Abstract
The aim of this study was to analyze whether dermal exposure to benzophenone 3 (BP-3) during pregnancy affects critical parameters of pregnancy, and whether this exposure may affect the outcome of a second pregnancy in mice. Pregnant mice were exposed to 50-mg BP-3/kg body weight/day or olive oil (vehicle) from gestation day (gd) 0 to gd6 by dermal exposure. High-frequency ultrasound imaging was used to follow up fetal and placental growth in vivo. Blood flow parameters in uterine and umbilical arteries were analyzed by Doppler measurements. Mice were killed at gd5, gd10, and gd14 on the first pregnancy, and at gd10 and 14 on the second pregnancy. The weight of the first and second progenies was recorded, and sex ratio was analyzed. BP-3 levels were analyzed in serum and amniotic fluid. BP-3 reduced the fetal weight at gd14 and feto-placenta index of first pregnancy, with 16.13% of fetuses under the 5th percentile; arteria uterina parameters showed altered pattern at gd10. BP-3 was detected in serum 4 h after the exposure at gd6, and in amniotic fluid at gd14. Offspring weight of first progeny was lower in BP-3 group. Placenta weights of BP-3 group were decreased in second pregnancy. First and second progenies of mothers exposed to BP-3 showed a higher percentage of females (female sex ratio). Dermal exposure to low dose of BP-3 during early pregnancy resulted in an intrauterine growth restriction (IUGR) phenotype, disturbed sex ratio and alterations in the growth curve of the offspring in mouse model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endocrine disrupting chemicals (EDCs) are substances with the potential to interfere with processes that are controlled by hormones. Due to the increasing incidence of endocrine disorders worldwide, and because of the continuous exposure of humans and wildlife to EDCs, the World Health Organization (WHO) urged to study the effects of EDCs in different aspects of health, including their effects on the reproductive system (Bergman et al. 2013) and the underlying mechanisms. Particularly, increasing attention is moving toward EDCs present in personal care products (PCPs). This is because PCPs are applied topically and multiple times daily. Thus, several PCPs are leave-on products, exposing a big proportion of the skin for prolonged time periods (Darbre and Harvey 2015).
Sunscreen products are one of the most used PCPs around the world. Benzophenone (BP)-type ultraviolet (UV) light filters are present in many sunscreen products. Among this family of compounds, BP-3—IUPAC name: (2-hydroxy-4-methoxyphenyl)-phenylmethanone—is the most prominent. It has been classified as an agonist through estrogen action pathways (Schlumpf et al. 2001) as well as anti-androgenic and anti-progestagenic (Schreurs et al. 2005). Humans are highly exposed to BP-3 on a regular basis not only by its topical application but also because of its presence in drinking water or food (Hayden et al. 1997; Janjua et al. 2004, 2008; Stackelberg et al. 2004; Loraine and Pettigrove 2006; Kim and Choi 2014). The monitoring results of BP-3 and/or its derivatives in urine performed in different countries like the USA, Australia, China, Denmark and Spain confirmed that the exposure to BP-3 is consistent in different world regions and calls for careful studies of its long-term effects on human health (Calafat et al. 2008; Casas et al. 2011; Frederiksen et al. 2014; Gao et al. 2015; Heffernan et al. 2015).
The potential danger of BP-3 contained in products used by pregnant and lactating women needs to be assessed. BP-3 and its metabolite, 2,4-dihydroxybenzophenone, were both detected in placenta and breast milk (Vela-Soria et al. 2014; Molins-Delgado et al. 2018). Besides, BP-3 presence was confirmed in amniotic fluid, fetal- and cord blood (Krause et al. 2018). Previous work suggests that BP-3 presence is associated with reproductive toxicity in humans and animals (Ghazipura et al. 2017). Accordingly, we have found that BP-3 alters early follicular assembly in rat whole ovary cultures, indicating that the negative effects are possible at very early stages (Santamaría et al. 2019). However, other publications doubt about the potential of BP-3 to negatively affect fertility (Ghazipura et al. 2017). Thus, it is critical to develop study models to precisely delineate the effect of BP-3 in reproductive-related processes and more importantly during pregnancy. Here, we aim to analyze whether and how a limited dermal exposure to a low dose of BP-3 during early gestation, affects critical parameters of pregnancy. This is relevant in particular because it includes the critical period of implantation when pregnancy is usually still not confirmed. Additionally, we studied whether the exposure during the first pregnancy may affect the outcome of a second one.
Materials and methods
Animals
Female C57BL/6J and male BALB/c mice were purchased from Janvier Labs (France). Females were kept in groups of 2–4 animals per cage. Mice received water and food ad libitum and were maintained in a controlled environment (22 ± 2 ℃; 12-h light/dark cycle, 7am–7pm; air humidity of 40–60%). Experiments started after 2 weeks of acclimatization.
Experimental design and sample collection
Animal experiments were performed according to the institutional guidelines upon ministerial approval (Landesverwaltungsamt Sachsen Anhalt: 42502-21296UniMD, Magdeburg, Germany and approved by the Ethical Committee of the Facultad de Bioquímica y Ciencias Biológicas, UNL, Santa Fe, Argentina: CE2018-62). The experiments were conducted by authorized persons according to the Guide for Care and Use of Animals in Agriculture Research and Teaching. C57BL/6J female and BALB/c male mice were paired to have a physiologically relevant allogeneic mating. The day of plug was defined as gestation day (gd) 0. Pregnant mice were distributed in three groups:
-
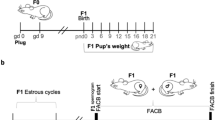
Group 1: C57BL/6J pregnant mice were shaved on the back on gd0 and topical applications of 50-mg BP-3 per kg of body weight (bw) per day (d) or olive oil (vehicle, Extra virgin olive oil Tres Marías, San Juan, Argentina) were given daily for 7 days (BP-3-treated mice: n = 19, olive oil-treated mice: n = 19). Pregnant females were followed up throughout pregnancy by high-frequency ultrasound at gd5, gd8, gd10, gd12 and gd14, and killed on gd5, gd10 or gd14 (Fig. 1a). The number of implantations was recorded for animals killed on gd5 (BP-3-treated mice: n = 4, olive oil-treated mice: n = 4). For those animals killed on gd10 (BP-3-treated mice: n = 6, olive oil-treated mice: n = 6) and gd14 (BP-3-treated mice: n = 4, olive oil-treated mice: n = 5), implantation and abortions as well as the weight of fetuses and placentas were recorded. Uteri were dissected and a single implantation from each female at gd10 was dissected and fixed in a 4% paraformaldehyde (PFA) solution containing sucrose.
