Abstract
Despite growing concern about adverse effects of bisphenol AF (BPAF) due to its endocrine disrupting properties, there is a lack of toxicity data from low-dose studies and direct evidence linking its adverse effects to endocrine disrupting properties. Here, we investigated the effects of gestational and postnatal exposure to BPAF through drinking water (0.15–15 μg/mL, equivalent to the daily intake of ~ 50 and 5 mg/kg/day) on testis development in mice. We found that like mestranol, 5 mg/kg/day BPAF resulted in remarkable decreases in multiple male reproductive parameters in adulthood, such as the sperm number and serum testosterone level. Notably, 50 μg/kg/day BPAF also caused significant decreases in anogenital distance (AGD), the luteinizing hormone level and spermatocyte number, along with declining trends in sperm number and the serum levels of testosterone and follicle-stimulating hormone. In line with the adverse outcomes observed in adulthood, on postnatal day (PND) 9, we also observed BPAF-caused dose-dependent alterations, including reduced AGD, seminiferous tubule area and numbers of total germ cells, spermatocytes and Leydig cells, coupled with down-regulated expression of male-biased genes in testes. Even when exposure to 5 mg/kg/day BPAF as well as MES was initiated from PND 0, similar alterations in male reproductive parameters were also found on PND 9, along with a decrease in the GnRH content in the hypothalamus; moreover, testicular alterations and the reduction in AGD were partly antagonized by the estrogen receptor (ER) antagonist ICI 182,780, but the reduction of GnRH production was not done, showing that the effects of BPAF on testis development may be partially mediated by ER signaling. In conclusion, all the findings demonstrate that low-dose BPAF can partly disrupt mammal testis development and cause adverse testicular outcomes in adulthood, indicating a potential reproductive risk to mammals including humans. Importantly, our finding that developmental alterations elicited by BPAF have been detectable on PND 9 provides important motivation for the development of effective methods for early detection of adverse effects of estrogenic chemicals on testis development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphenol AF (BPAF) is a chemical commonly used in the manufacturing of fluoroelastomers, polyimides, polyamides, polyesters, polycarbonate copolymers and other specialty polymers (ECHA 2020; NTP 2008). Due to its widespread use, BPAF has become ubiquitous in the environment, and even parts-per-billion levels of BPAF have been detected in water (15.3 μg/L) and indoor dust (739 ng/g) near manufacturing plants (Chen et al. 2016; Liu et al. 2021a, 2017; Song et al. 2012; Yang et al. 2014b). Unfortunately, BPAF has been also detected in human urine (up to 0.173 ng/mL), serum (up to 0.404 ng/mL), breast milk (up to 0.58 ng/mL) and even cord blood and placenta (Li et al. 2020; Yang et al. 2014a; Zhang et al. 2020). Especially, BPAF concentrations in cord plasma seem to be higher than those in paired maternal plasma (Pan et al. 2020), which agrees with the observations in experimental rodents (Waidyanatha et al. 2021). The presence of BPAF in humans has raised great concern about its adverse health effects, especially during gestational and lactational periods.
BPAF has been demonstrated to possess estrogenic activity mediated by estrogen receptors (ERα and ERß), with a higher potency than bisphenol A (BPA) (Kitamura et al. 2005; Matsushima et al. 2010), while several studies suggested its non-genomic estrogenic property mediated by G protein-coupled estrogen receptor (GPER) (Cao et al. 2017; Liu et al. 2021b; Yang et al. 2020). In addition, BPAF was also reported to act as androgen receptor (AR) antagonist in in vitro cells and in Hershberger assay (Teng et al. 2013; Yamasaki et al. 2003). Meanwhile, there are increasing data reporting adverse effects of BPAF on both adult and developing mammals (Escriva et al. 2021), yet most data are based on high-dose exposure. Take reproductive toxicity as an example, relevant data were generally from BPAF exposure at higher levels than 2 mg/kg body weight (bw)/day (Feng et al. 2012; Li et al. 2016; Umano et al. 2012; Wu et al. 2019; Yu et al. 2022), which is much higher than the human-relevant level. Additionally, despite growing data on adverse effects of BPAF, there is a lack of direct evidence linking adverse effects to its endocrine disrupting properties.
