Abstract
The organophosphorus nerve agents sarin, soman, cyclosarin and tabun phosphylate a tyrosine residue on albumin in human blood. These adducts may offer relatively long-lived biological markers of nerve agent exposure that do not ‘age’ rapidly, and which are not degraded by therapy with oximes. Sensitive methods for the detection of these adducts have been developed using liquid chromatography-tandem mass spectrometry. Adducts of all four nerve agents were detected in the blood of exposed guinea pigs being used in studies to improve medical countermeasures. The tyrosine adducts with soman and tabun were detected in guinea pigs receiving therapy 7 days following subcutaneous administration of five times the LD50 dose of the respective nerve agent. VX also forms a tyrosine adduct in human blood in vitro but only at high concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most chemical warfare (CW) agents are reactive electrophiles that are rapidly metabolised in vivo, and that form covalent adducts with nucleophilic sites on proteins and other macromolecules. These metabolites and adducts provide biological markers of exposure (hereafter referred to as biomarkers) (Black and Noort 2005; Noort et al. 2002; Noort and Black 2005). The analysis of biomedical samples for the presence of biomarkers may provide evidence of exposure in the event of a military or terrorist release of a CW agent. Biomedical sample analysis may also find application in diagnosis to ensure that appropriate medical countermeasures are administered, and in exposure monitoring, e.g. in individuals engaged in demilitarization activities. The ideal biomarker occurs in a readily accessible matrix, e.g. blood and urine, has a relatively long lifetime in the body, and provides unequivocal identification of the agent.
Organophosphorus (OP) nerve agents and the blister agent sulphur mustard are the CW agents of greatest concern. Tabun (GA) was used in the Iraq–Iran conflict in the 1980s (United Nations 1984). Sarin (GB) was used against Kurdish communities in Northern Iraq in the 1980s (Black et al. 1994) and by terrorists in Japan in the 1990s (e.g. Fidder et al. 2002; Nagao et al. 1997; Noort et al. 1998; Polhuijs et al. 1997). VX was used for an assassination in Japan (Tsuchihashi et al. 1998). Nerve agents are rapidly degraded on entering the body, and analysis for the presence of intact agents in blood is inappropriate unless samples are collected very soon after the exposure. More useful is the analysis of blood, and particularly urine, for alkyl methylphosphonic acids, the hydrolysis products of nerve agents (Riches et al. 2005; Black and Noort 2005; and references therein). These provide detectable biomarkers up to about 2 weeks following an exposure, depending on the dose absorbed and the sensitivity of the analytical method. Alternatively, covalent adducts with blood proteins may provide biomarkers with potential for retrospective identification up to several weeks post exposure, depending on the chemical or metabolic stability of the adduct, and the turnover of the protein (Noort et al. 2002; Noort and Black 2005).
The biochemical target for nerve agents is the enzyme acetylcholinesterase (AChE), which rapidly hydrolyses the neurotransmitter acetylcholine. Inhibition of AChE causes an excess of acetylcholine to remain within nerve synapses, leading to over-stimulation and eventual paralysis of the cholinergic nervous system. Traditionally the inhibition of AChE present in red blood cells, or the related enzyme butyrylcholinesterase (BuChE) (whose functional role remains unknown) present in serum and plasma, has been used to monitor nerve agent exposure using the Ellman colorimetric/photometric method (Ellman et al. 1961) or modifications thereof (Worek et al. 2004). This method is routinely used in occupational health monitoring, but detection of low-level exposure requires individual baseline measurements, and results are non-specific for the cause of cholinesterase inhibition.
Much more specific biomarkers of exposure to nerve agents (and OP pesticides) are the adducts formed by phosphylation of the active site serine residue in AChE and BuChE (Fidder et al. 2002; Nigg and Knaak 2000; Polhuijs et al. 1997). BuChE, which is much more abundant in blood than AChE, can be isolated from plasma, and adducts liberated as phosphylated nonapeptides encompassing the active site serine by digestion with pepsin (Fidder et al. 2002). Liquid chromatography–tandem mass spectrometry (LC-MS-MS) provides a versatile method for the detection and identification of these adducts. Alternatively, an experimentally less demanding method displaces the OP moiety as a fluoridate that can be extracted and detected using gas chromatography with mass spectrometric, flame photometric or nitrogen-phosphorus detection (Adams et al. 2004; Degenhardt et al. 2004; Jakubowski et al. 2001, 2004; Van Helden et al. 2003; Polhuijs et al. 1997). Fluoride reactivation is currently the most sensitive method for detecting cholinesterase (ChE) inhibition by some nerve agents, enabling detection at <1% inhibition of BuChE (Degenhardt et al. 2004).
Acetylcholinesterase or BuChE have one major disadvantage as biomarkers. With certain nerve agents (and pesticides) a dealkylation of the phosphylated serine occurs known as ‘ageing’. This results in a loss of structural information on the nerve agent concerned, and imparts a resistance to fluoride displacement of the OP moiety. Ageing occurs particularly rapidly with soman-inhibited ChE, and more slowly with cyclosarin, tabun and sarin. Another possible disadvantage of ChE as a biomarker is where the casualty has received therapy with oximes, which like fluoride also displace the OP moiety from the active site serine residue.
