Abstract
Tetrabromobisphenol A-bis(2,3-dibromopropyl ether) (TBBPA-BDBPE), a commonly used brominated flame retardant as a decabromodiphenyl ether substitute, has been detected in various environmental compartments, but its health hazards remain largely unknown. Our recent study showed that low-dose exposure of male mice to TBBPA-BDBPE from postnatal day (PND) 0 to 56 caused remarkable damage to the microtubule skeleton in Sertoli cells and the blood-testis barrier (BTB) but exerted little effect on conventional reproductive endpoints in adulthood. To investigate whether TBBPA-BDBPE may cause severe reproductive impairments at late reproductive age, here, we extended exposure of historically administrated male mice to 8-month age and allowed them to mate with non-treated females for the evaluation of fertility, followed by a general examination for the reproductive system. As expected, we found that 8-month exposure to 50 μg/kg/d as well as 1000 μg/kg/d TBBPA-BDBPE caused severe damage to the reproductive system, including reduced sperm counts, increased sperm abnormality, histological alterations of testes. Moreover, microtubule damage and BTB-related impairment were still observed following 8-month exposure. Noticeably, high-dose TBBPA-BDBPE-treated mice had fewer offspring with a female-biased sex ratio. All results show that long-term exposure to TBBPA-BDBPE caused severe reproductive impairment, including poor fertility at late reproductive age. It is therefore concluded that slight testicular injuries in early life can contribute to reproductive impairment at late reproductive age, highlighting that alterations in certain non-conventional endpoints should be noticed as well as conventional endpoints in future reproductive toxicity studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetrabromobisphenol A bis(2,3-dibromopropyl) ether (TBBPA-BDBPE), a TBBPA derivative widely used as an additive flame retardant in a variety of products, is an emerging pollutant that has been detected in various environmental compartments (Ali et al. 2011; Nyholm et al. 2013; Shi et al. 2009; Tao et al. 2016; Qu et al. 2013). In particular, TBBPA-BDBPE is frequently detected in indoor dust (Zuiderveen et al. 2020), with up to the level of 49,000 ng/g, as reported by Tao et al. (2016). Moreover, TBBPA-BDBPE is predicted to be persistent and bio-accumulative in humans and wildlife, similar to the properties of other banned brominated flame retardants such as decabromodiphenyl ether and hexabromocyclododecane (EPA 2014). Nevertheless, information on the toxicity of TBBPA-BDBPE is limited to date (Ren et al. 2020; Yao et al. 2021).
Recently, we found that postnatal exposure to 1000 μg/kg/d TBBPA-BDBPE disrupted testis development in mice and thereby caused declined sperm count and testicular alterations in young adulthood, including a reduced percentage of stage VIII seminiferous tubules, shrunk seminiferous tubules with decreased spermatogonia number, along with microtubule damage in Sertoli cells (Li et al. 2023). Strikingly, exposure to TBBPA-BDBPE as low as 50 μg/kg/d also led to microtubule damage and blood-testis barrier (BTB) impairment in mice, despite no alterations in conventional reproductive endpoints. Further molecular docking analysis, tubulin polymerization assay, and in vitro cell assay indicate that TBBPA-BDBPE could interact with tubulin and disrupt its polymerization, which may be partly responsible for testicular impairment (Li et al. 2023). Given the crucial functions of the microtubule cytoskeleton in Sertoli Cells and the BTB in spermatogenesis (Gerber et al. 2016; O’Donnell and O’Bryan 2014; Wang et al. 2020), we speculated that extended exposure to low-dose TBBPA-BDBPE may cause severe testicular impairment and even low fertility, especially at the late productive age. Indeed, aging is a risk factor for reproductive health, and delayed parenthood is associated with reproductive dysfunction and adverse health consequences in offspring (Albani et al. 2019; Balasch and Gratacós, 2012; Dong et al. 2022; Tarín et al. 1998). Thus, it is likely that a chemical may lead to more severe reproductive impairment in the late reproductive age than in vigorous reproductive age. This is an issue worthy of investigation because parenthood delay is becoming a more and more common phenomenon worldwide (Safdari‑Dehcheshmeh et al. 2023).
Thus, we performed a follow-up study of our recent study (Li et al. 2023) to investigate whether TBBPA-BDBPE may cause severe reproductive impairments in mammals at late reproductive age. Briefly, historically administrated male mice were continuously administrated with TBBPA-BDBPE until 8-month age and then allowed to mate with non-treated females for the fertility evaluation, followed by a general examination of the reproductive system. Overt testicular dysfunction and reduced fertility are anticipated. Our findings are expected to improve the understanding of male reproductive toxicity of low-dose TBBPA-BDBPE and to highlight the indicative significance of changes in non-traditional endpoints for reproductive impairment at late reproductive age.