Fig. 1 Short- term exposure to BP-3 or vehicle and sample collection. a Group 1: topical applications of 50 mg BP-3/kg bw d or olive oil (vehicle) were given to pregnant females from gd0 to gd6. Pregnant females were killed at gd5, gd10 and gd14. Females killed at gd10 and gd14 were analyzed at gd8 and gd8 and gd12 by high-frequency ultrasound, respectively. b Group 2: pregnant females were exposed to BP-3 dissolved in olive oil as it was described for Group 1. After weaning, they were mated with BALB/c male mice and killed at gd10 and gd14 of their second pregnancy. c Group 3: the weight of the pups neonatally exposed to BP-3 or olive oil was recorded from PND1 to PND19 every 3 days. Females exposed to BP-3 or olive oil during their first pregnancy were again mated with BALB/c male mice, and the weights of the pups were recorded from PDN1-19 every 3 days. PND postnatal day, gd gestation day. Arrows indicate the day of killing. Square indicates the day of high-frequency ultrasound analyses
-
Group 2: C57BL/6 pregnant mice were exposed to BP-3 or olive oil from gd0 to gd6 as it was previously described for group 1 (BP-3-treated mice: n = 9, olive oil-treated mice: n = 7). After weaning, the mothers were mated again with BALB/c males and were killed on gd10 (BP-3-treated mice: n = 5, olive oil-treated mice: n = 3) and gd14 (BP-3-treated mice: n = 4, olive oil-treated mice: n = 4) of their second pregnancy (Fig. 1b). Samples were obtained at gd10 and gd14 as we previously described.
-
Group 3: C57BL/6 pregnant mice exposed to BP-3 or olive oil from gd0 to gd6 of their first pregnancy was assigned to evaluate the weight of the pups from birth until weaning during the first and the second pregnancy (Fig. 1c) (BP-3-treated mice: n = 4, olive oil-treated mice: n = 5).
Thus, after the first pregnancy, the same mothers were subjected to a second pregnancy, without re-exposing them to BP-3 (multiparous study).
Selection of the dose
We selected the dose of 50 mg/kd bw/day because it represents the calculated dose after controlled whole-body dermal application in humans, according to Janjua et al. (2008). In that study, 2 mg of cream formulation at 10% (w/w) of BP-3 per cm2 of body surface was applied. Females displayed an average body area of 1.73 m2, resulting in a total average amount of 34,600 mg of cream each (see Table 1 in Janjua et al. 2008). Thus, with an average body weight of 68 kg, the BP-3 dose of Janjua’s study was 50.88 mg/kg bw in females. Based on the fact that an allometric conversion is not recommended for drugs administered by topical, nasal, subcutaneous, or intramuscular routes (Nair and Jacob 2016), we used a direct mg/kg conversion (50 mg/kg bw/day in mice).
Selection of window of exposure
We exposed the pregnant mice during the first seven days of pregnancy (gd0–gd6), because resembles the first trimester of a human pregnancy and includes the critical period of implantation where many women may not even have recognized that they are pregnant (Hill 2020).
Detection of BP-3 in serum and amniotic fluid
Short-term exposure to BP-3 or vehicle, from gd0 to gd6 was achieved and blood was collected by cardiac puncture under anesthesia 4 h after the last topical application of BP-3 on gd6. In addition, blood and amniotic fluid were collected at gd14 after the exposure to BP-3 or vehicle from gd0–gd6. Serum was obtained by centrifugation and stored at − 20 °C until measurement of BP-3 concentration. Amniotic fluid samples from the fetuses of one mother were pooled, and 3 different pools obtained from 3 mothers were used to measure BP-3 During the second pregnancy, serum samples were collected at gd10 and gd14. The detection of BP-3 was performed by ultra-high-performance liquid chromatography enhanced by chemometrics, following a methodology previously validated by our group (Teglia et al. 2019). The limit of detection (LOD) and quantification (LOQ) of the method are 0.7 ng/mL and 2.0 ng/mL, respectively.
High-frequency ultrasound examination
To analyze intrauterine embryonic and fetal development, ultrasound examinations were performed at gd5, gd8, gd10, gd12 and gd14 employing a Vevo 2100 System (Fuji Film Visualsonics Inc). Mice were anesthetized with isoflurane and fixed in dorsal position. Depilatory cream was used for ventral hair removal. Implantation size was determined by measuring the area of the implantation at gd5, gd8, gd10 and gd12. Placental area, thickness, and diameter (gd10, gd14) or blood flow in the arteria uterina (UA; gd5, gd8, gd10, gd12, 1gd4) were recorded. Peak systolic velocity (PSV) and end diastolic velocity (EDV) were measured. Moreover, the resistance index (RI) and the pulsatility index (PI) were estimated. Vevo 2100 software was used to analyze the recorded parameters.
Measurement of fetal and placental weight
The bicornial uterus of each mouse was collected and opened lengthwise opposite to the placentas. Fetuses and placentas were separated from surrounding decidual tissue and yolk sac. The weight of each fetus and each corresponding placenta was determined using an analytical balance (Kern & Sohn GmbH, minimum: 0.02 g, sensitivity: 0.001 g). Fetal growth restriction was defined as the weight below the 10th percentile when compared to weights of control animals (Faraci et al. 2011; Müller et al. 2018).