We previously reported the formation of an alternative protein biomarker for nerve agents (Black et al. 1999). Incubation of mass and radio-labelled sarin and soman with human plasma, and digestion with Pronase (which hydrolyses proteins to their constituent amino acids), liberated phosphonylated tyrosine residues (Fig. 1). As reported below, fractionation of blood proteins and digestion with trypsin indicated (but not unequivocally) that the adduct is with the tyrosine 411 residue on the abundant blood protein albumin. This is consistent with early studies, before the advent of modern mass spectrometry, which indicated that diisopropyl fluorophosphate (DFP) reacted with a tyrosine residue on albumin (Means and Wu 1979; Murachi 1963). More recent studies by Lockridge et al. (Peeples et al. 2005; Schopfer et al. 2005) have shown that FP-biotin, a biotinylated fluorophosphate, reacts with tyrosine on bovine and human serum albumin, and that a number of OP pesticides compete with FP-biotin for binding to this site. Our preliminary experiments indicated that the tyrosine adducts with sarin and soman do not age rapidly. This is supported by fluoride reactivation experiments, which have demonstrated unexpected regeneration of soman from human plasma proteins, and from soman treated albumin (Adams et al. 2004).
This present paper reports the formation of tyrosine adducts in vitro with an extended range of nerve agents. The development of sensitive LC-MS-MS methodology has enabled adducts to be detected in the blood of guinea pigs following exposure to sarin, soman, cyclosarin (GF) and tabun.
Experimental
Materials
Spectrometric grade trifluoroacetic acid (TFA) 99+%, HPLC grade acetonitrile, and protease (Pronase) from Streptomyces griseus, were purchased from Sigma-Aldrich (Gillingham, UK). Formic acid was obtained from Fluka (Gillingham, UK) and sodium hydroxide pellets from BDH (VWR, Poole, UK). BondElut C18 (500 mg, 3 ml) and Nexus (60 mg, 3 ml) solid phase extraction (SPE) cartridges were purchased from Varian (Oxford, UK), and Isolute C8 end-capped (500 mg, 3 ml) cartridges from Kinesis (Bolnhurst, Bedfordshire, UK). Water was purified using a Milli-Q system (Millipore). All other chemicals were purchased from Sigma-Aldrich.
Human blood samples were donated by one of the authors. Samples were collected in heparin tubes, centrifuged (4,500 rpm, 10 min) and the upper plasma layer removed and either used immediately or stored at −20°C until required. Blood samples from guinea pigs were collected up to 7 days following exposure to nerve agent as an adjunct to studies undertaken within the Biomedical Sciences Department, Dstl, aimed at developing improved medical countermeasures against nerve agent poisoning. This work was conducted under the conditions of a project licence issued under the Animals (Scientific Procedures) Act 1986. Sarin, soman, cyclosarin, tabun and VX, their corresponding tyrosine adducts, and [14/12CH3] (specific activity 185 MBq/mmol) and [CD3]-sarin, were synthesised by the Chemistry and Decontamination Group, Dstl. Standard solutions of nerve agents were made up in isopropanol (IPA).
Synthesis of O-(O-alkyl methylphosphonyl) tyrosine adducts
O-alkyl methylphosphonochloridates
O-ethyl and O-isopropyl methylphosphonochloridates were prepared from the corresponding O,O-dialkyl methylphosphonate and phosgene by standard methods (Bryant et al. 1960). O-Cyclohexyl and O-pinacolyl methylphosphonochloridates were prepared by the controlled addition of the lithium salt of the appropriate alcohol to methylphosphonic dichloride (Briseno-Roa et al. 2006).
N-carbobenzyloxy-L-tyrosine benzyl ester
N-carbobenzyloxy-L-tyrosine benzyl ester was prepared from N-carbobenzyloxy-L-tyrosine and benzyl bromide by the method of Kitas et al. (1990).
Protected phosphonylated tyrosine
O-(O-alkyl methylphosphonyl)tyrosine analogues (alkyl = ethyl, isopropyl, cyclohexyl and pinacolyl) were synthesised by condensation of the appropriate O-alkyl methylphosphonochloridate with the sodium salt of N-carbobenzyloxy-L-tyrosine benzyl ester in acetonitrile, with subsequent removal of the protecting groups by catalytic hydrogenation. A similar reaction with tabun itself was used for the tabun tyrosine adduct. The synthesis of O-(O-isopropyl methylphosphonyl)tyrosine is described as an example.