Materials and methods
Animals
Pregnant ICR (CD-1) mice and adult females were purchased from the Vital River Laboratories (Beijing, China) and housed as described in our previous study (Li et al. 2022). Briefly, dams were kept individually in an animal room with relatively stable conditions, including a 12-h light: dark cycle, controlled temperature (23 ± 1 °C), and 40–50% humidity. Food and water were available ad libitum. All experimental animal protocols were approved by the Animal Ethics Committee at the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (AEWC-RCEES-2022019).
Chemical treatments
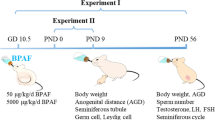
As a follow-up study, chemical treatments of animals were described in our recent study (Li et al. 2023). Briefly, fifteen dams (each with ten pups) after delivery were randomly assigned into three groups (n = 5) and were given TBBPA-BDBPE of 0 (as control), 150 or 3000 ng/mL TBBPA-BDBPE (CAS number 21850–44-2; > 98%; MREDA, China) in drinking water. On postnatal day (PND) 21, pups were weaned, and dams were sacrificed. Meanwhile, one male pup from each litter per group was randomly chosen into one cage (five mice per cage) for continuous exposure through drinking water, with three cages for each group. On PND 56, two cages of mice were sacrificed for systemic reproductive examination, in which no significant changes in testis weight, serum testosterone, and testicular histology were found (Li et al. 2023), and the remaining cage (5 mice) was continuously exposed until 8-month age when the fertility of male mice began to decline.
During the exposure, the drinking water was renewed, and its consumption was measured every other day. Body weight was measured twice per week. The actual TBBPA-BDBPE concentrations in drinking water within a renewal cycle were confirmed to be equivalent to the nominal concentrations. Based on the daily water consumption and body weight, the average daily intakes of TBBPA-BDBPE for dams and weaned males were estimated to be approximately 50, and 1000 μg/kg/d in two dose groups. The dose of 50 μg/kg for mice could be translated into 3.5 μg/kg for humans according to a guide for dose conversion between animals and humans (Nair and Jacob 2016), which is relevant to occupational population exposure to some brominated flame retardants (Malliari and Kalantzi 2017), and 1000 μg/kg/d is the tolerable daily intake (TDI) for the structural analog of TBBPA-BDBPE, TBBPA, recommended by the EU (EFSA 2011).
Mating test
After 8-month exposure, a mating test was conducted. Each male was allowed to mate with two non-treated female ICR mice (29.0 g ± 1.0 g) in estrus for seven consecutive days. Pregnancy was confirmed by the presence of a vaginal plug, and then pregnant mice were housed one per cage. After delivery, the litter size and survival rate of offspring were examined, and then on PND 7 pups were examined for body weight, sex, and gross anomalies. The uterus of each dam was also examined to confirm the number of implantation sites.
Sample collection
After mating, all male mice were sacrificed to evaluate the effects of long-term exposure to TBBPA-BDBPE on the reproductive system. Specifically, testes were collected for RNA extraction, histological examination, and immunofluorescence (IF) staining. For each individual, one epididymis was collected for histological examination, with the other for sperm counting, parameter analysis, and sperm DNA extraction.
Histological examination
Testes and epididymis were fixed in Bouin’s fixative for 48 h, dehydrated through an ethanol series, and routinely embedded in paraffin. The middle piece of the testis along the longitudinal axis was sectioned crossly at 5 μm and then stained with periodic Acid-Schiff (PAS) or hematoxylin and eosin (H&E). Finally, sections were examined for histological changes under an Axioskop microscope (Carl Zeiss, Germany). The entire testis sections were scanned by whole-slide digitalization (Pannoramic MIDI II, 3DHISTECH, Budapest, Hungary) and were analyzed in a blind assay.
Assessment of histological parameters
Histological evaluation was randomly selected from three non-serial cross sections per sample with a distance of a minimum of 50 µm among the sections. Staging of the spermatogenic cycle was conducted following the method reported by Ahmed and de Rooij (2009), and the percentage of seminiferous tubules at stage VIII was calculated. Then, round seminiferous tubules (the ratio of long- to short-axis diameter < 1.2) at stages VII-VIII were selected for morphological analysis involving the tubule area and spermatogenic epithelium height. For statistical analysis, at least 200 seminiferous tubules on three cross-sections per testis from each group were examined. The caput, corpus, and cauda of the epididymis were histologically examined (Mishra and Singh 2005).
Sperm counting
The caudal epididymis was collected and cut into pieces in 1 mL phosphate-buffered saline (PBS) and then incubated at 37 ℃ aqueous baths for 10 min. After homogenization and dilution ten times with PBS, the number of spermatozoa was counted using a hemocytometer under an inverted microscope.
Sperm morphological analysis
Sperm smears were prepared according to the standard protocol for the sperm morphology assay. Briefly, smears were immediately fixed in 95% ethanol for 5 min, rinsed with running water, stained with hematoxylin for 5 min, and rinsed with running water again. Then, smears were stained with eosin, dehydrated, transparentized, and mounted. For each mouse, at least 1000 spermatozoa were examined under an Axioskop microscope (Carl Zeiss, Germany) with 100 × 10 magnification. Sperm abnormalities were determined according to Wyrobek and Bruce (1975).