Analysis of uterine spiral arteries by histology
Implantations from gd10 were fixed in 4% PFA for 6 h at room temperature (RT) and embedded in paraffin, following protocols previously described (Zenclussen et al. 2010; Müller et al. 2018). Implantations were serially sectioned (5-µm thick) and stained with hematoxylin–eosin (H/E) for morphological analyses of the uterine spiral arteries (uSAs). Parameters defining uSA remodeling were analyzed using the Zeiss light microscope, and 3–8 uSAs were identified and analyzed in the decidua basalis of one implantation per mouse using the AxioVision Rel. 4.8 program. The outer and the lumen circumferences of 3–8 arteries per animal were measured and the diameter (diameter = circumference/π), the wall–lumen ratio (uSA diameter/lumen diameter) as well the wall thickness [(uSA diameter − lumen diameter)/2] were calculated. Mean values of the parameters mentioned above were calculated for each animal.
Weight curve
The weight of the pups was recorded from postnatal day 1 (PND1) to PND19 every three days using an analytical balance (OHAUS model CS 200, sensitivity: 0.1 g). The day of the delivery was considered as PND0. Pups were sexed and weighted individually every 3 days. It was done blind each time. We followed females and males individually, but they were not identified by pup.
Statistics
Normal distribution of the data was assessed with the D’Agostino–Pearson omnibus test. Data are presented as means ± S.E.M or means. Number of mice, samples, or experiments performed as well as the used statistical test and the according p values are indicated in the corresponding figure legends. GraphPad Prism 5.0 was used to perform the statistical analyses. MEDCALC® easy-to-use statistical software was used to compared proportions (https://www.medcalc.org/calc/comparison_of_proportions.php).
Results
Outcome obtained from the first pregnancy
BP-3 levels detected in serum and amniotic fluid
Table 1 provides BP-3 levels detected using our recently developed methodology (Teglia et al. 2019). We were able to detect BP-3 in serum of pregnant females exposed during gd0–gd6 when measured four hours after the last dermal application (n = 3; BP-3 mean concentration = 22.4 ± 2.3 ng/mL). Regarding the control group, BP-3 was not detectable at gd6 (n = 3). The levels of BP-3 in amniotic fluid and serum of pregnant females at gd14 were analyzed in paired samples. We found two samples of serum with non-detectable levels of BP-3 and one sample of serum with a concentration of 16.8 ng/mL of BP-3. Furthermore, BP-3 was present in the amniotic fluid (n = 3; BP-3 mean concentration = 22.63 ± 10.8 ng/mL) confirming that BP-3 can cross the placental barrier and directly affect the developing fetus. Non-detectable levels of BP-3 were found neither in the serum nor the amniotic fluid of control samples at gd14 (n = 3).
Implantation and placental parameters
The areas of the implantation measured at gd5, gd8, gd10 and gd12 showed no differences between BP-3-treated and control animals (Fig. S1a) Number of implantations, abortions and abortion rates were not affected by the BP-3 treatment (Fig. S2). In addition, placental parameters such as placental area, diameter and thickness, measured via ultrasound at gd10 and gd12, were comparable among the groups (Fig. S3).
Fetal weight and feto-placenta index
Fetal weight from BP-3-treated mice was significantly reduced at gd14 (p < 0.01) when compared to the controls (Fig. 2a). The weight of the placentas was not affected by BP-3 exposure (Fig. 2b). The feto-placenta index was decreased (p < 0.0001) as consequence of the diminished fetal weight in BP-3-treated mice (Fig. 2c). Additionally, 16.13% of fetuses in the BP-3 group were under the 5th percentile (Fig. 2d), meaning they are affected by intrauterine growth restriction. The 10th and 5th percentiles correspond to 200 mg and 195.95 mg, respectively.
Fetal and placental weight in control and BP-3-treated mice at gd14. Fetal weight (a), placental weight (b), and feto-placental index (c) from the progeny of control (mice: n = 5; fetuses/placentas: n = 40) or BP-3-treated (mice: n = 4; fetuses/placentas: n = 31) mice at gd14 d) Percentage of fetuses from BP-3-treated mice from whose weight was below the 5th percentile (red) or above the 5th percentile (green). The 5th percentile corresponds 195.95 mg. Results are presented as individual values and means. Statistical differences were determined using Mann–Whitney U test. BP-3 benzophenone-3, gd gestation day, FPI feto-placental index. †p < 0.01, §p < 0.0001 (color figure online)
Hemodynamic parameters (PSV, EDV, S/D ratio, RI, PI)
Blood velocity parameters of the maternal arteria uterina (UA) and arteria umbilicalis (UmA) were followed up by Doppler measurements at gd5, gd8, gd10, gd12 and gd14. Our results showed a decrease in end diastolic velocity (Fig. 3b) and an increase in the systolic/diastolic ratio (Fig. 3c), pulsatility and resistance index (Fig. 3d, e) of the UA in BP-3 treated mice at gd10. In addition, no negative effect of BP-3 exposure on flow velocity parameters of the UmA was observed (Fig. S4).
Flow velocity parameters of the UA in control and BP-3-treated mice at different gestational days. Peak systolic velocity (a), end diastolic velocity (b), systolic/diastolic ratio (c), resistance index (d) and pulsatility index (e) of UAs of control and BP-3-treated mice at gd5 (control: n = 4, BP-3: n = 4), gd8 (control: n = 4, BP-3: n = 4), gd10 (control: n = 6, BP-3: n = 6), gd12 (control: n = 3, BP-3: n = 4), and gd14 (control: n = 4, BP-3: n = 4) are shown. Data are presented as means and SEM. Statistical analysis was performed using the Mann–Whitney U test. BP-3 benzophenone-3, UA uterine artery, gd gestation day. *p < 0.05
Spiral artery remodeling
The analysis of uSA parameters in H/E-stained slides obtained from uterine tissue of BP-3-treated and control mice at gd10 revealed no statistically significant changes in wall thickness or wall–lumen ratio after exposure to BP-3 when compared to the controls (Fig. S5).
