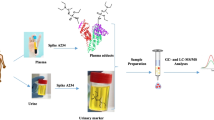
Abstract
We herein present for the first time the phosphylated (*) tetrapeptide (TP)-adduct GlyGluSer198*Ala generated from butyrylcholinesterase (BChE) with proteinase K excellently suited for the verification of exposure to toxic organophosphorus nerve agents (OPNA). Verification requires bioanalytical methods mandatory for toxicological and legal reasons. OPNA react with BChE by phosphonylation of the active site serine residue (Ser198) forming one of the major target protein adducts for verification. After its enzymatic cleavage with pepsin, the nonapeptide (NP) PheGlyGluSer*AlaGlyAlaAlaSer is typically produced as biomarker. Usually OPNA occur as racemic mixtures of phosphonic acid derivatives with the stereocenter at the phosphorus atom, e.g. (±)-VX. Both enantiomers react with BChE, but the adducted NP does not allow their chromatographic distinction. In contrast, the herein introduced TP-adducts appeared as two peaks when using a stationary reversed phase (1.8 µm) in micro-liquid chromatography–electrospray ionisation tandem-mass spectrometry (µLC–ESI MS/MS) analysis. These two peaks represent diastereomers of the (+)- and (−)-OPNA adducted to the peptide that comprises chiral L-amino acids exclusively. Concentration- and time-dependent effects of adduct formation with (±)-VX and its pure enantiomers (+)- and (−)-VX as well as with (±)-cyclosarin (GF) were investigated in detail characterising enantioselective adduct formation, stability, ageing and spontaneous reactivation. The method was also successfully applied to samples from a real case of pesticide poisoning as well as to samples of biomedical proficiency tests provided by the Organisation for the Prohibition of Chemical Weapons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The group of organophosphorus (OP) toxicants includes nerve agents (OPNA) and pesticides (Bajgar 2005; John et al. 2020). Their toxicity is due to the inhibition of acetylcholinesterase (AChE) after covalent binding (formation of adducts) to the active centre serine residue. Inhibited AChE causes a cholinergic crisis which might lead to death by respiratory paralysis (Grob and Harvey 1953; Namba 1971). The development, production, stockpiling and deployment of OPNA and chemical warfare agents (CWA) in general are banned by the Chemical Weapons Convention (CWC), which is supervised by the Organisation for the Prohibition of Chemical Weapons (OPCW) (Organisation for the Prohibition of Chemical Weapons 2020). Nevertheless, recent incidents document the deadly threat by these poisons in military conflicts and assassinations and attempted murder of individuals (Holmes and Philips 2017; John et al. 2018; Nakagawa and Tu 2018; Organisation for the Prohibition of Chemical Weapons 2018; Steindl et al. 2021). Therefore, selective and sensitive analytical methods are mandatory to prove exposure (verification). Accordingly, the continuous improvement of established methods and the development of complementary methods are indispensable (John and Thiermann 2021; John et al. 2016, 2022; Noort et al. 2021). Several procedures are established to analyse biological samples for the presence of e.g. the V-type OPNA VX (Fig. 1A), Chinese VX (CVX) and Russian VX (RVX) as well as the G-type OPNA tabun (GA), sarin (GB), soman (GD) and cyclosarin (GF, Fig. 1B). Furthermore, OP pesticides of the diethyl phosphate (DEP)-type (e.g. paraoxon-ethyl) and dimethyl phosphate (DMP)-type (e.g. malaoxon, Fig. 1C) are detected by quite similar methods. Very often hyphenated techniques like gas or liquid chromatography (LC) coupled to tandem mass spectrometry (MS/MS) are applied to detect the agent, its hydrolysis products as well as its adducts with endogenous proteins (Black 2008; John and Thiermann 2021; John et al. 2008, 2018, 2020; Noort et al. 2002). Adducts, resulting from the phosphylation (denominating both phosphonylation and phosphorylation) of the side chains of diverse proteinaceous amino acids, show beneficial stability and thus suitability as long-term biomarkers for post-exposure analysis (Ding et al. 2008; John and Thiermann 2021; John et al. 2020; Munkner et al. 1961; Peters 1996; Williams et al. 2007). Especially adducts of butyrylcholinesterase (BChE, EC 3.1.1.8; UniProtKB P06276) are of high forensic relevance (John and Thiermann 2021; John et al. 2020; Noort et al. 2021). Following BChE-adduct extraction from plasma and its subsequent proteolysis with pepsin, the phosphylated (*) nonapeptide (NP) PheGlyGluSer*AlaGlyAlaAlaSer is generated containing the active site serine residue Ser198 (Fidder et al. 2002; John and Thiermann 2021; John et al. 2020; Noort et al. 2021). Numbering of amino acids used herein does not include the signal peptide. As BChE-adducts often undergo an ageing process (hydrolysis of the P-bound alkoxy group), their non-aged as well as the aged variant of the NP-adduct are typically detected simultaneously by LC–MS/MS applying positive electrospray ionisation (ESI) (John and Thiermann 2021; John et al. 2015).
Scheme of BChE-adduct formation, its proteolysis and µLC-ESI MS/MS (SRM) analysis. The chiral (*) organophosphorus nerve agents (±)-VX (A) and (±)-cyclosarin, (±)-GF (B) as well as the non-chiral pesticide malaoxon (C) reacted with butyrylcholinesterase (BChE). The resulting BChE-adducts were proteolyzed with proteinase K (Prot K) yielding the tetrapeptide GlyGluSer*Ala (GESA) phosphylated (*) at its serine residue (D–F). Analysis by µLC–ESI MS/MS (SRM) showed two peaks for both nerve agents (G, H) and one peak for the pesticide (I). The two peaks represent two diastereomers consisting of the chiral phosphonyl moiety—either the (+)- or (−)-variant—that is bound to the tetrapeptide comprised natural L-amino acids exclusively. For reasons of clarity, only the traces of the respective most intense product ions (Qual I) are illustrated for µLC-ESI MS/MS (SRM) analysis (Table 1)
OPNA are usually produced as racemic mixtures with their phosphorus atom representing the stereocenter, e.g. (±)-VX (Fig. 1A) and (±)-GF (Fig. 1B) (John et al. 2020). It has been shown that the inhibition of cholinesterases as well as subsequent ageing are OPNA-enantioselective processes thus resulting in different toxicities of the enantiomers (John et al. 2020; Reiter et al. 2015). Whereas chromatographic methods suited for the enantioselective detection of OPNA were established (Reiter et al. 2007, 2011), no method has been introduced to differentiate BChE-adducts of enantiomers on the molecular protein or peptide level. Incubation of BChE with racemic mixtures of OPNA always yielded only one chromatographic peak for the NP-adducts after pepsin-mediated cleavage thus documenting the coelution of the adducts of (+)- and (−)-OPNA (Fidder et al. 2002; John and Thiermann 2021; John et al. 2015, 2020; Noort et al. 2021). Even though there is no need to detect the adducts with enantioselectivity for qualitative verification purposes, methods providing this specification will be very helpful to investigate mechanistic aspects of adduct formation and consecutive reactions like ageing and spontaneous reactivation.
Therefore, we developed a micro (µ)-LC–ESI MS/MS method working in the selected reaction monitoring mode (SRM) to detect a novel peptide-adduct biomarker with enantioselectivity. This method was applied to plasma samples of a real case of pesticide poisoning and to samples provided by the OPCW in the course of biomedical proficiency tests (BioPT).