Measurement of the serum testosterone level
The blood collected from the orbit was centrifuged at 3000 rpm for 20 min at 4 °C. Serum was carefully separated from plasma and immediately stored in a freezer at – 80 °C until analyzed. Serum testosterone was determined by the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (QiSong Bio). Briefly, 10 μL serum samples were diluted to 50 μL, then 100 μL of HRP-conjugate reagent was added to each sample in a 96-well plate, which was later covered with an adhesive strip and incubated for 60 min at 37 ℃. After washing each well five times, 50 μL chromogen solutions A and B were added to each well and the plate was incubated for 15 min at 37°C. The Optical Density (O.D.) at 450 nm wavelength was read using a multi-mode microplate reader (SpectraMax iD3). All samples were analyzed in the same assay to avoid variability.
IF staining
Testes were fixed for 48h at 4 °C in 4% paraformaldehyde (PFA). Samples were dehydrated by gradient ethanol and xylene, embedded with paraffin, and then sectioned crossly at 5 μm. After dewaxing and antigen retrieval, sections were immune-stained using the following antibodies: SOX9 (a Sertoli cell marker; 1:500; Sigma-Aldrich; #HPA001758), anti-KI67 (the spermatogonia marker; 1:200; Cell Signaling Technology; #9129S), anti-SYCP3 (a primary spermatocyte marker; 1:200; Abcam; #ab15093), anti-ACRV1 (a spermatid marker; 1:200; Proteintech; #14,040–1-AP), anti-TUBULIN βIII (1:200; Abcam; # ab18207) antibodies. Then, slides were treated with a Cy3-conjugated secondary antibody (1:500; Thermo Fisher Scientific; # A10520), followed by incubation with 4′,6-diami-dino-2-phenylindole (DAPI) (Thermo Fisher Scientific) for 2 min to visualize cell nuclei. Finally, slides were imaged using a laser scanning confocal inverted microscope (TCS SP5, Leica, Germany) or Thunder imager (DMi8, Leica, Germany). A negative control was also conducted. Based on IF images, the number of Sertoli cells (SOX9 +), spermatogonia (KI67), and spermatocytes (SYCP3 +) per seminiferous tubule were counted in round or slightly oblique seminiferous tubules (containing at least 100 seminiferous tubules blindly selected from three non-serial testicular cross section per animal). The amount for spermatids was calculated by the ACRV1 positive area per field (20X). The relative microtubule mount was represented by the percentage of TUBB3 positive area in the seminiferous tubule at stages VI-VII. For statistical analysis, at least 100 seminiferous tubules on three non-serial cross-sections per testis and five litters per group were used.
Tunel assay
After fixation for 48h at 4 °C in 4% PFA, testes were dehydrated by gradient sucrose, embedded in OCT compound, and cryo-sectioned at 10 μm. Then, sections were processed with the fluorescein (FITC) Tunel cell apoptosis detection kit (Servicebio, China, #G1501-50T) following instructions from the manufacturer. DNase I-treated slides were used as a positive control. Lastly, DAPI was used for nuclear staining. The TUNEL signals were examined under a fluorescent Axioskop standard microscope (Carl Zeiss, Germany).
RNA extraction and RT-qPCR
The total RNA of each tissue was extracted using the RNAprep Pure Tissue kit (TIANGEN, China, #DP431) following the protocol from the manufacturer. The concentration of total RNA for each sample was measured by a microspectrophotometer (Nanodrop 2000, ThermoFisher Scientific, America) and then the RNA was reversely transcribed into cDNA using reverse transcriptase named FastKing RT Kit with gDNase (TIANGEN, China, #KR116). Real-time quantitative fluorescence PCR of target genes was performed by the SYBR Green dye method using the SuperReal PreMix Plus (SYBR Green) kit (TIANGEN, China, #FP205). Prior to the assay for samples, the primer specificity was verified by melting curve analysis, and primer efficiencies of 90%–110% were confirmed. Primers for targeted genes were listed in Table 1, including Sertoli cell markers (Sox9 and Inhbb), spermatogonium markers (Ki67), spermatocyte markers (Ssxb1), Spermatid markers (Acrv1, and Prm2), apoptosis-related genes (Caspase3 and Caspase 9), and microtubule-related genes (Map9 and Map1a). The reaction reagent of 10 μL volume was prepared in a 384-well plate in duplicates. The prepared 384-well plate (Monad; Lot: 120,619) was put into the Roche LightCycler® 480 real-time PCR instrument (Roche, America) to run a three-step PCR amplification reaction using 60℃ for amplification (40 cycles). Gene expression was normalized to gapdh and finally quantified by the method of 2−ΔΔCt.
