Abstract
Limited studies in humans and in animal models have investigated the neurotoxic risks related to a gestational exposure to diesel exhaust particles (DEP) on the embryonic brain, especially those regarding monoaminergic systems linked to neurocognitive disorders. We previously showed that exposure to DEP alters monoaminergic neurotransmission in fetal olfactory bulbs and modifies tissue morphology along with behavioral consequences at birth in a rabbit model. Given the anatomical and functional connections between olfactory and central brain structures, we further characterized their impacts in brain regions associated with monoaminergic neurotransmission. At gestational day 28 (GD28), fetal rabbit brains were collected from dams exposed by nose-only to either a clean air or filtered DEP for 2 h/day, 5 days/week, from GD3 to GD27. HPLC dosage and histochemical analyses of the main monoaminergic systems, i.e., dopamine (DA), noradrenaline (NA), and serotonin (5-HT) and their metabolites were conducted in microdissected fetal brain regions. DEP exposure increased the level of DA and decreased the dopaminergic metabolites ratios in the prefrontal cortex (PFC), together with sex-specific alterations in the hippocampus (Hp). In addition, HVA level was increased in the temporal cortex (TCx). Serotonin and 5-HIAA levels were decreased in the fetal Hp. However, DEP exposure did not significantly modify NA levels, tyrosine hydroxylase, tryptophan hydroxylase or AChE enzymatic activity in fetal brain. Exposure to DEP during fetal life results in dopaminergic and serotonergic changes in critical brain regions that might lead to detrimental potential short-term neural disturbances as precursors of long-term neurocognitive consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, epidemiological studies have highlighted a possible role of gestational exposure to traffic-related air pollution in the emergence of neurodevelopmental disorders, along with neuropathological and neurobehavioral alterations including early attention deficits, impulsivity, anxiety and learning disturbances (Guxens et al. 2018; Harris et al. 2016; Suades-González et al. 2015). In addition, experimental studies have shown that gestational exposure to traffic-related air pollutants, mostly ascribed to diesel exhaust (DE) induces molecular, structural and functional alterations in brain tissues (Bolton et al. 2017; Costa et al. 2020; Ehsanifar et al. 2019; Klocke et al. 2017; Nway et al. 2017; Sugamata et al. 2006a, b). Interestingly, mice exposed in utero to DE display abnormal levels in dopamine (DA) or serotonin (5-HT) and their metabolites in various regions of the brain, namely the prefrontal cortex (PFC), the hippocampus (Hp), the striatum (Str) and the cerebellum (Cb), and underline monoaminergic-related neurocognitive disorders (Haghani et al. 2020; Suzuki et al. 2010; Yokota et al. 2009, 2013, 2015, 2016a, b).
Nevertheless, there are still many uncertainties regarding the nature of the link between particle inhalation and associated neuropathologies, as exposure conditions, animal models, exposure windows and perspectives of the study are all factors of variation to be taken into consideration. Most data on neurochemical disturbances result from mice models following maternal subcutaneous or whole-body exposures to high levels of DEP and have explored long-lasting effects in juveniles or adults. Only a few have investigated fetuses or neonates, despite interesting morphological and molecular alterations suggesting early damages (Bolton et al. 2017; Chang et al. 2019; Tachibana et al. 2015), none of which having discussed possible monoaminergic damages at this age. Yet, fetal life is a sensitive and critical period during which neural structures are being formed, but are already functional and highly plastic regarding the environment, therefore, representing a vulnerable stage of brain development (Costa et al. 2020; Heusinkveld et al. 2016; Hougaard et al. 2015). Understanding how in utero exposure to air pollution affects fetal brain development could, thus, help to identify the neural pathways that participate in the emergence of neurodevelopmental and neurodegenerative diseases later in life (Calderón-Garcidueñas et al. 2020a; Costa et al. 2020; Heusinkveld et al. 2016).
Furthermore, several of these studies have neglected sex-specific vulnerability to air pollution, despite the fact that important periods of sexually dimorphic neurodevelopment take place during fetal life. Indeed, neural differentiation (Chung and Auger 2013; Turano et al. 2018) and placental function (Chavatte-Palmer and Tarrade 2016; Valentino et al. 2016) display gender differences which could participate to the differential vulnerability of males and females observed in both epidemiological (Baio et al. 2018; Chiu et al. 2016) and experimental studies (Bernal-Meléndez et al. 2019; Bolton et al. 2013, 2017; Haghani et al. 2020; Nway et al. 2017; Patten et al. 2020; Sobolewski et al. 2018).
Finally, given that both the experimental conditions and the physiology of the placenta strongly influence the way fetuses are exposed, the development of experimental models mimicking a controlled nose-only maternal inhalation with an hemochorial placenta similar to that of humans is of utmost importance (Fischer et al. 2012). Using a rabbit model developed to analyze feto-placental development (Valentino et al. 2016), we have reported that gestational exposure to diesel exhaust enriched in particles (DEP) alters olfactory tissue morphology, affects dopaminergic and serotonergic neurotransmission in fetal olfactory bulbs (OBs), and is associated to variations in olfactory-based behaviors at birth (Bernal-Meléndez et al. 2019). Considering the anatomical and functional continuum between the olfactory system and other brain structures, these data raised the question of the origin of the monoaminergic damages, either locally, centrally, or both. Indeed, the observed neurochemical alterations in the OB may not only relate to DEP-mediated local injury on olfactory processes, but may also be indicative of disturbance in more integrative brain structures (Calderón-Garcidueñas et al. 2008a, b; Doty 2012), as olfactory sensory neurons (OSN) sensitivity and odor processing in the OB are modulated by centrifugal and centripetal neuromodulatory processes (Harvey and Heinbockel 2018; Lizbinski and Dacks 2018).
To fully characterize the DEP-exposed fetal brain, we further quantify the monoaminergic modulation in different fetal brain regions of exposed and control fetuses, via both an HPLC dosage of DA, 5-HT, NA, and their metabolites and an histochemical study of Tyrosine Hydroxylase (TH) and Tryptophan Hydroxylase (TrpH) expression, two rate-limiting enzyme of DA and 5-HT biosynthesis, respectively. The latter analyses were completed with a histochemical study of the enzymatic activity of acetylcholinesterase (AChE), used here as a marker to estimate the cytoarchitectural brain integrity (Gordon and Finch, 1984).