Materials and methods
Chemicals
Water (LCMS grade), acetonitrile (ACN, hypergrade for LCMS), isopropanol (iPrOH; for gas chromatography), formic acid (FA, ≥ 98%) and proteinase K (Prot K, from Tritirachium album) were from Merck (Darmstadt, Germany); ammonium hydrogen carbonate (≥ 99.5%), phosphate-buffered saline, 5,5-dithio-bis-2-nitrobenzoic acid, S-butyrylthiocholine iodide, Tween 20 and pepsin from porcine gastric mucosa (≥ 2.5 units/mg protein) from Sigma (Steinheim, Germany); mouse monoclonal anti-human BChE antibody (clone 3E8, HAH002-01, 1.07 mg/mL) from Statens Serum Institut (Kopenhagen, DK); protein G-coated magnetic beads (Dynabeads Prot G) from ThermoFisher Scientific (Waltham, MA, USA); NaOCl (12% Cl) from Carl Roth (Karlsruhe, Germany) and d3-atropine (d3-Atr) from CDN isotopes (Point Claire, Quebec, Canada). Paraoxon-ethyl (> 97%) and malaoxon (> 97%) were from Labor Dr. Ehrenstorfer-Schäfers (Augsburg, Germany); pooled human EDTA plasma from Dunn Labortechnik GmbH (Asbach, Germany) and EDTA plasma from individual donors from in.vent Diagnostica (Henningsdorf, Germany). The nerve agents GA, GB, GD, GF, VX, RVX and CVX were made available by the German Ministry of Defense. Enantiopure (+)- and (−)-VX were prepared from the racemic mixture as already described (Reiter et al. 2008). Purity and integrity of OPNA were tested in-house by nuclear magnetic resonance spectroscopy. Stock solutions (0.1% v/v, 1 mg/mL) and working solutions (1.25–25 µM) of OPNA and pesticides were prepared in iPrOH.
Incubation of plasma
Plasma (500 µL) was separately mixed with stock solutions (2 µL) of all OPNA and pesticides listed above to produce references (100 nM) with completely inhibited BChE. Plasma standards of (±)-VX and (±)-GF were prepared using working solutions resulting in concentrations of 5 nM, 10 nM, 15 nM, 20 nM, 40 nM, 50 nM, 60 nM, 75 nM and 100 nM. Spiked plasma was incubated (1 h, 37 °C) under gentle shaking prior to sample preparation or storage at − 20 °C. Inhibition of BChE was determined by a modified Ellman assay.
Ellman assay
A modified Ellman assay was used to determine the BChE activity as described before (Ellman et al. 1961; Worek et al. 1999).
Sample preparation
Plasma sample preparation according to the standard protocol included initial extraction of BChE and its adducts (immunomagnetic separation, IMS) followed by their proteolysis and final ultrafiltration (UF). Filtrates were subjected to analysis by µLC–ESI MS/MS (SRM) and by high-resolution tandem mass spectrometry (MS/HR MS) using µLC–ESI MS/HR MS working in the product ion scan (PIS) mode.
IMS of BChE
IMS was carried out based on the method described by Sporty et al. (Sporty et al. 2010) to extract BChE adducts from plasma (200 µL) (John et al. 2022).
Proteolysis of extracted BChE
Extracted bead-bound BChE was proteolyzed either with pepsin to generate the NP or with Prot K to produce the tetrapeptide (TP). For proteolysis with pepsin (30 min, 37 °C), water (75 µL) and pepsin solution (10 µL, 2 mg/mL in 5% v/v FA) were added to BChE immobilised on the beads (John et al. 2021, 2022). For proteolysis with Prot K (1 h, 50 °C), NH4HCO3 buffer (75 µL, 50 mM, pH 8.0) and Prot K solution (10 µL, 2 mg/mL in 50 mM NH4HCO3) were used.
After these proteolyses and subsequent magnetic separation, an aliquot of the liquid layer (70 µL) was mixed with d3-Atr solution (130 µL, 3 ng/mL in 0.5% v/v FA) in an UF device (Amicon Ultra-0.5 centrifugal filter unit 0.5 mL, 10 kDa molecular weight cut-off, MWCO, Merck) for immediate UF (15 min, 15 °C, 14,000 RCF). The filtrate (150 µL) was analysed either by µLC–ESI MS/HR MS (PIS) or by µLC–ESI MS/MS (SRM).
µLC–ESI MS/HR MS (PIS) analysis of TP-adducts
TP-adduct analysis (method 1)
The µLC system comprised a micro-LC 200 pump (Eksigent Technologies, Dublin, CA, USA), a HTC-xt DLW autosampler (CTC Analytics, Zwingen, Switzerland) kept at 15 °C and a 20 µL sample loop (Sunchrom, Friedrichsdorf, Germany). Separation of a sample (20 µL) was done on a Waters Acquity UPLC® HSS T3 column (C18, 100 × 1.0 mm I.D., 1.8 µm, 100 Å, Waters, Eschborn, Germany) protected by a precolumn (Security Guard™ Ultra Cartridges UHPLC C18-peptide 2.1 mm I.D., Phenomenex, Aschaffenburg, Germany) by gradient elution (30 µL/min, 30 °C) with solvent A (0.05% v/v FA) and solvent B (ACN/H2O 80:20 v/v, 0.05% v/v FA): t [min]/B [%] 0/2, 15/35, 15.5/95, 18/95, 18.5/2, 20/2 (gradient 1), including a 5 min equilibration period. Mass spectrometric detection in PIS mode was carried out with a TT5600+ (Sciex, Darmstadt, Germany) equipped with a time-of-flight (TOF) mass analyzer after positive ESI (Turbo V source, Sciex) in the mass range from m/z 50 to m/z 700. The following MS parameters were applied: curtain gas (CUR) 30 psi (2.07 × 105 Pa), ion source gas 1 (GS1) 40 psi (2.76 × 105 Pa), ion source gas 2 (GS2) 50 psi (3.45 × 105 Pa), ion spray voltage floating (ISVF) + 4500 V, ion source temperature (TEM) 200 °C, declustering potential (DP) 60 V, collision energy spread (CES) 5 V, ion release delay (IRD) 67 ms and ion release width (IRW) 25 ms. Mass calibration was performed after every fifth run using an atmospheric pressure chemical ionisation (APCI) interface coupled to a calibrant delivery system (CDS) using the APCI positive calibration solution (500 µL/min, Sciex). Specific collision energy (CE) values were applied for adducts of the following OPNA: 20 V for GA, GF, VX, DMP- and DEP-pesticides; 25 V for GB, CVX and RVX; 15 V for the non-adducted TP and 42 V for d3-Atr. In addition, a CE of 20 V was used for the aged adducts of GA, DMP and DEP pesticides, and 25 V was applied for aged adducts of G- and V-type OPNA. The accumulation time was 75 ms with a maximum cycle time of 975 ms. The system was controlled by the Analyst TF 1.8.1 software in combination with the Eksigent control software 4.3 (both Sciex). Precursor masses of the TP and its adducts are listed in Table 1. Product ion traces were extracted with ± 0.005 Th to generate extracted ion chromatograms (XIC).
µLC–ESI MS/MS (SRM) analysis of NP- and TP-adducts
TP-adduct analysis (method 2)
Separation of the sample (5 µL) was carried out with a M5 µLC-system (Sciex) equipped with an autosampler kept at 10 °C applying the same chromatography with gradient 1 as described above for µLC–ESI MS/HR MS (PIS). The µLC-system was online coupled to a QTrap 6500+ triple quadrupole mass spectrometer (Sciex) via positive ESI with an Optiflow source (Sciex) operating in SRM mode. The following MS parameters were applied: CUR 30 psi (2.07 × 105 Pa), GS1 50 psi (3.45 × 105 Pa), GS2 60 psi (4.14 × 105 Pa), ion spray voltage (IS) + 5000 V, TEM 200 °C, DP 60 V, entrance potential (EP) 10 V, cell exit potential (CXP) 10 V and dwell time 15 ms. Protonated single charged precursor and product ions (qualifier I-III, Qual I—Qual III) and corresponding CE values are given in Table 1. The system was controlled by the Analyst 1.71 and the Eksigent control software 4.3 (both Sciex).