Sperm DNA extraction and qPCR for sex chromosome-linked genes
To test whether TBBPA-BDBPE exposure caused damage to Y-bearing sperm in its quantity or quality, we attempted to separate Y-bearing sperm from X-bearing sperm but did not succeed. Then, we tried to characterize the Y-bearing sperm population versus the X-bearing sperm population by qPCR analysis for sex chromosome-linked genes. Specifically, sperm DNA was extracted using the Magnetic Animal Tissue Genomic DNA kit (TIANGEN, China, #DP341) following the protocol from the manufacturer. PCR for Y chromosome-linked genes Sry and Zfy, as well as the X chromosome-linked Tsx was performed as described in 2.12. The primers for these genes are shown in Table 1. The Ct value ratio for Sry or Zfy/Tsx was measured to represent the Y sperm population versus the X sperm population.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 (SPSS Inc., Chicago) software, and figures were made by the GraphPad Prism version 8.0 (GraphPad Software, La Jolla). Data are shown as mean ± standard deviation (SD). If homogeneity, data were analyzed by one-way analysis of variance (ANOVA) followed by Duncan (equal variances assumed) and Dennett’s T3 (equal variances not assumed). The Chi-square test was used for a statistical difference in the sex ratio of the offspring. Images were analyzed by ImageJ software (National Institutes of Health, USA; version 1.53c). A p < 0.05 was considered statistically significant.
Results
Changes in sperm count and morphology
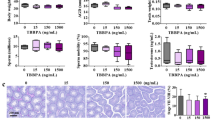
Following the 8-month exposure to 50 and 1000 μg/kg/d TBBPA-BDBPE, body weight, AGD, and serum testosterone levels were not significantly altered in mice (Fig. 1A). Testis weight showed a decreasing trend in both low-dose and high-dose TBBPA-BDBPE exposure groups compared to the control, despite a lack of statistical difference (Fig. 1B). Reduced epididymal sperm count was observed in the high-dose group (p < 0.05), and even the low-dose group displayed a decrease with a p-value of 0.056 compared with the control group (Fig. 1B). Meanwhile, morphologically abnormal spermatozoa increased with increased doses of TBBPA-BDBPE, with the abnormal percentages being approximately 3 and 9 times higher in 50 and 1000 μg/kg/d groups than that in the control group, respectively (p < 0.05; Fig. 1B). The morphological abnormalities of spermatozoa included an amorphous head, no hook, folded neck, and curly or bent tails (Fig. 1C). These findings show that long-term exposure to TBBPA-BDBPE negatively affected mouse sperm quantity and quality.
Changes in body weight and conventional reproductive parameters in male mice following exposure to 50 and 1000 μg/kg/d TBBPA-BDBPE from birth to 8-month age (n = 5). A Body weight, anogenital distance, and serum testosterone level. B Testis weight, sperm count, and sperm malformation rate. C Morphological abnormalities of sperm. For statistical analysis, at least 1000 sperm per cauda epididymidis of each mouse per group were used. Asterisk (*) indicates a significant difference from control (p < 0.05)
Changes in histology
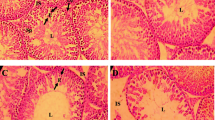
Histological analysis revealed testicular changes following long-term exposure to TBBPA-BDBPE. The percentage of stages VII-VIII seminiferous tubules was significantly reduced by TBBPA-BDBPE, even in the low-dose group (p < 0.05; Fig. 2A and B). Remarkably, TBBPA-BDBPE-treated testes showed reduced seminiferous epithelium height and seminiferous area in stages VII–VIII seminiferous tubules (Fig. 2A and B). Strikingly, immature germ cells improperly appeared in the lumen in the TBBPA-BDBPE groups (Fig. 2A); correspondingly, cell debris was also observed in the epididymis, particularly in the caput epididymidis (Fig. 3). All observations indicate that long-term exposure to as low as 50 μg/kg/d TBBPA-BDBPE damaged testicular histology.
Alterations in the testicular histology in male mice following exposure to 50 and 1000 μg/kg/d TBBPA-BDBPE from birth to 8-month age (n = 5). A Testicular histological images under 20 × –40 × objectives. Red arrows indicate exfoliated immature germ cells; Red short lines indicate the height of seminiferous epithelium. B Statistical results for the percentage of stage VII-VIII seminiferous tubules, seminiferous tubule area, the height of epithelium. Asterisk (*) indicates a significant difference from control (p < 0.05)
Changes of spermatogenic cells in seminiferous tubules
We further evaluated the effect of long-term exposure to TBBPA-BDBPE on spermatogenic cells in seminiferous tubules. Spermatogonia, spermatocytes, and spermatids in seminiferous tubules were marked by IF staining for their marker proteins (Fig. 4A–C). The positive signals for KI67, SYCP3, and ACRV1 were significantly reduced in the high-dose TBBPA-BDBPE groups (Fig. 4A–C), indicating decreases in the numbers of spermatogenic cells at different developmental stages. Even in the low-dose TBBPA-BDBPE group, spermatogonia and spermatids were significantly fewer compared to the control. Consistent with the IF results, RT-qPCR analysis revealed down-regulated expression of Ki67, Ssxb1, Acrv1, and Prm2, markers for spermatogonia, spermatocytes, spermatids, and spermatozoa, respectively, in the 1000 μg/kg/d TBBPA-BDBPE group (Fig. 4D). Of note, exposure to low-dose TBBPA-BDBPE also resulted in significant down-regulation of Acrv1 and Prm2 expression.