Weight curves of the progeny from the first pregnancy
The results from the weight curves of male- and female offspring from the first pregnancy are illustrated in Fig. 4. As it can be observed, male offspring was more affected than females. Male offspring from mothers exposed to BP-3 was lighter in PND4 and then since PND10 onwards. On the other hand, females born to BP3-exposed mothers showed decreased weight at PND1, PND10 and PND13, but then recovered their normal weight since PND16 forward. Thus, decreased weight persist until weaning only in males.
Weight curves of the first progeny after BP-3 or vehicle treatment from gd0 until gd6. The weight of the male progenies was lower from the middle of lactation until weaning in the BP-3 treated group (BP-3-treated: n = 7) compared to the control group (control: n = 17). The weight of the female progenies was lower after birth and at the middle of lactation in the BP-3-treated group (BP-3-treated: n = 12) compared to the control group (control: n = 11). Results are presented as the average weight of all offspring per group per day and were analyzed by Mann–Whitney U test. BP-3 benzophenone-3. *p < 0.05, †p < 0.01, ‡p < 0.001, §p < 0.0001
Outcome obtained after the second pregnancy
BP-3 serum levels
Females treated with BP-3 or vehicle during gd0–gd6 of their first pregnancy were mated again and serum was collected at gd10 and gd14 of the second pregnancy. During the second pregnancy, there was no further exposure to BP-3. We found no detectable levels of BP-3 in serum at gd10 or gd14 (see Table 1).
Implantations and abortion numbers
We found comparable numbers of implantations, abortions and abortion rates between BP-3-treated and control mice at gd10 and 14 of the second pregnancy (Fig. S6).
Placenta and fetal weights of second pregnancies
The placenta weights from the second pregnancy of BP-3-treated mothers and controls were comparable at gd10 (Fig. 5a). At day 14, however, the placenta weights from BP-3-treated mothers were significantly reduced (p < 0.05) compared to the controls (Fig. 5a). Fetal weight and fetus/placenta index showed no differences between controls and BP-3-treated mice (Fig. 5b, c).
Placental and fetal weight at gd14 of the second pregnancy. Placental weight (a), fetal weight (b), and feto-placental index (c) from the second pregnancy of control (gd10 mice: n = 3/placentas: n = 14; gd14 mice: n = 4, gd14 fetuses/placentas: n = 24) or BP-3-treated (gd10 mice: n = 5/placentas: 34; gd14 mice: n = 4, fetuses/placentas: n = 25) mice. Results are presented as individual values and means. Statistical differences were determined using unpaired t test. BP-3 benzophenone-3, gd gestation day, FPI feto-placental index. *p < 0.05
Weight curve of the offspring from the second pregnancies whose mothers were exposed to BP-3 during the first pregnancy
As shown in Fig. S7, neither male nor female offspring from the second pregnancy was affected by BP-3 treatment of mother during its first pregnancy.
Sex ratio from both pregnancies
The percentage of females (sex ratio) was higher in first and second pregnancies of mothers exposed to BP-3 during the first pregnancy compared to the vehicle-treated controls. (Fig. 6).
Percentage of males and females of the first and second pregnancies. Data are presented as percentage of females and males of mothers exposed to BP-3 or oil during the first and second pregnancies (control males: n = 24, BP-3-treated males: n = 13; control females: n = 26, BP-3 treated females: n = 18). Statistical differences were determined using N-1 Chi-squared test from the online software MEDCALC®. *p < 0.05
Discussion
Several compounds are used to absorb UV light in personal care products (PCPs), not only to protect the skin but also to protect the product itself during storage (Krause et al. 2012; Darbre and Harvey 2015). BP-3 is one of the most widely used UV filters in sunscreen, being detectable in urine of humans from different countries, including vulnerable populations such as pregnant women, teenagers, and children of 6–11 years old (Calafat et al. 2008; Zhang et al. 2013; Frederiksen et al. 2013; Vela-Soria et al. 2014; Buck Louis et al. 2014). More recently, a clinical trial using commercially available sunscreens at maximal use conditions (i.e., applied at least every 2 h) resulted in plasma concentrations of active UV filters exceeding 0.5 ng/mL. Considering that 0.5 ng/mL is the threshold of the FDA for waiving some nonclinical toxicology studies for sunscreens, the clinical effect of plasma concentrations of BP-3 exceeding 0.5 ng/mL is unknown (Matta et al. 2019). Thus, this study and several others (Janjua et al. 2008; Krause et al. 2012, 2018; Darbre and Harvey 2015; Ghazipura et al. 2017), support the concern that has arisen about the potential implications of UV filters for human health, especially on reproductive outcomes. No experimental models address the immediate and midterm effect of UV filters applied topically on pregnancy outcome. In the present study we exposed pregnant mice to BP-3 at a daily-use dose. We have chosen a dose of 50 mg/kg bw day to mimic a realistic scenario of daily sunscreen use in spring and summer time (Janjua et al. 2008; Matta et al. 2019). We did so during the first seven days of pregnancy. This early application resembles the topical use of sunscreen even before pregnancy is noticed as well as during the first trimester. We studied the consequences of BP-3 on implantation outcome, implantation size throughout pregnancy, fetal and placental size as well as fetal and placental weight. We further recorded the postnatal weight development of the progeny after birth. The serum concentrations reported here after the last topical application of BP-3 on gd6 (internal dose) was lower than the plasma concentration reported in humans at the same time (Janjua et al. 2008; Matta et al. 2019). Our results suggest that dermal exposure to BP-3 at a low internal dose during early pregnancy could impair fetal development, resulting in growth-restricted fetuses. The intrauterine growth restriction (IUGR) phenotype of the fetuses was associated with transient changes in hemodynamic parameters at maternal uterine artery, reduced weight of male offspring and persistence of significant levels of BP-3 in amniotic fluid until gd14. After the first pregnancy, the same mothers were mated again refraining BP-3 exposure to understand whether the observed BP-3 effects are sustained long-term. In the second pregnancy, no changes in fetal or placental weights were recorded on gd10 or gd14. The placenta weights were, however, decreased compared to the values observed for the offspring of vehicle-treated mothers. A very interesting observation done in our studies was an alteration of the sex ratio of the progeny, with females predominating over males when analyzing both pregnancies. Thus, our results show that dermal exposure to BP-3 at a low internal dose during early pregnancy affects fetal growth and this has a long-lasting impact at least in males as they have both a lower weight than the controls and decreased % of male offspring when analyzed after birth.