NP-adduct analysis (method 3)
The chromatographic and mass spectrometric systems and solvents used were the same as described above for TP-adduct SRM analysis, but the stationary phase and gradient were different. For chromatography, the trap-elute mode was used with a Luna® Omega Polar C18 trap column (20 × 0.5 mm I.D., 5 µm, 100 Å, Phenomenex) combined with a nanoEase™ M/Z HSS T3 (50 × 0.3 mm I.D., 1.8 µm, 100 Å, Waters) separation column. After 3 min of equilibration under starting conditions, the sample (5 µL) was injected and transferred onto the trap column for concentration (50 µL/min, t [min]/B [%] 0/2, 2/2). After 0.8 min, the following gradient was applied (10 µL/min, 30 °C): t [min]/B [%] 0/2, 6/35, 6.1/95, 8/95, 8.1/2, 9/2 (gradient 2). MS parameters were the same as described above for TP-adduct analysis (method 2) with the exception of the following: GS1 15 psi (1.03 × 105 Pa) and DP 100 V. Three product ions (m/z 778.3, m/z 673.3, m/z 602.2) of each protonated single charged precursor ion were monitored in SRM mode as already described by John et al. (2015).
Reaction time-dependent adduct formation of VX and GF
Blank plasma was separately spiked with solutions of enantiopure (+)- and (−)-VX as well as with the racemic mixtures of (±)-VX and (±)-GF (n = 3, each) causing only partial inhibition of BChE as determined by Ellman assay. The samples were incubated at 37 °C under gentle shaking to take aliquots (100 µL) after 5 min, 2 h, 6 h, 24 h, 48 h and 168 h. Following sample preparation by IMS and proteolysis with Prot K, analysis was performed by µLC–ESI MS/MS (SRM) (method 2). Relevant peak areas of the XIC of Qual I were plotted versus the reaction time.
Time-dependent generation of TP-adducts during Prot K-catalysed proteolysis
Plasma was incubated with (±)-VX (100 nM, 24 h) and 1.6 mL was subjected to IMS followed by proteolysis with Prot K using appropriately adjusted amounts of beads and volumes of buffer and enzyme solution (n = 3). During proteolysis aliquots of the mixture (70 µL) were taken after 15 min, 30 min, 45 min, 60 min, 90 min and 120 min to be immediately mixed with d3-Atr solution (130 µL) and subjected to UF. The filtrate was analysed by µLC–ESI MS/MS (SRM) (method 2). Peak areas obtained from XIC of Qual I were plotted against the time of proteolysis.
Dose–response study and limit of identification
Plasma incubated either with (±)-VX or (±)-GF (100 nM, 1 h, each, causing 100% inhibition of BChE) and blank plasma (0% inhibition) were prepared by IMS and proteolysis with Prot K according to the standard protocol. Resulting ultrafiltrates of inhibited and non-inhibited plasma were mixed in different volumes simulating samples of 1%, 2%, 3%, 4%, 5%, 10%, 25% and 50% inhibition (n = 3, each). Mixtures were analysed by µLC–ESI MS/MS (SRM) (method 2) to plot peak areas of TP-adducts versus the grade of inhibition. The limit of identification (LOI) was found at the lowest grade of inhibition at which the quality criteria for ion ratios fixed by the OPCW (Organisation for the Prohibition of Chemical Weapons 2023) were fulfilled in all triplicates and all relevant peaks had a signal-to-noise ratio (S/N) ≥ 5. Ion ratios representing the ratio of peak areas obtained from qualifying ions of a certain adduct peak were calculated and compared to those values deduced from respective references as summarised in Table 1. For determination of the LOI values, the following ratios were considered for (±)-VX Qual II/Qual I (peak 1 and 2) and for (±)-GF Qual II/Qual I (peak 1) and Qual III/Qual I (peak 2).
Comparison of mass spectrometric response of TP- and NP-adducts
Blank plasma and plasma separately inhibited either with (±)-VX or (±)-GF was prepared by IMS and proteolysis either with Prot K or pepsin as described above. Ultrafiltrates of non-inhibited and inhibited plasma were mixed simulating samples of 12.5%, 25%, 50%, and 100% inhibition, each. Samples were analysed by µLC–ESI MS/MS (SRM) using either method 3 for the NP-adduct or method 2 for the TP-adducts to compare the resulting peak areas.
Stability in the autosampler
Prepared plasma references (100 nM, 1 of all OPNA) and pesticides ready for analysis were stored at 10 °C in the autosampler and analysed hourly over 24 h. Peak areas of Qual I were plotted against the storage time to characterise the stability.
Application of the method to a real case of pesticide poisoning and BioPT samples
Plasma samples of a real case of human pesticide poisoning with parathion-ethyl and samples provided by the OPCW for the 4th (2019) and the 5th BioPT (2020) were analysed. Details on the pesticide case were already provided by our group before (Eyer et al. 2009; John et al. 2021; von der Wellen et al. 2018).
Safety considerations
OPNA are highly toxic substances that may only be handled under a fume hood whilst wearing suitable personal protective equipment. All materials that had contact to OPNA were decontaminated in a bleach solution containing NaOCl for at least 24 h.
Results and discussion
According to the guideline of the OPCW regulating quality criteria for verification (Organisation for the Prohibition of Chemical Weapons 2023), at least two biomarkers are to be detected to verify exposure to a certain CWA. In addition, peptide-adducts with individual structures but containing the same adducted amino acid residue are considered as different biomarkers when different enzymes were used for proteolysis of the same protein-adduct. Therefore, Prot K was tested for BChE-adduct hydrolysis promising to obtain adducts shorter than the NP-adducts. Prot K has already proven its suitability for verification in the generation of an adducted albumin-derived tripeptide biomarker (CysProPhe) documenting exposure to alkylating blister agents (Blum et al. 2020; John et al. 2019; Noort et al. 1999).
Initially, the postulated TP-adducts were detected by MS/HR MS in PIS mode to yield product ions detected with an appropriate accuracy and mass spectrometric resolution to confirm their structural assignment.
Detection and identification of TP-adducts by µLC–ESI MS/HR MS (PIS)
After protein-adduct extraction and proteolysis with Prot K, malaoxon (Fig. 1C) yielded one single peak (tR 6.1 min, Fig. 1I) representing the GlyGluSer(-DMP)Ala adduct. Only one peak was also obtained for the non-adducted TP as well as the aged adducts of G- and V-type nerve agents. Non-aged GD adducts could not be detected due to rapid ageing in the incubation buffer at pH 8.0. In contrast, all OPNA tested typically yielded two baseline separated TP-adduct peaks as exemplarily shown for (±)-VX (peak 1: tR 6.6 min and peak 2: tR 6.8 min, Fig. 1G) and (±)-GF (peak 1: tR 12.4 min and peak 2: tR 12.7 min, Fig. 1H). MS/HR MS spectra extracted from both peaks each were identical and enabled the identification of the adducted TP as exemplarily illustrated for the TP phosphonylated with an O-ethyl methylphosphonyl moiety (EMP) GlyGluSer(-EMP)Ala (Fig. 2A) derived from VX as well as a TP phosphonylated with an O-cyclohexyl methylphosphonyl moiety (cHMP) GlyGluSer(-cHMP)Ala (Fig. 2B) derived from GF.