Changes of spermatogenic cells in seminiferous tubules in male mice following exposure to 50 and 1000 μg/kg/d TBBPA-BDBPE from birth to 8-month age (n = 5). A spermatogonium marked by KI67 immunofluorescence (IF) and its number per area. B Spermatocyte marked by SYCP3 IF staining and its number per area. C Sperm marked by ACRV1 IF staining and its percentage per area. Display channel was set to red for the second antibody labeled with Cy3. For statistical analysis, at least 20 cross sections (under 20 × objectives) per testis, and five mice per group were used. D Alterations in the relative expression of markers for spermatogonium (Ki67), spermatocyte (Ssxb1), sperm (Acrv1 and Prm2). Asterisk (*) indicates a significant difference from control (p < 0.05)
The TUNEL assay revealed a few apoptotic spermatogenic cells occurring in TBBPA-BEBPE treatments as well as the control (Fig. 5A). However, both high-dose and low-dose TBBPA-BDBPE led to higher expression of apoptosis marker genes (Caspase 3, Caspase 9, Bax, and Bcl2) compared to the control (Fig. 5B), suggesting a mild apoptosis-promoting effect that cannot be detected by the TUNEL assay due to its low sensitivity. All data demonstrate that long-term exposure to low-dose TBBPA-BDBPE disrupted spermatogenesis in mice.
Apoptosis in testes of male mice following exposure to 50 and 1000 μg/kg/d TBBPA-BDBPE from birth to 8-month age (n = 5). A Apoptotic cells (green arrows) in testes identified by the TUNEL assay using a 20 × objectives. Display channel was set to green for the signal labeled with Alexa 488. B Alterations in the relative expression of markers for apoptosis. Asterisk (*) indicates a significant difference from control (p < 0.05)
Damage of sertoli cells and microtubule cytoskeleton in seminiferous tubules
Except for germ cells, the effects of TBBPA-BDBPE on Sertoli cells were assessed. By IF staining for SOX9, we observed fewer Sertoli cells per seminiferous tubule in TBBPA-BDBPE-treated testes than controls (Fig. 6A). Moreover, TUBB3-marked microtubule tracks of Sertoli cells became shorter and thinner following long-term exposure to TBBPA-BDBPE, with a dose-dependent decrease in fluorescence intensity (Fig. 6B), indicating microtubule damage.
Alterations in Sertoli cells in testes of male mice following exposure to 50 and 1000 μg/kg/d TBBPA-BDBPE from birth to 8-month age (n = 5). A The Sertoli cell marked by SOX9 IF and its number per tubule. For statistical analysis, at least 100 seminiferous tubules per testis and five mice per group were used. B The microtubule cytoskeleton, labeled by TUBB3 IF and its mean gray value per area. C Relative expression of blood-testis barrier marker genes (Tjp1, Ocln, and Jam2). Asterisk (*) indicates a significant difference from control (p < 0.05). Display channel was set to red for the second antibody labeled with Cy3. The 20 × and 40 × objectives were used for A and B, respectively
Reproductive outcomes following long-term exposure
After mating with either control or TBBPA-BDBPE-treated males for one week, all non-treated females became pregnant. There was no difference in the gestation period between groups (Fig. 7A). No treatment-related adverse symptoms were observed in any dams during the gestation and lactation periods. However, the litter size at birth was significantly reduced in the 1000 μg/kg/d TBBPA-BDBPE group compared to the control, with a decreasing trend in the low-dose group (Fig. 7A). Particularly, two females mating with the same male from the high-dose group produced only two and five pups, respectively, far below the average litter size in the control group. No death occurred within the first week after birth for all pups. On PND 7, there were no significant differences in body weight and the gender-matched AGD between the TBBPA-BDBPE groups and the control group (Fig. 7B). Strikingly, the male-to-female sex ratio of the offspring of the TBBPA-BDBPE-treated males was significantly reduced in the high-dose group (Fig. 7A). To explain the female-biased sex ratio, we measured the Ct value ratios for Sry/Tsx and Zfy/Tsx to represent the ratio of X/Y bearing sperm. As shown in Fig. 7C, the Ct value ratios for Sry/Tsx and Zfy/Tsx appeared to decrease with increased doses of TBBPA-BDBPE, suggesting a decreasing trend of the Y-bearing sperm population in TBBPA-BDBPE-treated mice.