Synthesis of O-(O-isopropyl methylphosphonyl)tyrosine
A solution of N-carbobenzyloxy-L-tyrosine benzyl ester (0.85 g, 2.1 mmol) in acetonitrile (20 ml) was stirred and cooled in an ice-water bath whilst sodium hydride (60% suspension in oil, 100 mg, 2.5 mmol) was added. After an initially vigorous reaction, the mixture was allowed to warm to room temperature and stirred for 30 min. Isopropyl methylphosphonochloridate (400 mg, 2.5 mmol) was added by syringe and the mixture stirred for a further 30 min. Thin layer chromatography (silica/chloroform saturated with ammonia) demonstrated a single component. The reaction mixture was concentrated to half volume using a rotary evaporator, water (20 ml) added and the product extracted into chloroform (2 × 20 ml). The chloroform extract was washed with water (10 ml), dried with magnesium sulphate, concentrated and the residue chromatographed over silica gel using petroleum ether (b.pt. 60–80°C) (70% v/v)–acetone (30% v/v) as eluent. The product, N-carbobenzyloxy-O-(O-isopropyl methylphosphonyl)tyrosine benzyl ester, was isolated as a pair of diastereoisomers as a colourless syrup, yield 1.03 g.
The benzyl and N-carbobenzyloxy protecting groups were removed by adding a catalytic amount of 10% palladium on charcoal (30 mg) to N-carbobenzyloxy-O-(O-isopropyl methylphosphonyl)tyrosine benzyl ester (0.3 g, 0.57 mmol) in ethanol (20 ml) and stirring at room temperature overnight under an atmosphere of hydrogen. The reaction mixture was centrifuged and the supernatant concentrated to a colourless viscous oil (219 mg) containing the tyrosine adduct with purity >95% by NMR. Further purification was undertaken by preparative reversed-phase C18 HPLC eluting with a 0.1%TFA/acetonitrile/water gradient.
Isolation and identification of a sarin-tripeptide adduct
Incubation of [14/12CH3]/[CD3]-sarin with human plasma
A stock solution of [14/12CH3]/[CD3]-sarin was prepared in IPA (12.5 mg/ml) with a [CD3]-sarin to [14/12CH3]-sarin ratio of 1:1. An aliquot of this solution (200 μl) was added to fresh human plasma (10 ml) to give a nominal sarin concentration of 250 μg/ml. The incubate was stirred at room temperature for 5 h and then kept overnight at room temperature. Analysis of the incubate by reverse phase HPLC (radiochemical detector) showed a poorly retained major component with retention time corresponding to isopropyl methylphosphonic acid, plus two minor components with longer retention times. The incubate was acidified to pH 2.5 with 10% TFA in water, centrifuged and filtered prior to clean-up in several aliquots by preparative reverse phase HPLC. The fractions were assayed for radioactivity and appropriate fractions combined and retained for digestion with trypsin.
Trypsin (8 mg) was added to the combined fractions in water (15 ml) at ambient temperature, and the solution adjusted to pH 9 with 1 M aqueous sodium hydroxide. Incubation was continued for 24 h, maintaining the pH between 7 and 9 with further additions of sodium hydroxide. Additional trypsin (8 mg) was added after 18 h. The course of the hydrolysis was monitored by HPLC and the major radioactive component isolated by repetitive preparative HPLC. The purified fractions were freeze dried prior to reconstitution in Milli-Q water (200 μl) for LC-MS analysis.
LC-MS
Capillary LC was performed using a Haisil 300 C18 column, 100 mm × 300 μm i.d., 5 μm particle size (Presearch, Hitchin, UK). Mobile phase was delivered by an ABI 140B dual syringe pump operating at 150 μl/min, with the flow split 50:1 by an LC Packings Accurate AC30 mixer/flow–splitter (Presearch) to give a flow through the column of 3 μl/min. The mobile phase consisted of A: 0.05% TFA in water and B: 0.05% TFA in acetonitrile. Elution was performed with a gradient of 5% B (0–2 min) to 90% B (20–25 min). Injections (1 μl) were made with a Valco C14W injector fitted with a 1 μl internal loop.
The LC system was interfaced to a Finnigan TSQ700 triple quadrupole mass spectrometer operating in positive electrospray ionization (ESI) mode. ESI conditions were as follows: spray 5 kV, heated capillary 200°C, sheath gas (nitrogen) 60 psi, auxiliary gas (nitrogen) 20 (arbitrary units). The source octapole (Q0) offset was set at −5 V. Scan range was m/z 100 to m/z 600 in 1 s. Tuning (tube lens and capillary offsets) was optimized on m/z 524 [MH+] from a solution of 1 μg/ml MRFA (MetArgPheAlaOH) in acetonitrile/water 50:50 + 0.05% TFA infused into the source at a flow rate of 3 μl/min.
Isolation and identification of tyrosine adducts
In vitro incubations with human plasma
Method development and positive control samples were prepared by incubation of agent with human plasma. Fresh human plasma (1–5 ml) was incubated with nerve agent (in IPA) at 37°C for 24–48 h. Samples were analysed for residual agent by LC-MS. Samples with residual agent were dialysed for 16 h (overnight) against 50 mM ammonium bicarbonate using a 10 kDa MWCO Slide-a-Lyzer dialysis cassette (Perbio Science UK Ltd, Northumberland, UK) prior to enzymatic digestion. Plasma BuChE inhibition measurements were performed using the Ellman method (Ellman et al. 1961).