Given the high susceptibility of male reproductive development to estrogenic chemicals (Martin et al. 2008; Toppari et al. 1996), we aimed to reveal the effects of pre- and postnatal exposure to BPAF at nominal doses of ~ 50 and ~ 5000 μg/kg/day with mestranol (MES) as a positive control, on testis development in mammals. According to the bioavailability (6%) of BPAF in male mice (Waidyanatha et al. 2019), dosing of 50 μg/kg/day was estimated to result in a plasma concentration close to the serum concentrations reported in humans (Li et al. 2020). Following exposure from gestational day (GD) 10.5 to postnatal day (PND) 56, male mice were examined for testicular alterations at multiple levels at the end of exposure. In addition, we also dissected the effects of BPAF on testis development in infancy (PND 9) to explore the possibility of early detecting adverse effects of chemicals on testis development. Importantly, we investigated the antagonistic actions of the ER antagonist ICI 182,780 on BPAF- and MES-caused alternations to determine whether BPAF-caused effects were associated with its estrogenic property. This study would highlight potential reproductive risk of low-dose BPAF to mammals including humans, and establish a direct link between its adverse effects and estrogenic activity.
Materials and methods
Animals
Time-bred pregnant CD-1 mice at GD 8.5 obtained from the Charles River (Beijing, China) were allowed to acclimatize for two days prior to exposure. All animals were housed in a temperature-controlled facility with a 12-h light/dark cycle. Food and water were available ad libitum. Animal use was approved by the Animal Ethics Committee at the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (AEWC-RCEES-2022002).
Exposure method and sample collection
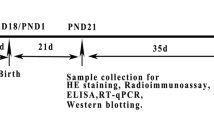
The study design is shown in Fig. 1. For experiment I, beginning on GD 10.5 and continuing through postpartum day 21, dams (n = 5) were given 0.1% dimethyl sulfoxide (DMSO as the solvent control), 0.15 μg/mL or 15 μg/mL BPAF (Sigma-Aldrich, USA), or 0.15 μg/mL MES (APExBIO, USA) in drinking water. Given the rapid development of the mammal testis within the first two weeks after birth, we anticipate that the effects of BPAF are detectable at this early stage, and thereby examined testicular alterations on PND 9. Three male pups per litter (n = 5 litters, 15 pups in total) were killed after euthanasia on PND 9. The testes were collected for RNA extraction and subsequent RT-qPCR analysis (left testis) or histological and immunofluorescence (IF) staining (right testis). Remaining male pups were housed five per cage after weaning with a follow-up exposure. On PND 56, 15 animals from 5 litters were killed for blood collection and anogenital distance (AGD) measurement, and then testes and cauda epididymis were collected for further analysis. Based on the daily dose of water consumption and body weight, the average daily intake of BPAF were estimated to be approximately 53.11 μg/kg/day and 5780 μg/kg/day for dams in 0.15 μg/mL and 15 μg/mL groups, with 42.26 μg/kg/d and 3960 μg/kg/day for weaned pups, respectively. Hereinafter, we defined the two BPAF groups as 50 μg/kg/day and 5000 μg/kg/day groups according to the doses for dams. According to the 6% bioavailability of BPAF in male mice (Waidyanatha et al. 2019), oral ingestion of 50 μg/kg per day was estimated to result in 3 μg/kg (6% × 50 μg/kg) entering in the blood, thereby be calculating to a maximum plasma concentration of 0.6 μg/kg (ng/ml) (0.2 × 3 μg/kg) based on the BPAF toxicokinetics.
Study design. Experiment I was designed for studying the effects of bisphenol AF (BPAF) on testis development in male mice (n = 5 litters, 3 male mice/litter on PNDs 9 and 56). Experiment II was designed for investigating whether the effects of BPAF on the testis development were due to its genomic estrogenic property (n = 3 litters, 3 male mice/litter). LH, luteinizing hormone; FSH, follicle-stimulating hormone
Experiment II was conducted to determine whether the effects of BPAF on testis development were due to its genomic estrogenic property. Newborn male pups (PND 0) were divided into six groups by given 0.1% DMSO, 5 mg/kg/day BPAF, or 50 μg/kg/day MES to their mothers by drinking water and injected intraperitoneally with 25 μL of olive oil or ICI 182,780 (Meilunbio, Dalian, China) at the dose of 0.5 mg/kg/day, referring to the previous study by Lee (1998). On PND 9, testes of male pups were collected (n = 3 litters including 9 male pups) for further analysis. In addition, hypothalamus tissue was also collected for analysis for GnRH content and Gnrh expression, given crucial roles of GnRH in the production of luteinizing hormone (LH) and testosterone and subsequent testicular development and spermatogenesis.
