-ipomeanol by CYP4B1: adduct characterization and evidence for an enedial intermediate. Chem Res Toxicol 18(5):855–864. https://doi.org/10.1021/tx0496993
Baer BR, Wienkers LC, Rock DA (2007) Time-dependent inactivation of P450 3A4 by raloxifene: identification of Cys239 as the site of apoprotein alkylation. Chem Res Toxicol 20(6):954–964. https://doi.org/10.1021/tx700037e
Bahari A, Mehrzad J, Mahmoudi M, Bassami MR, Dehghani H (2014) Cytochrome P450 isoforms are differently up-regulated in aflatoxin B1-exposed human lymphocytes and monocytes. Immunopharmacol Immunotoxicol 36(1):1–10. https://doi.org/10.3109/08923973.2013.850506
Bai J, Cederbaum AI (2004) Adenovirus mediated overexpression of CYP2E1 increases sensitivity of HepG2 cells to acetaminophen induced cytotoxicity. Mol Cell Biochem 262(1–2):165–176. https://doi.org/10.1023/b:mcbi.0000038232.61760.9e
Bao Z, He XY, Ding X, Prabhu S, Hong JY (2005) Metabolism of nicotine and cotinine by human cytochrome P450 2A13. Drug Metab Dispos 33(2):258–261. https://doi.org/10.1124/dmd.104.002105
Barbosa-Sicard E, Markovic M, Honeck H, Christ B, Muller DN, Schunck WH (2005) Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem Biophys Res Commun 329(4):1275–1281. https://doi.org/10.1016/j.bbrc.2005.02.103
Barceló S, Macé K, Pfeifer AM, Chipman JK (1998) Production of DNA strand breaks by N-nitrosodimethylamine and 2-amino-3-methylimidazo[4,5-f]quinoline in THLE cells expressing human CYP isoenzymes and inhibition by sulforaphane. Mutat Res 402(1–2):111–120. https://doi.org/10.1016/s0027-5107(97)00288-1
Baum M, Amin S, Guengerich FP, Hecht SS, Köhl W, Eisenbrand G (2001) Metabolic activation of benzo[c]phenanthrene by cytochrome P450 enzymes in human liver and lung. Chem Res Toxicol 14(6):686–693. https://doi.org/10.1021/tx000240s
Bell LC, Guengerich FP (1997) Oxidation kinetics of ethanol by human cytochrome P450 2E1. Rate-limiting product release accounts for effects of isotopic hydrogen substitution and cytochrome b5 on steady-state kinetics. J Biol Chem 272(47):29643–29651. https://doi.org/10.1074/jbc.272.47.29643
Bell-Parikh LC, Guengerich FP (1999) Kinetics of cytochrome P450 2E1-catalyzed oxidation of ethanol to acetic acid via acetaldehyde. J Biol Chem 274(34):23833–23840. https://doi.org/10.1074/jbc.274.34.23833
Bendaly J, Zhao S, Neale JR et al (2007) 2-Amino-3,8-dimethylimidazo-[4,5-f]quinoxaline-induced DNA adduct formation and mutagenesis in DNA repair-deficient Chinese hamster ovary cells expressing human cytochrome P450 1A1 and rapid or slow acetylator N-acetyltransferase 2. Cancer Epidemiol Biomarkers Prev 16(7):1503–1509. https://doi.org/10.1158/1055-9965.Epi-07-0305
Berson A, Wolf C, Chachaty C et al (1993) Metabolic activation of the nitroaromatic antiandrogen flutamide by rat and human cytochromes P-450, including forms belonging to the 3A and 1A subfamilies. J Pharmacol Exp Ther 265(1):366–372
Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD (2007) CYP2D6 polymorphisms and the impact on tamoxifen therapy. J Pharm Sci 96(9):2224–2231. https://doi.org/10.1002/jps.20892
Bezerra LS, Santos-Veloso MAO, Bezerra Junior NDS, Fonseca LCD, Sales WLA (2018) Impacts of cytochrome P450 2D6 (CYP2D6) genetic polymorphism in tamoxifen therapy for breast cancer. Rev Bras Ginecol Obstet 40(12):794–799. https://doi.org/10.1055/s-0038-1676303
Black GP, Collins KS, Blacquiere DP, Forkert PG (2006) Formation of N-alkylprotoporphyrin IX from metabolism of diallyl sulfone in lung and liver. Drug Metab Dispos 34(6):895–900. https://doi.org/10.1124/dmd.106.009928
Bohnenstengel F, Hofmann U, Eichelbaum M, Kroemer HK (1996) Characterization of the cytochrome P450 involved in side-chain oxidation of cyclophosphamide in humans. Eur J Clin Pharmacol 51(3–4):297–301. https://doi.org/10.1007/s002280050201
Bolt HM (1979) Metabolism of estrogens–natural and synthetic. Pharmacol Ther 4(1):155–181. https://doi.org/10.1016/0163-7258(79)90018-4
Bolt HM, Bolt M, Kappus H (1977) Interaction of rifampicin treatment with pharmacokinetics and metabolism of ethinyloestradiol in man. Acta Endocrinol (Copenhagen) 85(1):189–197. https://doi.org/10.1530/acta.0.0850189
Bolton JL (2002) Quinoids, quinoid radicals, and phenoxyl radicals formed from estrogens and antiestrogens. Toxicology 177(1):55–65. https://doi.org/10.1016/s0300-483x(02)00195-6
Bolton JL, Thatcher GR (2008) Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol 21(1):93–101
Bolton JL, Acay NM, Vukomanovic V (1994) Evidence that 4-allyl-o-quinones spontaneously rearrange to their more electrophilic quinone methides: potential bioactivation mechanism for the hepatocarcinogen safrole. Chem Res Toxicol 7(3):443–450. https://doi.org/10.1021/tx00039a024
Bond JA, Medinsky MA (2001) Insights into the toxicokinetics and toxicodynamics of 1,3-butadiene. Chem-Biol Interact 135–136:599–614. https://doi.org/10.1016/s0009-2797(01)00199-5
Boocock DJ, Brown K, Gibbs AH, Sanchez E, Turteltaub KW, White IN (2002) Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis 23(11):1897–1901. https://doi.org/10.1093/carcin/23.11.1897
Borges S, Desta Z, Li L et al (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80(1):61–74. https://doi.org/10.1016/j.clpt.2006.03.013
Bort R, Macé K, Boobis A, Gómez-Lechón MJ, Pfeifer A, Castell J (1999) Hepatic metabolism of diclofenac: role of human CYP in the minor oxidative pathways. Biochem Pharmacol 58(5):787–796. https://doi.org/10.1016/s0006-2952(99)00167-7
Borzelleca JF (2000) Profiles in toxicology–paracelsus: herald of modern toxicology. Toxicol Sci 53:2–4
Boysen G, Scarlett CO, Temple B et al (2007) Identification of covalent modifications in P450 2E1 by 1,2-epoxy-3-butene in vitro. Chem-Biol Interact 166(1–3):170–175. https://doi.org/10.1016/j.cbi.2007.01.007
Brandon EF, Meijerman I, Klijn JS et al (2005) In-vitro cytotoxicity of ET-743 (trabectedin, yondelis), a marine anti-cancer drug, in the Hep G2 cell line: influence of cytochrome P450 and phase II inhibition, and cytochrome P450 induction. Anticancer Drugs 16(9):935–943. https://doi.org/10.1097/01.cad.0000180121.16407.38
Brandon EF, Sparidans RW, Guijt KJ et al (2006) In vitro characterization of the human biotransformation and CYP reaction phenotype of ET-743 (yondelis, trabectidin), a novel marine anti-cancer drug. Invest New Drugs 24(1):3–14. https://doi.org/10.1007/s10637-005-4538-9
Brian WR, Sari M-A, Iwasaki M, Shimada T, Kaminsky LS, Guengerich FP (1990) Catalytic activities of human liver cytochrome P-450 IIIA4 expressed in Saccharomyces cerevisiae. Biochemistry 29(51):11280–11292. https://doi.org/10.1021/bi00503a018
Buening MK, Fortner JG, Kappas A, Corney AH (1978) 7,8-Benzoflavone stimulates the metabolic activation of aflatoxin B1 to mutagens by human liver. Biochem Biophys Res Commun 82(1):348–355. https://doi.org/10.1016/0006-291x(78)90616-2
Buratti FM, Volpe MT, Fabrizi L, Meneguz A, Vittozzi L, Testai E (2002) Kinetic parameters of OPT pesticide desulfuration by c-DNA expressed human CYPs. Environ Toxicol Pharmacol 11(3–4):181–190. https://doi.org/10.1016/s1382-6689(02)00010-8
Buratti FM, Volpe MT, Meneguz A, Vittozzi L, Testai E (2003) CYP-specific bioactivation of four organophosphorothioate pesticides by human liver microsomes. Toxicol Appl Pharmacol 186(3):143–154. https://doi.org/10.1016/s0041-008x(02)00027-3
Buratti FM, Leoni C, Testai E (2006) Foetal and adult human CYP3A isoforms in the bioactivation of organophosphorothionate insecticides. Toxicol Lett 167(3):245–255. https://doi.org/10.1016/j.toxlet.2006.10.006
Buters J, Quintanilla-Martinez L, Schober W et al (2003) CYP1B1 determines susceptibility to low doses of 7,12-dimethylbenz[a]anthracene-induced ovarian cancers in mice: correlation of CYP1B1-mediated DNA adducts with carcinogenicity. Carcinogenesis 24(2):327–334. https://doi.org/10.1093/carcin/24.2.327
Butler AM, Murray M (1997) Biotransformation of parathion in human liver: participation of CYP3A4 and its inactivation during microsomal parathion oxidation. J Pharmacol Exp Ther 280(2):966–973
Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF (1989) Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci USA 86(20):7696–7700. https://doi.org/10.1073/pnas.86.20.7696
Bylund J, Ericsson J, Oliw EH (1998a) Analysis of cytochrome P450 metabolites of arachidonic and linoleic acids by liquid chromatography-mass spectrometry with ion trap MS. Anal Biochem 265(1):55–68. https://doi.org/10.1006/abio.1998.2897
Bylund J, Kunz T, Valmsen K, Oliw EH (1998b) Cytochromes P450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. J Pharmacol Exp Ther 284(1):51–60
Cabaret O, Puel O, Botterel F et al (2010) Metabolic detoxication pathways for sterigmatocystin in primary tracheal epithelial cells. Chem Res Toxicol 23(11):1673–1681. https://doi.org/10.1021/tx100127b
Cabaret O, Puel O, Botterel F, Pean M, Bretagne S, Delaforge M (2011) Contribution of uniformly 13C-enriched sterigmatocystin to the study of its pulmonary metabolism. Rapid Commun Mass Spectrom 25(19):2704–2710. https://doi.org/10.1002/rcm.5068
Calinski DM, Zhang H, Ludeman S, Dolan ME, Hollenberg PF (2015) Hydroxylation and N-dechloroethylation of ifosfamide and deuterated ifosfamide by the human cytochrome P450s and their commonly occurring polymorphisms. Drug Metab Dispos 43(7):1084–1090. https://doi.org/10.1124/dmd.115.063628
Cameron MD, Wen B, Roberts AG, Atkins WM, Campbell AP, Nelson SD (2007) Cooperative binding of acetaminophen and caffeine within the P450 3A4 active site. Chem Res Toxicol 20(10):1434–1441. https://doi.org/10.1021/tx7000702
Carlson JR, Breeze RG (1984) Ruminal metabolism of plant toxins with emphasis on indolic compounds. J Anim Sci 58(4):1040–1049. https://doi.org/10.2527/jas1984.5841040x
Carlson ES, Upadhyaya P, Hecht SS (2016) Evaluation of nitrosamide formation in the cytochrome P450-mediated metabolism of tobacco-specific nitrosamines. Chem Res Toxicol 29(12):2194–2205. https://doi.org/10.1021/acs.chemrestox.6b00384
Caro AA, Cederbaum AI (2004) Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol 44:27–42. https://doi.org/10.1146/annurev.pharmtox.44.101802.121704
Cavalieri E, Rogan E (2006) Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture. Ann NY Acad Sci 1089:286–301. https://doi.org/10.1196/annals.1386.042
Cavalieri E, Chakravarti D, Guttenplan J et al (2006) Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta 1766(1):63–78. https://doi.org/10.1016/j.bbcan.2006.03.001
Chan W, Cui L, Xu G, Cai Z (2006) Study of the phase I and phase II metabolism of nephrotoxin aristolochic acid by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 20(11):1755–1760. https://doi.org/10.1002/rcm.2513
Chang TK, Weber GF, Crespi CL, Waxman DJ (1993) Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53(23):5629–5637
Chang TK, Yu L, Maurel P, Waxman DJ (1997) Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res 57(10):1946–1954
Chen Q, Galleano M, Cederbaum AI (1997) Cytotoxicity and apoptosis produced by arachidonic acid in HepG2 cells overexpressing human cytochrome P4502E1. J Biol Chem 272(23):14532–14541. https://doi.org/10.1074/jbc.272.23.14532
Chen W, Koenigs LL, Thompson SJ et al (1998) Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol 11(4):295–301. https://doi.org/10.1021/tx9701687
Chen Q, Ngui JS, Doss GA et al (2002) Cytochrome P450 3A4-mediated bioactivation of raloxifene: irreversible enzyme inhibition and thiol adduct formation. Chem Res Toxicol 15(7):907–914. https://doi.org/10.1021/tx0200109
Chen ZH, Hurh YJ, Na HK et al (2004) Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 25(10):2005–2013. https://doi.org/10.1093/carcin/bgh183
Chen CS, Jounaidi Y, Waxman DJ (2005) Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expressed cytochromes P450. Drug Metab Dispos 33(9):1261–1267. https://doi.org/10.1124/dmd.105.004788
Chen C, Meng L, Ma X et al (2006a) Urinary metabolite profiling reveals CYP1A2-mediated metabolism of NSC686288 (aminoflavone). J Pharmacol Exp Ther 318(3):1330–1342. https://doi.org/10.1124/jpet.106.105213
Chen Q, Doss GA, Tung EC et al (2006b) Evidence for the bioactivation of zomepirac and tolmetin by an oxidative pathway: identification of glutathione adducts in vitro in human liver microsomes and in vivo in rats. Drug Metab Dispos 34(1):145–151. https://doi.org/10.1124/dmd.105.004341
Chen PH, Mahmood Q, Mariottini GL, Chiang TA, Lee KW (2017) Adverse health effects of betel quid and the risk of oral and pharyngeal cancers. Biomed Res Int 2017:3904098. https://doi.org/10.1155/2017/3904098
Cheung C, Ma X, Krausz KW et al (2005a) Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem Res Toxicol 18(9):1471–1478. https://doi.org/10.1021/tx050136g
Cheung C, Yu AM, Ward JM et al (2005b) The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos 33(3):449–457. https://doi.org/10.1124/dmd.104.002402
Chiang HC, Wang CY, Lee HL, Tsou TC (2011) Metabolic effects of CYP2A6 and CYP2A13 on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced gene mutation–a mammalian cell-based mutagenesis approach. Toxicol Appl Pharmacol 253(2):145–152. https://doi.org/10.1016/j.taap.2011.03.022
Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB (2004) Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos 32(8):840–847. https://doi.org/10.1124/dmd.32.8.840
Christmas P, Carlesso N, Shang H et al (2003) Myeloid expression of cytochrome P450 4F3 is determined by a lineage-specific alternative promoter. J Biol Chem 278(27):25133–25142. https://doi.org/10.1074/jbc.M302106200
Chugh R, Wagner T, Griffith KA et al (2007) Assessment of ifosfamide pharmacokinetics, toxicity, and relation to CYP3A4 activity as measured by the erythromycin breath test in patients with sarcoma. Cancer 109(11):2315–2322. https://doi.org/10.1002/cncr.22669
Chun Y-J, Kim D (2016) Cancer activation and polymorphisms of human cytochrome P450 1B1. Toxicol Res 32(2):89–93. https://doi.org/10.5487/tr.2016.32.2.089
Chun Y-J, Kim S, Kim D, Lee SK, Guengerich FP (2001) A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res 61(22):8164–8170
Chun Y-J, Lee SK, Kim MY (2005) Modulation of human cytochrome P450 1B1 expression by 2,4,3´,5´-tetramethoxystilbene. Drug Metab Dispos 33(12):1771–1776. https://doi.org/10.1124/dmd.105.006502