Based on a teratogenicity study performed in rats, the Scientific Committee on Consumer Products (SCCP) of European Union deducted that the No-Observed-Adverse-Effect Level (NOAEL)-value for maternal and developmental toxicity is 200 mg/kg bw/day (SCCP 2008). This value was obtained from a prenatal developmental toxicity study performed following a valid test guideline and under GLP conditions upon oral gavage. In the same document, the NOAELs referred from a subchronic dermal reproduction toxicity screening test (SCCP 2008) for reproductive toxicity were 200 mg/kg bw/day for rats and 364 mg/kg bw/day for mice. The dose of BP-3 that we have used to apply on pregnant mice is lower than the NOAELs adopted by SCCP. Besides, the dose we tested in the present study is similar to the dose applied in humans by topical application of sunscreens on skin (Janjua et al. 2008; Matta et al. 2019). Janjua et al. (2008) applied an estimated/average dose of 51 mg/kg bw/day in 32 healthy volunteers and have shown that the maximum median plasma concentrations of BP-3 were 187 ng/mL for females and 238 ng/mL for men after a whole-body dermal application. More recently, the reported mean maximum plasma concentrations of BP-3 after sunscreen application under maximal use conditions (ie, applied at least every 2 h) were 209.6 ng/mL, 194.9 ng/mL or 169.3 ng/mL depending on formulation tested (Matta et al. 2019). Taking advantage of an analytical method able to measure BP-3 in biological fluids that we recently developed (Teglia et al. 2019), we determined an average serum concentration of 16.8 ng/mL of BP-3 in pregnant females 4 h after the last application at gd6. Thus, the internal dose in animals of our experiment was lower than the values reported in humans by Janjua et al. (2008) or Matta et al. (2019). Moreover, except for one animal, we found no measurable serum levels of BP-3 at gd14 but, instead, we found that BP-3 is measurable in amniotic fluid at gd14 (average concentration of 22.63 ng/mL, last application took place at gd6). This is in accordance with other studies in humans that detected BP-3 in 61% of amniotic fluid samples (Claire et al. 2013) or even in amniotic fluid and cord blood paired samples (Krause et al. 2012, 2018; Zhang et al. 2013; Buck Louis et al. 2014). Thus, our results add important evidence of BP-3 presence in amniotic fluid, suggesting its ability to reach feto-maternal compartments, which confirms a risk for direct fetal exposure.
Despite the widespread exposure to BP-3, there are few studies evaluating its effects on human reproductive outcomes (Ghazipura et al. 2017). Some studies reported associations between BP-3 levels in maternal urine samples and reduced duration of pregnancy or alterations in infant birth weight (Wolff et al. 2008; Claire et al. 2012; Tang et al. 2013). Even considering that the body of literature regarding effects of BP-3 on reproductive outcomes is larger in rodent species, the reported results are often conflicting and, particularly, studies performed by dermal route are scarce (French 1992; Ghazipura et al. 2017).
In the present work, we evaluated the impact of a dermal exposure to BP-3 during early murine pregnancy on pregnancy outcome. To do that, we have studied implantation outcome, implantation size throughout pregnancy, fetal and placental size as well as fetal and placental weight in a setting where BP-3 was applied from gd0 to gd6. We found that dermal exposure to BP-3 had no negative impact on implantation and no increased abortion was observed after BP-3 treatment. These results are in agreement with those referred by Nakamura et al. (2015), who observed no differences in the number of implantation sites/litter, resorptions/litter, number and weights of live fetuses in pregnant Harlan Sprague–Dawley rats after treatment with different doses of BP-3. To gain insights whether fetal growth was normal after implantation on day 5, high-frequency ultrasound was performed at gd8, gd10 and gd12. Measurements of the implantation areas revealed no differences in implantation size between BP-3-treated and control animals. Hence, we conclude that, in our experimental setting, BP-3 did not negatively influence implantation and fetal growth at early to mid-pregnancy. Moreover, we also observed that placental parameters were unaffected after BP-3 exposure. Concordantly with normal placental parameters observed in gd10 and gd12, the placenta weight was also unaffected in gd14. However, we observed a significant decrease in fetal weight and consequently a reduced FPI at the same day. In humans, fetal growth restriction (FGR), usually referred to as IUGR, is defined as the estimated fetal weight being below the 10th percentile of the normal value for a determined population. FGR or IUGR calls for extensive medical attention as the fetus may be undernourished as a consequence of a maternal or pregnancy pathology. Besides, fetuses with measurements below the 5th percentile are considered severely growth restricted (Zhang‐Rutledge et al. 2018). Doppler abnormalities usually precede IUGR, as they are the first indication that the blood supply from the mother to the fetus is inadequate. Gordijn SJ et al. (2016) define IUGR as estimated fetal weight below the 10th percentile and uterine artery PI above the 95th percentile (Gordijn et al. 2016). Dall'Asta et al. (2017) and Krishna and Bhalero (2011) showed that high resistance or pulsatility indices are signs for IUGR (Krishna and Bhalerao 2011; Dall'Asta et al. 2017). Our results showed that dermal exposition to a low dose of BP-3 leads to 16.3% of all fetuses being below the 5th percentile. This is further accompanied by transient changes in Doppler parameters of the UA at mid-pregnancy. Specifically, we found an increase of PI, S/D ratio and RI at gd10. Therefore, our results suggest that a short-term exposure to BP-3 leads to a clear IUGR phenotype in mice, with 16.3% of fetuses having a severe growth restriction. This alarming fact should not stay unnoticed as the health of the fetuses is in danger.