RNA extraction and RT-qPCR
The total RNA was extracted from testes or hypothalamus according to methods described in Supporting Information. RT-qPCR was conducted to analyze the expression of genes we concerned, including hormones or their receptors (Gnrh, Ar, Fshr, Esr1, Esr2, and Gper), Leydig cell marker genes (Cyp17a1 and 3β-Hsd), germ cell marker genes (Sycp3, γH2ax, Dmc1 and Pou5f2), and ovarian marker genes (Cyp19a1, Foxl2, and Rspo1).
Histological examination and IF staining
Histological examination and IF staining are described in Supporting Information. Based on IF images, the germ cell number per seminiferous tubule as well as the numbers of spermatocytes and Leydig cells per 200 × field were counted using ImageJ software (National Institutes of Health, USA; version 1.53c). Based on the images of LAMININ1 IF for the lamina of the seminiferous tubule, the tubule area was measured. Staging of seminiferous epithelium cycle was performed based on PAS-stained sections.
Measurement of the testosterone, LH, FSH, and GnRH
The content of GnRH (Qisong Bio, QS43291) in the tissue homogenate of the hypothalamus on PND 9 and levels of serum testosterone (Cayman Chemical, Cat NO. 582701), LH (Qisong Bio, QS43318), follicle-stimulating hormone, follicle-stimulating hormone (FSH) (Qisong Bio, QS43242) on PND 56 were determined by the enzyme-linked immunosorbent assay (ELISA) according to the manufacture’s protocol. All the samples were analyzed in the same assay in duplicates.
Sperm analysis
Spermatozoa were squeezed out from the caudal epididymis and incubated for 30 min at 37 °C as previous reported (Li et al. 2022). The incubated sperm medium was then diluted 1:500 and transferred to a hemocytometer for counting. Sperm motility evaluation was performed by the same person throughout the study by visual estimation (100 spermatozoa per animal, in duplicate) under a phase-contrast microscope (Carl Zeiss, Germany) at 200 × magnification. Then sperm number was counted using a hemocytometer.
Statistical analysis
Data are shown as mean ± standard deviation (SD). Data were analyzed by SPSS (version 16.0) or GraphPad Prism 8 software. If homogeneity, data were analyzed by independent t test (between two groups) or one-way ANOVA (treatment groups relative to the control group) followed by Duncan (equal variances assumed) and Dennett’s T3 (equal variances not assumed). When samples were heterogeneous, nonparametric analysis of variance was applied. Testis weight was analyzed using analysis of covariance with the body weight as a covariate. A p < 0.05 was considered statistically significant.
Results
Effects of BPAF on testis development in male mice on PND 9
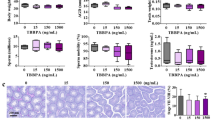
Given rapid testis development within the first two weeks after birth, we examined testicular changes of treated pups on PND 9. No difference in body weight was observed between BPAF-treated animals and controls, while the MES-treated pups showed slighter body weights compared to controls (Fig. 2A). Pups in the 5000 μg/kg/day BPAF group displayed higher liver weights compared to controls, with a decrease in the MES group (Fig. 2A). Expectedly, MES treatment resulted in reduced AGD in male pups; similarly, the 5000 μg/kg/day BPAF group also exhibited smaller AGD than the control group (p < 0.05, Fig. 2A). The cross-sectional area of the seminiferous tubule was significantly smaller in all BPAF and MES groups than that in the control group (p < 0.05, Fig. 2B, F). While MES-treated males exhibited fewer total germ cells (marked by DAZL IF), spermatocytes (marked by SYCP3 IF), and Leydig cells (marked by 3β-HSD IF) compared to controls, similar alterations were also observed in the 5000 μg/kg/day BPAF group (p < 0.05, Fig. 2C–F). Remarkably, AGD, the seminiferous tubule area and the spermatocyte number per tubule in the 50 μg/kg/day BPAF group were also significantly decreased compared to the control group (p < 0.05, Fig. 2D, F).