Structure and product ion spectra of GlyGluSer(-EMP)Ala and GlyGluSer(-cHMP)Ala. A Tetrapeptide-adduct of VX bearing the O-ethyl methylphosphonyl (EMP) moiety, GlyGluSer(-EMP)Ala, [M + H]+ m/z 469.169. B Tetrapeptide-adduct of cyclosarin (GF) bearing the O-cyclohexyl methylphosphonyl (cHMP)-moiety, GlyGluSer(-cHMP)Ala, [M + H]+ m/z 523.216. For reasons of clarity, the one letter code GESA is used for GlyGluSerAla. Spectra were extracted from the respective most intense peak 2 of µLC–ESI MS/HR MS runs monitored in product ion scan (PIS) mode after collision-induced dissociation of the single-protonated tetrapeptide-adducts. The cleavage sites of the most intense product ions are indicated. The loss of the respective phosphonyl moiety including the former O-atom of the serine residue (-pm) is indicated. Product ion spectra of the respective diastereomers obtained from ( +)- and (−)-enantiomers of the agents were identical. The m/z-values of product ions of GlyGluSer(-EMP)Ala are given in Table 2 and those of GlyGluSer(-cHMP)Ala in Table S1 of the supplementary material
For all adducts tested, the product ion at m/z 345.141 resulting from the loss of the entire phosphyl moiety also including the oxygen atom of the serine residue (pm) was the most intense one, [M + H]+loss of pm. Figure 2 indicates the cleavage sites resulting from collision-induced dissociation. The preferred cleavage of the pm moiety is also known from the fragmentation of the adducted NP (Fidder et al. 2002; John et al. 2015, 2021, 2022; Sporty et al. 2010). The second and third most intense ions were detected at m/z 159.076 (a2) and m/z 327.130 (b4,loss of pm) (Fig. 2). OPNA-specific product ions resulting from the loss of GlyGlu thus still containing the respective phosphyl moiety (y2) were detected as well for all agents tested. These ions were found for the VX-adducts at m/z 283.105 (Fig. 2A) and at m/z 337.152 for the GF-adducts (Fig. 2B). For GlyGluSer(-cHMP)Ala, an additional product ion resulting from the loss of the O-bound alkyl moiety was also detected with high intensity (m/z 441.138, [M + H]+loss of alkyl) (Fig. 2B). All product ions obtained from GlyGluSer(-EMP)Ala were structurally assigned as summarised in Table 2 and those of GlyGluSer(-cHMP)Ala in Table S1 of the supplementary material. Therefore, the peptide backbone of the TP in both peaks was unambiguously identified as GlyGluSer*Ala excluding the isobaric BChE-derived peptide GluSer198*AlaGly.
Even though the presence of the TP-adduct was expected, its appearance as two peaks was quite surprising. Identical product ion spectra obtained from the TP-adducts of both peaks suggested a phenomenon of chirality.
OPNA enantiomers cause the ´two peak phenomenon´
Due to their non-chiral synthesis, OPNA are typically present as racemic mixtures of two enantiomers, e.g. ( +)-VX and (−)-VX or ( +)-GF and (−)-GF based on the chirality at the P-atom (Fig. 1A, B) (John et al. 2020). The respective ( +)- and (−)-enantiomers exhibit different reactivity towards cholinesterases and thus different toxicity (Jiang et al. 2013; John et al. 2020; Ordentlich et al. 1999; Reiter et al. 2007; van der Schans et al. 2003; Wandhammer et al. 2011). Following BChE adduction, the phosphonyl moiety of the OPNA still contains a stereocenter at the P-atom (marked with an asterisk, Fig. 1D, E) as exemplarily shown for VX and GF. In contrast, the aged adducts and common DEP and DMP pesticides do not possess a chirality centre at the P-atom (Fig. 1C). Adducts of OPNA enantiomers with natural endogenous peptides, which comprised chiral L-amino acids (α-C-atom as stereocenter), thus represent diastereomers. Whereas in principle the chromatographic separation of enantiomers requires chiral columns, the separation of diastereomers is possible using non-chiral columns. Therefore, we assumed that the adducted TP GlyGluSer*Ala (Fig. 1D, E) allowed enantiomer-dependent chromatographic separation when using high-resolving reversed-phase material of 1.8 µm particle size as illustrated in Fig. 1G, H. In contrast, to TP-adducts, the NP-adducts are not separated with enantioselectivity as the relative impact of the phosphyl moiety on the entire hydrophobicity of the nine amino acid peptide is much smaller than on the four amino acid peptide. Accordingly, resulting differences do not affect the retention times significantly.
Accordingly, we investigated potential enantiomeric effects on TP adduction. For this purpose and due to internal laboratory operational reasons, we first developed a sensitive and selective µLC–ESI MS/MS (SRM) method. This method targeted the most intense product ions of the adducted and non-adducted TP (Qual I–Qual III) simultaneously as listed in Table 1.
Incubation with racemic and enantiopure VX
To confirm that the ´two peak phenomenon´ observed in our study was due to the separation of diastereomeric adducts, we incubated plasma separately with the racemic mixture (±)-VX as well as their enantiopure variants (+)-VX and (−)-VX. Added amounts of the agent resulted in lower molar concentration than that present for BChE (40–80 nM with a large individual variance, Black and Read 2013; Browne et al. 1998) and thus caused incomplete inhibition of 70.6 ± 12.2% for (+)-VX, 66.0 ± 0.8% for (−)-VX and 77.7 ± 3.7% for (±)-VX as measured by Ellman assay. The molar excess of BChE was chosen to provide potential binding sites for all agent molecules, thus avoiding enantioselective competition.
The use of (±)-VX yielded two peaks as already illustrated above (Fig. 1G). When incubating with (+)-VX, only peak 1 (tR 6.6 min) was obtained (Fig. 3A) and incubation with (−)-VX resulted in the dominant peak 2 (tR 6.8 min) and a much smaller peak 1 (Fig. 3B). Therefore, we clearly demonstrated that the two peaks obtained from the (±)-VX mixture corresponded to the enantioselective reaction products of the TP and thus approved our assumption of a chirality phenomenon.
Chromatograms of GlyGluSer(-EMP)Ala obtained after plasma incubation with (+)- and (−)-VX. Plasma was incubated with A enantiopure (+)-VX and B enantiopure (−)-VX prior to BChE-adduct extraction and proteolysis with proteinase K yielding the diastereomeric tetrapeptide GlyGluSerAla phosphonylated by the O-ethyl methylphosphonic acid moiety (EMP). Samples were analysed by µLC–ESI MS/MS (SRM). For reasons of clarity, only the trace of Qual I (m/z 345.1, Table 1) is shown
The occurrence of peak 1 after addition of (−)-VX was unexpected and not caused by impurities of (+)-VX as the purity of enantiopure VX had been confirmed by µLC–ESI MS/MS prior to use (Reiter et al. 2008). It might be argued that a certain low portion of the phosphonyl moiety underwent inversion of the configuration during the phosphonylation reaction or that repeated reactivation and re-inhibition of the enzyme effected the confirmation of the active site serine residue in BChE.
(±)-VX and (±)-GF concentration-dependent adduct formation
Incubation of plasma with rising concentrations of (±)-VX and (±)-GF in the nanomolar range resulted in concentration-dependent relative peak areas of peak 1, A(1), and peak 2, A(2) for each agent. Incubations with (±)-VX at concentrations from 5–60 nM yielded peak area ratios A(1):A(2) of 1:1.26 ± 0.04, whereas 100 nM VX resulted in a 1:1.70 ratio. Incubations with (±)-GF showed the same effect of the relative peak areas but to a much more prominent extent. Whereas at lowest (±)-GF concentrations tested (5 nM–20 nM), both peaks were of equal areas, A(1):A(2) 1:1.06 ± 0.04, and at higher concentrations, the second peak became more and more dominant resulting in an area ratio A(1):A(2) of 1:10 after incubation with 100 nM. Figure 4 exemplarily shows the chromatographic results obtained from four (±)-GF concentrations (20 nM, 40 nM, 50 nM and 100 nM, Fig. 4A–D) and illustrates the concentration-dependent individual areas of peak 1 and peak 2 resulting from incubations with (±)-GF (Fig. 4E) and (±)-VX (Fig. 4F).
Peak profiles of TP-adducts in dependence of the (±)-GF and (±)-VX concentrations in plasma. Plasma was incubated with indicated concentrations of (±)-GF for enantioselective monitoring of GlyGluSer(-cHMP)Ala peaks A 20 nM, B 40 nM, C 50 nM and D 100 nM. The peak area profiles of E GlyGluSer(-cHMP)Ala and F GlyGluSer(-EMP)Ala resulting from incubations with diverse concentrations of (±)-GF and (±)-VX document concentration-dependent effects. Peak areas were obtained from XIC of the respective Qual I (Table 1) from µLC–ESI MS/MS (SRM) analyses
These quantitative shifts were most presumably due to enantioselective chemical reactivities towards BChE adduction. At OPNA concentrations lower or quite similar to the BChE concentration, the entire amounts of both enantiomers could react with BChE not undergoing any competition. Accordingly, areas of peak 1 and peak 2 grew steadily exhibiting quite equal peak areas, A(1):A(2) 1:1. Increasing the agent concentrations to values higher than that of BChE caused a competition between both enantiomers. The more reactive enantiomer was adducted with preference more rapidly than the less reactive one and thus caused a shift of the relative peak area ratio. It is known that the (−)-enantiomer of OPNA is typically the more reactive variant of the molecule and thus causes higher inhibition rate constants (ki) (Benschop and De Jong 1988). Therefore, peak 2 resulting from the reaction of BChE with the (−)-enantiomer became dominant and its area was much higher than that of the (+)-enantiomer (peak 1). According to Barakat et al. (2009), the ki of a thiocholine analogue of (−)-GF is about 16 times higher than that of the corresponding (+)-GF variant and thus explains the tremendous shift of the peak area ratio A(1):A(2) to 1:10 observed in our study. In contrast, the ki of (−)-VX is only 2.4 times higher than that of (+)-VX (Reiter et al. 2008) and thus explains the rather moderate shift of the peak area ratio A(1):A(2) to 1:1.7.