Chung YT, Chen CL, Wu CC, Chan SA, Chi CW, Liu TY (2008) Safrole-DNA adduct in hepatocellular carcinoma associated with betel quid chewing. Toxicol Lett 183(1–3):21–27. https://doi.org/10.1016/j.toxlet.2008.09.013
Ciolino HP, Yeh GC (1999) The steroid hormone dehydroepiandrosterone inhibits CYP1A1 expression in vitro by a post-transcriptional mechanism. J Biol Chem 274(49):35186–35190. https://doi.org/10.1074/jbc.274.49.35186
Clarke TA, Waskell LA (2003) The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos 31(1):53–59. https://doi.org/10.1124/dmd.31.1.53
Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang TK, Waxman DJ (1997) Human cytochrome P450 2B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug Metab Dispos 25(8):985–993
Coller JK, Krebsfaenger N, Klein K et al (2004) Large interindividual variability in the in vitro formation of tamoxifen metabolites related to the development of genotoxicity. Br J Clin Pharmacol 57(1):105–111. https://doi.org/10.1046/j.1365-2125.2003.01970.x
Coulet M, Dacasto M, Eeckhoutte C et al (1998) Identification of human and rabbit cytochromes P450 1A2 as major isoforms involved in thiabendazole 5-hydroxylation. Fundam Clin Pharmacol 12(2):225–235. https://doi.org/10.1111/j.1472-8206.1998.tb00946.x
Coulet M, Eeckhoutte C, Larrieu G et al (2000) Evidence for cytochrome P450 1A2-mediated protein covalent binding of thiabendazole and for its passive intestinal transport: use of human and rabbit derived cells. Chem-Biol Interact 127(2):109–124. https://doi.org/10.1016/s0009-2797(00)00167-8
Crane AL, Klein K, Olson JR (2012a) Bioactivation of chlorpyrifos by CYP2B6 variants. Xenobiotica 42(12):1255–1262. https://doi.org/10.3109/00498254.2012.702246
Crane AL, Klein K, Zanger UM, Olson JR (2012b) Effect of CYP2B6*6 and CYP2C19*2 genotype on chlorpyrifos metabolism. Toxicology 293(1–3):115–122. https://doi.org/10.1016/j.tox.2012.01.006
Crespi CL, Penman BW, Steimel DT, Smith T, Yang CS, Sutter TR (1997) Development of a human lymphoblastoid cell line constitutively expressing human CYP1B1 cDNA: substrate specificity with model substrates and promutagens. Mutagenesis 12(2):83–89. https://doi.org/10.1093/mutage/12.2.83
Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EM (2002) Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4´-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 30(8):869–874. https://doi.org/10.1124/dmd.30.8.869
Cribb AE, Knight MJ, Dryer D et al (2006) Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol Biomarkers Prev 15(3):551–558. https://doi.org/10.1158/1055-9965.Epi-05-0801
Crofts FG, Strickland PT, Hayes CL, Sutter TR (1997) Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by human cytochrome P4501B1. Carcinogenesis 18(9):1793–1798. https://doi.org/10.1093/carcin/18.9.1793
Crofts FG, Sutter TR, Strickland PT (1998) Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P450 1A1, P450 1A2 and P450 1B1. Carcinogenesis 19(11):1969–1973. https://doi.org/10.1093/carcin/19.11.1969
Croom EL, Wallace AD, Hodgson E (2010) Human variation in CYP-specific chlorpyrifos metabolism. Toxicology 276(3):184–191. https://doi.org/10.1016/j.tox.2010.08.005
Czerwinski M, McLemore TL, Philpot RM et al (1991) Metabolic activation of 4-ipomeanol by complementary DNA-expressed human cytochromes P-450: evidence for species-specific metabolism. Cancer Res 51(17):4636–4638
Dai Y, Rashba-Step J, Cederbaum AI (1993) Stable expression of human cytochrome P450 2E1 in HepG2 cells: characterization of catalytic activities and production of reactive oxygen intermediates. Biochemistry 32(27):6928–6937. https://doi.org/10.1021/bi00078a017
Dai D, Tang J, Rose R et al (2001a) Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther 299(3):825–831
Dai D, Zeldin DC, Blaisdell JA et al (2001b) Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 11(7):597–607. https://doi.org/10.1097/00008571-200110000-00006
Dai J, Zhang F, Zheng J (2010) Retrorsine, but not monocrotaline, is a mechanism-based inactivator of P450 3A4. Chem-Biol Interact 183(1):49–56. https://doi.org/10.1016/j.cbi.2009.10.001
Daikh BE, Lasker JM, Raucy JL, Koop DR (1994) Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J Pharmacol Exp Ther 271(3):1427–1433
de Groene EM, Hassing IG, Blom MJ, Seinen W, Fink-Gremmels J, Horbach GJ (1996) Development of human cytochrome P450-expressing cell lines: application in mutagenicity testing of ochratoxin A. Cancer Res 56(2):299–304
Dehal SS, Kupfer D (1999) Cytochrome P-450 3A and 2D6 catalyze ortho-hydroxylation of 4-hydroxytamoxifen and 3-hydroxytamoxifen (droloxifene) yielding tamoxifen catechol: involvement of catechols in covalent binding to hepatic proteins. Drug Metab Dispos 27(6):681–688
Delannée V, Langouët S, Théret N, Siegel A (2017) A modeling approach to evaluate the balance between bioactivation and detoxification of MeIQx in human hepatocytes. PeerJ 5:e3703. https://doi.org/10.7717/peerj.3703
den Braver MW, den Braver-Sewradj SP, Vermeulen NP, Commandeur JN (2016) Characterization of cytochrome P450 isoforms involved in sequential two-step bioactivation of diclofenac to reactive p-benzoquinone imines. Toxicol Lett 253:46–54. https://doi.org/10.1016/j.toxlet.2016.04.022
Deng J, Zhao L, Zhang NY et al (2018) Aflatoxin B1 metabolism: regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat Res 778:79–89. https://doi.org/10.1016/j.mrrev.2018.10.002
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310(3):1062–1075. https://doi.org/10.1124/jpet.104.065607
Dicke KE, Skrlin SM, Murphy SE (2005) Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-butanone metabolism by cytochrome P450 2B6. Drug Metab Dispos 33(12):1760–1764. https://doi.org/10.1124/dmd.105.006718
Doehmer J, Holtkamp D, Soballa V et al (1995) Cytochrome P450 mediated reactions studied in genetically engineered V79 Chinese hamster cells. Pharmacogenetics 5:S91-96. https://doi.org/10.1097/00008571-199512001-00008
Dong H, Haining RL, Thummel KE, Rettie AE, Nelson SD (2000) Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab Dispos 28(12):1397–1400
Driscoll JP, Kornecki K, Wolkowski JP, Chupak L, Kalgutkar AS, O’Donnell JP (2007) Bioactivation of phencyclidine in rat and human liver microsomes and recombinant P450 2B enzymes: evidence for the formation of a novel quinone methide intermediate. Chem Res Toxicol 20(10):1488–1497. https://doi.org/10.1021/tx700145k
Duescher RJ, Elfarra AA (1994) Human liver microsomes are efficient catalysts of 1,3-butadiene oxidation: evidence for major roles by cytochromes P450 2A6 and 2E1. Arch Biochem Biophys 311(2):342–349. https://doi.org/10.1006/abbi.1994.1246
Duisken M, Benz D, Peiffer TH, Blömeke B, Hollender J (2005) Metabolism of ∆3-carene by human cytochrome P450 enzymes: identification and characterization of two new metabolites. Curr Drug Metab 6(6):593–601. https://doi.org/10.2174/138920005774832614
Durant JL, Lafleur AL, Busby WF Jr, Donhoffner LL, Penman BW, Crespi CL (1999) Mutagenicity of C24H14 PAH in human cells expressing CYP1A1. Mutat Res 446(1):1–14. https://doi.org/10.1016/s1383-5718(99)00135-7
Eaton DL (2000) Biotransformation enzyme polymorphism and pesticide susceptibility. Neurotoxicology 21(1–2):101–111
Edwards RJ, Murray BP, Murray S et al (1994) Contribution of CYP1A1 and CYP1A2 to the activation of heterocyclic amines in monkeys and human. Carcinogenesis 15(5):829–836. https://doi.org/10.1093/carcin/15.5.829
Einolf HJ, Story WT, Marcus CB et al (1997) Role of cytochrome P450 enzyme induction in the metabolic activation of benzo[c]phenanthrene in human cell lines and mouse epidermis. Chem Res Toxicol 10(5):609–617. https://doi.org/10.1021/tx960174n
Ekhart C, Doodeman VD, Rodenhuis S, Smits PH, Beijnen JH, Huitema AD (2009) Polymorphisms of drug-metabolizing enzymes (GST, CYP2B6 and CYP3A) affect the pharmacokinetics of thiotepa and tepa. Br J Clin Pharmacol 67(1):50–60. https://doi.org/10.1111/j.1365-2125.2008.03321.x
Ekins S, Wrighton SA (1999) The role of CYP2B6 in human xenobiotic metabolism. Drug Metab Rev 31(3):719–754. https://doi.org/10.1081/dmr-100101942
El Adlouni C, Pinelli E, Azémar B, Zaoui D, Beaune P, Pfohl-Leszkowicz A (2000) Phenobarbital increases DNA adduct and metabolites formed by ochratoxin A: role of CYP 2C9 and microsomal glutathione-S-transferase. Environ Mol Mutagen 35(2):123–131. https://doi.org/10.1002/(sici)1098-2280(2000)35:2%3c123::aid-em7%3e3.3.co;2-c
Elfarra AA, Krause RJ, Selzer RR (1996) Biochemistry of 1,3-butadiene metabolism and its relevance to 1,3-butadiene-induced carcinogenicity. Toxicology 113(1–3):23–30. https://doi.org/10.1016/0300-483x(96)03423-3
Ellison CA, Tian Y, Knaak JB, Kostyniak PJ, Olson JR (2012) Human hepatic cytochrome P450-specific metabolism of the organophosphorus pesticides methyl parathion and diazinon. Drug Metab Dispos 40(1):1–5. https://doi.org/10.1124/dmd.111.042572
Eun CY, Han S, Lim YR et al (2010) Bioactivation of aromatic amines by human CYP2W1, an orphan cytochrome P450 enzyme. Toxicol Res 26(3):171–175. https://doi.org/10.5487/tr.2010.26.3.171
Fan F, Roman RJ (2017) Effect of cytochrome P450 metabolites of arachidonic acid in nephrology. J Am Soc Nephrol 28(10):2845–2855. https://doi.org/10.1681/asn.2017030252
Fan T, Sun G, Zhao L, Cui X, Zhong R (2019) Metabolic activation and carcinogenesis of tobacco-specific nitrosamine N´-nitrosonornicotine (NNN): a density function theory and molecular docking study. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16020178
Fang J, Baker GB, Silverstone PH, Coutts RT (1997) Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol 17(2):227–233. https://doi.org/10.1023/a:1026317929335
Fang J, McKay G, Song J, Remillrd A, Li X, Midha K (2001) In vitro characterization of the metabolism of haloperidol using recombinant cytochrome P450 enzymes and human liver microsomes. Drug Metab Dispos 29(12):1638–1643
Fashe MM, Juvonen RO, Petsalo A, Vepsäläinen J, Pasanen M, Rahnasto-Rilla M (2015) In silico prediction of the site of oxidation by cytochrome P450 3A4 that leads to the formation of the toxic metabolites of pyrrolizidine alkaloids. Chem Res Toxicol 28(4):702–710. https://doi.org/10.1021/tx500478q
Finn RD, Henderson CJ, Scott CL, Wolf CR (2009) Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway. Biochem J 417(1):43–54. https://doi.org/10.1042/bj20080740
Fishman J, Guzik H, Hellman L (1970) Aromatic ring hydroxylation of estradiol in man. Biochemistry 9(7):1593–1598. https://doi.org/10.1021/bi00809a018
Fleming CM, Branch RA, Wilkinson GR, Guengerich FP (1992) Human liver microsomal N-hydroxylation of dapsone by cytochrome P-450 3A4. Mol Pharmacol 41(5):975–980
Forkert PG, Premdas PD, Bowers RJ (2000) Epoxide formation from diallyl sulfone is associated with CYP2E1 inactivation in murine and human lungs. Am J Respir Cell Mol Biol 23(5):687–695. https://doi.org/10.1165/ajrcmb.23.5.4149
Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR (2007) Human hepatic cytochrome P450-specific metabolism of parathion and chlorpyrifos. Drug Metab Dispos 35(2):189–193. https://doi.org/10.1124/dmd.106.012427
Frei E, Bieler CA, Arlt VM, Wiessler M, Stiborová M (2002) Covalent binding of the anticancer drug ellipticine to DNA in V79 cells transfected with human cytochrome P450 enzymes. Biochem Pharmacol 64(2):289–295. https://doi.org/10.1016/s0006-2952(02)01072-9
Fujita K, Kamataki T (2001a) Predicting the mutagenicity of tobacco-related N-nitrosamines in humans using 11 strains of Salmonella typhimurium YG7108, each coexpressing a form of human cytochrome P450 along with NADPH-cytochrome P450 reductase. Environ Mol Mutagen 38(4):339–346. https://doi.org/10.1002/em.10036
Fujita K, Kamataki T (2001b) Role of human cytochrome P450 (CYP) in the metabolic activation of N-alkylnitrosamines: application of genetically engineered Salmonella typhimurium YG7108 expressing each form of CYP together with human NADPH-cytochrome P450 reductase. Mutat Res 483(1–2):35–41. https://doi.org/10.1016/s0027-5107(01)00223-8
Fussell KC, Udasin RG, Smith PJ, Gallo MA, Laskin JD (2011) Catechol metabolites of endogenous estrogens induce redox cycling and generate reactive oxygen species in breast epithelial cells. Carcinogenesis 32(8):1285–1293. https://doi.org/10.1093/carcin/bgr109
Gallagher EP, Wienkers LC, Stapleton PL, Kunze KL, Eaton DL (1994) Role of human microsomal and human complementary DNA-expressed cytochromes P450 1A2 and P450 3A4 in the bioactivation of aflatoxin B1. Cancer Res 54(1):101–108
Gallagher EP, Kunze KL, Stapleton PL, Eaton DL (1996) The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol Appl Pharmacol 141(2):595–606. https://doi.org/10.1006/taap.1996.0326
Ganesan S, Sahu R, Walker LA, Tekwani BL (2010) Cytochrome P450-dependent toxicity of dapsone in human erythrocytes. J Appl Toxicol 30(3):271–275. https://doi.org/10.1002/jat.1493
Garcia V, Gilani A, Shkolnik B et al (2017) 20-HETE signals through G-protein-coupled receptor GPR75 (G(q)) to affect vascular function and trigger hypertension. Circ Res 120(11):1776–1788. https://doi.org/10.1161/circresaha.116.310525
Gardner I, Wakazono H, Bergin P et al (1997) Cytochrome P450 mediated bioactivation of methyleugenol to 1´-hydroxymethyleugenol in Fischer 344 rat and human liver microsomes. Carcinogenesis 18(9):1775–1783. https://doi.org/10.1093/carcin/18.9.1775
Gautier JC, Lecoeur S, Cosme J et al (1996) Contribution of human cytochrome P450 to benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol metabolism, as predicted from heterologous expression in yeast. Pharmacogenetics 6(6):489–499. https://doi.org/10.1097/00008571-199612000-00002
Gelhaus SL, Harvey RG, Penning TM, Blair IA (2011) Regulation of benzo[a]pyrene-mediated DNA- and glutathione-adduct formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human lung cells. Chem Res Toxicol 24(1):89–98. https://doi.org/10.1021/tx100297z
Gervot L, Rochat B, Gautier JC et al (1999) Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics 9(3):295–306
Gill HJ, Tingle MD, Park BK (1995) N-Hydroxylation of dapsone by multiple enzymes of cytochrome P450: implications for inhibition of haemotoxicity. Br J Clin Pharmacol 40(6):531–538. https://doi.org/10.1111/j.1365-2125.1995.tb05797.x