Effects of prenatal and postnatal exposure to bisphenol AF (BPAF) on testis development in male mice on postnatal day 9 (n = 5 litters, 3 male mice/litter). A The body weight, liver weight and anogenital distance of male mice. B The seminiferous tubule marked by immunostaining for LAMININ1. C Germ cells marked by immunostaining for DAZL. D Spermatocytes marked by immunostaining for SYCP3. E Leydig cells marked by immunostaining for 3β-HSD. F the seminiferous tubule area, germ cell number per tubule, number of spermatocytes and Leydig cells in the 200 × field. For seminiferous tubule area and germ cell number per tubule, at least 100 seminiferous tubule cross sections per testis and 5 litters per group were used for statistical analysis. For spermatocyte number and Leydig cell number, at least three 200 × field per testis and five litters per group were used for statistical analysis. Asterisk (*) indicates significant difference from control (p < 0.05). CON, control; BPAF-50, 50 μg/kg/day BPAF; BPAF-5000, 5000 μg/kg/day BPAF; MES, 50 μg/kg/day mestranol as positive control
In agreement with IF results, RT-qPCR analysis revealed that MES treatment led to lower expression of spermatocyte marker genes (Sycp3, γH2ax, Dmc1, and Pou5f2), Leydig cell marker genes (3β-Hsd and Cyp17a1) and hormone receptors (Ar and Fshr), along with higher expression of female-biased genes (Cyp19a1, Rspo1, Foxl2 and Esr2) compared to the control group (p < 0.05, Fig. 3A–C). Similarly, significant alterations in these endpoints were also observed in the 5000 μg/kg/day BPAF-treated animals. In addition, Cyp19a1 expression in the 50 μg/kg/day BPAF group was significantly lower than that in the control group (p < 0.05, Fig. 3C).
Effects of prenatal and postnatal exposure to bisphenol AF (BPAF) on relative expression of spermatocyte marker genes (A), Leydig cell marker genes and hormone receptors (B), and ovarian biased genes (C) on postnatal day 9 (n = 5 litters, 3 male mice/litter). Data are shown as mean ± standard deviation (n = 5). Asterisk (*) indicates significant difference from control (p < 0.05). CON, control; BPAF-50, 50 μg/kg/day BPAF; BPAF-5000, 5000 μg/kg/day BPAF; MES, 50 μg/kg/day mestranol as positive control
Taken together, these alterations in molecular, cellular, histological and morphological parameters demonstrate that gestational and lactational exposure to BPAF even at 50 μg/kg/day, like MES, affected testis development. In addition, up-regulated expression of female-biased genes indicates that BPAF exposure caused ovary-like characteristics in mouse testes to some degree.
Effects of BPAF on testis development in male mice on PND 56
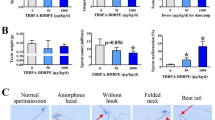
When exposure continued to adulthood, MES-treated mice displayed reduced AGD, testis weight, sperm number in cauda epididymis and serum levels of testosterone, FSH and LH as well as lower body weight and liver weight compared with controls (p < 0.05, Fig. 4A). Decreases in body weight, AGD, sperm number and hormone levels were also observed in BPAF-treated mice, despite the lack of significant differences in the sperm number, testosterone level (reduced by nearly 1/2; p = 0.06), and FSH (p = 0.06) between the 50 μg/kg/day group and the control group (Fig. 4A). Correspondingly, we observed significant decreases in the percentage of stage VII-VIII seminiferous tubules, spermatocytes (SYCP3+) per 200 × field, and expression of Sycp3 in the 5000 μg/kg/day BPAF group as well as the MES group compared to the control group (p < 0.05, Fig. 4B–E). Importantly, 50 μg/kg/day BPAF also caused significant decreases in spermatocyte number and Sycp3 expression, along with a declining trend in the percentage of stage VII–VIII seminiferous tubules (Fig. 4B–E). All alterations in conventional reproductive endpoints and the spermatocyte number show that like MES, BPAF even at 50 μg/kg/day affected testis development and caused adverse testicular outcomes in adulthood.