Enzymatic digestion of plasma samples and SPE
For enzymatic digestion, plasma (≤1 ml) was diluted with four times its volume of water and adjusted to pH 9 with 2% w/v aqueous sodium hydroxide. The resultant solution was treated with Pronase (15 mg per ml of plasma) and maintained at pH 9 for approximately 4 h. Plasma digest solutions were centrifuged and subjected to SPE as summarized in Table 1. SPE was optimised using plasma samples (1 ml) spiked with various concentrations of synthetic tyrosine adduct in water (10–50 μl). Once eluted, samples were concentrated to dryness at 45°C using a centrifugal evaporator (Speedvac SPD121P, Thermo Savant, USA), and the residues dissolved in Milli-Q water (50 μl) prior to LC-MS/MS analysis.
Samples from guinea pigs
Blood samples were collected from guinea pigs that were being used in studies directed towards improving medical countermeasures against nerve agent poisoning. The nerve agent had been administered either by subcutaneous injection or percutaneously to the close-clipped shoulder of the animals; blood samples were withdrawn up to 7 days post exposure. Control samples were collected from non-exposed animals. Doses of nerve agents ranged from 0.5 to 5 × LD50. LD50 doses relevant to this study are sarin 49 μg/kg, soman 27 μg/kg and tabun 125 μg/kg (subcutaneous), and cyclosarin 3 mg/kg (percutaneous). In most cases guinea pigs had received pre-treatment with pyridostigmine or physostigmine/hyoscine, plus therapy immediately after exposure with oxime (P2S, HI-6 or toxogonin), anticholinergic (atropine or hyoscine) and anticonvulsant (avizafone or midazolam). With some exposures to sarin and soman, guinea pigs received no medical countermeasures. The relevant in-vivo sample history is given in the respective figure captions contained within the “Results and discussion” section and in Table 2.
LC-MS
A Finnigan Surveyor LC was fitted with a Jupiter (Phenomenex, Macclesfield, UK) C18 column, 250 × 2 mm i.d., particle size 5 μm and a C18 wide pore guard cartridge. A linear gradient elution was performed at a flow rate of 0.2 ml/min, with a mobile phase of 0.05% formic acid in water (solvent A) and 0.05% formic acid in acetonitrile (solvent B). The gradient employed was 5% B (0–5 min) to 90% B (15–20 min). Injection volumes were 10 or 20 μl. The retention times of the tyrosine adducts were: sarin 15.1 min, soman 17.6 min, cyclosarin 14.9 min, tabun 13.4 min and VX 14.3 min.
MS analysis was performed using a Finnigan TSQ Quantum mass spectrometer fitted with an ESI source. The instrument was operated in positive ion mode with the spray voltage set at 3.5 kV, capillary temperature at 350°C and the source CID at 15 V. The sheath gas (nitrogen) was maintained at 30 (arbitrary units) and the auxiliary gas at 10 (arbitrary units). The capillary and tube lens offset voltages were optimised for each agent-tyrosine adduct by infusion of a synthetic standard. LC-MS analysis of residual agent in plasma samples was performed in selected ion monitoring (SIM) mode, monitoring the ions: sarin m/z 141 [MH+], m/z 99 [M-C3H6]+; soman m/z 183 [MH]+; tabun m/z 163 [MH+], m/z 135 [M-HCN]+; cyclosarin m/z 181 [MH+], m/z 99 [M-C6H10]+; VX m/z 268 [MH+]. Full scan mass spectra of synthetic tyrosine adducts were acquired over the scan range m/z 50–450. Full scan product ion spectra of protonated molecules, MH+, were obtained with a Q2 collision energy of 25 eV and collision gas (argon) pressure of 1.5 mTorr. Multiple reaction monitoring (MRM) was optimised for each agent-tyrosine adduct, selecting two fragmentations of the MH+ ion with a Q2 collision gas pressure of 0.8 mTorr and scan time 0.2 s. Fragmentations used for MRM of tyrosine adducts were: sarin m/z 302 → m/z 260 and m/z 214; soman m/z 344 → m/z 260 and m/z 214; tabun m/z 317 → m/z 243 and m/z 198; cyclosarin m/z 342 → m/z 214 and m/z 197.
Results and discussion
Identification of tyrosine adducts in vitro
Our previous identification of sarin and soman-tyrosine adducts (Black et al. 1999) has been extended to demonstrate the formation of similar adducts with tabun, cyclosarin and VX in human plasma. Initial identification was undertaken by full scan ESI mass spectrometry following isolation of adducts from incubations of high concentrations of agent in plasma (approximately 100 μg agent/ml plasma). The ESI LC-MS and LC-MS-MS spectra of the isolated adducts are summarised in Table 3. Representative spectra are shown for the adducts with cyclosarin and tabun in Figs. 2 and 3 respectively. The LC-MS spectra are dominated by the protonated molecules MH+, with water adducts [M + H3O]+ and varying degrees of fragmentation due to in-source CID. The soman, sarin and cyclosarin tyrosine adducts each show a loss of the O-alkyl substituent (isopropyl, pinacolyl and cyclohexyl respectively) to give common fragment ions at m/z 260, with further loss of HCO2H (from the tyrosine moiety) to give m/z 214. In contrast, LC-MS spectra of the tabun and VX adducts, showed minimal fragmentation under similar conditions. The LC-MS-MS product ion spectra from CID of the protonated molecules for sarin, soman and cyclosarin showed common product ions, the most abundant, m/z 214, being from loss of alkene + HCO2H. Additional weak fragment ions were observed at m/z 197, from further loss of 17 amu (NH3), m/z 136 (loss of MeP(O)OR + HCO2H) and m/z 118.