Gillam EMJ, Guo Z, Ueng Y-F et al (1995) Expression of cytochrome P450 3A5 in Escherichia coli: effects of 5´ modification, purification, spectral characterization, reconstitution conditions, and catalytic activities. Arch Biochem Biophys 317(2):374–384. https://doi.org/10.1006/abbi.1995.1177
Gillam EMJ, Wunsch RM, Ueng Y-F et al (1997) Expression of cytochrome P450 3A7 in Escherichia coli: effects of 5´ modification and catalytic characterization of recombinant enzyme expressed in bicistronic format with NADPH-cytochrome P450 reductase. Arch Biochem Biophys 346(1):81–90. https://doi.org/10.1006/abbi.1997.0286
Goda R, Nagai D, Akiyama Y et al (2006) Detection of a new N-oxidized metabolite of flutamide, N-[4-nitro-3-(trifluoromethyl)phenyl]hydroxylamine, in human liver microsomes and urine of prostate cancer patients. Drug Metab Dispos 34(5):828–835. https://doi.org/10.1124/dmd.105.008623
Gökmen MR, Cosyns JP, Arlt VM et al (2013) The epidemiology, diagnosis, and management of aristolochic acid nephropathy: a narrative review. Ann Intern Med 158(6):469–477. https://doi.org/10.7326/0003-4819-158-6-201303190-00006
Gonzalez FJ, Gelboin HV (1994) Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev 26(1–2):165–183. https://doi.org/10.3109/03602539409029789
Granvil CP, Madan A, Sharkawi M, Parkinson A, Wainer IW (1999) Role of CYP2B6 and CYP3A4 in the in vitro N-dechloroethylation of (R)- and (S)-ifosfamide in human liver microsomes. Drug Metab Dispos 27(4):533–541
Grollman AP, Shibutani S, Moriya M et al (2007) Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci USA 104(29):12129–12134. https://doi.org/10.1073/pnas.0701248104
Guengerich FP (1988) Ofxidation of 17a-ethynylestradiol by human liver cytochrome P-450. Mol Pharmacol 33(5):500–508
Guengerich FP (1990a) Inhibition of oral contraceptive steroid-metabolizing enzymes by steroids and drugs. Am J Obstet Gynecol 163(6 Pt 2):2159–2163
Guengerich FP (1990b) Mechanism-based inactivation of human liver microsomal cytochrome P-450 IIIA4 by gestodene. Chem Res Toxicol 3(4):363–371
Guengerich FP (1993) The 1992 Bernard B. Brodie Award Lecture. Bioactivation and detoxication of toxic and carcinogenic chemicals. Drug Metab Dispos 21(1):1–6
Guengerich FP (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17. https://doi.org/10.1146/annurev.pharmtox.39.1.1
Guengerich FP (2020) A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol Res. https://doi.org/10.1007/s43188-020-00056-z
Guengerich FP, Avadhani NG (2018) Roles of cytochrome P450 in metabolism of ethanol and carcinogens. Adv Exp Med Biol 1032:15–35. https://doi.org/10.1007/978-3-319-98788-0_2
Guengerich FP, Kim D-H (1991) Enzymatic oxidation of ethyl carbamate to vinyl carbamate and its role as an intermediate in the formation of 1, N6-ethenoadenosine. Chem Res Toxicol 4(4):413–421. https://doi.org/10.1021/tx00022a003
Guengerich FP, Rendic S (2010) Update information on drug metabolism systems–2009, part I. Curr Drug Metab 11(1):1–3. https://doi.org/10.2174/138920010791110908
Guengerich FP, Shimada T (1998) Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res 400(1–2):201–213. https://doi.org/10.1016/s0027-5107(98)00037-2
Guengerich FP, Kim DH, Iwasaki M (1991) Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol 4(2):168–179. https://doi.org/10.1021/tx00020a008
Guengerich FP, Parikh A, Turesky RJ, Josephy PD (1999) Inter-individual differences in the metabolism of environmental toxicants: cytochrome P450 1A2 as a prototype. Mutat Res 428(1–2):115–124. https://doi.org/10.1016/s1383-5742(99)00039-3
Guengerich FP, Chun Y-J, Kim D, Gillam EMJ, Shimada T (2003) Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat Res 523–524:173–182. https://doi.org/10.1016/s0027-5107(02)00333-0
Guo J, Johansson I, Mkrtchian S, Ingelman-Sundberg M (2016) The CYP2W1 enzyme: regulation, properties and activation of prodrugs. Drug Metab Rev 48(3):369–378. https://doi.org/10.1080/03602532.2016.1188939
Hamitouche S, Poupon J, Dreano Y, Amet Y, Lucas D (2006) Ethanol oxidation into acetaldehyde by 16 recombinant human cytochrome P450 isoforms: role of CYP2C isoforms in human liver microsomes. Toxicol Lett 167(3):221–230. https://doi.org/10.1016/j.toxlet.2006.09.011
Hammons GJ, Milton D, Stepps K, Guengerich FP, Tukey RH, Kadlubar FF (1997) Metabolism of carcinogenic heterocyclic and aromatic amines by recombinant human cytochrome P450 enzymes. Carcinogenesis 18(4):851–854. https://doi.org/10.1093/carcin/18.4.851
Han J, Xian Z, Zhang Y, Liu J, Liang A (2019) Systematic overview of aristolochic acids: nephrotoxicity, carcinogenicity, and underlying mechanisms. Front Pharmacol 10:648. https://doi.org/10.3389/fphar.2019.00648
Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF (2000) Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res 60(13):3440–3444
Hansten PD (2018) The underrated risks of tamoxifen drug interactions. Eur J Drug Metab Pharmacokinet 43(5):495–508. https://doi.org/10.1007/s13318-018-0475-9
Harleton E, Webster M, Bumpus NN, Kent UM, Rae JM, Hollenberg PF (2004) Metabolism of N, N´, N´´-triethylenethiophosphoramide by CYP2B1 and CYP2B6 results in the inactivation of both isoforms by two distinct mechanisms. J Pharmacol Exp Ther 310(3):1011–1019. https://doi.org/10.1124/jpet.104.069112
Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR (1996) 17b-Estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA 93(18):9776–9781. https://doi.org/10.1073/pnas.93.18.9776
Hazai E, Vereczkey L, Monostory K (2002) Reduction of toxic metabolite formation of acetaminophen. Biochem Biophys Res Commun 291(4):1089–1094. https://doi.org/10.1006/bbrc.2002.6541
He K, Woolf TF, Kindt EK, Fielder AE, Talaat RE (2001) Troglitazone quinone formation catalyzed by human and rat CYP3A: an atypical CYP oxidation reaction. Biochem Pharmacol 62(2):191–198. https://doi.org/10.1016/s0006-2952(01)00653-0
He K, Talaat RE, Pool WF et al (2004a) Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. Drug Metab Dispos 32(6):639–646. https://doi.org/10.1124/dmd.32.6.639
He XY, Shen J, Ding X, Lu AYH, Hong JY (2004b) Identification of critical amino acid residues of human CYP2A13 for the metabolic activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific carcinogen. Drug Metab Dispos 32(12):1516–1521. https://doi.org/10.1124/dmd.104.001370
He XY, Tang L, Wang SL, Cai QS, Wang JS, Hong JY (2006) Efficient activation of aflatoxin B1 by cytochrome P450 2A13, an enzyme predominantly expressed in human respiratory tract. Int J Cancer 118(11):2665–2671. https://doi.org/10.1002/ijc.21665
Hecht SS (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 11(6):559–603. https://doi.org/10.1021/tx980005y
Held-Warmkessel J (2003) Ecteinascidin-743. Clin J Oncol Nurs 7(3):313–319. https://doi.org/10.1188/03.Cjon.313-319
Hellmold H, Rylander T, Magnusson M, Reihnér E, Warner M, Gustafsson J-Å (1998) Characterization of cytochrome P450 enzymes in human breast tissue from reduction mammaplasties. J Clin Endocrinol Metab 83(3):886–895. https://doi.org/10.1210/jcem.83.3.4647
Hirani V, Yarovoy A, Kozeska A, Magnusson RP, Lasker JM (2008) Expression of CYP4F2 in human liver and kidney: assessment using targeted peptide antibodies. Arch Biochem Biophys 478(1):59–68. https://doi.org/10.1016/j.abb.2008.06.025
Ho PC, Abbott FS, Zanger UM, Chang TK (2003) Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogenomics J 3(6):335–342. https://doi.org/10.1038/sj.tpj.6500210
Hollenberg PF, Kent UM, Bumpus NN (2008) Mechanism-based inactivation of human cytochromes P450s: experimental characterization, reactive intermediates, and clinical implications. Chem Res Toxicol 21(1):189–205. https://doi.org/10.1021/tx7002504
Hu Y, Dehal SS, Hynd G, Jones GB, Kupfer D (2003) CYP2D6-mediated catalysis of tamoxifen aromatic hydroxylation with an NIH shift: similar hydroxylation mechanism in chicken, rat and human liver microsomes. Xenobiotica 33(2):141–151. https://doi.org/10.1080/0049825021000042733
Hu L, Wu F, He J, Zhong L, Song Y, Shao H (2019) Cytotoxicity of safrole in HepaRG cells: studies on the role of CYP1A2-mediated ortho-quinone metabolic activation. Xenobiotica 49(12):1504–1515. https://doi.org/10.1080/00498254.2019.1590882
Huang Z, Fasco MJ, Figge HL, Keyomarsi K, Kaminsky LS (1996) Expression of cytochromes P450 in human breast tissue and tumors. Drug Metab Dispos 24(8):899–905
Huang Z, Guengerich FP, Kaminsky LS (1998) 16a-Hydroxylation of estrone by human cytochrome P4503A4/5. Carcinogenesis 19(5):867–872. https://doi.org/10.1093/carcin/19.5.867
Huang Z, Roy P, Waxman DJ (2000) Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol 59(8):961–972. https://doi.org/10.1016/s0006-2952(99)00410-4
Huang YS, Chern HD, Su WJ et al (2003) Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 37(4):924–930. https://doi.org/10.1053/jhep.2003.50144
Hyland R, Gescher A, Thummel K et al (1992) Metabolic oxidation and toxification of N-methylformamide catalyzed by the cytochrome P450 isoenzyme CYP2E1. Mol Pharmacol 41(2):259–266
Ioannides C, Parke DV (1993) Induction of cytochrome P450 1 as an indicator of potential chemical carcinogenesis. Drug Metab Rev 25(4):485–501. https://doi.org/10.3109/03602539308993983
Irshaid Y, Branch RA, Adedoyin A (1996) Metabolic interactions of putative cytochrome P450 3A substrates with alternative pathways of dapsone metabolism in human liver microsomes. Drug Metab Dispos 24(2):164–171
Iscan M, Klaavuniemi T, Coban T, Kapucuoglu N, Pelkonen O, Raunio H (2001) The expression of cytochrome P450 enzymes in human breast tumours and normal breast tissue. Breast Cancer Res Treat 70(1):47–54. https://doi.org/10.1023/a:1012526406741
Iyer R, Coles B, Raney KD, Thier R, Guengerich FP, Harris TM (1994) DNA adduction by the potent carcinogen aflatoxin B1: mechanistic studies. J Am Chem Soc 116:1603–1609
Jacobson PA, Green K, Birnbaum A, Remmel RP (2002) Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of thioTEPA to TEPA. Cancer Chemother Pharmacol 49(6):461–467. https://doi.org/10.1007/s00280-002-0453-3
Jalas JR, Ding X, Murphy SE (2003) Comparative metabolism of the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol by rat cytochrome P450 2A3 and human cytochrome P450 2A13. Drug Metab Dispos 31(10):1199–1202. https://doi.org/10.1124/dmd.31.10.1199
Jan YH, Richardson JR, Baker AA et al (2016) Novel approaches to mitigating parathion toxicity: targeting cytochrome P450-mediated metabolism with menadione. Ann NY Acad Sci 1378(1):80–86. https://doi.org/10.1111/nyas.13156
Jefcoate CR, Liehr JG, Santen RJ et al (2000) Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr 27:95–112. https://doi.org/10.1093/oxfordjournals.jncimonographs.a024248
Jerabek P, Martinek V, Stiborova M (2012) Theoretical investigation of differences in nitroreduction of aristolochic acid I by cytochromes P450 1A1, 1A2 and 1B1. Neuro Endocrinol Lett 33(Suppl 3):25–32
Jeurissen SM, Bogaards JJ, Awad HM et al (2004) Human cytochrome P450 enzyme specificity for bioactivation of safrole to the proximate carcinogen 1´-hydroxysafrole. Chem Res Toxicol 17(9):1245–1250. https://doi.org/10.1021/tx040001v
Jeurissen SM, Bogaards JJ, Boersma MG et al (2006) Human cytochrome P450 enzymes of importance for the bioactivation of methyleugenol to the proximate carcinogen 1´-hydroxymethyleugenol. Chem Res Toxicol 19(1):111–116. https://doi.org/10.1021/tx050267h
Jeurissen SM, Punt A, Boersma MG et al (2007) Human cytochrome P450 enzyme specificity for the bioactivation of estragole and related alkenylbenzenes. Chem Res Toxicol 20(5):798–806. https://doi.org/10.1021/tx700012d
Jiang H, Shen YM, Quinn AM, Penning TM (2005) Competing roles of cytochrome P450 1A1/1B1 and aldo-keto reductase 1A1 in the metabolic activation of (±)-7,8-dihydroxy-7,8-dihydro-benzo[a]pyrene in human bronchoalveolar cell extracts. Chem Res Toxicol 18(2):365–374. https://doi.org/10.1021/tx0497245
Jiang H, Vudathala DK, Blair IA, Penning TM (2006) Competing roles of aldo-keto reductase 1A1 and cytochrome P4501B1 in benzo[a]pyrene-7,8-diol activation in human bronchoalveolar H358 cells: role of AKRs in P450 1B1 induction. Chem Res Toxicol 19(1):68–78. https://doi.org/10.1021/tx0502488
Johnson WW, Ueng Y-F, Widersten M et al (1997) Conjugation of highly reactive aflatoxin B1exo-8,9-epoxide catalyzed by rat and human glutathione transferases: estimation of kinetic parameters. Biochemistry 36(11):3056–3060. https://doi.org/10.1021/bi962537o
Johnson MD, Zuo H, Lee KH et al (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85(2):151–159. https://doi.org/10.1023/B:BREA.0000025406.31193.e8
Jorga KM, Fotteler B, Gasser R, Banken L, Birnboeck H (2000) Lack of interaction between tolcapone and tolbutamide in healthy volunteers. J Clin Pharmacol 40(5):544–551. https://doi.org/10.1177/00912700022009161
Josephy PD, Batty SM, Boverhof DR (2001) Recombinant human P450 forms 1A1, 1A2, and 1B1 catalyze the bioactivation of heterocyclic amine mutagens in Escherichia coli lacZ strains. Environ Mol Mutagen 38(1):12–18. https://doi.org/10.1002/em.1045
Juchau MR, Lee QP, Fantel AG (1992) Xenobiotic biotransformation/bioactivation in organogenesis-stage conceptual tissues: implications for embryotoxicity and teratogenesis. Drug Metab Rev 24(2):195–238. https://doi.org/10.3109/03602539208996293
Jushchyshyn MI, Wahlstrom JL, Hollenberg PF, Wienkers LC (2006) Mechanism of inactivation of human cytochrome P450 2B6 by phencyclidine. Drug Metab Dispos 34(9):1523–1529. https://doi.org/10.1124/dmd.106.010579
Kabler SL, Seidel A, Jacob J, Doehmer J, Morrow CS, Townsend AJ (2009) Differential protection by human glutathione S-transferase P1 against cytotoxicity of benzo[a]pyrene, dibenzo[a, l]pyrene, or their dihydrodiol metabolites, in bi-transgenic cell lines that co-express rat versus human cytochrome P450 1A1. Chem-Biol Interact 179(2–3):240–246. https://doi.org/10.1016/j.cbi.2009.01.010
Kalgutkar AS, Taylor TJ, Venkatakrishnan K, Isin EM (2003) Assessment of the contributions of CYP3A4 and CYP3A5 in the metabolism of the antipsychotic agent haloperidol to its potentially neurotoxic pyridinium metabolite and effect of antidepressants on the bioactivation pathway. Drug Metab Dispos 31(3):243–249. https://doi.org/10.1124/dmd.31.3.243