One recent study reported a decrease in the body weights of pups born to mothers orally treated with BP-3 at the highest dose at PND14 (50,000 ppm, equivalent to 7178.5 mg/kg bw/animal at PND14) (Nakamura et al. 2015). We found a decrease of male offspring weight after birth at PND 4, 10, 13, 16 and 19 by BP-3 exposure. On the contrary, female offspring showed a transitory decrease, showing normal weights at weaning. Taking into consideration that we also found transient alterations of Doppler parameters of UA at mid-pregnancy, is interesting to note that the early detection of an altered hemodynamical profile of UA is typical of pregnancies with high risk for development of small fetuses for gestational age (Barati et al. 2014).
After the first pregnancy, the same mothers were subjected to a second pregnancy, but without exposing them to BP-3. In their second pregnancy, we observed a decrease of placenta’s weight but no alterations in fetal or pups growth. Placental growth/development is regulated by epigenetic pattern (Vaiman 2017; McAninch et al. 2017). Taking into consideration that EDCs have been shown to affect epigenetic marks such as DNA methylation and histone modifications, and an epigenetic response of placenta to EDCs had been very well established (Grindler et al. 2018; Strakovsky and Schantz 2018; Leppert et al. 2020), epigenetic changes might be part of the underlying mechanisms altering placental growth. The impact of BP-3 in fetal growth prompted the question about the mechanisms leading to IUGR phenotype. Whereas alterations in Doppler flow studies of the UmA can reflect abnormalities in the fetal side of placental resistance, those observed in UA reflect the maternal side of placental resistance and are associated with a poor blood supply from the mother to the fetus with negative consequences (Sciscione and Hayes 2009). Despite the low fetal weight, in the BP-3-treated group, no differences were detected in placenta parameters (area, diameter and thickness) compared with the animals exposed to vehicle. We also evaluated SA remodeling, a crucial process to ensure that maternal blood reaches the fetus. In our experimental model, SA remodeling was unchanged in BP-3-exposed mothers. Future studies including the evaluation of cellular and molecular pathways will shed light to the underlying mechanisms.
To understand whether the impact of maternal exposure to BP-3 was different in female vs male fetuses, pups were sexed and weighted every 3 days starting on PND1 until weaning in first and second pregnancies. Analyzing both pregnancies, we determined that the percentage of females (female sex ratio) increased after BP-3 exposure (84%) compared to the control group (64%). We consider this result as a very important event, because altered sex ratio in a given population is considered an indicator of endocrine disruption (Mackenzie et al. 2005; Larebeke et al. 2008). For instance, it was established a significant decrease in the male–female sex ratio in a community living amid of petrochemical plants (Mackenzie et al. 2005). Other study shows that sex ratio at birth has declined significantly in USA population over the past four decades (Davis et al. 2007). Changes in sex ratio have been emphasized as a useful indicator of public health (Larebeke et al. 2008), because it is used to assess the reproduction of populations with demonstrated exposures to EDCs (Mocarelli et al. 1996), as well as in communities near hazardous chemical sites (Williams et al. 1995). In summary, independent of in which direction the sex ratio is skewed, any deviation of normal ratio is considered as sign of alarm. Among the lot of factors that could influence sex ratio, EDCs are one of them. Thus, our results suggest that exposure to BP-3 at a low dose dose could be one contributing factor to biased sex ratio.
This should urgently call for more experimental studies analyzing the underlying mechanisms behind these observations. Further, as the exposure to BP-3 took place in a narrow window of pregnancy, we also call for settings where BP-3 exposure takes place through a longer period and in combination with further EDCs contained in PCPs so to have a more realistic picture of the situation.
Conclusions
Using a mouse model, we show that the exposure to BP-3 as contained in sunscreens during early pregnancy was associated with an IUGR phenotype, disturbed sex ratio and alterations in the growth curve of male offspring. This is in accordance with other studies showing the higher vulnerability of male fetuses to environmental conditions (Rosenfeld and Roberts 2004; Pongou 2013). This alarming fact should not remind unnoticed and future studies will help to confirm these effects and understanding the underlying mechanisms.
To our knowledge, this is the first time that IUGR phenotype and disturbed sex ratio caused by exposure to the UV filter BP-3 at a low internal dose are reported. Based on that sunscreens have not been subjected to standard drug safety testing (Califf and Shinkai 2019), our results reinforce the need of performing more studies designed to evaluate the safety and effectiveness of these products.
References
Barati M, Shahbazian N, Ahmadi L, Masihi S (2014) Diagnostic evaluation of uterine artery Doppler sonography for the prediction of adverse pregnancy outcomes. J Res Med Sci 19:515–519
Bergman Å, United Nations Environment Programme, World Health Organization (2013) State of the science of endocrine disrupting chemicals - 2012 an assessment of the state of the science of endocrine disruptors. WHO, UNEP, Geneva
Buck Louis GM, Kannan K, Sapra KJ et al (2014) Urinary Concentrations of Benzophenone-Type Ultraviolet Radiation Filters and Couples’ Fecundity. Am J Epidemiol 180:1168–1175. https://doi.org/10.1093/aje/kwu285
Calafat AM, Wong L-Y, Ye X et al (2008) Concentrations of the Sunscreen Agent Benzophenone-3 in Residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ Health Perspect 116:893–897. https://doi.org/10.1289/ehp.11269