Reproductive impairment and altered sex ratio of the offspring in male mice following exposure to 50 and 1000 μg/kg/d TBBPA-BDBPE from birth to 8-month age (n = 5). A The gestational length (day) of pregnant mice (n = 10), litter size, and the sex ratio (male/female) of offspring. The numbers on columns indicates the number of offspring. B Body weight and anogenital distance (AGD) of male and female offspring. C Relative Ct value of genes in the Y sperm (Sry and Zfy) versus a gene in the X sperm (Tsx) identified by qPCR. Asterisk (*) indicates a significant difference from control (p < 0.05)
Discussion
Recently, we found that postnatal exposure of male mice to 50 μg/kg/d TBBPA-BDBPE from birth to PND 56 caused remarkable damage to the microtubule skeleton in Sertoli cells and the BTB but exerted little effect on conventional reproductive endpoints in young adulthood (Li et al. 2023). In this follow-up study, we found that extended exposure to low-dose TBBPA-BDBPE to 8-month age led to pathological changes of the testis and sperm parameter abnormalities, besides microtubule damage in Sertoli cells. Moreover, while 8-month-aged mice in the control group can mate with females and produce normal offspring, the males treated with high-dose TBBPA-BDBPE produced significantly fewer offspring, and the sex ratio of the offspring was biased towards females. And even the skewed sex ratio in the offspring was also observed in low-dose treatment, despite a lack of statistical significance, possibly due to relatively small sample size in this study. Taken together, all the findings demonstrate that extended exposure to low-dose TBBPA-BDBPE can cause severe reproductive impairments in male mice at the late reproductive age, despite slight injuries except for microtubule damage in early life. It is therefore concluded that slight testicular injuries in early life can lead to reproductive impairment at late reproductive age, highlighting that the alterations in certain non-conventional endpoints should be noticed as well as conventional endpoints in future reproductive toxicity studies. Given the crucial role of the Sertoli cell microtubule cytoskeleton in testicular structure and spermatogenesis (Gerber et al. 2016; O’Donnell and O’Bryan 2014; Wang et al. 2020), we propose that microtubule damage may be responsible for reproductive impairment following long-term exposure, as discussed in our recent study (Li et al. 2023).
Additionally, our results, together with our previous findings, also indicate that aging is a stressful factor leading to increased susceptibility of the testis to chemicals. Indeed, aging is considered as a significant risk factor for sperm quality (Albani et al. 2019), and delayed parenthood is known to be associated with adverse reproductive outcomes, with limited data concerning fathers (Albani et al. 2019; Dong et al. 2022; Tarín et al. 1998). From this perspective, it is understandable that the testis in late reproductive age is more susceptible to chemicals than in early reproductive age. Considering the fact that delayed parenthood is becoming a more and more common phenomenon worldwide, our study highlights the importance of understanding reproductive toxicity of chemicals to male mammals at late reproductive age. This seems to pose a challenge to conventional reproductive toxicity studies in which endpoints are generally examined at vigorous reproductive age but not late reproductive age. It is more feasible to employ early toxicological endpoints to predict possible reproductive impairment in late life than to directly detect the impairment caused by extended exposure to late reproductive age. Based on our results, microtubule damage could be an ideal early endpoint indicative of reproductive impairments in late life.
In this study, it is noteworthy that male mice received long-term exposure to 1000 μg/kg/d TBBPA-BDBPE produced offspring with a female-biased sex ratio, and the ratio in the offspring of low-dose treated males was also skewed towards females, despite a lack of statistical significance. Although increasing studies reported that maternal and fetal exposure to some chemicals caused female-biased sex ratios in mammals (Bevan et al. 1997; Dobrzynska et al. 2017; Gaukler et al. 2016; Rodríguez and Sanchez 2010; Santamaria et al. 2020), there are few studies investigating the effects of paternal exposure on the sex ratio of offspring except for a study by Dobrzynska et al. (2017), who reported that an 8-week exposure to 500 and 2000 mg/kg/d di-n-butyl phthalate caused a female-biased sex ratio of the offspring. Our finding, together with the study by Dobrzynska et al. (2017), highlights a need to investigate the effects of paternal exposure to chemicals on the sex ratio of offspring. Indeed, there are epidemiological data showing that occupational exposure of fathers is associated with the reduced male-to-female sex ratio of children (Davis et al. 1998, 2007; Goldsmith et al. 1984; Milham 1993; Mocarelli et al. 2000; Whorton et al. 1994), which encourages more efforts to understand the sex ratio bias caused by paternal exposure. In the present study, we noticed that despite the female-biased sex ratio at birth, there was an equal number of live pups and implantation sites in TBBPA-BDBPE-treated mice. This observation means that the female-biased sex ratio was due to fertilization or implantation of fewer male embryos rather than the death of male fetuses, which essentially results from lower number and/or poorer quality of Y-bearing sperm than X-bearing sperm. Thus, it is inferred that TBBPA-BDBPE exposure preferentially caused damage to Y-bearing sperm. To test our inference, we attempted to separate Y-bearing sperm from X-bearing sperm for further analysis but not succeed. However, the decreasing trend in the Ct value ratios for Sry/Tsx and Zfy/Tsx suggests a possibility of a lower Y-bearing sperm population than a Y-bearing sperm population, which needs further investigation. In previous studies, the female-biased sex ratio caused by chemical exposure is generally explained by the public-health maxim the males that have shorter life expectancies than females at every age from conception onward (Clapp and Ozonoff 2000). Here, our finding is expected to guide future research in chemical-caused sex ratio bias aiming to identify different susceptibility to chemicals between Y-bearing sperm and X-bearing sperm.