Reaction time-dependent adduct formation of (±)-VX and (±)-GF
To characterise the reaction time-dependent enantiomeric adduct yield, plasma was separately incubated with (±)-VX and (±)-GF. These experiments were carried out with an excess of BChE, thus allowing all poison molecules to react independent of a certain enantioselective reactivity. Figure 5 summarises the results for GlyGluSer(-EMP)Ala (left column) and GlyGluSer(-cHMP)Ala (right column) showing the respective peak areas of the non-adducted TP (Fig. 5A, B), peak 1 (Fig. 5C, D), peak 2 (Fig. 5E, F), the aged adduct (Fig. 5G, H) and the inhibition rate of BChE determined by Ellman assay (Fig. 5I, J).
Adduct formation and stability of TP-adducts in plasma. Plasma was spiked with (±)-VX (left column) or (±)-GF (right column) and stored at 37 °C. At time points indicated samples were taken for BChE extraction, proteolysis and monitoring of the relative concentration–time profiles of phosphonylated (*) GlyGluSer*Ala biomarkers by µLC–ESI MS/MS (SRM). (±)-VX caused the adduction of the O-ethyl methylphosphonic acid moiety (EMP) and (±)-GF of the O-cyclohexyl methylphosphonic acid moiety (cHMP). A, B Peak areas of the non-adducted GlyGluSerAla. C, D Peak areas of the respective peak 1 of the phosphonylated, non-aged GlyGluSer*Ala biomarkers formed by reaction with the respective (+)-enantiomer of the agent (Fig. 1). E, F Peak areas of the respective peak 2 of the phosphonylated, non-aged GlyGluSer*Ala biomarkers formed by reaction with the respective (−)-enantiomer of the agent (Fig. 1). G, H Peak areas of the respective phosphonylated, aged form of the biomarker GlyGluSer(-MPA)Ala (MPA: methyl phosphonic acid). I, J Activity of BChE in plasma determined by Ellman assay. Peak areas were deduced from extracted ion chromatograms of the respective most intense product ions (Qual I, Table 1). Data points represent the mean (M) and standard deviation (SD) of triplicate measurements
EMP-adducts of VX
Immediately after mixing, the amount of the non-adducted TP was quite high but decreased during the first phase reaching its minimum at time point 2 h (Fig. 5A) documenting initial enzyme inhibition. Afterwards, the concentration of the non-adducted TP continuously increased (Fig. 5A) indicating the release of non-adducted BChE resulting from spontaneous reactivation of adducts. The area of peak 1 rapidly increased and reached its stable plateau after about 6 h (Fig. 5C) indicating phosphonylation by the (+)-enantiomer yielding stable adducts. In contrast, the area of peak 2 also reached its maximum after 6 h but decreased afterwards to about one-third of the maximum after 168 h (Fig. 5E). This decrease documents the degradation of the (−)-enantiomer adduct most presumably caused by spontaneous reactivation and ageing. Accordingly, the yield of the aged adduct increased steadily (Fig. 5G) obviously resulting from the degradation of the (−)-VX adduct corresponding to peak 2. In addition, the entire inhibition rate of BChE activity was maximum after 6 h (78% inhibition of initial BChE activity) and slightly decreased until 168 h. This concentration profile documents that a certain portion of the adducts-most presumably that of the (−)-VX adduct (peak 2)- underwent spontaneous reactivation resulting in the active enzyme again (Fig. 5I) (Worek et al. 1998). These results clearly documented that following a rapid adduct formation process, the reaction products exhibited enantioselective stability. Whereas the adduct of (+)-VX (peak 1) remained stable within the test period, the adduct of (−)-VX (peak 2) was degraded to its aged product as well as to the non-adducted BChE again. The same study performed with enantiopure VX clearly documented that only the (−)-VX adduct (peak 2) underwent spontaneous reactivation and ageing, whereas high stability was observed for the (+)-VX adduct (peak 1) (results not shown). This difference is caused by the spatial proximity of the ethoxy substituent in the (−)-VX adduct to the catalytic His438 residue of BChE, which supports hydrolysis by its nucleophilicity (Wandhammer et al. 2011). In contrast, in the adduct of (+)-VX, the ethoxy substituent is located and protected in the acyl-binding pocket preventing hydrolysis (Wandhammer et al. 2011) and thus no ageing was observed.
cHMP-adducts of GF
(±)-GF showed the similar but much more rapid phenomenon. Peak areas of the non-adducted TP increased rapidly reaching a plateau after 48 h (Fig. 5B) indicating spontaneous reactivation of BChE (Worek et al. 1998). Peak 1 reached a stable plateau after about 2 h (Fig. 5D), whereas the area of peak 2 showed an immediate and rapid decrease nearly causing its complete disappearance after about 48 h (Fig. 5F). In contrast, the concentration of the aged adduct rapidly increased reaching its maximum after about 48 h (Fig. 5H) correlating to the decreasing peak areas of peak 2 (Fig. 5F). The inhibition rate of BChE reached its maximum immediately after (±)-GF addition and did not show any reactivation afterwards (Fig. 5J). These findings are in accordance to previous reports documenting that the ageing process of the cHMP-adduct happens much faster than spontaneous reactivation and much faster than the ageing process of the EMP-adduct (Aurbek et al. 2009; Worek et al. 1998).
Time-dependent generation of TP-adducts during Prot K-catalysed proteolysis
After about 30 min of proteolysis, maximum concentrations of both GlyGluSer(-EMP)Ala diastereomers corresponding to peak 1 (Fig. 6, open circles) and peak 2 (Fig. 6, filled circles) were obtained. No concentration decrease was observed until the end of the test period (120 min), thus documenting the stability of the TP biomarker with respect to the adducted phosphonyl moiety as well as the peptide backbone. Accordingly, these novel biomarkers appeared robust and stable and thus well suited for reliable verification.
Production of GlyGluSer(-EMP)Ala during proteinase K-catalysed proteolysis of the BChE-adduct. BChE-adducts of plasma incubated with (±)-VX were extracted and subjected to proteolysis (n = 3). At time points indicated the tetrapeptide GlyGluSerAla phosphonylated at its serine residue by a chiral O-ethyl methylphosphonic acid moiety (EMP) was monitored by µLC–ESI MS/MS (SRM). Data points represent the M ± SD (RSD ≤ 3.3%, n = 3) of peak areas of peak 1 (resulting from reaction with (+)-VX, open circles, Fig. 3A) and peak 2 (reaction with (−)-VX, filled circles, Fig. 3B) obtained from traces of Qual I (m/z 345.1, Table 1).
Selectivity
Typically, no interferences were found for the TP-adducts. Representative chromatograms are shown in Fig. 7A–D. For GlyGluSer(-cHMP)Ala and GlyGluSer(-EMP)Ala, the best selectivity especially for peak 2 was obtained for Qual III and Qual II, respectively.