The product ion spectra derived from the protonated molecules of tabun and VX tyrosine adducts showed additional fragment ions, reflecting losses of HCO2H, e.g. m/z 271 (tabun) and m/z 242 (VX) [MH–HCO2H]+. The tabun adduct showed an ion at m/z 198 corresponding to loss of Me2NH from m/z 243.
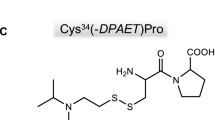
Tyrosine adducts were observed with sarin, soman, cyclosarin and tabun following in-vitro incubation with human plasma; a less abundant adduct with VX was also detected. Assuming that the reaction with tyrosine is purely chemical (i.e. no catalysis occurs), the lower abundance of the VX adduct is consistent with the relative reactivities of the nerve agents with nucleophiles. The SCH2CH2N(iPr)2 substituent in VX is a much weaker leaving group than F− or CN− in a non-catalysed reaction.
Identification of a phosphonylated tripeptide
The chromatogram obtained from a tryptic digest of the albumin fraction from plasma incubated with a 50:50 mixture of [14CH3]/[CD3]-sarin contained a single significant peak with a retention time of approximately 14.5 min. The ESI spectrum showed prominent H3/D3 doublets at m/z 531/534, m/z 489/492 and m/z 214/217. Less intense non-labelled ions were present at m/z 130 and m/z 248. Product ion spectra were obtained for the MH+ ions m/z 531 and m/z 534 (collision energy −25 eV, 3 mTorr argon). The major product ions from m/z 531 were: m/z 129, m/z 147 (Lys), m/z 248 (Thr-Lys), m/z 214 [MeP(O)OiPr-Ph]+, m/z 256 [MeP(O)(OiPr)-Tyr]+ and m/z 489 [MH-C3H6]+. The corresponding product ions from the D3 labelled m/z 534 MH+ ion were m/z 129, 147, 248, 217, 259 and 492. These product ions were consistent with the expected Y and A series product ions from MeP(O)(OiPr)-Tyr-Thr-LysOH (Table 4) and with the fragmentation of the isopropyl methylphosphonyl moiety.
The association of the adduct with the albumin fraction of blood, plus the identification of the phosphonylated Tyr-Thr-LysOH tripeptide, was consistent with the site of interaction being tyrosine 411 on human albumin. The tyrosine adducts were identified in albumin fractions isolated either by precipitation (Bechtold et al. 1992) or by using a ligand based purification column (Noort et al. 2004) from in-vitro exposed plasma. However, this tripeptide sequence is ubiquitous in proteins and the identification of albumin as the source was regarded as tentative. Lockeridge et al. (Peeples et al. 2005; Schopfer et al. 2005) have since confirmed that FP-biotin phosphonylates the analogous tyrosine 410 residue in bovine serum albumin.
Analytical methods
Sensitive analytical methods were developed for the detection of sarin, soman, cyclosarin and tabun tyrosine adducts in human plasma, based on Pronase digestion, concentration of the adducts by SPE, and analysis by LC-MS-MS. A range of SPE cartridges were investigated for recovery, including Isolute C18 (end-capped), C8, C8 (end capped), phenyl, phenyl (end-capped), Oasis HLB, BondElut C18 and Nexus. The best recoveries were obtained with C18 for the sarin and tabun adducts, and with C8 (end-capped) for the more hydrophobic soman and cyclosarin.
One or two product ions were selected and optimized for MRM, as shown in Table 1. The detection limits for pure standards on the Finnigan TSQ Quantum LC-MS/MS system were 0.2–0.5 pg on-column, and calibration graphs were linear over several orders (e.g. sarin: 0.2–100 pg, y = 116,406x + 2,536, R 2 = 0.9997; soman: 0.5–250 pg, y = 2,431x − 21, R 2 = 0.9998; cyclosarin: 0.5–10 pg, y = 79,588x − 1142, R 2 = 0.9999 and tabun: 0.2–100 pg, y = 179,706x + 2,872, R 2 = 0.9995).
Application to in vitro incubations
The potential sensitivity of the adducts as biomarkers was assessed in-vitro by determining the adducts in plasma after incubation with agent to produce a range of BuChE inhibition levels. Human plasma incubated with agent concentrations from 1–25 ng/ml, provided samples with BuChE inhibition from 10 to 100%. The tyrosine adduct was detected at 10–20% plasma BuChE inhibition for tabun (5 ng agent/ml plasma), soman (1 ng agent/ml plasma) and cyclosarin (2 ng agent/ml plasma). For sarin the detection limit was higher, ≥70% BuChE inhibition (10 ng/ml plasma). It has been reported that symptoms following an exposure to sarin typically appear at ∼85% BuChE depression (Nigg and Knaak 2000).