Alterations in testicular and relevant parameters in adult mice following prenatal and postnatal exposure to bisphenol AF (BPAF) (n = 5 litters, 3 male mice/litter). A Conventional reproductive endpoints including anogenital distance, testis weight, sperm number in cauda epididymis, and serum hormones. B Testicular histology. C Spermatocytes marked by immunostaining for SYCP3. D Seminiferous epithelium cycle. E Spermatocyte number in the 200 × field and relative expression of spermatocyte marker gene (Sycp3). For seminiferous epithelium cycle, at least 100 seminiferous tubule cross-sections per testis and five litters male mice per group were used for statistical analysis. For spermatocyte number, at least three 200 × field per testis and five litters male mice per treatment group were used for statistical analysis. CON, control; BPAF-50, 50 μg/kg/day BPAF; BPAF-5000, 5000 μg/kg/day BPAF; MES, 50 μg/kg/day mestranol as positive control
Link between adverse effects of BPAF on testis development and its estrogenic activity
In line with the results of exposure from GD 10.5, postnatal exposure to MES during PND 0–9 also led to lower body weight and AGD, and fewer spermatocytes and Leydig cells compared with the control group. Similarly, decreased AGD, spermatocyte number, and Leydig cell number were also observed in the 5000 μg/kg/day BPAF-treated mice on PND 9 (Fig. 5A–D). While ICI 182,780 by itself had no effects on these endpoints, it antagonized BPAF- and MES-caused decreases in AGD (Fig. 5A–D). IF staining showed that ICI 182,780 seemed not to significantly rescue BPAF- and MES-caused reductions in the spermatocyte number and Leydig cell number, but an antagonistic trend was observable. Notably, RT-qPCR analysis revealed significant antagonistic effects of ICI 182,780 on expression of their marker genes (γH2ax and 3β-Hsd) (Fig. 5D). In the hypothalamus, both the GnRH content and its gene expression were lower in both BPAF and MES groups than those in the control group, but these effects were not abolished by co-exposure with ICI 182,780. By further expression analysis, we observed a high level of Gper expression in the hypothalamus and its up-regulation in MES treatment compared with the control group, while expression of Esr1 and Esr2 was non-detectable (Fig. 5A). These observations show that BPAF, like MES, exerted its effects on testis development partially through the ER-mediated signaling pathway.
Effects of the ER antagonist, ICI 182,780, on bisphenol AF (BPAF)-caused impairment of testis development (n = 3 litters, 3 male mice/litter). A The body weight, anogenital distance and liver weight of male mice. B Spermatocytes marked by immunostaining for SYCP3, its number in the 200 × field, and expression of a spermatocyte marker gene (γH2ax). C Leydig cells marked by immunostaining for 3β-HSD, its number in the 200 × field, and expression of a Leydig cell marker gene (3β-Hsd). D GnRH level and relative expression of Gnrh and Gper in hypothalamus. For spermatocyte number and Leydig cell number, at least three 200 × field per testis and three litters per group were used for statistical analysis. Data are shown as mean ± standard deviation. Asterisk (*) indicates significant difference from control (p < 0.05). CON, control; BPAF, 5000 μg/kg/day BPAF; MES, 50 μg/kg/day mestranol as positive control
Discussion
Here, we for the first time reported that prenatal and postnatal exposure to BPAF disrupted testis development and caused adverse testicular outcomes in adulthood to a certain extent. Like MES, 5000 μg/kg/day BPAF significantly reduced AGD, sperm number, percentage of stage VII–VIII seminiferous tubules, the spermatocyte number, and serum levels of testosterone, FSH and LH in male mice on PND 56. Strikingly, 50 μg/kg/day BPAF also caused significant decreases in AGD, LH level and spermatocyte number, along with a declining trend in sperm number, the serum levels of testosterone, and FSH. In fact, evidence from both animal experiments and epidemiological observations has shown that reduced AGD is correlated with low testosterone level, sperm count, and genital size (Dean and Sharpe 2013), and AGD is believed to be a marker for the development of the male reproductive system in clinical diagnosis (Schwartz et al. 2019). In terms of reduced AGD, LH level and spermatocyte number combined with the declining trend of the sperm number and serum testosterone level, therefore, the effects of 50 μg/kg/day BPAF on testis development are meaningful and should not be ignored. Especially, oral ingestion of 50 μg/kg/day BPAF was estimated to result in a maximum plasma concentration of 0.6 ng/ml, which is comparable to the reported concentrations of BPAF in human serum (Waidyanatha et al. 2019; Li et al. 2020), meaning that the dose we used is human-relevant. Previously, the effective doses leading to male reproductive damages were generally higher than 5 mg/kg/day (Feng et al. 2012; Li et al. 2016; Umano et al. 2012; Yu et al. 2022). Our study, for the first time, uncovers the adverse effects of BPAF at as low as 50 μg/kg/day on testis development, highlighting its potential reproductive risks to mammals including humans. Certainly, whether the effects of low-dose BPAF would lead to reproductive dysfunction or increased susceptibility to other reproductive toxicants needs further studies.