Materials and methods
Animal procedure
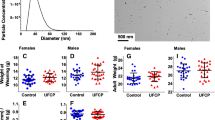
The experimental procedure for animal exposure has been described elsewhere (Bernal-Meléndez et al. 2019; Valentino et al. 2016). Briefly, 28 pregnant New-Zealand white female rabbits (INRA1077 line, 1-year old) were exposed by nose-only inhalation to either clean air (control group; n = 14) or diluted DE (1 mg/m3, average size of 69 nm) (exposed group; n = 14) for 2 h/day, 5 days/week, from gestational day 3 (GD3) to GD27 (i.e., 20 days over a 31-day gestation).
At GD28, 12 dams (n = 5 controls; n = 7 exposed) were euthanized and their fetuses counted, weighed individually and euthanized by decapitation. Fetuses were sexed by visual observation of their internal genital organs (confirmed by a second sexing by qPCR approach). Whole brains were dissected and weighed. Random samples of the latter structure were dedicated to subsequent anatomical or chemical measures.
Brain HPLC dosage
The levels of DA, 5-HT, NA, and their respective metabolites were quantified using high-performance liquid chromatography (HPLC) on crushed brain structures samples.
Brain tissues of the fetuses GD28 were randomly selected and frozen as half hemispheres. Brain sub-regions were then dissected using a reproducible protocol for micro-dissection on frozen tissue. The latter consisted in attaching the half hemisphere of the brain to the cryostat, making 20-μm-thick sagittal sections and staining them with cresyl violet to compare the samples with the similarly colored images of the Paxinos and Watson stereotaxic atlas of the rat brain (6th edition, 2007). Once the structure of interest was identified, we performed micro-dissections directly on the tissue using a disposable scalpel to select five brain structures (the prefrontal cortex (PFC), the hippocampus (Hp), the temporal and entorhinal cortex (TCx), the mesencephalon (Mes) and the rest of the brainstem corresponding to the Pons and the Medulla Oblongata (Bs). They were stored at − 80 °C until use.
The latter structures (n = 4 male and n = 4 female per group, i.e.: n = 8 control fetuses and n = 8 exposed fetuses) were crushed in 400 µL of 0.2 M perchloric acid and centrifugated at 22,000g for 20 min at 4 °C. The supernatants were collected and filtered through a 10 kDa membrane (Nanosep, Pall) by centrifugation at 7000g (30 min). Then, a 20 µL aliquot of each sample was analyzed for 5-HT and 5-HIAA by fluorometric detection (Kema). The amounts of catecholamines (DA and NA) and of their main metabolites (DOPAC and HVA for DA; MHPG for NA) were measured by electrochemical detection on a serial array of coulometric flow-through graphite electrodes (CoulArray, ESA). Analysis, data reduction, and peak identification were fully automated. Results were expressed as femtomoles/milligram of fresh tissues (Gamache et al. 1993; Kema et al. 1993). Results are expressed as mean ± SD. Ratios between the monoamine of interest and its main metabolite(s) were calculated, and used as an estimator of the monoaminergic activity, providing information on the catabolic rate or on the particular enzymatic activity.
Brain histological and histochemical measurements
Tissue preparation
Randomly chosen fetal brains from different litters (n = 5 male and n = 4 female control fetuses; n = 4 male and n = 4 female exposed fetuses) were rapidly frozen in cooled isopentane at − 34 °C and subsequently stored at − 80 °C. 20-μm-thick coronal sections were serially cut on a cryostat, collected on gelatin-chrome alum-coated slides and stored at − 80 °C until histological and histochemical processing.
Brain tyrosine hydroxylase and tryptophan hydroxylase immunohistochemistry and image analysis
The slices were dried at room temperature for 20 min. The endogenous peroxidase activity was inhibited by H2O2 (0.3%) for 30 min. Sections were then blocked with 1% BSA and normal horse serum for 30 min and incubated overnight at 4 °C in the presence of mouse monoclonal primary anti-tyrosine hydroxylase antibody (TH, catalogue #T1299; Sigma, St. Louis, MO, 1:5000 in 0.3% Triton X-100) or in the presence of a mouse monoclonal primary anti-tryptophan hydroxylase antibody (TrpH, catalogue #T0678; Sigma, St. Louis, MO, 1:2000 in 0.3% Triton X-100). After extensive washing (3 × 15 min), samples were incubated for 30 min at room temperature with biotinylated anti-mouse IgG (Vectastain Elite ABC Kit, PK-6102, Vector laboratories, Burlingame, USA), followed by avidin–biotin–peroxidase complex (Vectastain Elite ABC Kit, PK-6102, Vector laboratories, Burlingame, USA) according to the supplier’s instructions. The slices were revealed for 3 min using 3′,3-diaminobenzidine (DAB) substrate kit for visualization (SK-4100, Vector laboratories, Burlingame, USA), resulting in a gray–black reaction product, then stopped with deionized water. The sections were then dried, coverslipped with Eukitt mounting medium (catalogue #03989; Sigma, St. Louis, MO) and kept in the dark until image analysis.
Analysis of TH and TrpH stained sections was carried out with a BIOCOM computer-assisted image analysis system (Les Ulis, France), in which optical densities were automatically read. The total labeling was measured by taking the mean of two optical densities on the same slice and on three successive slices (i.e., 6 optical density readings per animal). The mean non-specific labeling (measured on a region without TH or TrpH labeling) was subtracted to the total labeling to have the specific labeling of each structure of interest.
TH+ measures were carried out on the mesencephalon (substantia nigra, SN, and ventral tegmental area, VTA), the striatum (Str) and the prefrontal cortex (PFC). TrpH+ measures concerned the Raphe nuclei. All anatomical structures were defined according to the Paxinos and Watson stereotaxic atlas of the rat brain (6th edition, 2007).
Histochemical measurement of acetylcholinesterase activity
Slides were incubated for 12 h in 180 mL of stock solution (50 mM sodium acetate buffer, 4 mM copper sulfate, 16 mM glycine, pH 5.0 with HCl) to which 208 mg of S-acetylthiocholine iodide and 5.4 mg ethopropazine were freshly added. The slides were rinsed with deionized water and developed for 10 min in 1% sodium sulfide in phosphate buffer 0.1 M at pH 7.5. They were then rinsed three times for 5 min each with deionized water and immersed in 4% paraformaldehyde in phosphate buffer 0.1 M for 30 min, and then rinsed with distilled water (3 times; 5 min each). Subsequently, slides were dehydrated in series of ethanol baths (50, 70, 96, and 100%) for 3 min each, and cleared with toluene twice for 5 min. Slides were then coverslipped with Eukitt mounting medium (catalogue #03989; Sigma, St. Louis, MO).