In conclusion, our study demonstrates that extended exposure to low-dose TBBPA-BDBPE, which only caused damage to the microtubule skeleton in Sertoli cells and the BTB on PND 56, led to severe male reproductive impairment, including altered fertility at the late reproductive age. This implies that mammal testes in late reproductive age are more susceptible to TBBPA-BDBPE than in early reproductive age, highlighting the importance of understanding male reproductive toxicity to chemicals in late reproductive age.
Data availability
Data is available within the article or on request from the corresponding author.
References
Ahmed EA, de Rooij DG (2009) Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol 558:263–277. https://doi.org/10.1007/978-1-60761-103-5_16
Albani E, Castellano S, Gurrieri B et al (2019) Male age: negative impact on sperm DNA fragmentation. Aging 11(9):2749–2761. https://doi.org/10.18632/aging.101946
Ali N, Harrad S, Muenhor D, Neels H, Covaci A (2011) Analytical characteristics and determination of major novel brominated flame retardants (NBFRs) in indoor dust. Anal Bioanal Chem 400(9):3073–3083. https://doi.org/10.1007/s00216-011-4966-7
Balasch J, Gratacós E (2012) Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 24(3):187–193. https://doi.org/10.1097/GCO.0b013e3283517908
Bevan C, Tyl RW, Neeper-Bradley TL et al (1997) Developmental toxicity evaluation of methyl tertiary-butyl ether (MTBE) by inhalation in mice and rabbits. J Appl Toxicol 17(Suppl 1):S21-29. https://doi.org/10.1002/(sici)1099-1263(199705)17:1+%3cs21::aid-jat407%3e3.3.co;2-5
Clapp R, Ozonoff D (2000) Where the boys aren’t: dioxin and the sex ratio. Lancet (london, England) 355(9218):1838–1839. https://doi.org/10.1016/s0140-6736(00)02280-7
Davis DL, Gottlieb MB, Stampnitzky JR (1998) Reduced ratio of male to female births in several industrial countries: a sentinel health indicator? JAMA 279(13):1018–1023. https://doi.org/10.1001/jama.279.13.1018
Davis DL, Webster P, Stainthorpe H, Chilton J, Jones L, Doi R (2007) Declines in sex ratio at birth and fetal deaths in Japan, and in U.S. whites but not African Americans. Environ Health Perspect 115(6):941–946. https://doi.org/10.1289/ehp.9540
Dobrzynska MM, Tyrkiel EJ, Gajowik A (2017) Three generation study of reproductive and developmental toxicity following exposure of pubescent F0 male mice to di-n-butyl phthalate. Mutagenesis 32(4):445–454. https://doi.org/10.1093/mutage/gex011
Dong S, Chen C, Zhang J, Gao Y, Zeng X, Zhang X (2022) Testicular aging, male fertility and beyond. Front Endocrinol (lausanne) 13:1012119. https://doi.org/10.3389/fendo.2022.1012119
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2011) Scientific Opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA J 9(12):2477. https://doi.org/10.2903/j.efsa.2011.2477
EPA (2014) An alternative assessment for the flame retardant decabromodiphenyl ether (DecaBDE)
Gaukler SM, Ruff JS, Potts WK (2016) Paroxetine exposure skews litter sex ratios in mice suggesting a Trivers-Willard process. Behav Ecol 27(4):1113–1121. https://doi.org/10.1093/beheco/arw017
Gerber J, Heinrich J, Brehm R (2016) Blood-testis barrier and Sertoli cell function: lessons from SCCx43KO mice. Reproduction 151(2):R15-27. https://doi.org/10.1530/REP-15-0366
Goldsmith JR, Potashnik G, Israeli R (1984) Reproductive outcomes in families of DBCP-exposed men. Arch Environ Health 39(2):85–89. https://doi.org/10.1080/00039896.1984.10545840
Li Y, Dong M, Xiong Y et al (2022) Effects of postnatal exposure to tetrabromobisphenol A on testis development in mice and early key events. Arch Toxicol 96(6):1881–1892. https://doi.org/10.1007/s00204-022-03259-5
Li YY, Xiong YM et al (2023) Tetrabromobisphenol A-bis(2,3-dibromopropyl ether) impairs postnatal testis development in mice: the microtubule cytoskeleton as a sensitive target. Environ Health. https://doi.org/10.1021/envhealth.3c00044
Malliari E, Kalantzi OI (2017) Children’s exposure to brominated flame retardants in indoor environments - a review. Environ Int 108:146–169. https://doi.org/10.1016/j.envint.2017.08.011
Milham S Jr (1993) Unusual sex ratio of births to carbon setter fathers. Am J Ind Med 23(5):829–831. https://doi.org/10.1002/ajim.4700230516
Mishra RK, Singh SK (2005) Effect of aqueous leaf extract of Azadirachta indica on the reproductive organs in male mice. Indian J Exp Biol 43:1093–1103