Importantly, we found that in line with the adverse testicular outcomes observed in adulthood following pre- and postnatal exposure to BPAF, some alterations in testicular and associated parameters have already been detectable in developing testes on PND 9. These alterations included reduced seminiferous tubule area and decreased numbers of total germ cells, spermatocytes and Leydig cells, coupled with down-regulated expression of male-biased genes. Particularly, a noteworthy alteration is reduced AGD by 50 μg/kg/day BPAF, indicating the high responsiveness of this parameter to BPAF. In the literature, similar alterations in male reproductive parameters have been detected in developing animals treated with estrogenic chemicals (Ikeda et al. 2008). Typically, gestational exposure to diethylstilbestrol (DES) was showed to reduce intratesticular testosterone level and seminiferous tubule diameter in fetal or infancy rodents, along with down-regulated expression of steroidogenesis-associated genes (Guyot et al. 2004; Ikeda et al. 2008; Sharpe et al. 1998). In the present study, we found that even if exposure began from PND 0, both BPAF and MES still reduced AGD and the populations of spermatocytes and Leydig cells in mouse testes on PND 9. Therefore, we conclude that the effects of estrogenic chemicals on testis development have been detectable as early as the first 2 weeks after birth, meaning a possibility of early detection of potential adverse effects of estrogenic chemicals on testis development, instead of the conventional detection in adulthood. This conclusion is of significance for developing effective methods for early detection of adverse effects of chemicals on testis development.
In the present study, we found that BPAF-caused alterations in testicular and associated parameters are in line with MES-caused changes on both PND 9 and PND 56, suggesting that BPAF may disrupt testicular development by estrogen-like mechanisms, genomic and/or nongenomic estrogenic pathways. Further findings that the ER-specific antagonist ICI 182,780 antagonized 5000 μg/kg/day BPAF-caused decreases in AGD, the numbers of spermatocytes and Leydig cells and expression of some marker genes to different degree support the involvement of the ER-mediated pathway in BPAF-caused alterations in testicular development (Fig. 6). However, given the observations that ICI 182,780 did not significantly antagonize all the BPAF-caused alterations, we assume that BPAF could exert its effects possibly through certain non-ER-mediated mechanism(s), in addition to the ER-mediated signaling pathway. As anticipated, we found that postnatal exposure to BPAF as well as MES reduced the GnRH content and its expression in the hypothalamus on PND 9, agreeing with the reductions in serum LH and testosterone levels in adult mice following exposure from GD 10.5. These observations indicate that BPAF as well as MES could disrupt testis development by reducing the production of GnRH (Fig. 6). This result is in consistence with previous reports that estrogens can negatively regulate the hypothalamus and thereby lead to testicular dysplasia or dysfunction in rodents and fish (D'Souza et al. 2005; Qin et al. 2014). In the literature, several studies reported that BPA and its analogues disrupted the HPG axis, but their results have remained inconsistent and even contradictory (Shamhari et al. 2021). Our study provides a support that bisphenols may negatively regulate the HPG axis and elicit adverse effects on the male reproductive system. Importantly, we found that ICI 182,780 had no effects on BPAF- and MES-caused decreases in the GnRH content and its gene expression in the hypothalamus. Considering our findings of high Gper expression and non-detectable Esr1 and Esr2 expression in the hypothalamus, together with relevant reports (Chimento et al. 2014; Shamhari et al. 2021), we propose that BPAF and MES could act on the hypothalamus possibly via a non-ER mediated pathway. In fact, there are several studies supporting the involvement of GPER in estrogen regulation on the hypothalamus in males despite this question being under investigation (Chimento et al. 2014). Collectedly, these observations indicate that the adverse effects of BPAF on mammal testis development could be partly mediated by the ER signaling pathway, coupled with the involvement of the hypothalamus possibly via GPER.
In conclusion, all the findings demonstrate that BPAF even at 50 μg/kg/day can partly disrupt mouse testis development and cause adverse testicular outcomes in adulthood, indicating a potential reproductive risk to mammals including humans. Our study also has provided evidence that adverse effects of BPAF mechanistically may be mediated via the ER (Fig. 6). Importantly, our finding that developmental alterations elicited by BPAF have been detectable on PND 9 provides important motivation for the development of effective methods for early detection of adverse effects of estrogenic chemicals on testis development.