Standards for specific acetylcholinesterase (AChE) activity were prepared from whole brain homogenates of adult rabbits. Briefly, brain homogenate was diluted with 5–5’-dithiobis-2-nitrobenzoate acid (DTNB, 0.01 M) and acetylthiocholine iodide (75 mM). The thio-choline, formed during hydrolysis of acetylthiocholine, rapidly reacts with DTNB to release a colored 5-thio-2-nitrobenzoate anion with maximum absorption at 412 nm. The specific enzymatic activity of AChE was calculated from the linear slope of the curve obtained and was 8.41 µmol/min/g of tissue. Brain homogenate sections of variable thickness were used to cover the entire range of activity measured in the different brain structures. Enzyme activity of brain homogenates was biochemically measured by spectrophotometry according to the protocol described by Ellman et al. and modified by Dumont et al. (Dumont et al. 2006; Ellman et al. 1961). Under our experimental conditions, the staining intensity was highly correlated to the thickness of the standard sections (linear function with r = 0.997). Analysis of AChE-stained sections was carried out with a BIOCOM computer-assisted image analysis system (Les Ulis, France), in which optical density readings were converted by means of standards into enzymatic activity values expressed in μmol/min/g of tissue. Based on the Paxinos and Watson stereotaxic atlas of rat brain (6th edition, 2007), two optical densities on the same slice and on three successive slices (i.e., 6 optical density readings per animal) were taken to obtain a homogeneous evaluation.
Statistical analysis
For each variable, a Levene test for homogeneity of variances was performed. Given that homogeneity of variance was assumed for most of them, a general linear model (GLM) was used for statistical analysis, adjusting the litter as a confounding variable. Significance was set at p < 0.05 and a near-significant tendency was considered at 0.05 < p < 0.1, as we avoided using statistical significance threshold of p < 0.05 as the sole criterion for interpreting our findings. However, tendencies were only considered in the discussion regarding other significant values. All statistical analyses were carried out using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Repeated gestational DEP exposure effects on fetal catecholamine
Neurochemical approach
GD28 brain tissue levels of dopamine (DA), its main metabolites (DOPAC and HVA) and their turnover ratios are presented in Fig. 1. We observed an 89% increase of DA levels in the prefrontal cortex (PFC) of DEP-exposed fetuses compared to control ones (F(1,15) = 7.045, p = 0.022), in both sexes (F(1,15) = 0.002, p = 0.962) (Fig. 1a). An interaction between exposure and sex was observed (F(1,15) = 7.166, p = 0.022), with a higher increase of DA levels in males (222%) than in females (15%) compared to controls of the same sex. In the hippocampus (Hp), DA levels were globally unaffected by DEP exposure (F(1,15) = 0.240, p = 0.634), but a tendency for a sex difference (F(1,15) = 4.400, p = 0.060) and an interaction between exposure and sex (F(1,15) = 5.220, p = 0.043) were noticed (Fig. 1b). DA level were increased by 75% in exposed females and decreased by 50% in exposed males compared to controls. No significant differences were found in the remaining analyzed brain regions, except for a tendency for an increase of DA levels in the temporal/entorhinal cortex (TCx) of exposed fetuses (F(1,15) = 3.636, p = 0.083) and of an interaction between exposure and sex in the brainstem (Bs) (F(1,15) = 3.419, p = 0.091) (Figs. 1c and S1).
Effects of gestational exposure to DEP on the central dopaminergic system of GD28 fetuses. Graphical representation of tissue levels of dopamine (DA), its main metabolites (DOPAC and HVA) and the turnover ratios in the PFC (a), the Hp (b) and the TCx (c) from control fetuses (n = 4 males; n = 4 females; in white) and exposed fetuses (n = 4 males; n = 4 females; in gray). Results were expressed as femtomoles/milligram of fresh tissues (fmol/mg) and shown as mean ± SD. *p < 0.05 represented a significant difference between control and exposed fetuses. §p < 0.05 represented a significant interaction between exposure and sex. DA dopamine, DAto dopamine turnover (i.e., (DOPAC + HVA)/DA), DOPAC 3,4-Dihydroxyphenylacetic acid, Hp hippocampus, HVA homovanillic acid, PFC prefrontal cortex, TCx temporal/entorhinal cortex
Regarding DOPAC levels in the PFC (Fig. 1a), neither exposure (F(1,15) = 1.893, p = 0.196), nor sex effects (F(1,15) = 1.076, p = 0.322) were noticed. An interaction between exposure and sex was, however, observed (F(1,15) = 6.784, p = 0.024), with a 67% decrease in the level of DOPAC in exposed females and a 52% increase in exposed males compared to controls of the same sex. No significant differences were observed between the two groups for the remaining analyzed structures, except a tendency for an increase of DOPAC levels in the TCx (F(1,15) = 3.569, p = 0.085) whatever the sex (F(1,15) = 0.887, p = 0.367) with no interaction between exposure and sex (F(1,15) = 0.753, p = 0.403) (Figs. 1 and S1).
DEP exposure increased HVA levels in the TCx by 109% (F(1,15) = 5.782, p = 0.035), irrespective of sex (F(1,15) = 1.840, p = 0.202) and with no interaction between exposure and sex (F(1,15) = 0.101, p = 0.757) (Fig. 1c). No difference was observed in the other analyzed structures (Figs. 1 and S1).
Regarding the DOPAC/DA ratio in the PFC (Fig. 1a), DEP exposure was associated with a 62% decrease in exposed fetuses compared to controls (F(1,15) = 18.150, p = 0.001). Neither sex difference (F(1,15) = 0.002, p = 0.968) nor interaction between exposure and sex (F(1,15) = 0.247, p = 0.629) were observed. No significant difference was observed in other brain regions (Figs. 1 and S1).
In the PFC (Fig. 1a), the HVA/DA ratio was decreased by 71% in exposed fetuses compared to controls (F(1,15) = 15,693, p = 0.002) with no sex difference (F(1,15) = 0.375, p = 0.553), nor interaction between sex and exposure (F(1,15) = 0.020, p = 0.890). Regarding the HVA/DA ratio in the Hp (Fig. 1b), no DEP exposure effect was noticed (F(1,15) = 0.171, p = 0.687). A tendency for a sex difference (F(1,15) = 4.027, p = 0.070) was observed, as well as an interaction between exposure and sex (F(1,15) = 4.092, p = 0.068). No significant differences were observed in other structures (Figs. 1 and S1).
Finally, DA turnover (i.e., (HVA + DOPAC)/DA ratio, DAto) in the PFC was decreased by 64% in exposed fetuses compared to controls (F(1,15) = 23.134, p = 0.001) (Fig. 1a), with no sex difference (F(1,15) = 0.035, p = 0.855) and no interaction between exposure and sex (F(1,15) = 0.119, p = 0.736). In the Hp (Fig. 1b), no effect of DEP exposure on the DA turnover was observed (F(1,15) = 0.040, p = 0.845). A tendency for a sex difference (F(1,15) = 4.115, p = 0.067) and for an interaction between exposure and sex were observed (F(1,15) = 4.568, p = 0.056). No significant differences were observed in other structures (Figs. 1c and S1).