Califf RM, Shinkai K (2019) Filling in the Evidence About Sunscreen. JAMA 321:2077–2079. https://doi.org/10.1001/jama.2019.5528
Casas L, Fernández MF, Llop S et al (2011) Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int 37:858–866. https://doi.org/10.1016/j.envint.2011.02.012
Claire P, Marion M, Cécile C et al (2012) Exposure to Phthalates and Phenols during Pregnancy and Offspring Size at Birth. Environ Health Perspect 120:464–470. https://doi.org/10.1289/ehp.1103634
Claire P, Wolff MS, Calafat AM et al (2013) Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluid and Variability in Urinary Concentrations during Pregnancy. Environ Health Perspect 121:1225–1231. https://doi.org/10.1289/ehp.1206335
Dall’Asta A, Brunelli V, Prefumo F et al (2017) Early onset fetal growth restriction. Matern Health Neonatol Perinatol. https://doi.org/10.1186/s40748-016-0041-x
Darbre PD, Harvey PW (2015) Regulatory Considerations for Dermal Application of Endocrine Disrupters in Personal Care Products. In: Endocrine Disrruption and Human Health, Darbre Philippa D. Academic Press, pp 343–361
Davis DL, Webster P, Stainthorpe H et al (2007) Declines in Sex Ratio at Birth and Fetal Deaths in Japan, and in U.S. Whites but Not African Americans. Environ Health Perspect 115:941–946. https://doi.org/10.1289/ehp.9540
Faraci M, Renda E, Monte S et al (2011) Fetal growth restriction: current perspectives. J Prenat Med 5:31–33
Frederiksen H, Jensen TK, Jørgensen N et al (2014) Human urinary excretion of non-persistent environmental chemicals: an overview of Danish data collected between 2006 and 2012. Reproduction 147:555–565. https://doi.org/10.1530/REP-13-0522
Frederiksen H, Nielsen JK, Mørck TA et al (2013) Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int J Hyg Environ Health 216:772–783. https://doi.org/10.1016/j.ijheh.2013.02.006
French JE (1992) NTP technical report on the toxicity studies of 2-Hydroxy-4-methoxybenzophenone (CAS No. 131–57-7) Administered Topically and in Dosed Feed to F344/N Rats and B6C3F1 Mice. Toxic Rep Ser 21:1–E14
Gao C, Liu L, Ma W et al (2015) Benzonphenone-type UV filters in urine of Chinese young adults: Concentration, source and exposure. Environ Pollut 203:1–6. https://doi.org/10.1016/j.envpol.2015.03.036
Ghazipura M, McGowan R, Arslan A, Hossain T (2017) Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies. Reprod Toxicol 73:175–183. https://doi.org/10.1016/j.reprotox.2017.08.015
Gordijn SJ, Beune IM, Thilaganathan B et al (2016) Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 48:333–339. https://doi.org/10.1002/uog.15884
Grindler NM, Vanderlinden L, Karthikraj R et al (2018) Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci Rep. https://doi.org/10.1038/s41598-018-24505-w
Hayden CG, Roberts MS, Benson HA (1997) Systemic absorption of sunscreen after topical application. The Lancet 350:863–864. https://doi.org/10.1016/S0140-6736(05)62032-6
Heffernan AL, Baduel C, Toms LML et al (2015) Use of pooled samples to assess human exposure to parabens, benzophenone-3 and triclosan in Queensland, Australia. Environ Int 85:77–83. https://doi.org/10.1016/j.envint.2015.09.001
Hill M (2020) Implantation - Embryology. https://embryology.med.unsw.edu.au/embryology/index.php/Implantation.
Janjua NR, Kongshoj B, Andersson A-M, Wulf HC (2008) Sunscreens in human plasma and urine after repeated whole-body topical application. J Eur Acad Dermatol Venereol 22:456–461. https://doi.org/10.1111/j.1468-3083.2007.02492.x
Janjua NR, Mogensen B, Andersson A-M et al (2004) Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol 123:57–61. https://doi.org/10.1111/j.0022-202X.2004.22725.x
Kim S, Choi K (2014) Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ Int 70:143–157. https://doi.org/10.1016/j.envint.2014.05.015
Krause M, Frederiksen H, Sundberg K et al (2018) Presence of benzophenones commonly used as UV filters and absorbers in paired maternal and fetal samples. Environ Int 110:51–60. https://doi.org/10.1016/j.envint.2017.10.005
Krause M, Klit A, Jensen MB et al (2012) Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl 35:424–436. https://doi.org/10.1111/j.1365-2605.2012.01280.x
Krishna U, Bhalerao S (2011) Placental insufficiency and fetal growth restriction. J Obstet Gynaecol India 61:505–511. https://doi.org/10.1007/s13224-011-0092-x
Larebeke NAV, Sasco AJ, Brophy JT et al (2008) Sex Ratio Changes as Sentinel Health Events of Endocrine Disruption. International Journal of Occupational and Environmental Health 14:138–143. https://doi.org/10.1179/oeh.2008.14.2.138
Leppert B, Strunz S, Seiwert B et al (2020) Maternal paraben exposure triggers childhood overweight development. Nat Commun 11:561. https://doi.org/10.1038/s41467-019-14202-1
Loraine GA, Pettigrove ME (2006) Seasonal Variations in Concentrations of Pharmaceuticals and Personal Care Products in Drinking Water and Reclaimed Wastewater in Southern California. Environ Sci Technol 40:687–695. https://doi.org/10.1021/es051380x
Mackenzie CA, Lockridge A, Keith M (2005) Declining Sex Ratio in a First Nation Community. Environ Health Perspect 113:1295–1298. https://doi.org/10.1289/ehp.8479
Matta MK, Zusterzeel R, Pilli NR et al (2019) Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 321:2082–2091. https://doi.org/10.1001/jama.2019.5586
McAninch D, Roberts CT, Bianco-Miotto T (2017) Mechanistic Insight into Long Noncoding RNAs and the Placenta. Int J Mol Sci 18:1371. https://doi.org/10.3390/ijms18071371
Mocarelli P, Brambilla P, Gerthoux PM et al (1996) Change in sex ratio with exposure to dioxin. The Lancet 348:409. https://doi.org/10.1016/S0140-6736(05)65030-1
Molins-Delgado D, del Olmo-Campos M, Valeta-Juan G et al (2018) Determination of UV filters in human breast milk using turbulent flow chromatography and babies’ daily intake estimation. Environ Res 161:532–539. https://doi.org/10.1016/j.envres.2017.11.033
Müller JE, Meyer N, Santamaria CG et al (2018) Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Scientific Reports 8:9196. https://doi.org/10.1038/s41598-018-27575-y