References
Cao LY, Ren XM, Li CH et al (2017) Bisphenol AF and bisphenol B exert higher estrogenic effects than bisphenol A via G protein-coupled estrogen receptor pathway. Environ Sci Technol 51(19):11423–11430. https://doi.org/10.1021/acs.est.7b03336
Chen D, Kannan K, Tan H et al (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-a review. Environ Sci Technol 50(11):5438–5453. https://doi.org/10.1021/acs.est.5b05387
Chimento A, Sirianni R, Casaburi I, Pezzi V (2014) Role of estrogen receptors and G protein-coupled estrogen receptor in regulation of hypothalamus-pituitary-testis axis and spermatogenesis. Front Endocrinol 5:1. https://doi.org/10.3389/fendo.2014.00001
Dean A, Sharpe RM (2013) Clinical review: anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab 98(6):2230–2238. https://doi.org/10.1210/jc.2012-4057
D’Souza R, Gill-Sharma MK, Pathak S, Kedia N, Kumar R, Balasinor N (2005) Effect of high intratesticular estrogen on the seminiferous epithelium in adult male rats. Mol Cell Endocrinol 241(1–2):41–48. https://doi.org/10.1016/j.mce.2005.04.011
ECHA (2020) Mass of bisphenol AF and benzyl(diethylamino)diphenylphosphonium. https://www.echaeuropaeu/documents/10162/2842450/clh_rep_React+mass+of+bisphenol+AF+and+benzyl%28diethylamino%29diphenylphosphonium_15689_enpdf/23d8e2cc-5fbd-9d83-3d36-cde6cdeed223. Accessed 25 Dec 2021
Escriva L, Zilliacus J, Hessel E, Beronius A (2021) Assessment of the endocrine disrupting properties of bisphenol AF: a case study applying the European regulatory criteria and guidance. Environ Health Glob Acc Sci Sour 20(1):48. https://doi.org/10.1186/s12940-021-00731-0
Feng Y, Yin J, Jiao Z, Shi J, Li M, Shao B (2012) Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol Lett 211(2):201–209. https://doi.org/10.1016/j.toxlet.2012.03.802
Guyot R, Odet F, Leduque P, Forest MG, Le Magueresse-Battistoni B (2004) Diethylstilbestrol inhibits the expression of the steroidogenic acute regulatory protein in mouse fetal testis. Mol Cell Endocrinol 220(1–2):67–75. https://doi.org/10.1016/j.mce.2004.03.008
Ikeda Y, Tanaka H, Esaki M (2008) Effects of gestational diethylstilbestrol treatment on male and female gonads during early embryonic development. Endocrinology 149(8):3970–3979. https://doi.org/10.1210/en.2007-1599
Kitamura S, Suzuki T, Sanoh S et al (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci off J Soc Toxicol 84(2):249–259. https://doi.org/10.1093/toxsci/kfi074
Lee PC (1998) Disruption of male reproductive tract development by administration of the xenoestrogen, nonylphenol, to male newborn rats. Endocrine 9(1):105–111. https://doi.org/10.1385/endo:9:1:105
Li J, Sheng N, Cui R et al (2016) Gestational and lactational exposure to bisphenol AF in maternal rats increases testosterone levels in 23-day-old male offspring. Chemosphere 163:552–561. https://doi.org/10.1016/j.chemosphere.2016.08.059
Li A, Zhuang T, Shi W et al (2020) Serum concentration of bisphenol analogues in pregnant women in China. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.136100
Li Y, Dong M, Xiong Y et al (2022) Effects of postnatal exposure to tetrabromobisphenol A on testis development in mice and early key events. Arch Toxicol. https://doi.org/10.1007/s00204-022-03259-5
Liu Y, Zhang S, Song N et al (2017) Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake): Implications for ecological and human health risk. Sci Total Environ 599–600:1090–1098. https://doi.org/10.1016/j.scitotenv.2017.05.069
Liu J, Zhang L, Lu G, Jiang R, Yan Z, Li Y (2021a) Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment: a review. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2020.111481
Liu X, Xue Q, Zhang H, Fu J, Zhang A (2021b) Structural basis for molecular recognition of G protein-coupled estrogen receptor by selected bisphenols. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.148558
Martin OV, Shialis T, Lester JN, Scrimshaw MD, Boobis AR, Voulvoulis N (2008) Testicular dysgenesis syndrome and the estrogen hypothesis: a quantitative meta-analysis. Environ Health Perspect 116(2):149–157. https://doi.org/10.1289/ehp.10545
Matsushima A, Liu X, Okada H, Shimohigashi M, Shimohigashi Y (2010) Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta. Environ Health Perspect 118(9):1267–1272. https://doi.org/10.1289/ehp.0901819
NTP (2008) Chemical information profile for bisphenol AF [CAS No. 1478–61–1]. http://www.ntpniehsnihgov/. Accessed 21 Dec 2021
Pan Y, Deng M, Li J et al (2020) Occurrence and maternal transfer of multiple bisphenols, including an emerging derivative with unexpectedly high concentrations, in the human maternal-fetal-placental unit. Environ Sci Technol 54(6):3476–3486. https://doi.org/10.1021/acs.est.0c00206