Analysis of plasma samples obtained from OPCW BioPTs and a real case of pesticide poisoning. Plasma samples sent by the Organisation for the Prohibition of Chemical Weapons (OPCW) in the course of the 4th and 5th biomedical proficiency test (BioPT) as well as a plasma samples from a patient poisoned with the organophosphorus pesticide parathion-ethyl (Eyer et al. 2009; von der Wellen et al. 2018) were analysed by µLC–ESI MS/MS (SRM) to detect the respective phosphylated (*) tetrapeptides GlyGluSer*Ala as biomarkers of exposure. The detection of GlyGluSer(-IMP)Ala indicated exposure to the nerve agent sarin (GB, 4th BioPT 2019) causing phosphonylation by an O-iso-propyl methylphosphonyl moiety (IMP) (first column, A, E, I). GlyGluSer(-nBuMP)Ala documented the presence of the nerve agent Chinese VX (CVX, 5th BioPT 2020) after adduction of an O-n-butyl methylphosphonyl moiety (nBuMP) (second column, B,F,J). The aged variant of the GB-adduct GlyGluSer(-MPA)Ala was found in the 4th BioPT sample bearing a methyl phosphonic acid moiety (MPA) (third column, C, G, K). The phosphorylated biomarker GlyGluSer(-DEP)Ala containing a diethyl phosphoryl moiety (DEP) proved poisoning with the pesticide paraoxon-ethyl, which was produced in vivo from parathion-ethyl swallowed by the patient with suicidal intend (fourth column, D, H, L). For each biomarker, respective blanks (top row, A–D), references (middle row, E–H) and the sample (bottom row, I–L) are shown. The occurrence of two peaks documented the presence of a pair of diastereomers resulting from reaction of the ( +)- (peak 1) and (−)-variant (peak 2) of the agents. For reasons of clarity, only the traces of the respective Qual I (Table 1) are illustrated.
Dose–response study and LOI
Considering the ion ratio of Qual II/Qual I, the LOI of GlyGluSer(-EMP)Ala was found at 3% inhibition for peak 1 (ion ratio: 95.3 ± 3.1%) and at 2% for peak 2 (ion ratio: 109.8 ± 11.5%). Linearity was found over the entire range of inhibition for both peaks (R2 ≥ 0.9997). For GlyGluSer(-cHMP)Ala, the LOI was found to be at 2% inhibition for peak 1 (ion ratio: 58.5 ± 1.7% for Qual II/Qual I) and at 3% for peak 2 (ion ratio: 13.4 ± 2.3% for Qual III/Qual II). Linearity resulted over the entire range of inhibition for both peaks (R2 ≥ 0.9999). At the LOI level, the S/N was > 7 in all triplicates. However, the LOI values reported are more or less characteristic only for the conditions tested herein (1 h incubation, 100 nM racemic mixture of OPNA). In general, as discussed above, peak areas vary depending on several factors, such as the incubation time (determining the grade of adduction and ageing) and the OPNA concentration applied, determining the relative ratio of the diverse enantiomeric peaks. Nevertheless, it is concluded that the traceability of the TP-adduct is at least as good as that reported of the NP-adducts (Black and Read 2013).
Comparison of mass spectrometric response of TP- and NP-adducts
To characterise the suitability of the TP-adducts as biomarkers of exposure in comparison to the well-established NP-adducts, samples simulating diverse grades of BChE-inhibition were analysed monitoring either the TP- or the NP-adducts. Exemplarily, the relative mass spectrometric response of (±)-VX and (±)-GF adducts was investigated. The peak areas (XIC of Qual I) of only the predominant peak of the TP-adducts (peak 2 of the EMP-and cHMP-adduct) were already 1.5 times higher for the EMP-adduct and 4.7 times higher for the cHMP-adduct. In addition, the quality criteria set by the OPCW regarding the product ion ratios of biomarkers (Organisation for the Prohibition of Chemical Weapons 2023) was excellently fulfilled. These results strongly document that the TP-adducts are superior or at least as suitable for verification as the NP-adducts making them promising additional biomarkers for the investigation of future real samples and samples of BioPTs.
Stability in the autosampler
TP-adducts of all OPNA (except for GA) and pesticides tested were stable in the autosampler for at least 24 h showing no trend of degradation. Accordingly, the relative standard deviations (RSD) of peak areas over the entire test period for all OPNA-adducts were 1.8–7.8%. Therefore, these novel TP-adducts are very well suited for verification purposes even if larger sets of samples are to be analysed.
Merely the GA-derived biomarker phosphylated with an O-ethyl dimethylaminophosphonyl moiety (EDMAP), GlyGluSer(-EDMAP)Ala, showed an immediate and continuous linear decrease of the areas causing 60% loss after 24 h (results not shown). In parallel, the peak area of the aged adduct resulting from the loss of the dimethylamino-group linearly increased by 70%. The P-bound dimethylamino-group easily undergoes hydrolysis under acidic conditions at low pH values (Larsson et al. 1953) and thus causes a highly limited stability of the adduct. According to the experimental conditions applied in this study, the sample solution ready for analysis had pH 3, thus favouring the ageing process. Under more acidic conditions, even significantly faster degradation was observed causing, e.g. complete ageing within 8 h at pH 1. Consequently, GlyGluSer(-EDMAP)Ala containing samples should be analysed as soon as possible to minimise the effect of ageing or the pH has to be increased accordingly.
Application of the method to a real case of pesticide poisoning and BioPT samples
The µLC–ESI MS/MS (SRM) method simultaneously targeting diverse TP-adducts was successfully applied to OPCW samples of the 4th (2019) and 5th BioPT (2020) as well as to a real case of pesticide poisoning.
Pesticide poisoning
A real case of pesticide poisoning (suicide attempt by swallowing parathion-ethyl) (Eyer et al. 2009, John et al. 2021; von der Wellen et al. 2018) was investigated. A single peak of the GlyGluSer(-DEP)Ala adduct corresponding to the non-chiral DEP-adduct was detected at tR 9.1 min (Fig. 7L). In addition, the aged variant of the TP-DEP adduct phosphorylated by a mono-ethyl phosphate moiety (MEP) was found at tR 5.2 min (data not shown). Even though this aged variant seems to possess four different substituents, it only yielded a single peak. This is explained by the tautomerism between the OH-group and the double bond oxygen atom at the phosphorus atom. In a dynamic chemical equilibrium, a hydrogen atom permanently migrates converting one form into the other, thus eliminating the stereo-centre and causing only one peak.
As expected parathion-ethyl swallowed by the patient was converted in the liver and oxidised to its much more toxic paraoxon-ethyl biotransformation product (Eyer et al. 2009, von der Wellen et al. 2018). Paraoxon-ethyl induced not only the cholinergic crisis of the patient (inhibition of AChE) but also the formation of BChE-adducts that were successfully targeted herein for forensic analysis (Eyer et al. 2009, John et al. 2021; von der Wellen et al. 2018).
OPCW BioPT
As documented in Fig. 7I, in vitro exposure of plasma to (±)-GB was confirmed by the detection of the diastereomeric O-iso-propyl methylphosphonyl TP-adduct, GlyGluSer(-IMP)Ala. Peak 2 (tR 8.3 min, Fig. 7I) represented the predominant variant of the GB adduct. In addition, the aged variant of this adduct was also detected (Fig. 7K) appearing as a single peak (tR 4.3 min) as the methyl phosphonic acid moiety (MPA) does not possess a stereocenter anymore. The lack of enantiopure GB prevented us from the assignment of the relevant enantiomers experimentally. However, the evidence of GB exposure was unambiguous.
Exposure to (±)-CVX of a 5th BioPT sample was confirmed by one peak of the O-n-butyl methylphosphonyl TP-adduct, GlyGluSer(-nBuMP)Ala, eluting at tR 10.5 min (Fig. 7J). By comparison to a freshly prepared reference, this adduct corresponded to peak 1 of two diastereomers (Fig. 7F) presumably derived from (+)-CVX. Obviously, the adduct of the other enantiomer was completely degraded most presumably by ageing as described above for GF (Fig. 5F). Accordingly, the aged TP-adduct was also detected in this sample at tR 4.3 min (results not shown). The same phenomenon of the missing peak 2 was also observed after analysis of another (±)-CVX reference that had been spiked some years before in our laboratory and had underwent repeated freezing and thawing steps, thus supporting the assumption of enantioselective ageing.