Kalgutkar AS, Obach RS, Maurer TS (2007) Mechanism-based inactivation of cytochrome P450 enzymes: chemical mechanisms, structure-activity relationships and relationship to clinical drug-drug interactions and idiosyncratic adverse drug reactions. Curr Drug Metab 8(5):407–447. https://doi.org/10.2174/138920007780866807
Kamataki T, Fujita K, Nakayama K, Yamazaki Y, Miyamoto M, Ariyoshi N (2002) Role of human cytochrome P450 (CYP) in the metabolic activation of nitrosamine derivatives: application of genetically engineered Salmonella expressing human CYP. Drug Metab Rev 34(3):667–676. https://doi.org/10.1081/dmr-120005668
Kamdem LK, Meineke I, Gödtel-Armbrust U, Brockmöller J, Wojnowski L (2006) Dominant contribution of P450 3A4 to the hepatic carcinogenic activation of aflatoxin B1. Chem Res Toxicol 19(4):577–586. https://doi.org/10.1021/tx050358e
Kang P, Dalvie D, Smith E, Zhou S, Deese A (2007) Identification of a novel glutathione conjugate of flutamide in incubations with human liver microsomes. Drug Metab Dispos 35(7):1081–1088. https://doi.org/10.1124/dmd.107.014860
Kang P, Dalvie D, Smith E, Zhou S, Deese A, Nieman JA (2008) Bioactivation of flutamide metabolites by human liver microsomes. Drug Metab Dispos 36(7):1425–1437. https://doi.org/10.1124/dmd.108.020370
Kappers WA, Edwards RJ, Murray S, Boobis AR (2001) Diazinon is activated by CYP2C19 in human liver. Toxicol Appl Pharmacol 177(1):68–76. https://doi.org/10.1006/taap.2001.9294
Karlgren M, Miura S, Ingelman-Sundberg M (2005) Novel extrahepatic cytochrome P450s. Toxicol Appl Pharmacol 207(2 Suppl):57–61. https://doi.org/10.1016/j.taap.2004.12.022
Kartha JS, Yost GS (2008) Mechanism-based inactivation of lung-selective cytochrome P450 CYP2F enzymes. Drug Metab Dispos 36(1):155–162. https://doi.org/10.1124/dmd.107.017897
Kassahun K, Pearson PG, Tang W et al (2001) Studies on the metabolism of troglitazone to reactive intermediates in vitro and in vivo. Evidence for novel biotransformation pathways involving quinone methide formation and thiazolidinedione ring scission. Chem Res Toxicol 14(1):62–70. https://doi.org/10.1021/tx000180q
Kazui M, Nishiya Y, Ishizuka T et al (2010) Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 38(1):92–99. https://doi.org/10.1124/dmd.109.029132
Kerlan V, Dreano Y, Bercovici JP, Beaune PH, Floch HH, Berthou F (1992) Nature of cytochromes P450 involved in the 2-/4-hydroxylations of estradiol in human liver microsomes. Biochem Pharmacol 44(9):1745–1756. https://doi.org/10.1016/0006-2952(92)90068-t
Kharasch ED, Hankins DC, Fenstamaker K, Cox K (2000) Human halothane metabolism, lipid peroxidation, and cytochromes P450 2A6 and P450 3A4. Eur J Clin Pharmacol 55(11–12):853–859. https://doi.org/10.1007/s002280050707
Khojasteh-Bakht SC, Chen W, Koenigs LL, Peter RM, Nelson SD (1999) Metabolism of (R)-(+)-pulegone and (R)-(+)-menthofuran by human liver cytochrome P-450s: evidence for formation of a furan epoxide. Drug Metab Dispos 27(5):574–580
Kiang TK, Ho PC, Anari MR, Tong V, Abbott FS, Chang TK (2006) Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol Sci 94(2):261–271. https://doi.org/10.1093/toxsci/kfl096
Kim D, Guengerich FP (2004) Selection of human cytochrome P450 1A2 mutants with enhanced catalytic activity for heterocyclic amine N-hydroxylation. Biochemistry 43(4):981–988. https://doi.org/10.1021/bi035593f
Kim B-R, Oh HS, Kim D-H (1997) Differential inhibition of aflatoxin B1 oxidation by gestodene action on human liver microsomes. Biochem Mol Biol Int 43(4):839–846. https://doi.org/10.1080/15216549700204651
Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR (1998) Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis 19(10):1847–1853. https://doi.org/10.1093/carcin/19.10.1847
Kim SY, Suzuki N, Santosh Laxmi YR, Rieger R, Shibutani S (2003) a-Hydroxylation of tamoxifen and toremifene by human and rat cytochrome P450 3A subfamily enzymes. Chem Res Toxicol 16(9):1138–1144. https://doi.org/10.1021/tx0300131
Kim JY, Chung JY, Park JE et al (2007) Benzo[a]pyrene induces apoptosis in RL95-2 human endometrial cancer cells by cytochrome P450 1A1 activation. Endocrinology 148(10):5112–5122. https://doi.org/10.1210/en.2007-0096
King LC, Adams L, Allison J et al (1999) A quantitative comparison of dibenzo[a, l]pyrene-DNA adduct formation by recombinant human cytochrome P450 microsomes. Mol Carcinog 26(2):74–82
Kisselev P, Schwarz D, Platt KL, Schunck WH, Roots I (2002) Epoxidation of benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1 in reconstituted membranes. Effects of charge and nonbilayer phase propensity of the membrane. Eur J Biochem 269(7):1799–1805. https://doi.org/10.1046/j.1432-1033.2002.02848.x
Korzekwa KR, Krishnamachary N, Shou M et al (1998) Evaluation of atypical cytochrome P450 kinetics with two-substrate models: evidence that multiple substrates can simultaneously bind to cytochrome P450 active sites. Biochemistry 37(12):4137–4147. https://doi.org/10.1021/bi9715627
Krause RJ, Elfarra AA (1997) Oxidation of butadiene monoxide to meso- and (±)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch Biochem Biophys 337(2):176–184. https://doi.org/10.1006/abbi.1996.9781
Krishnan S, Hvastkovs EG, Bajrami B, Schenkman JB, Rusling JF (2009) Human cyt P450 mediated metabolic toxicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) evaluated using electrochemiluminescent arrays. Mol Biosyst 5(2):163–169. https://doi.org/10.1039/b815910f
Kudo S, Ishizaki T (1999) Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet 37(6):435–456. https://doi.org/10.2165/00003088-199937060-00001
Kuffel MJ, Schroeder JC, Pobst LJ et al (2002) Activation of the antitumor agent aminoflavone (NSC 686288) is mediated by induction of tumor cell cytochrome P450 1A1/1A2. Mol Pharmacol 62(1):143–153. https://doi.org/10.1124/mol.62.1.143
Kumar S, Samuel K, Subramanian R et al (2002) Extrapolation of diclofenac clearance from in vitro microsomal metabolism data: role of acyl glucuronidation and sequential oxidative metabolism of the acyl glucuronide. J Pharmacol Exp Ther 303(3):969–978. https://doi.org/10.1124/jpet.102.038992
Kurth MJ, Yokoi T, Gershwin ME (2014) Halothane-induced hepatitis: paradigm or paradox for drug-induced liver injury. Hepatology 60(5):1473–1475. https://doi.org/10.1002/hep.27253
Kushida H, Fujita K, Suzuki A et al (2000) Metabolic activation of N-alkylnitrosamines in genetically engineered Salmonella typhimurium expressing CYP2E1 or CYP2A6 together with human NADPH-cytochrome P450 reductase. Carcinogenesis 21(6):1227–1232
Lacassagne A (1932) Results of the treatment of cancer of the cervix uteri. Br Med J 2(3750):912–913. https://doi.org/10.1136/bmj.2.3750.912
Laine JE, Auriola S, Pasanen M, Juvonen RO (2009) Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 39(1):11–21. https://doi.org/10.1080/00498250802512830
Lalani AS, Alters SE, Wong A, Albertella MR, Cleland JL, Henner WD (2007) Selective tumor targeting by the hypoxia-activated prodrug AQ4N blocks tumor growth and metastasis in preclinical models of pancreatic cancer. Clin Cancer Res 13(7):2216–2225. https://doi.org/10.1158/1078-0432.Ccr-06-2427
Langouët S, Welti DH, Kerriguy N et al (2001) Metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in human hepatocytes: 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2. Chem Res Toxicol 14(2):211–221. https://doi.org/10.1021/tx000176e
Lanza DL, Yost GS (2001) Selective dehydrogenation/oxygenation of 3-methylindole by cytochrome P450 enzymes. Drug Metab Dispos 29(7):950–953
Lanza DL, Code E, Crespi CL, Gonzalez FJ, Yost GS (1999) Specific dehydrogenation of 3-methylindole and epoxidation of naphthalene by recombinant human CYP2F1 expressed in lymphoblastoid cells. Drug Metab Dispos 27(7):798–803
Larson AM, Polson J, Fontana RJ et al (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42(6):1364–1372
Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK (2000) Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem 275(6):4118–4126. https://doi.org/10.1074/jbc.275.6.4118
Lassila T, Mattila S, Turpeinen M, Pelkonen O, Tolonen A (2016) Tandem mass spectrometric analysis of S- and N-linked glutathione conjugates of pulegone and menthofuran and identification of P450 enzymes mediating their formation. Rapid Commun Mass Spectrom 30(7):917–926. https://doi.org/10.1002/rcm.7518
Lauer B, Tuschl G, Kling M, Mueller SO (2009) Species-specific toxicity of diclofenac and troglitazone in primary human and rat hepatocytes. Chem-Biol Interact 179(1):17–24. https://doi.org/10.1016/j.cbi.2008.10.031
Lecoeur S, Bonierbale E, Challine D et al (1994) Specificity of in vitro covalent binding of tienilic acid metabolites to human liver microsomes in relationship to the type of hepatotoxicity: comparison with two directly hepatotoxic drugs. Chem Res Toxicol 7(3):434–442. https://doi.org/10.1021/tx00039a023
Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ (1996) Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem 271(20):12063–12067. https://doi.org/10.1074/jbc.271.20.12063
Lee AJ, Kosh JW, Conney AH, Zhu BT (2001) Characterization of the NADPH-dependent metabolism of 17b-estradiol to multiple metabolites by human liver microsomes and selectively expressed human cytochrome P450 3A4 and 3A5. J Pharmacol Exp Ther 298(2):420–432
Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT (2003a) Characterization of the oxidative metabolites of 17b-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinology 144(8):3382–3398. https://doi.org/10.1210/en.2003-0192
Lee AJ, Conney AH, Zhu BT (2003b) Human cytochrome P450 3A7 has a distinct high catalytic activity for the 16a-hydroxylation of estrone but not 17b-estradiol. Cancer Res 63(19):6532–6536
Leemann T, Transon C, Dayer P (1993) Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4´-hydroxylation in human liver. Life Sci 52(1):29–34. https://doi.org/10.1016/0024-3205(93)90285-b
Levorato S, Dominici L, Fatigoni C et al (2018) In vitro toxicity evaluation of estragole-containing preparations derived from Foeniculum vulgare Mill. (fennel) on HepG2 cells. Food Chem Toxicol 111:616–622. https://doi.org/10.1016/j.fct.2017.12.014
Lewis CW, Smith JE, Anderson JG, Freshney RI (1999) Increased cytotoxicity of food-borne mycotoxins toward human cell lines in vitro via enhanced cytochrome P450 expression using the MTT bioassay. Mycopathologia 148(2):97–102. https://doi.org/10.1023/a:1007130923558
Lewis BC, Korprasertthaworn P, Miners JO (2016) Impaired dacarbazine activation and 7-ethoxyresorufin deethylation in vitro by polymorphic variants of CYP1A1 and CYP1A2: implications for cancer therapy. Pharmacogenet Genom 26(10):453–461. https://doi.org/10.1097/fpc.0000000000000236
Li Y, Wang E, Patten CJ, Chen L, Yang CS (1994) Effects of flavonoids on cytochrome P450-dependent acetaminophen metabolism in rats and human liver microsomes. Drug Metab Dispos 22(4):566–571
Li DN, Seidel A, Pritchard MP, Wolf CR, Friedberg T (2000) Polymorphisms in P450 CYP1B1 affect the conversion of estradiol to the potentially carcinogenic metabolite 4-hydroxyestradiol. Pharmacogenetics 10(4):343–353. https://doi.org/10.1097/00008571-200006000-00008
Li XQ, Björkman A, Andersson TB, Gustafsson LL, Masimirembwa CM (2003) Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol 59(5–6):429–442. https://doi.org/10.1007/s00228-003-0636-9
Li X, He X, Chen S et al (2020) Evaluation of pyrrolizidine alkaloid-induced genotoxicity using metabolically competent TK6 cell lines. Food Chem Toxicol 145:111662. https://doi.org/10.1016/j.fct.2020.111662
Liehr JG (2000) Is estradiol a genotoxic mutagenic carcinogen? Endocrinol Rev 21(1):40–54. https://doi.org/10.1210/edrv.21.1.0386
Lin HL, Hollenberg PF (2007) The inactivation of cytochrome P450 3A5 by 17a-ethynylestradiol is cytochrome b5-dependent: metabolic activation of the ethynyl moiety leads to the formation of glutathione conjugates, a heme adduct, and covalent binding to the apoprotein. J Pharmacol Exp Ther 321(1):276–287. https://doi.org/10.1124/jpet.106.117861
Lin HL, Kent UM, Hollenberg PF (2002) Mechanism-based inactivation of cytochrome P450 3A4 by 17a-ethynylestradiol: evidence for heme destruction and covalent binding to protein. J Pharmacol Exp Ther 301(1):160–167. https://doi.org/10.1124/jpet.301.1.160
Lin CY, Pan TS, Ting CC et al (2013) Cytochrome P450 metabolism of betel quid-derived compounds: implications for the development of prevention strategies for oral and pharyngeal cancers. Sci World J 2013:618032. https://doi.org/10.1155/2013/618032
Loaiza-Pérez AI, Kenney S, Boswell J et al (2004) Aryl hydrocarbon receptor activation of an antitumor aminoflavone: basis of selective toxicity for MCF-7 breast tumor cells. Mol Cancer Ther 3(6):715–725
López-Garcia MP, Dansette PM, Valadon P et al (1993) Human-liver cytochromes P-450 expressed in yeast as tools for reactive-metabolite formation studies. Oxidative activation of tienilic acid by cytochromes P-450 2C9 and 2C10. Eur J Biochem 213(1):223–232. https://doi.org/10.1111/j.1432-1033.1993.tb17752.x
López-Garcia MP, Dansette PM, Mansuy D (1994) Thiophene derivatives as new mechanism-based inhibitors of cytochromes P-450: inactivation of yeast-expressed human liver cytochrome P-450 2C9 by tienilic acid. Biochemistry 33(1):166–175. https://doi.org/10.1021/bi00167a022
Lozano JJ, Pastor M, Cruciani G et al (2000) 3D-QSAR methods on the basis of ligand-receptor complexes. Application of COMBINE and GRID/GOLPE methodologies to a series of CYP1A2 ligands. J Comput Aided Mol Des 14(4):341–353. https://doi.org/10.1023/a:1008164621650
Lu H, Wang JJ, Chan KK, Philip PA (2006) Stereoselectivity in metabolism of ifosfamide by CYP3A4 and CYP2B6. Xenobiotica 36(5):367–385. https://doi.org/10.1080/00498250600598486
Lu Y, Wong KY, Tan C, Ma J, Feng B, Lin G (2020) Establishment of a novel CYP3A4-transduced human hepatic sinusoidal endothelial cell model and its application in screening hepatotoxicity of pyrrolizidine alkaloids. J Environ Sci Health C Toxicol Carcinog 38(2):169–185. https://doi.org/10.1080/26896583.2020.1769409
Luch A, Coffing SL, Tang YM et al (1998) Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a, l]pyrene. Chem Res Toxicol 11(6):686–695. https://doi.org/10.1021/tx970236p