Regarding the noradrenalin system, only a neurochemical approach was realized. No significant differences were found in the analyzed brain regions, except for a tendency for interaction between exposure and sex (F(1,15) = 3.328, p = 0.095) on the level of NA in the brainstem (Fig. S2).
TH+ immunolabeling and AChE activity
Regarding the substantia nigra (SN) and the ventral tegmental area (VTA), the TH+ immunolabeling in GD28 fetuses’ brain did not reveal any difference between the two groups (Table 1). Only a tendency for a decrease in the relative intensity of TH+ labeling was observed in Cd (F(1,16) = 4.137, p = 0.065), AcbC (F(1,16) = 3.550, p = 0.084) and AcbS (F(1,16) = 4.438, p = 0.057) in exposed fetuses. DEP exposure did not impact TH+ intensity in any other studied structure (Table 1). The signals from the dorsolateral (LSD), the intermediolateral (LSI) and the medial (MS) nuclei of the septal area were too faint to be exploited.
The TH+ staining was complemented by a histochemical study of acetylcholinesterase (AChE) activity as an indicator of brain cytoarchitectural integrity (Table 2 and Fig. S3). There was no impact of DEP exposure on the AChE regardless of the fetal brain region examined. Only sex differences were observed, in which females displayed lower AChE activity levels than males in the Cd (F(1,16) = 7.382, p = 0.019) and in the Put (F(1,16) = 6,540, p = 0.025) together with a tendency in the AcbC (F(1,16) = 4.431, p = 0.057).
No significant variations were observed between the two groups for the remaining analyzed structures (Fig. S3), except for a sex difference in the LSI (F(1,15) = 7.476, p = 0.019), with female displaying a lower level of activity than males. Moreover, a tendency for an interaction between exposure and sex was observed in the Broca Diagonal Horizontal Band (HdB) (F(1,14) = 4.018, p = 0.070) (Fig. S3).
Repeated gestational DEP exposure effects on serotonergic neuromodulation
Neurochemical approach
Fetal GD28 brain regions were analyzed for serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA), the main metabolite of 5-HT (Fig. 2).
Effects of gestational exposure to DEP on the central serotonergic system of GD28 fetuses. Graphical representation of tissue levels of serotonin (5-HT), its main metabolite (5-HIAA) and the turnover ratio (5-HIAA/5-HT) in the PFC (a), the Hp (b) and the TCx (c) from control fetuses (n = 4 males; n = 4 females; in white) and exposed fetuses (n = 4 males; n = 4 females; in gray). Results were expressed as femtomoles/milligram of fresh tissues (fmol/mg) and shown as mean ± SD. *p < 0.05 represented a significant difference between control and exposed fetuses. §p < 0.05 represented a significant interaction between exposure and sex. 5-HT serotonin, 5-HIAA 5-hydroxyindoleacetic acid, Hp hippocampus, PFC prefrontal cortex, TCx temporal/entorhinal cortex
In the PFC, a tendency was observed for a decrease of 5-HT levels in exposed fetuses compared to control fetuses (F(1,15) = 3.499, p = 0.088) (Fig. 2a) with neither sex difference (F(1,15) = 0.158, p = 0.699) nor evidence of an interaction between exposure and sex (F(1,15) = 1.104, p = 0.316). No effects of DE exposure in 5-HIAA levels were observed in the PFC.
In the Hippocampus (Hp), a 33% decrease of 5-HT levels was observed in exposed fetuses (F(1,15) = 6.022, p = 0.032) (Fig. 2b) with neither sex effect (F(1,15) = 0.090, p = 0.769) nor an interaction between exposure and sex (F(1,15) = 0.315, p = 0.586). Regarding 5-HIAA, a 51% decrease was observed in exposed fetuses (F(1,15) = 6.333, p = 0.029) with neither sex effects (F(1,15) = 0.070, p = 0.796) nor an interaction between exposure and sex (F(1,15) = 1.154, p = 0.306) (Fig. 2b). For the other structures analyzed, there was no effect of DEP exposure on 5-HT and 5-HIAA levels (Figs. 2c and S4).
Regarding the 5-HIAA/5-HT ratio, used here as a serotonergic activity estimation, no difference was observed between the two groups of animals for the five structures (Figs. 2 and S4).
TrpH+ immunolabeling and AChE activity
TrpH+ labeling intensity of all Raphe nuclei (N.Rh) revealed no significant effect of DEP exposure (F(1,14) = 2.716, p = 0.130) or related to sex (F(1,14) = 0.002, p = 0.969) (Table 3). Nevertheless, a tendency for an interaction between fetal sex and exposure (F(1,14) = 3.975, p = 0.074) was observed, with a 29% decrease in intensity in exposed males versus control males, while only a faint increase (4%) was observed in females.
As the bulbar serotonergic innervation is derived from the fibers from the Raphe median and dorsal nuclei (MnR and DRD, respectively) (Doty 2012; Steinfeld et al. 2015), and given our previous results suggesting bulbar serotonergic denervation (Bernal-Meléndez et al. 2019), we summed the intensity values from these two structures to highlight possible alterations of the “bulbar serotonergic pathway”. The statistical analysis of the TrpH+ labeling intensity of the MnR + DRD (OBin) did not reveal a significant difference between the two groups of fetuses (Table 3).
Finally, TrpH+ labeling was supplemented by a histochemical study of AChE activity. No difference was observed between the two groups of animals in any of the analyzed structures (Fig. S5).
Discussion
Results from the present study demonstrate that a controlled daily maternal DEP exposure induces sex-specific neurochemical deregulations in the rabbit fetal brain. To our knowledge, this is the first study focused on monoaminergic alterations at the embryonic stage, as the current knowledge regarding the DEP neurotoxicity has been mostly dedicated to its long-term neural consequences (Bolton et al. 2013; Calderón-Garcidueñas, et al. 2008a; Ehsanifar et al. 2019; Haghani et al, 2020; Klocke et al. 2017; Patten et al. 2020; Suzuki et al. 2010; Takahashi et al. 2010; Yokota et al. 2009, 2013, 2015, 2016a, b), despite characterized neuroanatomical disorganization or molecular dysfunctions in brain around birth (Bolton et al. 2017; Chang et al. 2019; Sugamata et al. 2006a, b; Tachibana et al. 2015).