Blanks (Fig. 7A–D, top row) were free of interferences proving the selectivity and applicability of the method. OPCW quality criteria for verification (Organisation for the Prohibition of Chemical Weapons 2023) were fulfilled for all samples as the respective tR, and the ion ratios obtained for the samples were identical to those of the references within the allowed tolerance interval considering the ion ratio of Qual II/Qual I and peaks providing a S/N ≥ 5. Therefore, these BChE-derived TP-adducts represent important novel biomarkers of exposure to OPNA and pesticides and significantly expand the toolbox of methods for verification.
Conclusions
According to the OPCW guidelines (Organisation for the Prohibition of Chemical Weapons 2023), at least two biomarkers are to be detected in a sample to verify exposure to a certain agent. At least one of those must be a primary biomarker (adduct with endogenous molecules) that confirms the incorporation of the poison. Serum albumin as the most abundant protein in plasma is known to be adducted by several exogenous chemicals also including OPNA. From these protein adducts, several primary biomarkers are produced and targeted for verification. Very often the adducted albumin-derived tyrosine-residue is detected together with the adducted BChE-derived NP (John and Thiermann 2021). But in cases of exposure to OPNA that exhibit either no or only minimum reactivity with serum albumin, such biomarkers are hardly accessible. Therefore, the method presented herein will be highly beneficial as it provides a second primary biomarker for verification in addition to the NP-adduct both generated from the highly reactive BChE. Furthermore, our method will be adaptable to other BChE-inhibiting agents like carbamates or those OPNA, that were recently added to the annex of CWC in schedule 1.A.13–A.1.16 (e.g. Novichok agents). Even though carbamates often do not add a chiral moiety to BChE, the resulting non-diastereomeric TP-adducts will also be detectable by our procedure similar to the non-chiral DEP and DMP pesticides as shown herein for the paraoxon-ethyl poisoning case.
Apart from the use of the method for qualitative detection of TP-adducts with any OP poison, it will be very useful to investigate enantioselective processes of adduction, ageing and reactivation and will thus help to unravel and understand processes of molecular toxicology in more detail. Therefore, our method allowing the simultaneous TP-adduct analysis significantly extents the toolbox of bioanalytical methods highly suited for biomedical verification.
Abbreviations
- AChE:
-
Acetylcholinesterase
- ACN:
-
Acetonitrile
- BChE:
-
Butyrylcholinesterase
- CE:
-
Collision energy
- cHMP:
-
O-cyclohexyl methylphosphonyl moiety
- CVX:
-
Chinese VX
- CWA:
-
Chemical warfare agents
- CWC:
-
Chemical Weapons Convention
- d 3-Atr:
-
Triple deuterated atropine
- EDMAP:
-
O-ethyl-dimethylaminophosphonyl moiety
- EMP:
-
O-ethyl-methylphosphonyl moiety
- ESI:
-
Electrospray ionisation
- FA:
-
Formic acid
- GA:
-
Nerve agent tabun
- GB:
-
Nerve agent sarin
- GD:
-
Nerve agent soman
- GF:
-
Nerve agent cyclosarin
- HR:
-
High-resolution
- IMS:
-
Immunomagnetic separation
- iPrOH:
-
Iso-propanol
- k i :
-
Inhibition rate constant
- LC:
-
Liquid chromatography
- LOI:
-
Limit of identification
- M :
-
Mean
- MS:
-
Mass spectrometry
- MS/MS:
-
Tandem mass spectrometry
- NP:
-
Nonapeptide
- OP:
-
Organophosphorus
- OPCW:
-
Organisation for the Prohibition of Chemical Weapons
- OPNA:
-
Organophosphorus nerve agents
- PIS:
-
Product ion scan
- pm :
-
Phosphyl moiety
- Prot K:
-
Proteinase K
- Qual:
-
Qualifying ion
- RVX:
-
Russian VX
- RSD:
-
Relative standard deviation
- SD:
-
Standard deviation
- SRM:
-
Selected reaction monitoring
- TP:
-
Tetrapeptide
- UF:
-
Ultrafiltration
- VX:
-
Nerve agent O-ethyl-S-(2-diisopropylaminoethyl)methylphosphono thioate
- XIC:
-
Extracted ion chromatogram
References
Aurbek N, Thiermann H, Eyer F et al (2009) Suitability of human butyrylcholinesterase as therapeutic marker and pseudo catalytic scavenger in organophosphate poisoning: a kinetic analysis. Toxicology 259:133–139. https://doi.org/10.1016/j.tox.2009.02.014
Bajgar J (2005) Complex view on poisoning with nerve agents and organophosphates. Acta Med Hung 48:3–21. https://doi.org/10.14712/18059694.2018.23
Barakat NH, Zheng X, Gilley CB et al (2009) Chemical synthesis of two series of nerve agent model compounds and their stereoselective interaction with human acetylcholinesterase and human butyrylcholinesterase. Chem Res Toxicol 22:1669–1679. https://doi.org/10.1021/tx900096j
Benschop HP, De Jong LPA (1988) Nerve agent stereoisomers: analysis, isolation and toxicology. Acc Chem Res 21:368–374. https://doi.org/10.1021/ar00154a003
Black RM (2008) An overview of biological markers of exposure to chemical warfare agents. J Anal Toxicol 32:2–9. https://doi.org/10.1093/jat/32.1.2
Black RM, Read RW (2013) Biological markers of exposure to organophosphorus nerve agents. Arch Toxicol 87:421–437. https://doi.org/10.1007/s00204-012-1005-1
Blum MM, Richter A, Siegert M et al (2020) Adducts of the blistering warfare agent sesquimustard with human serum albumin and their mass spectrometric identification for biomedical verification of exposure. Anal Bioanal Chem 412:7723–7737. https://doi.org/10.1007/s00216-020-02917-w
Browne SP, Slaughter EA, Couch RA et al (1998) The influence of plasma butyrylcholinesterase concentration on the in vitro hydrolysis of cocaine in human plasma. Biopharm Drug Dispos 19:309–314. https://doi.org/10.1002/(SICI)1099-081X(199807)19:5%3c309::AID-BDD108%3e3.0.CO;2-9
Ding S-J, Carr J, Carlson JE et al (2008) Five tyrosines and two serines in human albumin are labeled by the organophosphorus agent FP-biotin. Chem Res Toxicol 21:1787–1794. https://doi.org/10.1021/tx800144z
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Eyer F, Worek F, Eyer P et al (2009) Obidoxime in acute organophosphate poisoning: 1–clinical effectiveness. Clin Toxicol 47:798–806. https://doi.org/10.1080/15563650903206828
Fidder A, Hulst AG, Noort D et al (2002) Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol 15:582–590. https://doi.org/10.1021/tx0101806
Grob D, Harvey AM (1953) The effects and treatment of nerve gas poisoning. Am J Med 14:52–63. https://doi.org/10.1016/0002-9343(53)90358-1
Holmes O, Phillips T (2017) Kim Jong-nam killed by VX nerve agent, say Malaysian police. The Guardian
Jiang W, Cashman JR, Nachon F et al (2013) Mass spectrometry method to identify aging pathways of Sp- and Rp-tabun adducts on human butyrylcholinesterase based on the acid labile P–N bond. Toxicol Sci 132:390–398. https://doi.org/10.1093/toxsci/kft011
John H, Thiermann H (2021) Poisoning by organophosphorus nerve agents and pesticides: an overview of the principle strategies and current progress of mass spectrometry-based procedures for verification. J Mass Spectrom Adv Clin Lab 19:20–31. https://doi.org/10.1016/j.jmsacl.2021.01.002
John H, Worek F, Thiermann H (2008) LC-MS based procedures for monitoring of toxic organophosphorus compounds and the verification of pesticide and nerve agent poisoning. Anal Bioanal Chem 391:97–116. https://doi.org/10.1007/s00216-008-1925-z
John H, Breyer F, Schmidt Ch et al (2015) Small scale purification of butyrylcholinesterase from human plasma and implementation of a µLC-UV/ESI MS/MS method to detect its organophosphorus adducts. Drug Test Anal 7:947–956. https://doi.org/10.1002/dta.1792