Luch A, Kishiyama S, Seidel A, Doehmer J, Greim H, Baird WM (1999a) The K-region trans-8,9-diol does not significantly contribute as an intermediate in the metabolic activation of dibenzo[a, l]pyrene to DNA-binding metabolites by human cytochrome P450 1A1 or 1B1. Cancer Res 59(18):4603–4609
Luch A, Schober W, Soballa VJ et al (1999b) Metabolic activation of dibenzo[a, l]pyrene by human cytochrome P450 1A1 and P450 1B1 expressed in V79 Chinese hamster cells. Chem Res Toxicol 12(4):353–364. https://doi.org/10.1021/tx980240g
Lundblad MS, Stark K, Eliasson E, Oliw E, Rane A (2005) Biosynthesis of epoxyeicosatrienoic acids varies between polymorphic CYP2C enzymes. Biochem Biophys Res Commun 327(4):1052–1057. https://doi.org/10.1016/j.bbrc.2004.12.116
Mahadevan B, Luch A, Atkin J, Haynes M, Nguyen T, Baird WM (2007) Inhibition of human cytochrome P450 1B1 further clarifies its role in the activation of dibenzo[a, l]pyrene in cells in culture. J Biochem Mol Toxicol 21(3):101–109. https://doi.org/10.1002/jbt.20168
Mammen JS, Pittman GS, Li Y et al (2003) Single amino acid mutations, but not common polymorphisms, decrease the activity of CYP1B1 against (-)benzo[a]pyrene-7R-trans-7,8-dihydrodiol. Carcinogenesis 24(7):1247–1255. https://doi.org/10.1093/carcin/bgg088
Mammen JS, Kleiner HE, DiGiovanni J, Sutter TR, Strickland PT (2005) Coumarins are competitive inhibitors of cytochrome P450 1B1, with equal potency for allelic variants. Pharmacogenet Genomics 15(3):183–188. https://doi.org/10.1097/01213011-200503000-00007
Mancy A, Antignac M, Minoletti C et al (1999) Diclofenac and its derivatives as tools for studying human cytochromes P450 active sites: particular efficiency and regioselectivity of P450 2Cs. Biochemistry 38(43):14264–14270. https://doi.org/10.1021/bi991195u
Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT (2000) Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther 67(3):275–282. https://doi.org/10.1067/mcp.2000.104736
Martínez-Cabot A, Morató A, Commandeur JN, Vermeulen NP, Messeguer A (2007) In vitro bioactivation of 3-(N-phenylamino)propane-1,2-diol by human and rat liver microsomes and recombinant P450 enzymes. Implications for toxic oil syndrome. Chem Res Toxicol 20(8):1218–1224. https://doi.org/10.1021/tx700209p
Martinkova E, Dontenwill M, Frei E, Stiborova M (2009) Cytotoxicity of and DNA adduct formation by ellipticine in human U87MG glioblastoma cancer cells. Neuro Endocrinol Lett 30(Suppl 1):60–66
Masubuchi Y, Nakano T, Ose A, Horie T (2001) Differential selectivity in carbamazepine-induced inactivation of cytochrome P450 enzymes in rat and human liver. Arch Toxicol 75(9):538–543. https://doi.org/10.1007/s002040100270
May DG, Porter J, Wilkinson GR, Branch RA (1994) Frequency distribution of dapsone N-hydroxylase, a putative probe for P450 3A4 activity, in a white population. Clin Pharmacol Ther 55(5):492–500. https://doi.org/10.1038/clpt.1994.62
McCarthy HO, Yakkundi A, McErlane V et al (2003) Bioreductive GDEPT using cytochrome P450 3A4 in combination with AQ4N. Cancer Gene Ther 10(1):40–48. https://doi.org/10.1038/sj.cgt.7700522
McCune JS, Risler LJ, Phillips BR, Thummel KE, Blough D, Shen DD (2005) Contribution of CYP3A5 to hepatic and renal ifosfamide N-dechloroethylation. Drug Metab Dispos 33(7):1074–1081. https://doi.org/10.1124/dmd.104.002279
McErlane V, Yakkundi A, McCarthy HO et al (2005) A cytochrome P450 2B6 meditated gene therapy strategy to enhance the effects of radiation or cyclophosphamide when combined with the bioreductive drug AQ4N. J Gene Med 7(7):851–859. https://doi.org/10.1002/jgm.728
McGill MR, Jaeschke H (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30(9):2174–2187. https://doi.org/10.1007/s11095-013-1007-6
McManus ME, Burgess WM, Veronese ME, Huggett A, Quattrochi LC, Tukey RH (1990) Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochromes P-450. Cancer Res 50(11):3367–3376
Megaraj V, Zhou X, Xie F, Liu Z, Yang W, Ding X (2014) Role of CYP2A13 in the bioactivation and lung tumorigenicity of the tobacco-specific lung procarcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: in vivo studies using a CYP2A13-humanized mouse model. Carcinogenesis 35(1):131–137. https://doi.org/10.1093/carcin/bgt269
Melet A, Assrir N, Jean P et al (2003) Substrate selectivity of human cytochrome P450 2C9: importance of residues 476, 365, and 114 in recognition of diclofenac and sulfaphenazole and in mechanism-based inactivation by tienilic acid. Arch Biochem Biophys 409(1):80–91. https://doi.org/10.1016/s0003-9861(02)00548-9
Milichovský J, Bárta F, Schmeiser HH et al (2016) Active site mutations as a suitable tool contributing to explain a mechanism of aristolochic acid I nitroreduction by cytochromes P450 1A1, 1A2 and 1B1. Int J Mol Sci 17(2):213. https://doi.org/10.3390/ijms17020213
Minoda Y, Kharasch ED (2001) Halothane-dependent lipid peroxidation in human liver microsomes is catalyzed by cytochrome P450 2A6 (CYP2A6). Anesthesiology 95(2):509–514. https://doi.org/10.1097/00000542-200108000-00037
Miranda CL, Reed RL, Guengerich FP, Buhler DR (1991) Role of cytochrome P450 IIIA4 in the metabolism of the pyrrolizidine alkaloid senecionine in human liver. Carcinogenesis 12(3):515–519. https://doi.org/10.1093/carcin/12.3.515
Miranda CL, Yang YH, Henderson MC et al (2000) Prenylflavonoids from hops inhibit the metabolic activation of the carcinogenic heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline, mediated by cDNA-expressed human CYP1A2. Drug Metab Dispos 28(11):1297–1302
Mitra AK, Thummel KE, Kalhorn TF, Kharasch ED, Unadkat JD, Slattery JT (1995) Metabolism of dapsone to its hydroxylamine by CYP2E1 in vitro and in vivo. Clin Pharmacol Ther 58(5):556–566. https://doi.org/10.1016/0009-9236(95)90176-0
Miyazaki M, Sugawara E, Yoshimura T, Yamazaki H, Kamataki T (2005) Mutagenic activation of betel quid-specific N-nitrosamines catalyzed by human cytochrome P450 coexpressed with NADPH-cytochrome P450 reductase in Salmonella typhimurium YG7108. Mutat Res 581(1–2):165–171. https://doi.org/10.1016/j.mrgentox.2004.12.002
Modugno F, Knoll C, Kanbour-Shakir A, Romkes M (2003) A potential role for the estrogen-metabolizing cytochrome P450 enzymes in human breast carcinogenesis. Breast Cancer Res Treat 82(3):191–197. https://doi.org/10.1023/b:Brea.0000004376.21491.44
Monien BH, Sachse B, Niederwieser B, Abraham K (2019) Detection of N-acetyl-S-[3´-(4-methoxyphenyl)allyl]-l-Cys (AMPAC) in human urine samples after controlled exposure to fennel tea: a new metabolite of estragole and trans-anethole. Chem Res Toxicol 32(11):2260–2267. https://doi.org/10.1021/acs.chemrestox.9b00287
Moore CD, Reilly CA, Yost GS (2010a) CYP3A4-Mediated oxygenation versus dehydrogenation of raloxifene. Biochemistry 49(21):4466–4475. https://doi.org/10.1021/bi902213r
Moore CD, Shahrokh K, Sontum SF, Cheatham TE 3rd, Yost GS (2010b) Improved cytochrome P450 3A4 molecular models accurately predict the Phe215 requirement for raloxifene dehydrogenation selectivity. Biochemistry 49(41):9011–9019. https://doi.org/10.1021/bi101139q
Mráz J, Jheeta P, Gescher A, Hyland R, Thummel K, Threadgill MD (1993) Investigation of the mechanistic basis of N, N-dimethylformamide toxicity. Metabolism of N, N-dimethylformamide and its deuterated isotopomers by cytochrome P450 2E1. Chem Res Toxicol 6(2):197–207. https://doi.org/10.1021/tx00032a009
Murray M, Butler AM, Stupans I (1994) Competitive inhibition of human liver microsomal cytochrome P450 3A-dependent steroid 6b-hydroxylation activity by cyclophosphamide and ifosfamide in vitro. J Pharmacol Exp Ther 270(2):645–649
Mutch E, Williams FM (2006) Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology 224(1–2):22–32. https://doi.org/10.1016/j.tox.2006.04.024
Mutch E, Blain PG, Williams FM (1999) The role of metabolism in determining susceptibility to parathion toxicity in man. Toxicol Lett 107(1–3):177–187. https://doi.org/10.1016/s0378-4274(99)00044-2
Mutch E, Daly AK, Leathart JB, Blain PG, Williams FM (2003) Do multiple cytochrome P450 isoforms contribute to parathion metabolism in man? Arch Toxicol 77(6):313–320. https://doi.org/10.1007/s00204-003-0452-0
Nakajima M, Komagata S, Fujiki Y et al (2007) Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenet Genom 17(6):431–445. https://doi.org/10.1097/FPC.0b013e328045c4fb
Nastainczyk W, Rendic S, Ullrich V, Krüger R (1982) Prevention by metyrapone of halothane induced liver necrosis in rats (author’s transl). Anaesthesist 31(4):181–184
Neal GE (1995) Genetic implications in the metabolism and toxicity of mycotoxins. Toxicol Lett 82–83:861–867. https://doi.org/10.1016/0378-4274(95)03600-8
Ngui JS, Tang W, Stearns RA et al (2000) Cytochrome P450 3A4-mediated interaction of diclofenac and quinidine. Drug Metab Dispos 28(9):1043–1050
Nieusma JL, Claffey DJ, Koop DR et al (1998) Oxidation of 1,3-butadiene to (R)- and (S)-butadiene monoxide by purified recombinant cytochrome P450 2E1 from rabbit, rat and human. Toxicol Lett 95(2):123–129. https://doi.org/10.1016/s0378-4274(98)00026-5
Nishida CR, Lee M, Ortiz de Montellano PR (2010) Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol Pharmacol 78(3):497–502. https://doi.org/10.1124/mol.110.065045
Nishigaki R, Totsuka Y, Takamura-Enya T, Sugimura T, Wakabayashi K (2004) Identification of cytochrome P-450s involved in the formation of APNH from norharman with aniline. Mutat Res 562(1–2):19–25. https://doi.org/10.1016/j.mrgentox.2004.05.003
Niwa T, Yabusaki Y, Honma K et al (1998) Contribution of human hepatic cytochrome P450 isoforms to regioselective hydroxylation of steroid hormones. Xenobiotica 28(6):539–547. https://doi.org/10.1080/004982598239290
Niwa T, Murayama N, Imagawa Y, Yamazaki H (2015) Regioselective hydroxylation of steroid hormones by human cytochromes P450. Drug Metab Rev 47(2):89–110. https://doi.org/10.3109/03602532.2015.1011658
Notley LM, De Wolf CJ, Wunsch RM, Lancaster RG, Gillam EMJ (2002) Bioactivation of tamoxifen by recombinant human cytochrome P450 enzymes. Chem Res Toxicol 15(5):614–622. https://doi.org/10.1021/tx0100439
Notley LM, Crewe KH, Taylor PJ, Lennard MS, Gillam EMJ (2005) Characterization of the human cytochrome P450 forms involved in metabolism of tamoxifen to its a-hydroxy and a,4-dihydroxy derivatives. Chem Res Toxicol 18(10):1611–1618. https://doi.org/10.1021/tx050140s
Novak RF, Woodcroft KJ (2000) The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch Pharm Res 23(4):267–282. https://doi.org/10.1007/bf02975435
Oda Y, Aryal P, Terashita T, Gillam EMJ, Guengerich FP, Shimada T (2001) Metabolic activation of heterocyclic amines and other procarcinogens in Salmonella typhimurium umu tester strains expressing human cytochrome P4501A1, 1A2, 1B1, 2C9, 2D6, 2E1, and 3A4 and human NADPH-P450 reductase and bacterial O-acetyltransferase. Mutat Res 492(1–2):81–90. https://doi.org/10.1016/s1383-5718(01)00154-1
O’Rourke M, Ward C, Worthington J et al (2008) Evaluation of the antiangiogenic potential of AQ4N. Clin Cancer Res 14(5):1502–1509. https://doi.org/10.1158/1078-0432.Ccr-07-1262
O’Shea D, Kim RB, Wilkinson GR (1997) Modulation of CYP2E1 activity by isoniazid in rapid and slow N-acetylators. Br J Clin Pharmacol 43(1):99–103. https://doi.org/10.1111/j.1365-2125.1997.tb00039.x
Palacharla RC, Uthukam V, Manoharan A et al (2017) Inhibition of cytochrome P450 enzymes by saturated and unsaturated fatty acids in human liver microsomes, characterization of enzyme kinetics in the presence of bovine serum albumin (0.1 and 1.0% w/v) and in vitro–in vivo extrapolation of hepatic clearance. Eur J Pharm Sci 101:80–89. https://doi.org/10.1016/j.ejps.2017.01.027
Pan LP, Wijnant P, De Vriendt C, Rosseel MT, Belpaire FM (1997) Characterization of the cytochrome P450 isoenzymes involved in the in vitro N-dealkylation of haloperidol. Br J Clin Pharmacol 44(6):557–564. https://doi.org/10.1046/j.1365-2125.1997.t01-1-00629.x
Pang S, Cao JQ, Katz BH, Hayes CL, Sutter TR, Spink DC (1999) Inductive and inhibitory effects of non-ortho-substituted polychlorinated biphenyls on estrogen metabolism and human cytochromes P450 1A1 and 1B1. Biochem Pharmacol 58(1):29–38. https://doi.org/10.1016/s0006-2952(99)00070-2
Paracchini V, Pedotti P, Raimondi S et al (2005) A common CYP1B1 polymorphism is associated with 2-OH E1/16-OH E1 urinary estrone ratio. Clin Chem Lab Med 43(7):702–706. https://doi.org/10.1515/cclm.2005.119
Parikh A, Josephy PD, Guengerich FP (1999) Selection and characterization of human cytochrome P450 1A2 mutants with altered catalytic properties. Biochemistry 38(17):5283–5289. https://doi.org/10.1021/bi990142+
Parkinson OT, Liggitt HD, Rettie AE, Kelly EJ (2013) Generation and characterization of a Cyp4b1 null mouse and the role of CYP4B1 in the activation and toxicity of ipomeanol. Toxicol Sci 134(2):243–250. https://doi.org/10.1093/toxsci/kft123
Patten CJ, Thomas PE, Guy RL et al (1993) Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 6(4):511–518. https://doi.org/10.1021/tx00034a019
Patten CJ, Smith TJ, Murphy SE et al (1996) Kinetic analysis of the activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by heterologously expressed human P450 enzymes and the effect of P450-specific chemical inhibitors on this activation in human liver microsomes. Arch Biochem Biophys 333(1):127–138. https://doi.org/10.1006/abbi.1996.0373
Patten CJ, Smith TJ, Friesen MJ, Tynes RE, Yang CS, Murphy SE (1997) Evidence for cytochrome P450 2A6 and 3A4 as major catalysts for N´-nitrosonornicotine a-hydroxylation by human liver microsomes. Carcinogenesis 18(8):1623–1630. https://doi.org/10.1093/carcin/18.8.1623
Patterson LH (1993) Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metastasis Rev 12(2):119–134. https://doi.org/10.1007/bf00689805
Patterson LH (2002) Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab Rev 34(3):581–592. https://doi.org/10.1081/dmr-120005659
Patterson LH, Murray GI (2002) Tumour cytochrome P450 and drug activation. Curr Pharm Des 8(15):1335–1347. https://doi.org/10.2174/1381612023394502
Patterson LH, McKeown SR, Robson T, Gallagher R, Raleigh SM, Orr S (1999) Antitumour prodrug development using cytochrome P450 (CYP) mediated activation. Anticancer Drug Des 14(6):473–486