At low concentrations it is expected that nerve agent interactions would be dominated by catalytic reactions with the enzymes BuChE and AChE. As the concentration of agent in plasma is raised, reactions with abundant non-catalytic proteins, such as albumin, are likely to increase. The results suggest that tyrosine adducts should be viable biomarkers in human blood at exposure levels that produce relatively mild symptoms, and that at exposures producing near 100% inhibition albumin adducts should be relatively abundant.
Application to exposed animals
A small number of blood samples were collected as an adjunct to guinea pig studies whose primary purpose was aimed at improving medical countermeasures to prevent or mitigate the effects of nerve agent poisoning. Analysis of these samples demonstrated the detection of albumin adducts after exposure to sub-lethal doses of sarin and soman, and lethal doses (in the absence of therapy) of cyclosarin and tabun; all control samples were negative. LC-MS/MS MRM chromatograms for a guinea pig administered 0.5 × LD50 sarin subcutaneously are shown in Fig. 4. Samples from three guinea pigs taken at 24 h post-exposure all gave a signal to noise ratio (S/N) > 3 for both transitions monitored, with retention times matching that of an authentic synthetic sample of the adduct. Figure 5 shows the detection of soman in guinea pig plasma taken 24 h after a similar dose; samples from two guinea pigs gave a signal (S/N > 3) for the soman-tyrosine adduct.
Electrospray LC-MS-MS chromatograms for the transition m/z 302 → m/z 260 showing the detection of sarin-Tyr in a 0.5 ml sample of plasma from a guinea pig exposed to 0.5 × LD50 of sarin (subcutaneous). Sample taken at 24 h post-exposure. Top: plasma extract from exposed guinea pig showing presence of sarin-Tyr at retention time 15.09 min. Concentration in extract estimated at 0.6 nM. Middle: 0.2 pg (injected) synthetic standard of sarin-Tyr (retention time 15.10 min.). Bottom: control guinea pig plasma
Electrospray LC-MS-MS chromatograms for the transition m/z 344 → m/z 260 showing the detection of soman-Tyr in a 0.5 ml sample of plasma from a guinea pig exposed to 0.5 × LD50 of soman (subcutaneous). Sample taken at 24 h post-exposure. Top: plasma extract from exposed guinea pig showing presence of soman-Tyr at retention time 17.65 min. Concentration in extract estimated at 0.2 nM. Middle: 0.5 pg (injected) synthetic standard of soman-Tyr (retention time 17.63 min.). Bottom: control guinea pig plasma
Figure 6 shows chromatograms derived from a guinea pig exposed percutaneously to 2 × LD50 cyclosarin, the sample being taken at 45 min. LC-MS/MS MRM analysis provided S/N > 3 for both transitions monitored for the tyrosine adduct and the retention time matched that of a synthetic standard.
Electrospray LC-MS-MS chromatograms for the transition m/z 342 → m/z 214 showing the detection of cyclosarin-Tyr in a 0.5 ml sample of plasma from a guinea pig exposed to 2 × LD50 of cyclosarin (percutaneous). Sample taken at 45 min post-exposure. Top: plasma extract from exposed guinea pig showing presence of cyclosarin-Tyr at retention time 14.92 min. Concentration in extract estimated at 6 nM. Middle: 1 pg (injected) synthetic standard of cyclosarin-Tyr (retention time 14.90 min.). Bottom: control guinea pig plasma
Analysis of samples from a guinea pig exposed subcutaneously to 5 × LD50 tabun successfully detected the tabun tyrosine adduct 7 days post exposure, as shown in Fig. 7. LC-MS/MS analysis provided a S/N > 3 for both transitions monitored for the tabun adduct and the retention time matched that of a synthetic standard.
Electrospray LC-MS-MS chromatograms for the transition m/z 317 → m/z 243 showing the detection of tabun-Tyr in a 0.5 ml sample of plasma from a guinea pig exposed to 5 × LD50 of tabun (subcutaneous) and receiving pre-treatment and therapy. Sample taken at 7 days post-exposure. Top: plasma extract from exposed guinea pig showing presence of tabun-Tyr at retention time 13.43 min. Concentration in extract estimated at 28 nM. Middle: 1 pg (injected) synthetic standard of tabun-Tyr (retention time 13.42 min.). Bottom: control guinea pig plasma
Oxime treatment and biomarker lifetime
Phosphylated acetyl- and butyrylcholinesterase may undergo a spontaneous enzyme-mediated dealkylation reaction called ageing, in which the O-alkyl group on phosphorus is cleaved. This results in a loss of structural information on the agent and renders the enzyme resistant to regeneration using fluoride ion or oximes. The rate of ageing is dependent on the phosphorus alkyl group and varies for each agent. For example soman ages extremely rapidly with loss of the pinacolyl moiety, with a reported half-life of 2–3 min (Worek et al. 2004). This leaves a methylphosphonyl group attached to the active-site serine, which could be derived from a number of nerve agents or possibly a fire retardant precursor. The tyrosine-adducts, as evidenced from in vitro studies and by their detection in guinea pigs up to at least a week following exposure to a nerve agent, do not appear to undergo rapid dealkylation. Tabun and soman tyrosine adducts were detected in guinea pigs (7 and 8 animals respectively) up to 7 days post-exposure, and up to 24 h post exposure for sarin (3 animals) (Table 2). Owing to the experimental design of the guinea pig studies samples collected 7 days post-exposure to sarin were not available. No samples were collected beyond 7 days.