Dopaminergic pathways are mainly affected, DEP exposure increasing DA levels in the fetal PFC and tending to in the temporal/entorhinal cortex (TCx), together with alterations of the levels of its two main metabolites (HVA and DOPAC) in the same regions. Sex effects were noticed in the PFC and the Hp for dopaminergic levels, with a tendency in the Bs. Along with these neurochemical variations, a tendency for a decrease in TH+ immunolabeling levels in the caudate and the nucleus accumbens was observed (Fig. 3a). Such dopaminergic modulations have been observed in PFC and in striatum (Str) of juvenile male mice exposed in utero to TiO2 nanoparticles (Takahashi et al. 2010) and in the PFC of juvenile male mice prenatally exposed to DE (Suzuki et al. 2010), but contrast with those of Yokota et al. (2013) showing a decrease in DA and its metabolite in the PFC of prenatally DE-exposed mice at 3 weeks. The tendency for a decrease in TH+ labeling intensity in the Cd and the Acb in exposed fetuses is consistent with the decrease in DA levels in the Acb and Str in male mice exposed in utero to DEP (Yokota et al. 2009). The modulation of DA level is suggestive of modifications in the mesocorticolimbic and/or the nigrostriatal pathways, both of which innervate the OB (Doty 2012; Höglinger et al. 2015) (Fig. 3a). Whether these changes are the consequence of dopaminergic local disturbances due to the presence of particle-like depositions in the OB (Bernal-Meléndez et al. 2019), and/or result from their physical presence in central regions remain to be established. This question is particularly relevant as we observed that the central dopaminergic neurochemical variations are comparable in level and direction of fluctuation to those observed at the OB level in the same animal model for which a particle-like transplacental transfer has been shown (Bernal-Meléndez et al. 2019; Valentino et al. 2016). Even though the physical presence of DE particles has not been explored here, other works have shown (i) a direct toxicity of particles on dopaminergic neurons in vitro (Block et al. 2004), (ii) a decrease in levels of DA and its metabolites in the PFC along with an increase in the Amy of adult mice exposed in utero to DEP (Yokota et al. 2009, 2013) and (iii) a correlation between neurochemical damages and the presence of particles in PFC and Str (Sugamata et al. 2006b). Alternatively, these dopaminergic alterations may result from indirect functional changes in other regions of the brain (Yokota et al. 2013). Only the localization of nanoparticles in the brain would help to have insight on the mechanisms by which fetal DA levels are affected by particles, all mechanisms not being exclusive.
Schematic representation of dopaminergic (a) and serotonergic (b) alterations observed in GD28 fetuses. Each key brain regions investigated in our study or referring to Bernal-Melendez et al. (2019) is figured by a circle, main projections for DA and 5-HT are indicated with arrows. The color scale represents the direction and intensity of variation of the alterations measured in the different brain regions in exposed rabbits compared to controls, nuclei left in gray display no modulation. Acb nucleus accumbens, Bs brainstem, Cb cerebellum, Cd caudate, Ho hypothalamus, Hp hippocampus, Mes mesencephalon, N.Rh Raphe nuclei, OB olfactory bulb, PFC prefrontal cortex, SN substantia nigra, Str striatum, TCx temporal/Entorhinal cortex, Th thalamus, Tu olfactory tubercle, VTA ventral tegmental area. Image inspired by that of Dos Santos Coura and Granon (2012)
These alterations of DA circuitry may have contributed to the behavioral consequences of DEP exposure on the rabbit mammary pheromone perception observed at birth (Bernal-Meléndez et al. 2019). Indeed, as DEP exposure has been consistently associated with reduced long-term locomotor activity and motor coordination (Suzuki et al. 2010; Yokota et al. 2009, 2013), pheromone-induced sucking movements may have been impaired, without any alteration of the peripheral sensitivity to odors. Alternatively, as both intrinsic and extrinsic DA release are involved in the processing of olfactory information in the OB and upper olfactory tracts (Cansler et al. 2020; Cave and Baker 2009); DA signal perturbations could also have modified the olfactory threshold for the pheromone (Bernal-Meléndez et al. 2019).
Regarding the serotonergic modulation, 5-HT and 5-HIAA levels were decreased in the Hp in DEP-exposed fetuses along with a tendency towards a decrease in the PFC for 5-HT levels; only a tendency to a decrease in TrpH+ immunolabeling levels in Raphe nuclei (N.Rh) was noted (Fig. 3b). Interestingly, as observed in the OB of exposed fetuses (Bernal-Meléndez et al. 2019), the Hp and the PFC are both regions where opposite regulations of DA and 5-HT levels were observed (Fig. 3). Concomitant sensitivity of these two systems to polluted environments has been previously noticed in the same regions in rodents exposed in utero to DEP, even if the direction of variation makes findings usually hard to interpret. Yokota et al. (2013) showed a decrease in levels of DA and its metabolites and an increase in 5-HT and 5-HIAA levels in the PFC of 3-week-old male mice. Along with it, increased levels of 5-HT and 5-HIAA in the Amy and the Ho were pinpointed at 3 and 6 weeks of age, respectively (Yokota et al. 2013). Moreover, increased DA levels in the PFC and the Acb, together with decreased 5-HT levels in the Acb, the Amy, and the Ho were observed in socially isolated DE-exposed adult male mice (Yokota et al. (2016a). Furthermore, Suzuki et al. (2010) found that DA and NA (along with their metabolites) were increased in PFC in DE-exposed 5-week-old male mice. Discrepancies between studies in the direction of variation of these monoamines may be due to the experimental conditions (timing and route of exposure) and to the age where observations have been done, as fast age-dependent variations could be observed and reflect the high plasticity of the brain during early life.
These findings highlight the complexity of the interactions between these monoaminergic systems and indicate that dopaminergic and serotonergic systems are already interconnected at the fetal stage. As 5-HT plays a key role in regulating social and non-social behaviors by modulating the activity of other neurotransmitters (Alex and Pehek 2007; Di Matteo et al. 2008; Doty 2012; Lucas et al. 2000), it could be suggested that the DEP-induced increase of DA levels in the brain results from a compensatory plastic response following serotonergic denervation as already evoked in the OB (Mundiñano et al. 2011). As the pollution-induced CNS changes data remain fragmented at the fetal stage and the investigation of the serotonergic system via immunolabeling of TrpH were not conclusive, further investigations are required before confirming a functional link between the serotonergic and the dopaminergic fetal systems, as well as the resulting neurodevelopmental repercussions. Nevertheless, such a combined imbalances of two key neurotransmitter’s homeostasis in the Hp and in the PFC, which are key elements in olfactory information processing (Kadohisa 2013; Li et al. 2010), could have influenced the olfactory-based behaviors at birth (Bernal-Meléndez et al. 2019).