Pearce RE, Uetrecht JP, Leeder JS (2005) Pathways of carbamazepine bioactivation in vitro: II. The role of human cytochrome P450 enzymes in the formation of 2-hydroxyiminostilbene. Drug Metab Dispos 33(12):1819–1826. https://doi.org/10.1124/dmd.105.004861
Pearce RE, Lu W, Wang Y, Uetrecht JP, Correia MA, Leeder JS (2008) Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine. Drug Metab Dispos 36(8):1637–1649. https://doi.org/10.1124/dmd.107.019562
Penman BW, Reece J, Smith T et al (1993) Characterization of a human cell line expressing high levels of cDNA-derived CYP2D6. Pharmacogenetics 3(1):28–39. https://doi.org/10.1097/00008571-199302000-00003
Philip PA, Ali-Sadat S, Doehmer J et al (1999) Use of V79 cells with stably transfected cytochrome P450 cDNAs in studying the metabolism and effects of cytotoxic drugs. Cancer Chemother Pharmacol 43(1):59–67. https://doi.org/10.1007/s002800050863
Powell PK, Wolf I, Jin R, Lasker JM (1998) Metabolism of arachidonic acid to 20-hydroxy-5,8,11,14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J Pharmacol Exp Ther 285(3):1327–1336
Quinn AM, Penning TM (2008) Comparisons of (±)-benzo[a]pyrene-trans-7,8-dihydrodiol activation by human cytochrome P450 and aldo-keto reductase enzymes: effect of redox state and expression levels. Chem Res Toxicol 21(5):1086–1094. https://doi.org/10.1021/tx700345v
Rahman MA, Kodidela S, Sinha N et al (2019) Plasma exosomes exacerbate alcohol- and acetaminophen-induced toxicity via CYP2E1 pathway. Sci Rep 9(1):6571. https://doi.org/10.1038/s41598-019-43064-2
Raleigh SM, Wanogho E, Burke MD, McKeown SR, Patterson LH (1998) Involvement of human cytochromes P450 (CYP) in the reductive metabolism of AQ4N, a hypoxia activated anthraquinone di-N-oxide prodrug. Int J Radiat Oncol Biol Phys 42(4):763–767. https://doi.org/10.1016/s0360-3016(98)00308-3
Ramsdell HS, Parkinson A, Eddy AC, Eaton DL (1991) Bioactivation of aflatoxin B1 by human liver microsomes: role of cytochrome P450 IIIA enzymes. Toxicol Appl Pharmacol 108(3):436–447. https://doi.org/10.1016/0041-008x(91)90090-2
Raney KD, Shimada T, Kim D-H, Groopman JD, Harris TM, Guengerich FP (1992) Oxidation of aflatoxins and sterigmatocystin by human liver microsomes: significance of aflatoxin Q1 as a detoxication product of aflatoxin B1. Chem Res Toxicol 5(2):202–210. https://doi.org/10.1021/tx00026a009
Raucy JL (1995) Risk assessment: toxicity from chemical exposure resulting from enhanced expression of CYP2E1. Toxicology 105(2–3):217–224. https://doi.org/10.1016/0300-483x(95)03216-3
Raucy JL, Lasker JM, Lieber CS, Black M (1989) Acetaminophen activation by human liver cytochromes P450 IIE1 and P450 IA2. Arch Biochem Biophys 271(2):270–283. https://doi.org/10.1016/0003-9861(89)90278-6
Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM (1999) Metabolic activation of dacarbazine by human cytochromes P450: the role of CYP1A1, CYP1A2, and CYP2E1. Clin Cancer Res 5(8):2192–2197
Reid JM, Kuffel MJ, Ruben SL et al (2002) Rat and human liver cytochrome P-450 isoform metabolism of ecteinascidin 743 does not predict gender-dependent toxicity in humans. Clin Cancer Res 8(9):2952–2962
Ren S, Yang JS, Kalhorn TF, Slattery JT (1997) Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res 57(19):4229–4235
Rendic S (2002) Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev 34(1–2):83–448. https://doi.org/10.1081/dmr-120001392
Rendic S, DiCarlo FJ (1997) Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev 29(1–2):413–580. https://doi.org/10.3109/03602539709037591
Rendic S, Guengerich FP (2010) Update information on drug metabolism systems–2009, part II: summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr Drug Metab 11(1):4–84. https://doi.org/10.2174/138920010791110917
Rendic S, Guengerich FP (2012) Contributions of human enzymes in carcinogen metabolism. Chem Res Toxicol 25(7):1316–1383. https://doi.org/10.1021/tx300132k
Rendic S, Guengerich FP (2015) Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol 28(1):38–42. https://doi.org/10.1021/tx500444e
Rendic SP, Guengerich FP (2018) Development and uses of offline and web-searchable metabolism databases–the case of benzo[a]pyrene. Curr Drug Metab 19(1):3–46. https://doi.org/10.2174/1389200219666171207123939
Rifkind AB, Lee C, Chang TK, Waxman DJ (1995) Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch Biochem Biophys 320(2):380–389. https://doi.org/10.1016/0003-9861(95)90023-3
Rivera SP, Wang F, Saarikoski ST et al (2007) A novel promoter element containing multiple overlapping xenobiotic and hypoxia response elements mediates induction of cytochrome P450 2S1 by both dioxin and hypoxia. J Biol Chem 282(15):10881–10893. https://doi.org/10.1074/jbc.M609617200
Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL (2001) Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther 296(2):537–541
Roe AL, Snawder JE, Benson RW, Roberts DW, Casciano DA (1993) HepG2 cells: an in vitro model for P450-dependent metabolism of acetaminophen. Biochem Biophys Res Commun 190(1):15–19. https://doi.org/10.1006/bbrc.1993.1003
Roellecke K, Virts EL, Einholz R et al (2016) Optimized human CYP4B1 in combination with the alkylator prodrug 4-ipomeanol serves as a novel suicide gene system for adoptive T-cell therapies. Gene Ther 23(7):615–626. https://doi.org/10.1038/gt.2016.38
Roman RJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82(1):131–185. https://doi.org/10.1152/physrev.00021.2001
Roy P, Yu LJ, Crespi CL, Waxman DJ (1999b) Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos 27(6):655–666
Roy P, Tretyakov O, Wright J, Waxman DJ (1999a) Stereoselective metabolism of ifosfamide by human P-450s 3A4 and 2B6. Favorable metabolic properties of R-enantiomer. Drug Metab Dispos 27(11):1309–1318
Ruan Q, Gelhaus SL, Penning TM, Harvey RG, Blair IA (2007) Aldo-keto reductase- and cytochrome P450-dependent formation of benzo[a]pyrene-derived DNA adducts in human bronchoalveolar cells. Chem Res Toxicol 20(3):424–431. https://doi.org/10.1021/tx060180b
Saarikoski ST, Rivera SP, Hankinson O, Husgafvel-Pursiainen K (2005) CYP2S1: a short review. Toxicol Appl Pharmacol 207(2 Suppl):62–69. https://doi.org/10.1016/j.taap.2004.12.027
Sabater Vilar M, Kuilman-Wahls ME, Fink-Gremmels J (2003) Inhibition of aflatoxin B1 mutagenicity by cyclopiazonic acid in the presence of human liver preparations. Toxicol Lett 143(3):291–299. https://doi.org/10.1016/s0378-4274(03)00196-6
Sacerdoti D, Gatta A, McGiff JC (2003) Role of cytochrome P450-dependent arachidonic acid metabolites in liver physiology and pathophysiology. Prostaglandins Other Lipid Mediat 72(1–2):51–71. https://doi.org/10.1016/s1098-8823(03)00077-7
Sadeque AJ, Fisher MB, Korzekwa KR, Gonzalez FJ, Rettie AE (1997) Human CYP2C9 and CYP2A6 mediate formation of the hepatotoxin 4-ene-valproic acid. J Pharmacol Exp Ther 283(2):698–703
Sakano K, Kawanishi S (2002) Metal-mediated DNA damage induced by curcumin in the presence of human cytochrome P450 isozymes. Arch Biochem Biophys 405(2):223–230. https://doi.org/10.1016/s0003-9861(02)00302-8
Salmela KS, Kessova IG, Tsyrlov IB, Lieber CS (1998) Respective roles of human cytochrome P-450 2E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol Clin Exp Res 22(9):2125–2132
Sams C, Mason HJ, Rawbone R (2000) Evidence for the activation of organophosphate pesticides by cytochromes P450 3A4 and 2D6 in human liver microsomes. Toxicol Lett 116(3):217–221. https://doi.org/10.1016/s0378-4274(00)00221-6
Sams C, Cocker J, Lennard MS (2004) Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP). Xenobiotica 34(10):861–873. https://doi.org/10.1080/00498250400017273
Sarich T, Kalhorn T, Magee S et al (1997) The effect of omeprazole pretreatment on acetaminophen metabolism in rapid and slow metabolizers of S-mephenytoin. Clin Pharmacol Ther 62(1):21–28. https://doi.org/10.1016/s0009-9236(97)90148-x
Satoh T, Fujita KI, Munakata H et al (2000) Studies on the interactions between drugs and estrogen: analytical method for prediction system of gynecomastia induced by drugs on the inhibitory metabolism of estradiol using Escherichia coli coexpressing human CYP3A4 with human NADPH-cytochrome P450 reductase. Anal Biochem 286(2):179–186. https://doi.org/10.1006/abio.1999.4775
Sausville LN, Gangadhariah MH, Chiusa M et al (2018) The cytochrome P450 slow metabolizers CYP2C9*2 and CYP2C9*3 directly regulate tumorigenesis via reduced epoxyeicosatrienoic acid production. Cancer Res 78(17):4865–4877. https://doi.org/10.1158/0008-5472.Can-17-3977
Schmeiser HH, Stiborovà M, Arlt VM (2009) Chemical and molecular basis of the carcinogenicity of Aristolochia plants. Curr Opin Drug Discov Devel 12(1):141–148
Schmidt R, Baumann F, Knüpfer H et al (2004) CYP3A4, CYP2C9 and CYP2B6 expression and ifosfamide turnover in breast cancer tissue microsomes. Br J Cancer 90(4):911–916. https://doi.org/10.1038/sj.bjc.6601492
Schwarz D, Roots I (2003) In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1. Biochem Biophys Res Commun 303(3):902–907. https://doi.org/10.1016/s0006-291x(03)00435-2
Schwarz D, Kisselev P, Cascorbi I, Schunck WH, Roots I (2001) Differential metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1 variants. Carcinogenesis 22(3):453–459. https://doi.org/10.1093/carcin/22.3.453
Schwarz D, Kisselev P, Roots I (2003) St John’s wort extracts and some of their constituents potently inhibit ultimate carcinogen formation from benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1. Cancer Res 63(22):8062–8068
Schwarz D, Kisselev P, Ericksen SS et al (2004) Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol 67(8):1445–1457. https://doi.org/10.1016/j.bcp.2003.12.023
Seaton MJ, Follansbee MH, Bond JA (1995) Oxidation of 1,2-epoxy-3-butene to 1,2:3,4-diepoxybutane by cDNA-expressed human cytochromes P450 2E1 and 3A4 and human, mouse and rat liver microsomes. Carcinogenesis 16(10):2287–2293. https://doi.org/10.1093/carcin/16.10.2287
Seidel A, Soballa VJ, Raab G et al (1998) Regio- and stereoselectivity in the metabolism of benzo[c]phenanthrene mediated by genetically engineered V79 Chinese hamster cells expressing rat and human cytochromes P450. Environ Toxicol Pharmacol 5(3):179–196. https://doi.org/10.1016/s1382-6689(97)10073-4
Sellers EM, Ramamoorthy Y, Zeman MV, Djordjevic MV, Tyndale RF (2003) The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine Tob Res 5(6):891–899. https://doi.org/10.1080/14622200310001615231
Shebley M, Jushchyshyn MI, Hollenberg PF (2006) Selective pathways for the metabolism of phencyclidine by cytochrome P450 2B enzymes: identification of electrophilic metabolites, glutathione, and N-acetylcysteine adducts. Drug Metab Dispos 34(3):375–383. https://doi.org/10.1124/dmd.105.007047
Shen S, Marchick MR, Davis MR, Doss GA, Pohl LR (1999) Metabolic activation of diclofenac by human cytochrome P450 3A4: role of 5-hydroxydiclofenac. Chem Res Toxicol 12(2):214–222. https://doi.org/10.1021/tx9802365
Shen C, Meng Q, Zhang G, Hu W (2008) Rifampicin exacerbates isoniazid-induced toxicity in human but not in rat hepatocytes in tissue-like cultures. Br J Pharmacol 153(4):784–791. https://doi.org/10.1038/sj.bjp.0707611
Shet MS, McPhaul M, Fisher CW, Stallings NR, Estabrook RW (1997) Metabolism of the antiandrogenic drug (flutamide) by human CYP1A2. Drug Metab Dispos 25(11):1298–1303
Shibutani S, Bonala RR, Rosenquist T et al (2010) Detoxification of aristolochic acid I by O-demethylation: less nephrotoxicity and genotoxicity of aristolochic acid Ia in rodents. Int J Cancer 127(5):1021–1027. https://doi.org/10.1002/ijc.25141
Shield KD, Ferlay J, Jemal A et al (2017) The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin 67(1):51–64. https://doi.org/10.3322/caac.21384
Shimada T (2006) Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet 21(4):257–276. https://doi.org/10.2133/dmpk.21.257
Shimada T, Fujii-Kuriyama Y (2004) Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci 95(1):1–6. https://doi.org/10.1111/j.1349-7006.2004.tb03162.x
Shimada T, Guengerich FP (1989) Evidence for cytochrome P-450NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci U S A 86(2):462–465. https://doi.org/10.1073/pnas.86.2.462
Shimada T, Guengerich FP (1991) Activation of amino-a-carboline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and a copper phthalocyanine cellulose extract of cigarette smoke condensate by cytochrome P-450 enzymes in rat and human liver microsomes. Cancer Res 51(19):5284–5291
Shimada T, Guengerich FP (2006) Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem Res Toxicol 19(2):288–294. https://doi.org/10.1021/tx050291v
Shimada T, Gillam EMJ, Sandhu P, Guo Z, Tukey RH, Guengerich FP (1994) Activation of procarcinogens by human cytochrome P450 enzymes expressed in Escherichia coli Simplified bacterial systems for genotoxicity assays. Carcinogenesis 15(11):2523–2529. https://doi.org/10.1093/carcin/15.11.2523
Shimada T, Hayes CL, Yamazaki H et al (1996) Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res 56(13):2979–2984
Shimada T, Gillam EM, Sutter TR, Strickland PT, Guengerich FP, Yamazaki H (1997b) Oxidation of xenobiotics by recombinant human cytochrome P450 1B1. Drug Metab Dispos 25(5):617–622
Shimada T, El-Bayoumy K, Upadhyaya P, Sutter TR, Guengerich FP, Yamazaki H (1997a) Inhibition of human cytochrome P450-catalyzed oxidations of xenobiotics and procarcinogens by synthetic organoselenium compounds. Cancer Res 57(21):4757–4764
Shimada T, Wunsch RM, Hanna IH, Sutter TR, Guengerich FP, Gillam EMJ (1998) Recombinant human cytochrome P450 1B1 expression in Escherichia coli. Arch Biochem Biophys 357(1):111–120. https://doi.org/10.1006/abbi.1998.0808
Shimada T, Watanabe J, Kawajiri K et al (1999) Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis 20(8):1607–1613. https://doi.org/10.1093/carcin/20.8.1607
Shimada T, Watanabe J, Inoue K, Guengerich FP, Gillam EMJ (2001b) Specificity of 17b-oestradiol and benzo[a]pyrene oxidation by polymorphic human cytochrome P4501B1 variants substituted at residues 48, 119 and 432. Xenobiotica 31(3):163–176. https://doi.org/10.1080/00498250110043490
Shimada T, Oda Y, Gillam EMJ, Guengerich FP, Inoue K (2001a) Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos 29(9):1176–1182