An important component of medical countermeasures against nerve agents is the use of oximes to reactivate inhibited AChE. Reactivation, which is agent and oxime dependent, displaces the OP moiety from the active-site serine, and may render AChE or BuChE ineffective as forensic biomarkers, depending on the extent of reactivation. The soman-tyrosine adduct was detected in a total of six guinea pigs that had been given various levels of pre-treatment and therapy, including oximes (Fig. 8), whilst tabun-tyrosine was detected in a total of seven guinea pigs that had undergone exposure and treatment. Thus the tyrosine biomarker does not appear to be grossly affected by oxime therapy.
Electrospray LC-MS-MS chromatograms for the summed transitions m/z 344 → m/z 260 and m/z 344 → 214 showing the detection of soman-Tyr in 0.5 ml samples of plasma from guinea pigs exposed to soman (subcutaneous) and receiving pre-treatment and/or therapy. Top: plasma extract from guinea pig exposed to 2 × LD50 and receiving oxime therapy, showing presence of soman-Tyr at retention time 17.40 min. Sample taken at 24 h post-exposure. Concentration in extract estimated at 0.4 nM. Middle: plasma extract from guinea pig exposed to 5 × LD50 and receiving pre-treatment and oxime therapy, showing presence of soman-Tyr at retention time 17.36 min. Sample taken at 7 days post-exposure. Concentration in extract estimated at 0.9 nM. Bottom: plasma extract from control guinea pig receiving pre-treatment and therapy
Summary
The nerve agents sarin, soman, cyclosarin and tabun have been shown to form adducts with a tyrosine residue on albumin when incubated with human plasma in-vitro. VX forms a similar adduct but only at high concentrations. Sensitive analytical methods based on SPE and LC-MS-MS were developed for the detection of tyrosine adducts. These methods could detect adducts with soman, cyclosarin and tabun at BuChE inhibition levels of 10–20% in vitro, and at inhibition levels of 70% for sarin.
Adducts with sarin and soman were detected in guinea pigs exposed to sub-lethal doses (0.5 × LD50 subcutaneously) of agent, and those with cyclosarin and tabun were detected in guinea pigs exposed to doses of twice the LD50. Estimation of the lowest doses that can be detected following in vivo exposures would require further animal studies. The tyrosine adducts have shown no signs of rapid ageing. The sarin adduct was detected in samples collected up to 24 h post exposure and soman and tabun adducts in samples collected up to 7 days post exposure. Soman and tabun adducts were also detected in guinea pigs that received therapy with oximes, demonstrating that tyrosine adducts should remain viable biomarkers in samples from casualties that have received medical treatment. The opportunities to undertake assessments ex vivo were constrained by the experimental design of the guinea pig studies, which had a different primary purpose, the outcome of which will be published elsewhere.
References
Adams TK, Capacio BR, Smith JR, Whalley CED, Korte WD (2004) The application of the fluoride reactivation process to the detection of sarin and soman nerve agent exposures in biological samples. Drug Chem Toxicol 27:77–91
Bechtold WE, Willis JK, Sun JD, Griffith WC, Reddy TV (1992) Biological markers of exposure to benzene: S-phenylcysteine in albumin. Carcinogenesis 13:1217–1220
Black RM, Noort D (2005) Methods for the retrospective detection of exposure toxic scheduled chemicals. Part A: analysis of free metabolites. In: Mesilaakso M (ed) Chemical weapons convention chemicals analysis (Sample collection, preparation and analytical methods). Wiley, Chichester, pp 403–431
Black RM, Clarke RJ, Read RW, Reid MTJ (1994) Application of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry to the analysis of chemical warfare samples, found to contain residues of the nerve agent sarin, sulphur mustard and their degradation products. J Chromatogr A 662:301–321
Black RM, Harrison JM, Read RW (1999) The interaction of sarin and soman with plasma proteins: the identification of a novel phosphonylation site. Arch Toxicol 73:123–126
Briseno-Roa L, Hill J, Notman S, Sellars D, Smith AP, Timperley CM, Wetherell J, Williams NH, Williams GR, Fersht AR, Griffiths AD (2006) Analogues with fluorescent leaving groups for screening and selection of enzymes that efficiently hydrolyze organophosphorus nerve agents. J Med Chem 49:246–255
Bryant PJR, Ford-Moore AH, Perry BJ, Wardrop AWH, Watkins TF (1960) The preparation and physical properties of isopropyl methylphosphonofluoridate (sarin). J Chem Soc 1553–1555
Degenhardt CEAM, Pleijsier K, Van der Schans MJ, Langenberg JP, Preston KE, Solano MI, Maggio VL, Barr JR (2004) Improvements of the fluoride reactivation method for the verification of nerve agent exposure. J Anal Toxicol 28:364–371