John H, Willoh S, Hörmann P et al (2016) Procedures for analysis of dried plasma using microsampling devices to detect sulfur mustard albumin adducts for verification of poisoning. Anal Chem 88:8787–8794. https://doi.org/10.1021/acs.analchem.6b02199
John H, van der Schans MJ, Koller M et al (2018) Fatal sarin poisoning in Syria 2013: forensic verification within an international laboratory network. Forensic Toxicol 36:61–71. https://doi.org/10.1007/s11419-017-0376-7
John H, Koller M, Worek F et al (2019) Forensic evidence of sulfur mustard exposure in real cases of human poisoning by detection of diverse albumin-derived protein adducts. Arch Toxicol 93:1881–1891. https://doi.org/10.1007/s00204-019-02461-2
John H, Balszuweit F, Steinritz D et al (2020) Toxicokinetic aspects of nerve agents and vesicants. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents, 3rd edn. Academic Press, London, pp 875–929
John H, Richter A, Siegert M et al (2021) Evidence of exposure to organophosphorus toxicants by detection of the propionylated butyrylcholinesterase-derived nonapeptide-adduct as a novel biomarker. Forensic Sci Int 323:110818. https://doi.org/10.1016/j.forsciint.2021.110818
John H, Dentzel M, Siegert M, Thiermann H (2022) A non-targeted high-resolution mass spectrometric workflow for the detection of butyrylcholinesterase-derived adducts with organophosphorus toxicants and structural characterization of their phosphyl-moiety after in-source fragmentation. Anal Chem 94:2048–2055. https://doi.org/10.1021/acs.analchem.1c04116
Larsson L, Rubinstein K, Zachariasen H et al (1953) The hydrolysis of dimethylamido-ethoxy-phosphoryl cyanide (Tabun). Acta Chem Scand 7:306–314. https://doi.org/10.3891/acta.chem.scand.07-0306
Munkner T, Matzke J, Videbaek A (1961) Cholinesterase activity of human plasma after intramuscular diisopropyl fluorophosphonate (DFP). Acta Pharmacol Tox 18:170–174. https://doi.org/10.1111/j.1600-0773.1961.tb00327.x
Nakagawa T, Tu AT (2018) Murders with VX: Aum Shinrikyo in Japan and the assassination of Kim Jong-Nam in Malaysia. Forensic Toxicol 36:542–544. https://doi.org/10.1007/s11419-018-0426-9
Namba T (1971) Cholinesterase inhibition by organophosphorus compounds and its clinical effects. Bull World Health Organ 44:289–307
Noort D, Hulst A, de Jong L, Benschop H (1999) Alkylation of human serum albumin by sulfur mustard in vitro and in vivo: mass spectrometric analysis of a cysteine adduct as a sensitive biomarker of exposure. Chem Res Toxicol 12:715–721
Noort D, Benschop HP, Black RM (2002) Biomonitoring of exposure to chemical warfare agents: a review. Toxicol Appl Pharm 184:116–126. https://doi.org/10.1006/taap.2002.9449
Noort D, Fidder A, van der Riet-van OD et al (2021) Verification of exposure to novichok nerve agents utilizing a semitargeted human butyrylcholinesterase nonapeptide assay. Chem Res Toxicol 34:1926–1932. https://doi.org/10.1021/acs.chemrestox.1c00198
Ordentlich A, Barak D, Kronman C et al (1999) Exploring the active center of human acetylcholinesterase with stereomers of an organophosphorus inhibitor with two chiral centers. Biochemistry 38:3055–3066. https://doi.org/10.1021/bi982261f
Organisation for the Prohibition of Chemical Weapons (2018) Summary of the report on activities carried out in support of a request for technical assistance by the UK of Great Britain and Northern Ireland (Technical Assistance Visit TAV/02/18) S/1612/2018. https://www.opcw.org/sites/default/files/documents/S_series/2018/en/s-1612-2018_e___1_.pdf. (accessed 14 August 2023). OPCW, The Hague. The Netherlands.
Organisation for the Prohibition of Chemical Weapons (2020) Chemical weapons convention: convention on the prohibition of the development, production, stockpiling and use of chemical weapons and on their destruction. https://www.opcw.org/sites/default/files/documents/CWC/CWC_en.pdf. (Accessed 14 August 2023). OPCW, The Hague. The Netherlands.
Organisation for the Prohibition of Chemical Weapons (2023) Work instruction for the reporting of the results of the OPCW biomedical proficiency tests. In: Quality Management System Document No. QDOC/LAB/WI/BioPT04. OPCW, The Hague. The Netherlands
Peters T Jr (1996) All about albumin: biochemistry, genetics, and medical applications. Academic Press, New York
Reiter G, Koller M, Thiermann H et al (2007) Development and application of procedures for the highly sensitive quantification of cyclosarin enantiomers in hemolysed swine blood samples. J Chromatogr B 859:9–15. https://doi.org/10.1016/j.jchromb.2007.08.040
Reiter G, Mikler J, Hill I et al (2008) Chromatographic resolution, characterisation and quantification of VX enantiomers in hemolysed swine blood samples. J Chromatogr B 873:86–94. https://doi.org/10.1016/j.jchromb.2008.08.00
Reiter G, Mikler J, Hill I et al (2011) Simultaneous quantification of VX and its toxic metabolite in blood and plasma samples and its application for in vivo and in vitro toxicological studies. J Chromatogr B 879:2704–2713. https://doi.org/10.1016/j.jchromb.2011.07.031
Reiter G, Müller S, Hill I et al (2015) In vitro and in vivo toxicological studies of V nerve agents: molecular and stereoselective aspects. Toxicol Lett 232:438–448. https://doi.org/10.1016/j.toxlet.2014.11.010
Sporty JLS, Lemire SW, Jakubowski EM et al (2010) immunomagnetic separation and quantification of butyrylcholinesterase nerve agent adducts in human serum. Anal Chem 82:6593–6600. https://doi.org/10.1021/ac101024z
Steindl D, Boehmerle W, Körner R et al (2021) Novichok nerve agent poisoning. The Lancet 397:249–252. https://doi.org/10.1016/S0140-6736(20)32644-1
van der Schans MJ, Lander BJ, van der Wiel H et al (2003) Toxicokinetics of the nerve agent (±)-VX in anesthetized and atropinized hairless guinea pigs and marmosets after intravenous and percutaneous administration. Toxicol Appl Pharm 191:48–62. https://doi.org/10.1016/S0041-008X(03)00216-3
von der Wellen J, Winterhalter P, Siegert M et al (2018) A toolbox for microbore liquid chromatography tandem-high-resolution mass spectrometry analysis of albumin-adducts as novel biomarkers of organophosphorus pesticide poisoning. Toxicol Lett 292:46–54. https://doi.org/10.1016/j.toxlet.2018.04.025
Wandhammer M, Carletti E, Van der Schans M et al (2011) Structural study of the complex stereoselectivity of human butyrylcholinesterase for the neurotoxic V-agents. J Biol Chem 286:16783–16789. https://doi.org/10.1074/jbc.M110.209569
Williams NH, Harrison JM, Read RW, Black RM (2007) Phosphylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch Toxicol 81:627–639. https://doi.org/10.1007/s00204-007-0191-8
Worek F, Eyer P, Szinicz L (1998) Inhibition, reactivation and aging kinetics of cyclohexylmethylphosphonofluoridate-inhibited human cholinesterases. Arch Toxicol 72:580–587. https://doi.org/10.1007/s002040050546
Worek F, Mast U, Kiderlen D et al (1999) Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta 288:73–90. https://doi.org/10.1016/S0009-8981(99)00144-8
Funding
Part of the work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, Grant/Award Number Research Training Group GRK 2338, P03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kranawetvogl, T., Siegert, M., Steinritz, D. et al. The phosphylated butyrylcholinesterase-derived tetrapeptide GlyGluSerAla proves exposure to organophosphorus agents with enantioselectivity. Arch Toxicol 98, 791–806 (2024). https://doi.org/10.1007/s00204-023-03657-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03657-3