Shimada T, Murayama N, Yamazaki H et al (2013) Metabolic activation of polycyclic aromatic hydrocarbons and aryl and heterocyclic amines by human cytochromes P450 2A13 and 2A6. Chem Res Toxicol 26(4):529–537. https://doi.org/10.1021/tx3004906
Shin JG, Kane K, Flockhart DA (2001) Potent inhibition of CYP2D6 by haloperidol metabolites: stereoselective inhibition by reduced haloperidol. Br J Clin Pharmacol 51(1):45–52. https://doi.org/10.1046/j.1365-2125.2001.01313.x
Shou M, Korzekwa KR, Crespi CL, Gonzalez FJ, Gelboin HV (1994) The role of 12 cDNA-expressed human, rodent, and rabbit cytochromes P450 in the metabolism of benzo[a]pyrene and benzo[a]pyrene trans-7,8-dihydrodiol. Mol Carcinog 10(3):159–168. https://doi.org/10.1002/mc.2940100307
Shou M, Krausz KW, Gonzalez FJ, Gelboin HV (1996c) Metabolic activation of the potent carcinogen dibenzo[a, l]pyrene by human recombinant cytochromes P450, lung and liver microsomes. Carcinogenesis 17(11):2429–2433. https://doi.org/10.1093/carcin/17.11.2429
Shou M, Gonzalez FJ, Gelboin HV (1996a) Stereoselective epoxidation and hydration at the K-region of polycyclic aromatic hydrocarbons by cDNA-expressed cytochromes P450 1A1, 1A2, and epoxide hydrolase. Biochemistry 35(49):15807–15813. https://doi.org/10.1021/bi962042z
Shou M, Korzekwa KR, Krausz KW et al (1996b) Specificity of cDNA-expressed human and rodent cytochrome P450s in the oxidative metabolism of the potent carcinogen 7,12-dimethylbenz[a]anthracene. Mol Carcinog 17(4):241–249. https://doi.org/10.1002/(sici)1098-2744(199612)17:4%3c241::Aid-mc8%3e3.0.Co;2-g
Shou M, Korzekwa KR, Brooks EN, Krausz KW, Gonzalez FJ, Gelboin HV (1997) Role of human hepatic cytochrome P450 1A2 and 3A4 in the metabolic activation of estrone. Carcinogenesis 18(1):207–214. https://doi.org/10.1093/carcin/18.1.207
Simarro Doorten Y, Nijmeijer S, de Nijs-Tjon L, Fink-Gremmels J (2006) Metabolism-mediated ochratoxin A genotoxicity in the single-cell gel electrophoresis (Comet) assay. Food Chem Toxicol 44(2):261–270. https://doi.org/10.1016/j.fct.2005.07.009
Sinclair J, Jeffery E, Wrighton S et al (1998) Alcohol-mediated increases in acetaminophen hepatotoxicity: role of CYP2E and CYP3A. Biochem Pharmacol 55(10):1557–1565. https://doi.org/10.1016/s0006-2952(97)00656-4
Smith TJ, Guo Z, Gonzalez FJ, Guengerich FP, Stoner GD, Yang CS (1992) Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human lung and liver microsomes and cytochromes P-450 expressed in hepatoma cells. Cancer Res 52(7):1757–1763
Smith TJ, Stoner GD, Yang CS (1995) Activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human lung microsomes by cytochromes P450, lipoxygenase, and hydroperoxides. Cancer Res 55(23):5566–5573
Smith TJ, Guo Z, Guengerich FP, Yang CS (1996) Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by human cytochrome P450 1A2 and its inhibition by phenethyl isothiocyanate. Carcinogenesis 17(4):809–813. https://doi.org/10.1093/carcin/17.4.809
Smith KS, Smith PL, Heady TN, Trugman JM, Harman WD, Macdonald TL (2003b) In vitro metabolism of tolcapone to reactive intermediates: relevance to tolcapone liver toxicity. Chem Res Toxicol 16(2):123–128. https://doi.org/10.1021/tx025569n
Smith GB, Bend JR, Bedard LL, Reid KR, Petsikas D, Massey TE (2003a) Biotransformation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in peripheral human lung microsomes. Drug Metab Dispos 31(9):1134–1141. https://doi.org/10.1124/dmd.31.9.1134
Spink DC, Spink BC, Cao JQ et al (1997) Induction of cytochrome P450 1B1 and catechol estrogen metabolism in ACHN human renal adenocarcinoma cells. J Steroid Biochem Mol Biol 62(2–3):223–232. https://doi.org/10.1016/s0960-0760(97)00024-1
Spink DC, Spink BC, Cao JQ et al (1998) Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis 19(2):291–298. https://doi.org/10.1093/carcin/19.2.291
Spink DC, Katz BH, Hussain MM et al (2002b) Induction of CYP1A1 and CYP1B1 in T-47D human breast cancer cells by benzo[a]pyrene is diminished by arsenite. Drug Metab Dispos 30(3):262–269. https://doi.org/10.1124/dmd.30.3.262
Spink BC, Pang S, Pentecost BT, Spink DC (2002a) Induction of cytochrome P450 1B1 in MDA-MB-231 human breast cancer cells by non-ortho-substituted polychlorinated biphenyls. Toxicol In Vitro 16(6):695–704. https://doi.org/10.1016/s0887-2333(02)00091-7
Spracklin DK, Kharasch ED (1998) Human halothane reduction in vitro by cytochrome P450 2A6 and 3A4: identification of low and high KM isoforms. Drug Metab Dispos 26(6):605–607
Spracklin DK, Thummel KE, Kharasch ED (1996) Human reductive halothane metabolism in vitro is catalyzed by cytochrome P450 2A6 and 3A4. Drug Metab Dispos 24(9):976–983
Spracklin DK, Hankins DC, Fisher JM, Thummel KE, Kharasch ED (1997) Cytochrome P450 2E1 is the principal catalyst of human oxidative halothane metabolism in vitro. J Pharmacol Exp Ther 281(1):400–411
Staretz ME, Murphy SE, Patten CJ et al (1997) Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N´- nitrosonornicotine in human hepatic microsomes. Drug Metab Dispos 25(2):154–162
Stearns V, Johnson MD, Rae JM et al (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95(23):1758–1764. https://doi.org/10.1093/jnci/djg108
Stiborová M, Bieler CA, Wiessler M, Frei E (2001a) The anticancer agent ellipticine on activation by cytochrome P450 forms covalent DNA adducts. Biochem Pharmacol 62(12):1675–1684. https://doi.org/10.1016/s0006-2952(01)00806-1
Stiborová M, Frei E, Wiessler M, Schmeiser HH (2001b) Human enzymes involved in the metabolic activation of carcinogenic aristolochic acids: evidence for reductive activation by cytochromes P450 1A1 and 1A2. Chem Res Toxicol 14(8):1128–1137. https://doi.org/10.1021/tx010059z
Stiborová M, Borek-Dohalská L, Hodek P, Mráz J, Frei E (2002) New selective inhibitors of cytochromes P450 2B and their application to antimutagenesis of tamoxifen. Arch Biochem Biophys 403(1):41–49. https://doi.org/10.1016/s0003-9861(02)00259-x
Stiborová M, Sejbal J, Borek-Dohalská L et al (2004) The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res 64(22):8374–8380. https://doi.org/10.1158/0008-5472.Can-04-2202
Stiborová M, Frei E, Hodek P, Wiessler M, Schmeiser HH (2005) Human hepatic and renal microsomes, cytochromes P450 1A1/2, NADPH: cytochrome P450 reductase and prostaglandin H synthase mediate the formation of aristolochic acid-DNA adducts found in patients with urothelial cancer. Int J Cancer 113(2):189–197. https://doi.org/10.1002/ijc.20564
Stiborová M, Levová K, Bárta F et al (2012a) Bioactivation versus detoxication of the urothelial carcinogen aristolochic acid I by human cytochrome P450 1A1 and 1A2. Toxicol Sci 125(2):345–358. https://doi.org/10.1093/toxsci/kfr306
Stiborová M, Poljaková J, Martínková E et al (2012b) Ellipticine oxidation and DNA adduct formation in human hepatocytes is catalyzed by human cytochromes P450 and enhanced by cytochrome b5. Toxicology 302(2–3):233–241. https://doi.org/10.1016/j.tox.2012.08.004
Stiborová M, Martínek V, Frei E, Arlt VM, Schmeiser HH (2013) Enzymes metabolizing aristolochic acid and their contribution to the development of aristolochic acid nephropathy and urothelial cancer. Curr Drug Metab 14(6):695–705. https://doi.org/10.2174/1389200211314060006
Stiborová M, Frei E, Arlt VM, Schmeiser HH (2014) Knockout and humanized mice as suitable tools to identify enzymes metabolizing the human carcinogen aristolochic acid. Xenobiotica 44(2):135–145. https://doi.org/10.3109/00498254.2013.848310
Stiborová M, Bárta F, Levová K et al (2015) A mechanism of O-demethylation of aristolochic acid I by cytochromes P450 and their contributions to this reaction in human and rat livers: experimental and theoretical approaches. Int J Mol Sci 16(11):27561–27575. https://doi.org/10.3390/ijms161126047
Styles JA, Davies A, Lim CK et al (1994) Genotoxicity of tamoxifen, tamoxifen epoxide and toremifene in human lymphoblastoid cells containing human cytochrome P450s. Carcinogenesis 15(1):5–9. https://doi.org/10.1093/carcin/15.1.5
Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X (2000) Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 60(18):5074–5079
Takada K, Arefayene M, Desta Z et al (2004) Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum 50(7):2202–2210. https://doi.org/10.1002/art.20338
Takagi T, Ishii H, Takahashi H et al (1983) Potentiation of halothane hepatotoxicity by chronic ethanol administration in rat: an animal model of halothane hepatitis. Pharmacol Biochem Behav 18(Suppl 1):461–465. https://doi.org/10.1016/0091-3057(83)90218-6
Takemoto K, Yamazaki H, Nakajima M, Yokoi T (2002) Genotoxic activation of benzophenone and its two metabolites by human cytochrome P450s in SOS/umu assay. Mutat Res 519(1–2):199–204. https://doi.org/10.1016/s1383-5718(02)00141-9
Tan SC, New LS, Chan EC (2008) Prevention of acetaminophen (APAP)-induced hepatotoxicity by leflunomide via inhibition of APAP biotransformation to N-acetyl-p-benzoquinone imine. Toxicol Lett 180(3):174–181. https://doi.org/10.1016/j.toxlet.2008.06.001
Tang W (2003) The metabolism of diclofenac–enzymology and toxicology perspectives. Curr Drug Metab 4(4):319–329. https://doi.org/10.2174/1389200033489398
Tang W, Stearns RA, Wang RW, Chiu SH, Baillie TA (1999) Roles of human hepatic cytochrome P450s 2C9 and 3A4 in the metabolic activation of diclofenac. Chem Res Toxicol 12(2):192–199. https://doi.org/10.1021/tx9802217
Tang J, Cao Y, Rose RL et al (2001) Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab Dispos 29(9):1201–1204
Teiber JF, Hollenberg PF (2000) Identification of the human liver microsomal cytochrome P450s involved in the metabolism of N-nitrosodi-n-propylamine. Carcinogenesis 21(8):1559–1566
Teitelbaum AM, McDonald MG, Kowalski JP et al (2019) Influence of stereochemistry on the bioactivation and glucuronidation of 4-ipomeanol. J Pharmacol Exp Ther 368(2):308–316. https://doi.org/10.1124/jpet.118.249771
Tettey JN, Maggs JL, Rapeport WG, Pirmohamed M, Park BK (2001) Enzyme-induction dependent bioactivation of troglitazone and troglitazone quinone in vivo. Chem Res Toxicol 14(8):965–974. https://doi.org/10.1021/tx0001981
Thornton-Manning J, Appleton ML, Gonzalez FJ, Yost GS (1996) Metabolism of 3-methylindole by vaccinia-expressed P450 enzymes: correlation of 3-methyleneindolenine formation and protein-binding. J Pharmacol Exp Ther 276(1):21–29
Thummel KE, Slattery JT, Ro H et al (2000) Ethanol and production of the hepatotoxic metabolite of acetaminophen in healthy adults. Clin Pharmacol Ther 67(6):591–599. https://doi.org/10.1067/mcp.2000.106574
Travica S, Pors K, Loadman PM et al (2013) Colon cancer-specific cytochrome P450 2W1 converts duocarmycin analogues into potent tumor cytotoxins. Clin Cancer Res 19(11):2952–2961. https://doi.org/10.1158/1078-0432.Ccr-13-0238
Tsou HH, Ko HT, Chen CT et al (2019) Betel quid containing safrole enhances metabolic activation of tobacco specific 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Environ Pollut 251:13–21. https://doi.org/10.1016/j.envpol.2019.04.080
Tsuchiya Y, Nakajima M, Yokoi T (2005) Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227(2):115–124. https://doi.org/10.1016/j.canlet.2004.10.007
Tu M, Li L, Lei H et al (2014) Involvement of organic cation transporter 1 and CYP3A4 in retrorsine-induced toxicity. Toxicology 322:34–42. https://doi.org/10.1016/j.tox.2014.04.007
Turesky RJ, Constable A, Richoz J et al (1998) Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2. Chem Res Toxicol 11(8):925–936. https://doi.org/10.1021/tx980022n
Turesky RJ, Constable A, Fay LB, Guengerich FP (1999) Interspecies differences in metabolism of heterocyclic aromatic amines by rat and human P450 1A2. Cancer Lett 143(2):109–112. https://doi.org/10.1016/s0304-3835(99)00137-8
Turesky RJ, Parisod V, Huynh-Ba T, Langouët S, Guengerich FP (2001) Regioselective differences in C(8)- and N-oxidation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline by human and rat liver microsomes and cytochromes P450 1A2. Chem Res Toxicol 14(7):901–911. https://doi.org/10.1021/tx010035s
Turesky RJ, Guengerich FP, Guillouzo A, Langouët S (2002) Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P450 1A2. Mutat Res 506–507:187–195. https://doi.org/10.1016/s0027-5107(02)00165-3
Ueng Y-F, Shimada T, Yamazaki H, Guengerich FP (1995) Oxidation of aflatoxin B1 by bacterial recombinant human cytochrome P450 enzymes. Chem Res Toxicol 8(2):218–225. https://doi.org/10.1021/tx00044a006
Ueng Y-F, Kuwabara T, Chun Y-J, Guengerich FP (1997) Cooperativity in oxidations catalyzed by cytochrome P450 3A4. Biochemistry 36(2):370–381. https://doi.org/10.1021/bi962359z
Ueng Y-F, Hsieh CH, Don MJ, Chi CW, Ho LK (2004) Identification of the main human cytochrome P450 enzymes involved in safrole 1’-hydroxylation. Chem Res Toxicol 17(8):1151–1156. https://doi.org/10.1021/tx030055p
Uno T, Obe Y, Ogura C et al (2013) Metabolism of 7-ethoxycoumarin, safrole, flavanone and hydroxyflavanone by cytochrome P450 2A6 variants. Biopharm Drug Dispos 34(2):87–97. https://doi.org/10.1002/bdd.1825
Usuki E, Van der Schyf CJ, Castagnoli N Jr (1998) Metabolism of haloperidol and its tetrahydropyridine dehydration product HPTP. Drug Metab Rev 30(4):809–826. https://doi.org/10.3109/03602539808996331
van Duursen MB, Sanderson JT, van der Bruggen M, van der Linden J, van den Berg M (2003) Effects of several dioxin-like compounds on estrogen metabolism in the malignant MCF-7 and nontumorigenic MCF-10A human mammary epithelial cell lines. Toxicol Appl Pharmacol 190(3):241–250. https://doi.org/10.1016/s0041-008x(03)00166-2
Van Vleet TR, Klein PJ, Coulombe RA Jr (2001) Metabolism of aflatoxin B1 by normal human bronchial epithelial cells. J Toxicol Environ Health A 63(7):525–540. https://doi.org/10.1080/15287390152410156
Van Vleet TR, Klein PJ, Coulombe RA Jr (2002a) Metabolism and cytotoxicity of aflatoxin B1 in cytochrome P-450-expressing human lung cells. J Toxicol Environ Health A 65(12):853–867. https://doi.org/10.1080/00984100290071216
Van Vleet TR, Macé K, Coulombe RA Jr (2002b) Comparative aflatoxin B1 activation and cytotoxicity in human bronchial cells expressing cytochromes P450 1A2 and 3A4. Cancer Res 62(1):105–112
von Weymarn LB, Chun JA, Hollenberg PF (2006) Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: potential for chemoprevention in smokers. Carcinogenesis 27(4):782–790. https://doi.org/10.1093/carcin/bgi301