Qin F, Wang X, Liu S et al (2014) Gene expression profiling of key genes in hypothalamus-pituitary-gonad axis of rare minnow Gobiocypris rarus in response to EE2. Gene 552(1):8–17. https://doi.org/10.1016/j.gene.2014.09.006
Schwartz CL, Christiansen S, Vinggaard AM, Axelstad M, Hass U, Svingen T (2019) Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch Toxicol 93(2):253–272. https://doi.org/10.1007/s00204-018-2350-5
Shamhari AA, Abd Hamid Z, Budin SB, Shamsudin NJ, Taib IS (2021) Bisphenol A and its analogues deteriorate the hormones physiological function of the male reproductive system: a mini-review. Biomedicines. https://doi.org/10.3390/biomedicines9111744
Sharpe RM, Atanassova N, McKinnell C et al (1998) Abnormalities in functional development of the Sertoli cells in rats treated neonatally with diethylstilbestrol: a possible role for estrogens in Sertoli cell development. Biol Reprod 59(5):1084–1094. https://doi.org/10.1095/biolreprod59.5.1084
Song S, Ruan T, Wang T, Liu R, Jiang G (2012) Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China. Environ Sci Technol 46(24):13136–13143. https://doi.org/10.1021/es303960k
Teng C, Goodwin B, Shockley K et al (2013) Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact 203(3):556–564. https://doi.org/10.1016/j.cbi.2013.03.013
Toppari J, Larsen JC, Christiansen P et al (1996) Male reproductive health and environmental xenoestrogens. Environ Health Perspect 104(Suppl 4):741–803. https://doi.org/10.1289/ehp.96104s4741
Umano T, Tanaka R, Yamasaki K (2012) Endocrine-mediated effects of 4,4’-(hexafluoroisopropylidene)diphenol in SD rats, based on a subacute oral toxicity study. Arch Toxicol 86(1):151–157. https://doi.org/10.1007/s00204-011-0731-0
Waidyanatha S, Black SR, Aillon K et al (2019) Toxicokinetics and bioavailability of bisphenol AF following oral administration in rodents: a dose, species, and sex comparison. Toxicol Appl Pharmacol 373:39–47. https://doi.org/10.1016/j.taap.2019.04.015
Waidyanatha S, Collins BJ, Cunny H et al (2021) An investigation of systemic exposure to bisphenol AF during critical periods of development in the rat. Toxicol Appl Pharmacol. https://doi.org/10.1016/j.taap.2020.115369
Wu D, Huang CJ, Jiao XF et al (2019) Bisphenol AF compromises blood-testis barrier integrity and sperm quality in mice. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.124410
Yamasaki K, Takeyoshi M, Sawaki M, Imatanaka N, Shinoda K, Takatsuki M (2003) Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology 183(1–3):93–115. https://doi.org/10.1016/s0300-483x(02)00445-6
Yang Y, Guan J, Yin J, Shao B, Li H (2014a) Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere 112:481–486. https://doi.org/10.1016/j.chemosphere.2014.05.004
Yang Y, Lu L, Zhang J, Yang Y, Wu Y, Shao B (2014b) Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A 1328:26–34. https://doi.org/10.1016/j.chroma.2013.12.074
Yang S, Cheng W, Li X et al (2020) Use of embryonic stem cell-derived cardiomyocytes to study cardiotoxicity of bisphenol AF via the GPER/CAM/eNOS pathway. Toxicology. https://doi.org/10.1016/j.tox.2020.152380
Yu Y, Xin X, Ma F et al (2022) Bisphenol AF blocks Leydig cell regeneration from stem cells in male rats. Environ Pollut. https://doi.org/10.1016/j.envpol.2022.118825
Zhang B, He Y, Zhu H et al (2020) Concentrations of bisphenol A and its alternatives in paired maternal-fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environ Int. https://doi.org/10.1016/j.envint.2019.105407
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0901103) and National Natural Science Foundation of China (22076211). The authors would like to thank Professor Wei Li and Dr. Chao Liu (State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences) for some assistance with sperm analysis. Figure 6 was created in BioRender.com (https://biorender.com/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
204_2022_3377_MOESM1_ESM.docx
Supplementary file1 Experimental section including RNA extraction and RT-qPCR, IF assay, quantitative analysis of seminiferous tubules. A table listing primer sequences of genes. (DOCX 23 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Xiong, Y., Lv, L. et al. Effects of low-dose bisphenol AF on mammal testis development via complex mechanisms: alterations are detectable in both infancy and adulthood. Arch Toxicol 96, 3373–3383 (2022). https://doi.org/10.1007/s00204-022-03377-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03377-0