DEP exposure had no effect on the cytoarchitectural integrity of neural circuits, estimated here with an AChE activity histoenzymological assay, except for evidence for sex differences in several basal forebrain structures. Variations in the fetal monoaminergic levels and AChE activity seem subtle when compared to what is observed in the OB (Bernal-Meléndez et al. 2019) or in other tissues (Bolton et al. 2013; Calderón-Garcidueñas et al. 2008a; Klocke et al. 2017; Yokota et al. 2009, 2016a, b), which highlights the relevance of studying various developmental stages and to dissociate short- and long-term effects. These faint variations in enzymatic and monoaminergic activities might originate from (i) a low brain activity at the end of gestation, which could mask faint variations, (ii) a fetal adaptive response due to repetitive exposure to DEP, which may have either reversed or attenuated the initial effect of the stressor, (iii) a rapid regulation of the neural circuitry from a brain region to another, and/or (iv) a missed acute neurochemical response, given that fetuses were collected 1 day after the last DEP exposure (at GD28) and as monoamines brain contents might rapidly vary between the exposure and the observation (Gonzalez-Pina et al. 2008; Tabatabaie et al. 2017).
The present work is among the few experimental studies to show a differential impact of DEP according to sex at the fetal stage (Bernal-Meléndez et al. 2019; Bolton et al. 2013, 2017). It reinforces other studies which concur in sex differences of air pollution on neurochemical, molecular and morphological outcomes in various brain regions at early postnatal stages (Bolton et al. 2013; Chang et al. 2019; Haghani et al. 2020; Klocke et al. 2017; Nway et al. 2017; Patten et al. 2020; Tachibana et al. 2015). In many, but not all, there is a male excess response, whose bases are still unknown (Bolton et al. 2017; Chang et al. 2019; Tachibana et al. 2015). Here, DEP exposure induces sex-dependent variations for dopaminergic pathways in the Hp and the PFC, with both direction and amplitude of variation being large. Whether the effects are more pronounced in males or females remain difficult to say. It would be interesting to follow the postnatal future of both male and female to see whether these early sex differences in DEP impact lasts over time.
In summary, imbalances in fetal dopaminergic and serotonergic pathways after gestational DEP exposure confirms that the prenatal stage should be considered as an important period, during which there is a non-negligible susceptibility of various brain regions to environmental insults. If and how these anatomical and functional alterations persist in adulthood (Costa et al. 2020; Heusinkveld et al. 2016), or predispose the progeny to neuropathologies later in life (Calderón-Garcidueñas et al. 2018, 2020a, b) is still unresolved. Furthermore, it highlights the importance of considering sex as a variable in the statistical models for future neurotoxicological studies. Finally, such experimental study only imperfectly fills in some of the gaps left by population studies, but should be considered by public authorities to prevent vulnerable populations, such as pregnant women, from regular contact with polluted environments.
Availability of data and material
All data generated or analyzed during this study are included in this article (and its supplementary information files). The datasets analyzed during the current study are available from the corresponding author upon request.
Code availability
Not applicable.
Abbreviations
- 4N:
-
Trochlear nerve
- 5-HT:
-
Serotonin
- 5-HIAA:
-
5-Hydroxyindoleacetic acid
- Acb:
-
Nucleus accumbens
- AcbC:
-
Nucleus accumbens, Core
- AcbS:
-
Nucleus accumbens, Shell
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- Amy:
-
Amygdala
- B:
-
Meynert basal nucleus
- Bs:
-
Brainstem
- Cb:
-
Cerebellum
- Cd:
-
Caudate
- CNS:
-
Central nervous system
- DA:
-
Dopamine
- DEP:
-
Diesel exhaust particles
- df:
-
Degrees of freedom
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- GD:
-
Gestational day
- GLM:
-
General linear model
- HdB:
-
Broca Diagonal Horizontal Band
- Ho:
-
Hypothalamus
- Hp:
-
Hippocampus
- HVA:
-
Homovanillic acid
- Ld:
-
Lambdoid septal area
- LSD:
-
Dorsolateral septal area
- LSI:
-
Intermediolateral septal area
- Mes:
-
Mesencephalon
- MHPG:
-
3-Methoxy-4-hydroxyphenylglycol
- MCPO/LOT1:
-
Magnocellular preoptic nucleus/Lateral nucleus of the olfactory tract: layer 1
- Mitg/PBG:
-
Microcellular tegmental nucleus/parabigeminal nucleus
- MS:
-
Medial septal area
- NA:
-
Noradrenaline
- NP:
-
Nanoparticles
- N.Rh:
-
Raphe nuclei
- OB:
-
Olfactory bulb
- OBin:
-
The “bulbar serotonergic pathway”
- OSN:
-
Olfactory sensory neurons
- PD:
-
Parkinson’s disease
- PFC:
-
Prefrontal cortex
- PM:
-
Particulate matter
- Ptg:
-
Pedonculopontin nucleus
- Put:
-
Putamen
- Rbd:
-
Rhabdoid nucleus
- SN:
-
Substantia nigra
- SNc:
-
Substantia nigra pars compacta
- SNr:
-
Substantia nigra pars reticulata
- Str:
-
Striatum
- TCx:
-
Temporal and entorhinal cortex
- Th:
-
Thalamus
- TH:
-
Tyrosine hydroxylase
- TRP:
-
Tryptophan
- TrpH:
-
Tryptophan hydroxylase
- ThV:
-
Ventral thalamus
- Tu:
-
Olfactory tubercle
- VP:
-
Ventral pallidum
- VTA:
-
Ventral tegmental area
- UFPM:
-
Ultrafine particle matter
References
Alex KD, Pehek EA (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113:296–320. https://doi.org/10.1016/j.pharmthera.2006.08.004
Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z et al (2018) Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 67(6):1–23. https://doi.org/10.15585/mmwr.ss6706a1
Bernal-Meléndez E, Lacroix M, Bouillaud P et al (2019) Repeated gestational exposure to diesel engine exhaust affects the fetal olfactory system and alters olfactory-based behavior in rabbit offspring. Part Fibre Toxicol 16(5):1–17. https://doi.org/10.1186/s12989-018-0288-7
Block ML, Wu X, Pei Z et al (2004) Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J 18(13):1618–1620. https://doi.org/10.1096/fj.04-1945fje
Bolton JL, Huff NC, Smith SH et al (2013) Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect 121(9):1075–1082. https://doi.org/10.1289/ehp.1306560
Bolton JL, Marinero S, Hassanzadeh T et al (2017) Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front Synapt Neurosci 9:1–16. https://doi.org/10.3389/fnsyn.2017.00010
Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C et al (2008a) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36(2):289–310. https://doi.org/10.1177/0192623307313011
Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E et al (2008b) Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn 68(2):117–127. https://doi.org/10.1016/j.bandc.2008.04.008
Calderón-Garcidueñas L, Gónzalez-Maciel A, Reynoso-Robles R et al (2018) Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤ 40 years of age. Environ Res 164:475–487. https://doi.org/10.1016/j.envres.2018.03.023
Calderón-Garcidueñas L, Torres-jardón R, Kulesza RJ et al (2020a) Alzheimer disease starts in childhood in polluted Metropolitan Mexico City. A major health crisis in progress. Environ Res. https://doi.org/10.1016/j.envres.2020.109137
Calderón-Garcidueñas L, Herrera-soto A, Jury N et al (2020b) Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution. Environ Res 183:109226. https://doi.org/10.1016/j.envres.2020.109226
Cansler HL, Wright KN, Stetzik LA, Wesson DW (2020) Neurochemical organization of the ventral striatum’s olfactory tubercle. J Neurochem. https://doi.org/10.1111/jnc.14919
Cave J, Baker H (2009) Dopamine systems in the forebrain. Adv Exp Med Biol 651:15–35. https://doi.org/10.1007/978-1-4419-0322-8_2
Chang YC, Daza R, Hevner R et al (2019) Prenatal and early life diesel exhaust exposure disrupts cortical lamina organization: evidence for a reelin-related pathogenic pathway induced by interleukin-6. Brain Behav Immun 78:105–115. https://doi.org/10.1016/j.bbi.2019.01.013
Chavatte-Palmer P, Tarrade A (2016) Placentation in different mammalian species. Annales D’endocrinologie 77(2):67–74. https://doi.org/10.1016/j.ando.2016.04.006
Chiu YHM, Hsu HHL, Coull BA et al (2016) Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int 87:56–65. https://doi.org/10.1016/j.envint.2015.11.010
Chung WCJ, Auger AP (2013) Gender differences in neurodevelopment and epigenetics. Pflug Arch/eur Physiol 465:573–584. https://doi.org/10.1007/s00424-013-1258-4
Costa LG, Cole TB, Dao K et al (2020) Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol Ther 107523:1–8. https://doi.org/10.1016/j.pharmthera.2020.107523
Di Matteo V, Pierucci M, Esposito E et al (2008) Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson’s disease and other motor disorders. Prog Brain Res 172(08):423–463. https://doi.org/10.1016/S0079-6123(08)00921-7
Dos Santos CR, Granon S (2012) Prefrontal neuromodulation by nicotinic receptors for cognitive processes. Psychopharmacology 221(1):1–18. https://doi.org/10.1007/s00213-011-2596-6
Doty RL (2012) Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis 46(3):527–552. https://doi.org/10.1016/j.nbd.2011.10.026
Dumont M, Lalonde R, Ghersi-Egea JF et al (2006) Regional acetylcholinesterase activity and its correlation with behavioral performances in 15-month old transgenic mice expressing the human C99 fragment of APP. J Neural Transm 113(9):1225–1241. https://doi.org/10.1007/s00702-005-0373-6
Ehsanifar M, Jonidi A, Nikzad H et al (2019) Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol Environ Saf 176:34–41. https://doi.org/10.1016/j.ecoenv.2019.03.090
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Fischer B, Chavatte-Palmer P, Viebahn C et al (2012) Rabbit as a reproductive model for human health. Reproduction 144(1):1–10. https://doi.org/10.1530/REP-12-0091
Gamache P, Ryan E, Svendsen C et al (1993) Simultaneous measurement of monoamines, metabolites and amino acids in brain tissue and microdialysis perfusates. J Chromatogr Biomed Sci Appl 614(2):213–220. https://doi.org/10.1016/0378-4347(93)80311-q
Gonzalez-Pina R, Escalante-Membrillo C, Alfaro-Rodriguez A, Gonzalez-Maciel A (2008) Prenatal exposure to ozone disrupts cerebellar monoamine contents in newborn rats. Neurochem Res 33(5):912–918. https://doi.org/10.1007/s11064-007-9534-3
Gordon MN, Finch CE (1984) Topochemical localization of choline acetyltransferase and acetylcholinesterase in mouse brain. Brain Res 308(2):364–368. https://doi.org/10.1016/0006-8993(84)91079-5
Guxens M, Lubczyńska MJ, Muetzel RL et al (2018) Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiat 84(4):295–303. https://doi.org/10.1016/j.biopsych.2018.01.016
Haghani A, Johnson RG, Woodward NC et al (2020) Adult mouse hippocampal transcriptome changes associated with long-term behavioral and metabolic effects of gestational air pollution toxicity. Transl Psychiatry 10(1):218. https://doi.org/10.1038/s41398-020-00907-1
Harris MH, Gold DR, Rifas-Shiman SL et al (2016) Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicol Teratol 57:60–70. https://doi.org/10.1016/j.ntt.2016.06.008
Harvey JD, Heinbockel T (2018) Neuromodulation of synaptic transmission in the main olfactory bulb. Int J Environ Res Public Health 15(10):2194. https://doi.org/10.3390/ijerph15102194
Heusinkveld HJ, Wahle T, Campbell A et al (2016) Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 56:94–106. https://doi.org/10.1016/j.neuro.2016.07.007
Höglinger GU, Fischer DA, Carrión OA et al (2015) A new dopaminergic nigro-olfactory projection. Acta Neuropathol 130(3):333–348. https://doi.org/10.1007/s00401-015-1451-y
Hougaard KS, Campagnolo L, Chavatte-Palmer P et al (2015) A perspective on the developmental toxicity of inhaled nanoparticles. Reprod Toxicol 56:118–140. https://doi.org/10.1016/j.reprotox.2015.05.015
Kadohisa M (2013) Effects of odor on emotion, with implications. Front Syst Neurosci 10(7):66. https://doi.org/10.3389/fnsys.2013.00066
Kema IP, Schellings AMJ, Hoppenbrouwers CJM et al (1993) High performance liquid chromatographic profiling of tryptophan and related indoles in body fluids and tissues of carcinoid patients. Clin Chim Acta 221(1–2):143–158. https://doi.org/10.1016/0009-8981(93)90029-4
Klocke C, Allen JL, Sobolewski M et al (2017) Neuropathological consequences of gestational exposure to concentrated ambient fine and ultrafine particles in the mouse. Toxicol Sci 156(2):492–508. https://doi.org/10.1093/toxsci/kfx010
Li W, Lopez L, Osher J et al (2010) Right orbitofrontal cortex mediates conscious olfactory perception. Psychol Sci 21(10):1454–1463. https://doi.org/10.1177/0956797610382121