Walker D, Flinois JP, Monkman SC et al (1994) Identification of the major human hepatic cytochrome P450 involved in activation and N-dechloroethylation of ifosfamide. Biochem Pharmacol 47(7):1157–1163. https://doi.org/10.1016/0006-2952(94)90387-5
Walsky RL, Obach RS (2007) A comparison of 2-phenyl-2-(1-piperidinyl)propane (PPP), 1,1´,1´´-phosphinothioylidynetrisaziridine (thioTEPA), clopidogrel, and ticlopidine as selective inactivators of human cytochrome P450 2B6. Drug Metab Dispos 35(11):2053–2059. https://doi.org/10.1124/dmd.107.015883
Wang H, Dick R, Yin H et al (1998) Structure-function relationships of human liver cytochromes P450 3A: aflatoxin B1 metabolism as a probe. Biochemistry 37(36):12536–12545. https://doi.org/10.1021/bi980895g
Wang YP, Yan J, Fu PP, Chou MW (2005) Human liver microsomal reduction of pyrrolizidine alkaloid N-oxides to form the corresponding carcinogenic parent alkaloid. Toxicol Lett 155(3):411–420. https://doi.org/10.1016/j.toxlet.2004.11.010
Ward S, Back DJ (1993) Metabolism of gestodene in human liver cytosol and microsomes in vitro. J Steroid Biochem Mol Biol 46(2):235–243. https://doi.org/10.1016/0960-0760(93)90299-c
Watanabe J, Shimada T, Gillam EMJ et al (2000) Association of CYP1B1 genetic polymorphism with incidence to breast and lung cancer. Pharmacogenetics 10(1):25–33. https://doi.org/10.1097/00008571-200002000-00004
Watanabe M, Watanabe N, Maruyama S, Kawashiro T (2015) Comparative metabolic study between two selective estrogen receptor modulators, toremifene and tamoxifen, in human liver microsomes. Drug Metab Pharmacokinet 30(5):325–333. https://doi.org/10.1016/j.dmpk.2015.05.004
Weems JM, Yost GS (2010) 3-Methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively. Chem Res Toxicol 23(3):696–704. https://doi.org/10.1021/tx9004506
Weems JM, Lamb JG, D’Agostino J, Ding X, Yost GS (2010) Potent mutagenicity of 3-methylindole requires pulmonary cytochrome P450-mediated bioactivation: a comparison to the prototype cigarette smoke mutagens B(a)P and NNK. Chem Res Toxicol 23(11):1682–1690. https://doi.org/10.1021/tx100147z
Wen W, Ren Z, Shu XO et al (2007) Expression of cytochrome P450 1B1 and catechol-O-methyltransferase in breast tissue and their associations with breast cancer risk. Cancer Epidemiol Biomark Prev 16(5):917–920. https://doi.org/10.1158/1055-9965.Epi-06-1032
White IN, De Matteis F (2001) The role of CYP forms in the metabolism and metabolic activation of HCFCs and other halocarbons. Toxicol Lett 124(1–3):121–128. https://doi.org/10.1016/s0378-4274(00)00288-5
White IN, De Matteis F, Gibbs AH et al (1995) Species differences in the covalent binding of [14C]tamoxifen to liver microsomes and the forms of cytochrome P450 involved. Biochem Pharmacol 49(8):1035–1042. https://doi.org/10.1016/0006-2952(95)98498-x
Williams JA, Stone EM, Millar BC, Gusterson BA, Grover PL, Phillips DH (1998) Determination of the enzymes responsible for activation of the heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline in the human breast. Pharmacogenetics 8(6):519–528. https://doi.org/10.1097/00008571-199812000-00009
Williams JA, Martin FL, Muir GH, Hewer A, Grover PL, Phillips DH (2000) Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis 21(9):1683–1689. https://doi.org/10.1093/carcin/21.9.1683
Williams JA, Ring BJ, Cantrell VE et al (2002) Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos 30(8):883–891. https://doi.org/10.1124/dmd.30.8.883
Williams JM, Murphy S, Burke M, Roman RJ (2010) 20-Hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56(4):336–344. https://doi.org/10.1097/FJC.0b013e3181f04b1c
Winter HR, Wang Y, Unadkat JD (2000) CYP2C8/9 mediate dapsone N-hydroxylation at clinical concentrations of dapsone. Drug Metab Dispos 28(8):865–868
Wolkenstein P, Tan C, Lecoeur S et al (1998) Covalent binding of carbamazepine reactive metabolites to P450 isoforms present in the skin. Chem-Biol Interact 113(1):39–50. https://doi.org/10.1016/s0009-2797(98)00021-0
Wong HL, Murphy SE, Hecht SS (2005a) Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N´-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem Res Toxicol 18(1):61–69. https://doi.org/10.1021/tx0497696
Wong HL, Zhang X, Zhang QY et al (2005b) Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem Res Toxicol 18(6):913–918. https://doi.org/10.1021/tx0500777
Wu J, Dong H, Cai Z, Yu Y (1997) Stable expression of human cytochrome CYP2B6 and CYP1A1 in Chinese hamster CHL cells: their use in micronucleus assays. Chin Med Sci J 12(3):148–155
Wu Z-L, Sohl CD, Shimada T, Guengerich FP (2006) Recombinant enzymes overexpressed in bacteria show broad catalytic specificity of human cytochrome P450 2W1 and limited activity of human cytochrome P450 2S1. Mol Pharmacol 69(6):2007–2014. https://doi.org/10.1124/mol.106.023648
Xiao Y, Shinkyo R, Guengerich FP (2011) Cytochrome P450 2S1 is reduced by NADPH-cytochrome P450 reductase. Drug Metab Dispos 39(6):944–946. https://doi.org/10.1124/dmd.111.039321
Xue L, Wang HF, Wang Q et al (2001) Influence of P450 3A4 SRS-2 residues on cooperativity and/or regioselectivity of aflatoxin B1 oxidation. Chem Res Toxicol 14(5):483–491. https://doi.org/10.1021/tx000218z
Yager JD (2000) Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr 27:67–73. https://doi.org/10.1093/oxfordjournals.jncimonographs.a024245
Yakkundi A, McErlane V, Murray M et al (2006) Tumor-selective drug activation: a GDEPT approach utilizing cytochrome P450 1A1 and AQ4N. Cancer Gene Ther 13(6):598–605. https://doi.org/10.1038/sj.cgt.7700933
Yamazaki H, Inui Y, Yun C-H, Guengerich FP, Shimada T (1992) Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis 13(10):1789–1794. https://doi.org/10.1093/carcin/13.10.1789
Yamazaki H, Mimura M, Oda Y et al (1993) Roles of different forms of cytochrome P450 in the activation of the promutagen 6-aminochrysene to genotoxic metabolites in human liver microsomes. Carcinogenesis 14(7):1271–1278. https://doi.org/10.1093/carcin/14.7.1271
Yamazaki H, Inui Y, Wrighton SA, Guengerich FP, Shimada T (1995) Procarcinogen activation by cytochrome P450 3A4 and 3A5 expressed in Escherichia coli and by human liver microsomes. Carcinogenesis 16(9):2167–2170. https://doi.org/10.1093/carcin/16.9.2167
Yamazaki H, Inoue K, Chiba K et al (1998a) Comparative studies on the catalytic roles of cytochrome P450 2C9 and its Cys- and Leu-variants in the oxidation of warfarin, flurbiprofen, and diclofenac by human liver microsomes. Biochem Pharmacol 56(2):243–251. https://doi.org/10.1016/s0006-2952(98)00133-6
Yamazaki H, Shaw PM, Guengerich FP, Shimada T (1998b) Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol 11(6):659–665. https://doi.org/10.1021/tx970217f
Yamazaki H, Shibata A, Suzuki M et al (1999) Oxidation of troglitazone to a quinone-type metabolite catalyzed by cytochrome P-450 2C8 and P-450 3A4 in human liver microsomes. Drug Metab Dispos 27(11):1260–1266
Yamazaki Y, Fujita K, Nakayama K et al (2004) Establishment of ten strains of genetically engineered Salmonella typhimurium TA1538 each co-expressing a form of human cytochrome P450 with NADPH-cytochrome P450 reductase sensitive to various promutagens. Mutat Res 562(1–2):151–162. https://doi.org/10.1016/j.mrgentox.2004.06.003
Yan Z, Zhong HM, Maher N et al (2005) Bioactivation of 4-methylphenol (p-cresol) via cytochrome P450-mediated aromatic oxidation in human liver microsomes. Drug Metab Dispos 33(12):1867–1876. https://doi.org/10.1124/dmd.105.006387
Yang MX, Cederbaum AI (1997) Characterization of cytochrome P450 2E1 turnover in transfected HepG2 cells expressing human CYP2E1. Arch Biochem Biophys 341(1):25–33. https://doi.org/10.1006/abbi.1997.9907
Yang CS, Chhabra SK, Hong JY, Smith TJ (2001) Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr 131(3s):1041s–1045s. https://doi.org/10.1093/jn/131.3.1041S
Yang XJ, Lu HY, Li ZY et al (2012) Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology 300(3):138–148. https://doi.org/10.1016/j.tox.2012.06.010
Yang G, Nowsheen S, Aziz K, Georgakilas AG (2013) Toxicity and adverse effects of tamoxifen and other anti-estrogen drugs. Pharmacol Ther 139(3):392–404. https://doi.org/10.1016/j.pharmthera.2013.05.005
Yang AH, Zhang L, Zhi DX, Liu WL, Gao X, He X (2018) Identification and analysis of the reactive metabolites related to the hepatotoxicity of safrole. Xenobiotica 48(11):1164–1172. https://doi.org/10.1080/00498254.2017.1399227
Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N (2016) Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol 4(2):131–142. https://doi.org/10.14218/jcth.2015.00052
Yueh MF, Nguyen N, Famourzadeh M et al (2001) The contribution of UDP-glucuronosyltransferase 1A9 on CYP1A2-mediated genotoxicity by aromatic and heterocyclic amines. Carcinogenesis 22(6):943–950. https://doi.org/10.1093/carcin/22.6.943
Yukinaga H, Takami T, Shioyama SH et al (2007) Identification of cytochrome P450 3A4 modification site with reactive metabolite using linear ion trap-Fourier transform mass spectrometry. Chem Res Toxicol 20(10):1373–1378. https://doi.org/10.1021/tx700165q
Yun C-H, Shimada T, Guengerich FP (1991) Purification and characterization of human liver microsomal cytochrome P-450 2A6. Mol Pharmacol 40(5):679–685
Yun C-H, Shimada T, Guengerich FP (1992) Roles of human liver cytochrome P450 2C and 3A enzymes in the 3-hydroxylation of benzo(a)pyrene. Cancer Res 52(7):1868–1874
Zaher H, Buters JT, Ward JM et al (1998) Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol 152(1):193–199. https://doi.org/10.1006/taap.1998.8501
Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E (2006) The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem Res Toxicol 19(1):164–172. https://doi.org/10.1021/tx050229y
Zahno A, Bouitbir J, Maseneni S, Lindinger PW, Brecht K, Krähenbühl S (2013) Hepatocellular toxicity of clopidogrel: mechanisms and risk factors. Free Radic Biol Med 65:208–216. https://doi.org/10.1016/j.freeradbiomed.2013.06.007
Zand R, Nelson SD, Slattery JT et al (1993) Inhibition and induction of cytochrome P450 2E1-catalyzed oxidation by isoniazid in humans. Clin Pharmacol Ther 54(2):142–149. https://doi.org/10.1038/clpt.1993.125
Zeldin DC, DuBois RN, Falck JR, Capdevila JH (1995) Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys 322(1):76–86. https://doi.org/10.1006/abbi.1995.1438
Zeldin DC, Moomaw CR, Jesse N et al (1996) Biochemical characterization of the human liver cytochrome P450 arachidonic acid epoxygenase pathway. Arch Biochem Biophys 330(1):87–96. https://doi.org/10.1006/abbi.1996.0229
Zhai Y, Wang L, Yang F et al (2016) The mechanism and risk factors of clopidogrel-induced liver injury. Drug Chem Toxicol 39(4):367–374. https://doi.org/10.3109/01480545.2015.1122606
Zhang X, Su T, Zhang QY et al (2002) Genetic polymorphisms of the human CYP2A13 gene: identification of single-nucleotide polymorphisms and functional characterization of an Arg257Cys variant. J Pharmacol Exp Ther 302(2):416–423. https://doi.org/10.1124/jpet.302.2.416
Zhang X, D’Agostino J, Wu H et al (2007) CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J Pharmacol Exp Ther 323(2):570–578. https://doi.org/10.1124/jpet.107.127068
Zhang Z, Yang X, Wang Y et al (2013) Cytochrome P450 2A13 is an efficient enzyme in metabolic activation of aflatoxin G1 in human bronchial epithelial cells. Arch Toxicol 87(9):1697–1707. https://doi.org/10.1007/s00204-013-1108-3
Zhang C, Booz GW, Yu Q, He X, Wang S, Fan F (2018) Conflicting roles of 20-HETE in hypertension and renal end organ damage. Eur J Pharmacol 833:190–200. https://doi.org/10.1016/j.ejphar.2018.06.010
Zhao Y, Wan D, Yang J, Hammock BD, Ortiz de Montellano PR (2016) Catalytic activities of tumor-specific human cytochrome P450 CYP2W1 toward endogenous substrates. Drug Metab Dispos 44(5):771–780. https://doi.org/10.1124/dmd.116.069633
Zhou L, Erickson RR, Hardwick JP, Park SS, Wrighton SA, Holtzman JL (1997) Catalysis of the cysteine conjugation and protein binding of acetaminophen by microsomes from a human lymphoblast line transfected with the cDNAs of various forms of human cytochrome P450. J Pharmacol Exp Ther 281(2):785–790
Zhou D, Lu Y, Steiner MS, Dalton JT (2000) Cytochrome P-450 2C9 sensitizes human prostate tumor cells to cyclophosphamide via a bystander effect. Antimicrob Agents Chemother 44(10):2659–2663. https://doi.org/10.1128/aac.44.10.2659-2663.2000
Zhou H, Josephy PD, Kim D, Guengerich FP (2004) Functional characterization of four allelic variants of human cytochrome P450 1A2. Arch Biochem Biophys 422(1):23–30. https://doi.org/10.1016/j.abb.2003.11.019
Zhu BT, Lee AJ (2005) NADPH-dependent metabolism of 17b-estradiol and estrone to polar and nonpolar metabolites by human tissues and cytochrome P450 isoforms. Steroids 70(4):225–244. https://doi.org/10.1016/j.steroids.2005.01.002
Zhu BT, Roy D, Liehr JG (1993) The carcinogenic activity of ethinyl estrogens is determined by both their hormonal characteristics and their conversion to catechol metabolites. Endocrinology 132(2):577–583. https://doi.org/10.1210/endo.132.2.8381068
Acknowledgements
We thank K. Trisler for assistance in the preparation of the manuscript.
Funding
F.P.G. acknowledges current support from the United States National Institutes of Health Grant R01 GM118122. The Alexander von Humboldt Foundation, Bon-Bad Godesberg, FR Germany, supported the research of S.R. in the field of drug metabolism and P450 enzymes on several occasions from 1975–2001 at the Faculty of Pharmacy, University of Muenster, Muenster, Germany; Institute for Physiological Chemistry, University of Saarland, Homburg, Saar, Germany; Faculty of Biology, University of Konstanz, Germany; and Institute of Biochemistry of the German Sport University Cologne, Cologne, Germany, and his collaboration with Prof. Dr. K.E. Schulte, Prof. Dr. Volker Ullrich and Prof. Dr. Manfred Donike.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rendic, S.P., Guengerich, F.P. Human Family 1–4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update. Arch Toxicol 95, 395–472 (2021). https://doi.org/10.1007/s00204-020-02971-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02971-4