Mocarelli P, Gerthoux PM, Ferrari E et al (2000) Paternal concentrations of dioxin and sex ratio of offspring. The Lancet 355(9218):1858–1863. https://doi.org/10.1016/s0140-6736(00)02290-x
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Nyholm JR, Grabic R, Arp HP, Moskeland T, Andersson PL (2013) Environmental occurrence of emerging and legacy brominated flame retardants near suspected sources in Norway. Sci Total Environ 443:307–314. https://doi.org/10.1016/j.scitotenv.2012.10.081
O’Donnell L, O’Bryan MK (2014) Microtubules and spermatogenesis. Semin Cell Dev Biol 30:45–54. https://doi.org/10.1016/j.semcdb.2014.01.003
Qu G, Liu A, Wang T et al (2013) Identification of tetrabromobisphenol A allyl ether and tetrabromobisphenol A 2,3-dibromopropyl ether in the ambient environment near a manufacturing site and in mollusks at a coastal region. Environ Sci Technol 47(9):4760–4767. https://doi.org/10.1021/es3049916
Ren XM, Yao L, Xue Q et al (2020) Binding and activity of tetrabromobisphenol A mono-ether structural analogs to thyroid hormone transport proteins and receptors. Environ Health Perspect 128(10):107008. https://doi.org/10.1289/EHP6498
Rodríguez PE, Sanchez MS (2010) Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in Wistar rat offspring. J Toxicol Environ Health A 73(24):1678–1688. https://doi.org/10.1080/15287394.2010.516241
Safdari-Dehcheshmeh F, Noroozi M, Taleghani F, Memar S (2023) Factors influencing the delay in childbearing: A narrative review. Iran J Nurs Midwifery Res 28(1):10–19. https://doi.org/10.4103/ijnmr.ijnmr_65_22
Santamaria CG, Meyer N, Schumacher A et al (2020) Dermal exposure to the UV filter benzophenone-3 during early pregnancy affects fetal growth and sex ratio of the progeny in mice. Arch Toxicol 94(8):2847–2859. https://doi.org/10.1007/s00204-020-02776-5
Shi T, Chen SJ, Luo XJ et al (2009) Occurrence of brominated flame retardants other than polybrominated diphenyl ethers in environmental and biota samples from southern China. Chemosphere 74(7):910–916. https://doi.org/10.1016/j.chemosphere.2008.10.047
Tao F, Abdallah MA, Harrad S (2016) Emerging and legacy flame retardants in UK indoor air and dust: evidence for replacement of PBDEs by emerging flame retardants? Environ Sci Technol 50(23):13052–13061. https://doi.org/10.1021/acs.est.6b02816
Tarín JJ, Brines J, Cano A (1998) Long-term effects of delayed parenthood. Hum Reprod 13(9):2371–2376. https://doi.org/10.1093/humrep/13.9.2371
Wang L, Yan M, Wu S et al (2020) Microtubule cytoskeleton and spermatogenesis-lesson from studies of toxicant models. Toxicol Sci 177(2):305–315. https://doi.org/10.1093/toxsci/kfaa109
Whorton MD, Haas JL, Trent L, Wong O (1994) Reproductive effects of sodium borates on male employees: birth rate assessment. Occup Environ Med 51(11):761–767. https://doi.org/10.1136/oem.51.11.761
Wyrobek AJ, Bruce WR (1975) Chemical induction of sperm abnormalities in mice. Proc Natl Acad Sci U S A 72(11):4425–4429. https://doi.org/10.1073/pnas.72.11.4425
Yao L, Wang Y, Shi J et al (2021) Toxicity of tetrabromobisphenol A and its derivative in the mouse liver following oral exposure at environmentally relevant levels. Environ Sci Technol 55(12):8191–8202. https://doi.org/10.1021/acs.est.1c01726
Zuiderveen EAR, Slootweg JC, de Boer J (2020) Novel brominated flame retardants - A review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere 255:126816. https://doi.org/10.1016/j.chemosphere.2020.126816
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0901103) and the National Natural Science Foundation of China (42277434).
Author information
Authors and Affiliations
Contributions
YYL: Conceptualization, Methodology, Investigation, Data curation, Writing–original draft, Writing– review & editing. YMK: Investigation, Visualization. XYC: Investigation. JYS: Experiment. LL: Experiment. XHL: Supervision. ZF: Conceptualization, Project administration, Supervision, Writing– review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YY., Xiong, YM., Chen, XY. et al. Extended exposure to tetrabromobisphenol A-bis(2,3-dibromopropyl ether) leads to subfertility in male mice at the late reproductive age. Arch Toxicol 97, 2983–2995 (2023). https://doi.org/10.1007/s00204-023-03589-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03589-y