Lizbinski KM, Dacks AM (2018) Intrinsic and extrinsic neuromodulation of olfactory processing. Front Cell Neurosci 11:424. https://doi.org/10.3389/fncel.2017.00424
Lucas G, De Deurwaerde P, Porras G, Spampinato U (2000) Endogenous serotonin enhances the release of dopamine in the striatum only when nigro-striatal dopaminergic transmission is activated. Neuropharmacology 39(11):1984–1995. https://doi.org/10.1016/s0028-3908(00)00020-4
Mundiñano IC, Caballero MC, Ordóñez C et al (2011) Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol 122(1):61–74. https://doi.org/10.1007/s00401-011-0830-2
Nway NC, Fujitani Y, Hirano S et al (2017) Role of TLR4 in olfactory-based spatial learning activity of neonatal mice after developmental exposure to diesel exhaust origin secondary organic aerosol. Neurotoxicology 63:155–165. https://doi.org/10.1016/j.neuro.2017.10.001
Patten KT, González EA, Valenzuela A et al (2020) Effects of early life exposure to traffic-related air pollution on brain development in juvenile Sprague-Dawley rats. Transl Psychiatry 10:166–177. https://doi.org/10.1038/s41398-020-0845-3
Sobolewski M, Anderson T, Conrad K et al (2018) Developmental exposures to ultrafine particle air pollution reduces early testosterone levels and adult male social novelty preference: risk for children’s sex-biased neurobehavioral disorders. Neurotoxicology 68:203–211. https://doi.org/10.1016/j.neuro.2018.08.009
Steinfeld R, Herb JT, Sprengel R et al (2015) Divergent innervation of the olfactory bulb by distinct raphe nuclei. J Comp Neurol 523(5):805–813. https://doi.org/10.1002/cne.23713
Suades-González E, Gascon M, Guxens M, Sunyer J (2015) Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology 156(10):3473–3482. https://doi.org/10.1210/en.2015-1403
Sugamata M, Ihara T, Sugamata M, Takeda K (2006a) Maternal exposure to diesel exhaust leads to pathological similarity to autism in newborns. J Health Sci 52(4):486–488. https://doi.org/10.1248/jhs.52.486
Sugamata M, Ihara T, Takano H et al (2006b) Maternal diesel exhaust exposure damages newborn murine brains. J Health Sci 52(1):82–84. https://doi.org/10.1248/jhs.52.82
Suzuki T, Oshio S, Iwata M et al (2010) In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part Fibre Toxicol 7(1):7. https://doi.org/10.1186/1743-8977-7-7
Tabatabaie SRF, Mehdiabadi B, Bakhtiari NM, Tabandeh MR (2017) Silver nanoparticle exposure in pregnant rats increases gene expression of tyrosine hydroxylase and monoamine oxidase in offspring brain. Drug Chem Toxicol 40(4):440–447. https://doi.org/10.1080/01480545.2016.1255952
Tachibana K, Takayanagi K, Akimoto A et al (2015) Prenatal diesel exhaust exposure disrupts the DNA methylation profile in the brain of mouse offspring. J Toxicol Sci 40(1):1–11. https://doi.org/10.2131/jts.40.1
Takahashi Y, Mizuo K, Shinkai Y et al (2010) Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostriatum of mice. J Toxicol Sci 35(5):749–756. https://doi.org/10.2131/jts.35.749
Turano A, Osborne BF, Schwarz JM (2018) Sexual differentiation and sex differences in neural development. Neuroendocr Regul Beh 43:69–110. https://doi.org/10.1007/7854_2018_56
Valentino SA, Tarrade A, Aioun J et al (2016) Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits. Part Fibre Toxicol 13:39. https://doi.org/10.1186/s12989-016-0151-7
Yokota S, Mizuo K, Moriya N et al (2009) Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neurosci Lett 449(1):38–41. https://doi.org/10.1016/j.neulet.2008.09.085
Yokota S, Moriya N, Iwata M et al (2013) Exposure to diesel exhaust during fetal period affects behavior and neurotransmitters in male offspring mice. J Toxicol Sci 38(1):13–23. https://doi.org/10.2131/jts.38.13
Yokota S, Sato A, Umezawa M et al (2015) In utero exposure of mice to diesel exhaust particles affects spatial learning and memory with reduced N-methyl-d-aspartate receptor expression in the hippocampus of male offspring. Neurotoxicology 50:108–115. https://doi.org/10.1016/j.neuro.2015.08.009
Yokota S, Oshio S, Moriya N, Takeda K (2016a) Social isolation-induced territorial aggression in male offspring is enhanced by exposure to diesel exhaust during pregnancy. PLoS ONE 11(2):1–15. https://doi.org/10.1371/journal.pone.0149737
Yokota S, Oshio S, Takeda K (2016b) In utero exposure to diesel exhaust particles induces anxiogenic effects on male offspring via chronic activation of serotonergic neuron in dorsal raphe nucleus. J Toxicol Sci 41(5):583–593. https://doi.org/10.2131/jts.41.583
Acknowledgements
We particularly thank Pr. S. Granon (University Paris-Saclay) for her interest in this work and for the critical reading of the manuscript.
Funding
The study was supported by a doctoral fellowship to EBM, given by ADEME and ANSES (PNREST 2014/1/190). Production of exposed and control animals was supported by ANR grant ANR-13-CESA-0011-EPAPP and ERC consolidator grant N°311765–E-DOHaD.
Author information
Authors and Affiliations
Contributions
Henri Schroeder, Christine Baly and Pascale Chavatte-Palmer designed the study. Estefania Bernal-Meléndez, Pascaline Bouillaud, Marie-Annick Persuy and Karine Badonnel performed the dissection of brain tissues. Jacques Callebert performed the monoaminergic neurochemical analysis by HPLC. Estefania Bernal-Meléndez, Pascaline Bouillaud and Benoit Olivier performed the immunohistochemistry experiments. Estefania Bernal-Meléndez performed the statistical analyses. Christine Baly and Estefania Bernal-Meléndez wrote the draft manuscript, which was critically read and approved by all the authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Animals were treated according to the ethical standards defined by the National Institute for Agronomic Research (INRA) for animal health and care with strict compliance with the EEC recommendations (no. 86/609) and the 2010/63/EU directive on the protection of animals used for scientific purposes. The local ethical committee (N°45 in the French National register) approved the experimentation under N°12/102.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bernal-Meléndez, E., Callebert, J., Bouillaud, P. et al. Dopaminergic and serotonergic changes in rabbit fetal brain upon repeated gestational exposure to diesel engine exhaust. Arch Toxicol 95, 3085–3099 (2021). https://doi.org/10.1007/s00204-021-03110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03110-3