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Nakamura N, Inselman AL, White GA et al (2015) Effects of maternal and lactational exposure to 2-hydroxy-4-methoxybenzone on development and reproductive organs in male and female rat offspring. Birth Defects Res B Dev Reprod Toxicol 104:35–51. https://doi.org/10.1002/bdrb.21137
Pongou R (2013) Why Is Infant Mortality Higher in Boys Than in Girls? A New Hypothesis Based on Preconception Environment and Evidence From a Large Sample of Twins. Demography 50:421–444. https://doi.org/10.1007/s13524-012-0161-5
Rosenfeld CS, Roberts RM (2004) Maternal Diet and Other Factors Affecting Offspring Sex Ratio: A Review. Biol Reprod 71:1063–1070. https://doi.org/10.1095/biolreprod.104.030890
Santamaría CG, Abud JE, Porporato MM et al (2019) The UV filter benzophenone 3, alters early follicular assembly in rat whole ovary cultures. Toxicol Lett 303:48–54. https://doi.org/10.1016/j.toxlet.2018.12.016
Schlumpf M, Cotton B, Conscience M et al (2001) In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect 109:239–244
Schreurs RHMM, Sonneveld E, Jansen JHJ et al (2005) Interaction of Polycyclic Musks and UV Filters with the Estrogen Receptor (ER), Androgen Receptor (AR), and Progesterone Receptor (PR) in Reporter Gene Bioassays. Toxicol Sci 83:264–272. https://doi.org/10.1093/toxsci/kfi035
Sciscione AC, Hayes EJ (2009) Uterine artery Doppler flow studies in obstetric practice. Am J Obstet Gynecol 201:121–126. https://doi.org/10.1016/j.ajog.2009.03.027
Stackelberg PE, Furlong ET, Meyer MT et al (2004) Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci Total Environ 329:99–113. https://doi.org/10.1016/j.scitotenv.2004.03.015
Strakovsky RS, Schantz SL (2018) Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ Epigenet. https://doi.org/10.1093/eep/dvy022
Tang R, Chen M, Ding G et al (2013) Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut 178:115–120. https://doi.org/10.1016/j.envpol.2013.03.023
Teglia CM, Santamaría CG, Rodriguez HA et al (2019) Determination of 2-hydroxy-4-methoxybenzophenone in mice serum and human plasma by ultra-high-performance liquid chromatography enhanced by chemometrics. Microchem J 148:35–41. https://doi.org/10.1016/j.microc.2019.04.032
Vaiman D (2017) Genes, epigenetics and miRNA regulation in the placenta. Placenta 52:127–133. https://doi.org/10.1016/j.placenta.2016.12.026
Vela-Soria F, Rodríguez I, Ballesteros O et al (2014) Simplified matrix solid phase dispersion procedure for the determination of parabens and benzophenone-ultraviolet filters in human placental tissue samples. J Chromatogr A 1371:39–47. https://doi.org/10.1016/j.chroma.2014.10.063
Williams FL, Ogston SA, Lloyd OL (1995) Sex ratios of births, mortality, and air pollution: can measuring the sex ratios of births help to identify health hazards from air pollution in industrial environments? Occup Environ Med 52:164–169
Wolff MS, Engel SM, Berkowitz GS et al (2008) Prenatal Phenol and Phthalate Exposures and Birth Outcomes. Environ Health Perspect 116:1092–1097. https://doi.org/10.1289/ehp.11007
Zenclussen ML, Thuere C, Ahmad N et al (2010) The persistence of paternal antigens in the maternal body is involved in regulatory T-cell expansion and fetal-maternal tolerance in murine pregnancy. Am J Reprod Immunol 63(3):200–208. https://doi.org/10.1111/j.1600-0897.2009.00793.x
Zhang T, Sun H, Qin X et al (2013) Benzophenone-type UV filters in urine and blood from children, adults, and pregnant women in China: Partitioning between blood and urine as well as maternal and fetal cord blood. Sci Total Environ 461–462:49–55. https://doi.org/10.1016/j.scitotenv.2013.04.074
Zhang-Rutledge K, Mack LM, Mastrobattista JM, Gandhi M (2018) Significance and Outcomes of Fetal Growth Restriction Below the 5th Percentile Compared to the 5th to 10th Percentiles on Midgestation Growth Ultrasonography. J Ultrasound Med 37:2243–2249. https://doi.org/10.1002/jum.14577
Acknowledgements
We are very thankful to Stefanie Langwisch, who was in charge of the mouse colonies and to Markus Scharm, Walter Nykolajczuk and Laura Bergero for his technical support and Abud Julián. This work was funded by grants from ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica) Projects PICT 2017-0340 and PICT 2017-1532; CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Project PIP-2015 Nº 0111) and Universidad Nacional del Litoral (Project CAI + D 2016-50120150100110LI) and grants 01DN16021 from DLR/BMBF and from the German Research Foundation (DFG, ZE 526/12-1) to ACZ. Mobility grants from MinCyT (Ministerio de Ciencia y Tecnología, Argentina AL//15/06) and BMBF (Germany) to HAR and ACZ enabled the interchange of scientists between Argentina and Germany. CGS and CMT are postdoctoral fellows from CONICET. MLZ, MJC and HAR are career Investigators of CONICET.
Author information
Authors and Affiliations
Contributions
Conceptualization: ACZ and HAR; Methodology: CGS, NM, AS, CMT, MJC. Full analysis and investigation: CGS, NM, AS; MLZ. Writing–original draft preparation: CGS, HAR, MLZ; NM. Writing–review and editing: ACZ, HAR. Funding acquisition: ACZ, HAR, MJC; CMT. Resources: ACZ, HAR. Supervision: ACZ, HAR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted: Landesverwaltungsamt Sachsen Anhalt: 42502-21296UniMD, Magdeburg, Germany; Ethical committee of the Facultad de Bioquímica y Ciencias Biológicas, UNL, Santa Fe, Argentina, CE2018-62. The experiments were conducted by authorized persons according to the Guide for Care and Use of Animals in Agriculture Research and Teaching. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santamaria, C.G., Meyer, N., Schumacher, A. et al. Dermal exposure to the UV filter benzophenone-3 during early pregnancy affects fetal growth and sex ratio of the progeny in mice. Arch Toxicol 94, 2847–2859 (2020). https://doi.org/10.1007/s00204-020-02776-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02776-5