Ellman GL, Courtney KD, Anders V, Featherstone RM (1961) A new and rapid colorometric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fidder A, Noort D, Hulst AG, De Ruiter R, Van der Schans MJ, Benschop HP, Langenberg JP (2002) Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol 15:582–590
Jakubowski EM, Heykamp LS, Durst HD, Thomson SA (2001) Preliminary studies in the formation of ethyl methylphosphonofluoridate from rat and human serum exposed to VX and treated with fluoride ion. Anal Lett 34:727–737
Jakubowski EM, McGuire JM, Evans RA, Edwards JL, Hulet SW, Benton BJ, Forster JS, Burnett DC, Muse WT, Matson K, Crouse CL, Mioduszewski RJ, Thomson SA (2004) Quantitation of fluoride ion released sarin in red blood cell samples by gas chromatography-chemical ionization mass spectrometry using isotope dilution and large-volume injection. J Anal Toxicol 28:357–363
Kitas EA, Perich JW, Tregear GW, Johns RB (1990) Synthesis of phosphotyrosine-containing peptides. 3. Synthesis of H-Pro-Tyr(P)-Val-OH via dimethylphosphate protection and the use of improved deprotection procedures. J Org Chem 55:4181–4187
Means GE, Wu HL (1979) The reactive tyrosine residue of human serum albumin: characterization of its reaction with diisopropylfluorophosphate. Arch Biochem Biophys 194:526–530
Murachi T (1963) A general reaction of diisopropylphosphofluoridate with proteins without direct effect on enzymic activities. Biochim Biophys Acta 71:239–241
Nagao M, Takatori T, Matsuda Y, Nakajima M, Iwase H, Iwadate K (1997) Definitive evidence for the acute sarin poisoning diagnosis in the Tokyo subway. Toxicol Appl Pharmacol 144:198–203
Nigg HN, Knaak JB (2000) Blood cholinesterases as biomarkers of organophosphorus pesticide exposure. Rev Environ Contam Toxicol 163:29–112
Noort D, Black RM (2005) Methods for the retrospective detection of exposure toxic scheduled chemicals. Part B: mass spectrometric and immunochemical analysis of covalent adducts to proteins and DNA. In: Mesilaakso M (ed) Chemical weapons convention chemicals analysis (sample collection, preparation and analytical methods). Wiley, Chichester, pp 433–451
Noort D, Hulst AG, Platenburg DHJM, Polhuijs M, Benschop HP (1998) Quantitative analysis of O-isopropyl methylphosphonic acid in serum samples of Japanese citizens allegedly exposed to sarin: estimation of internal dosage. Arch Toxicol 72:671–675
Noort D, Benschop HP, Black RM (2002) Biomonitoring of exposure to chemical warfare agents: a review. Toxicol Appl Pharmacol 184:116–126
Noort D, Fidder A, Hulst AG, Woolfit AR, Ash D, Barr JR (2004) Retrospective detection of exposure to sulfur mustard: improvements on an assay for liquid chromatography-tandem mass spectrometry analysis of albumin/sulfur mustard adducts. J Anal Toxicol 28:333–338
Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O (2005) Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci 83:303–312
Polhuijs M, Langenberg JP, Benschop HP (1997) New method for retrospective detection of exposure to organophosphorus anticholinesterases: application to alleged victims of Japanese terrorists. Toxicol Appl Pharmacol 146:156–161
Riches J, Morton I, Read RW, Black RM (2005) The trace analysis of alkyl alkylphosphonic acids in urine using gas chromatography-ion trap negative ion tandem mass spectrometry. J Chromatogr B 816:251–258
Schopfer LM, Champion MC, Tamblyn N, Thompson CM, Lockridge O (2005) Characteristic mass spectral fragments of the organophosphorus agent FP-biotin and FP-biotinylated peptides from trypsin and bovine albumin (Tyr410). Anal Biochem 345:122–132
Tsuchihashi H, Katagi M, Nishikawa M, Tatsuno M (1998) Identification of metabolites of nerve agent VX in serum collected from a victim. J Anal Toxicol 22:383–388
United Nations (1984) Report of the specialists appointed by the Secretary-General to investigate allegations by the Islamic Republic of Iran concerning the use of chemical weapons. Report number S/16433
Van Helden HPM, Trap HC, Oostdijk JP, Kuijpers WC, Langenberg JP, Benschop HP (2003) Long-term, low-level exposure of guinea pigs and marmosets to sarin vapor in air: lowest observable effect level. Toxicol Appl Pharmacol 189:170–179
Worek F, Thiermann H, Szinicz L, Eyer P (2004) Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 68:2237–2248
Acknowledgments
Biomedical Sciences Department, Dstl, Porton Down for guinea pig samples and to N. Bailey and E. Gosden, Dstl, Porton Down for cholinesterase determinations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, N.H., Harrison, J.M., Read, R.W. et al. Phosphylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch Toxicol 81, 627–639 (2007). https://doi.org/10.1007/s00204-007-0191-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0191-8